Hydrometallurgical Process for Tantalum Recovery from Epoxy-Coated Solid Electrolyte Tantalum Capacitors
Abstract
:1. Introduction
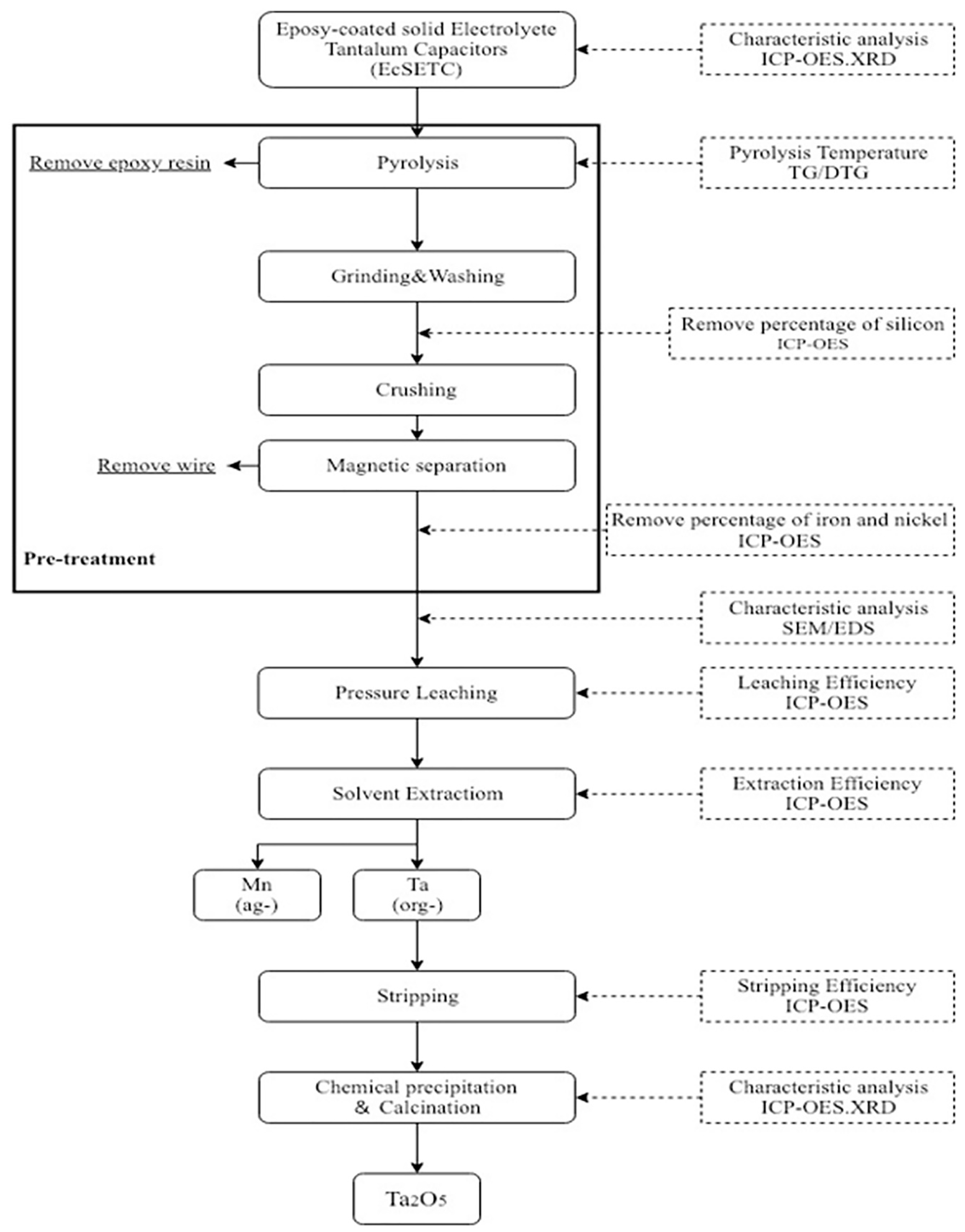
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
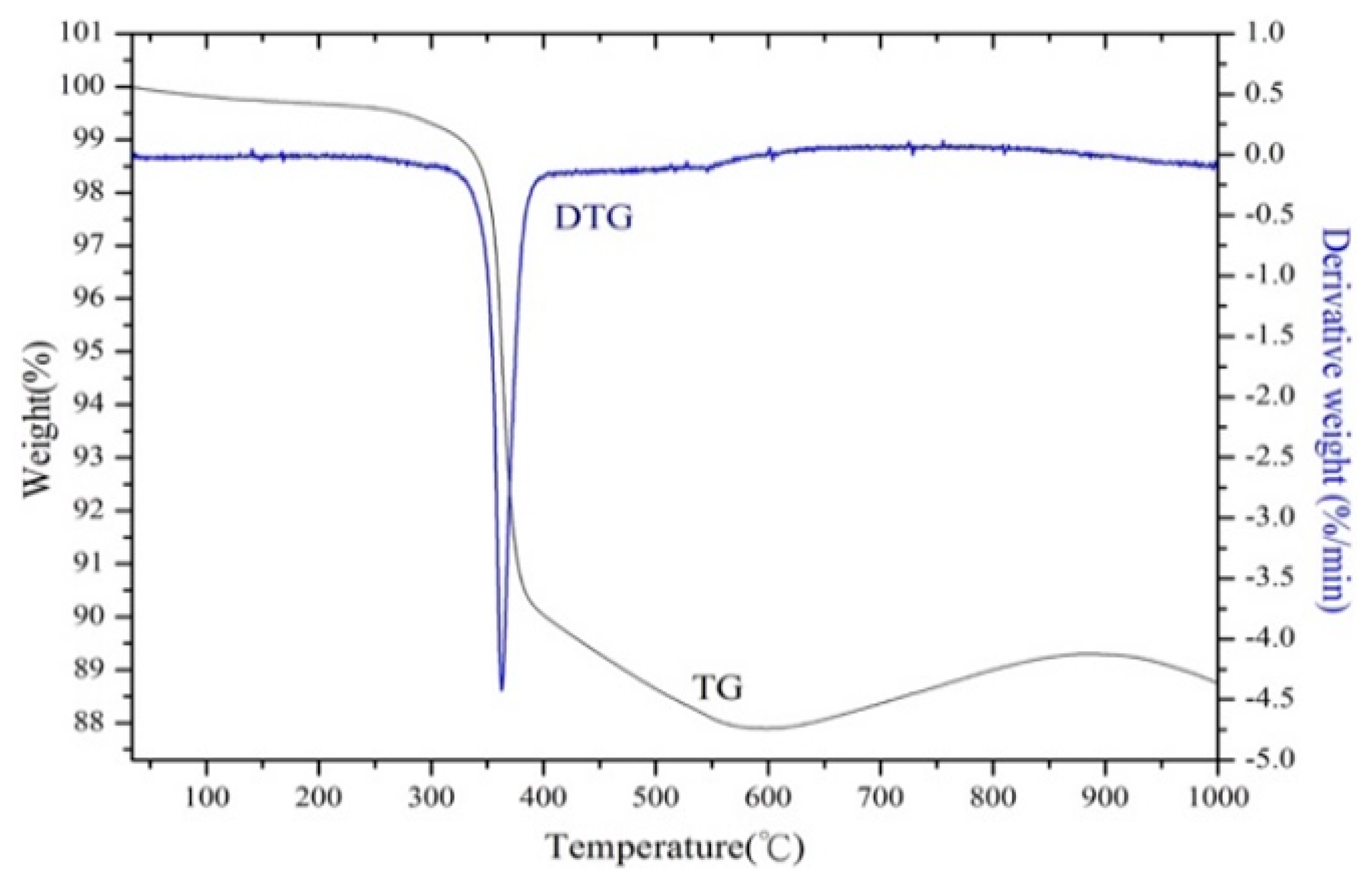
3.1. Pretreatment
3.2. Leaching
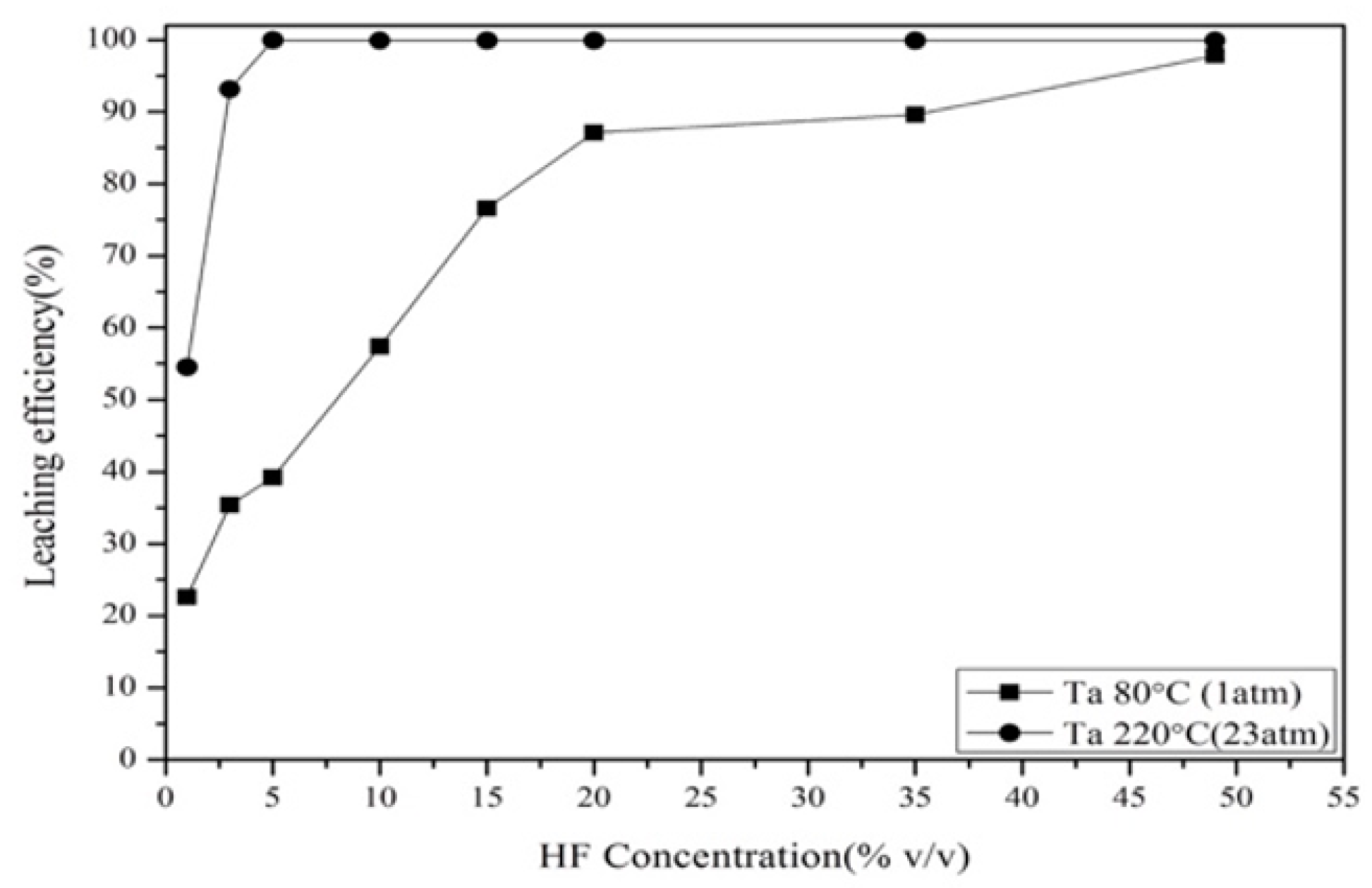
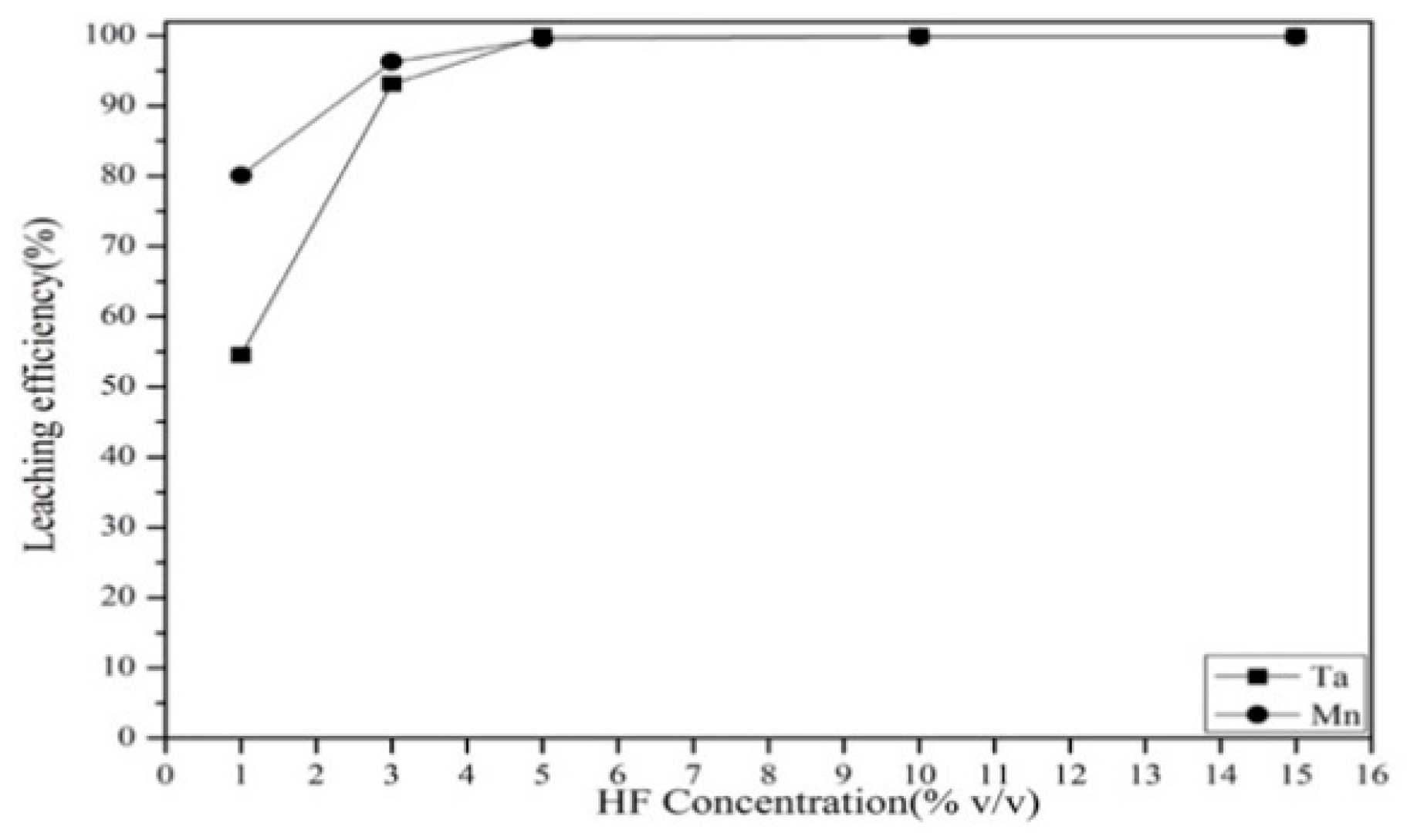
3.2.1. Effect of Hydrofluoric Acid Concentration
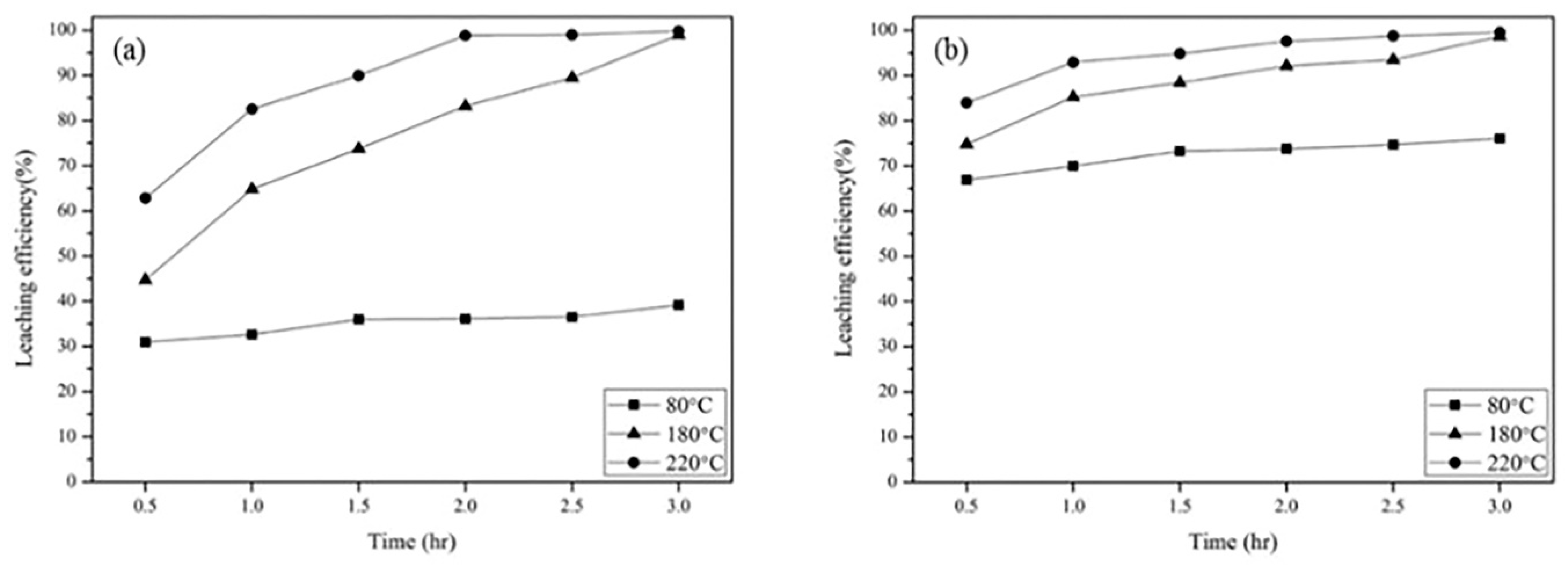
3.2.2. Effect of Temperature and Reaction Time
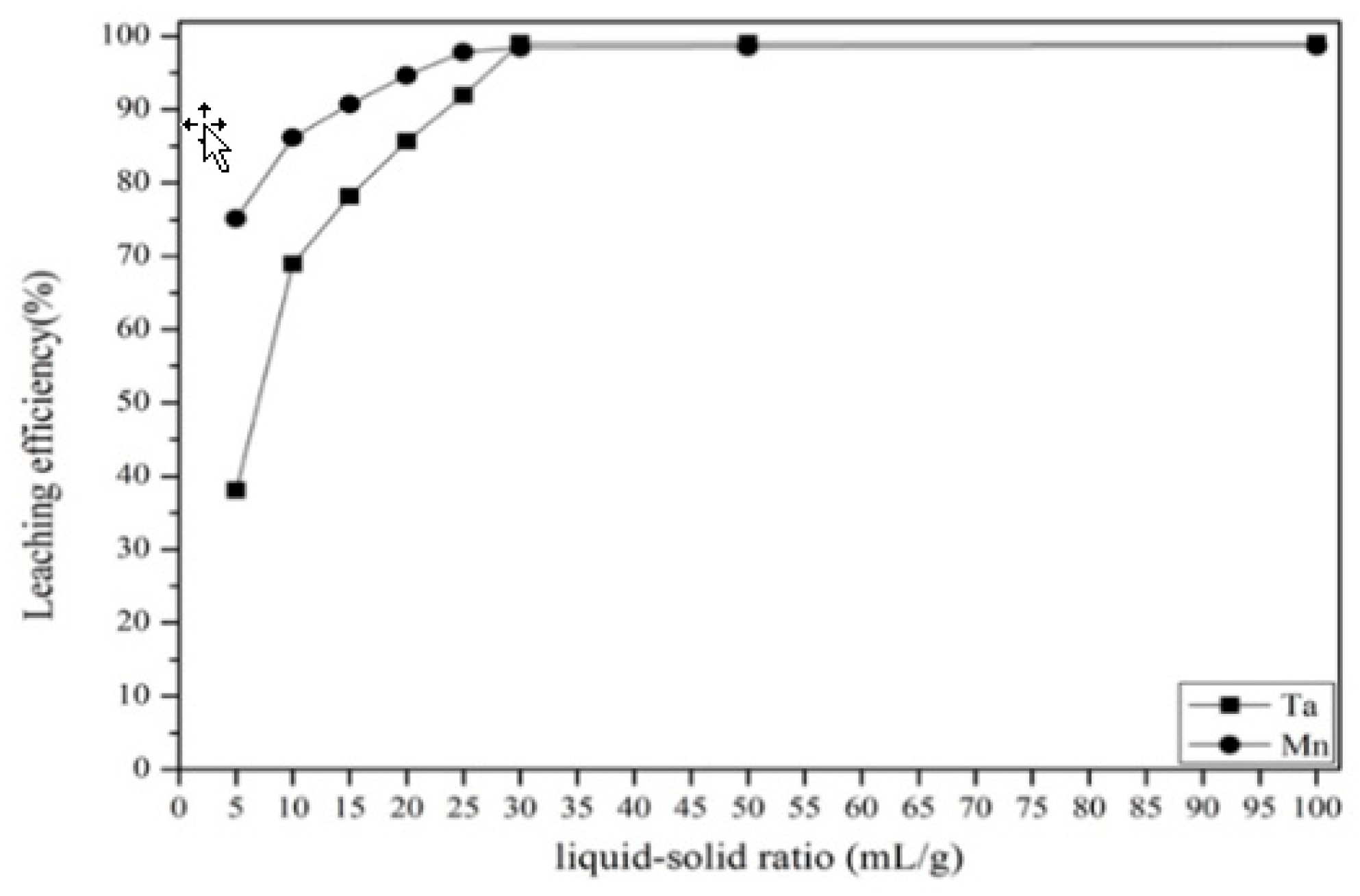
3.2.3. Effect of Liquid-to-Solid Ratio
3.3. Solvent Extraction and Stripping
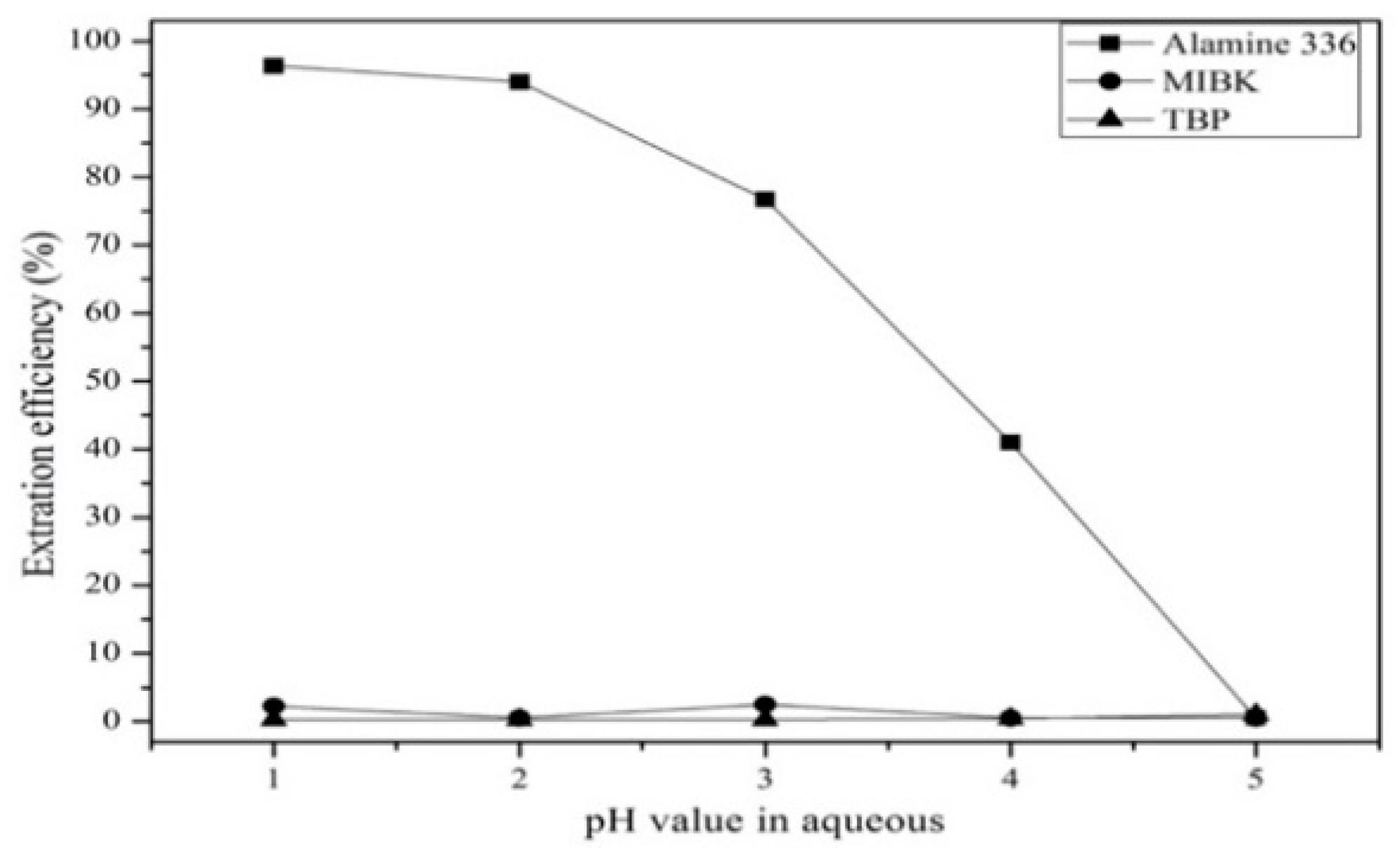
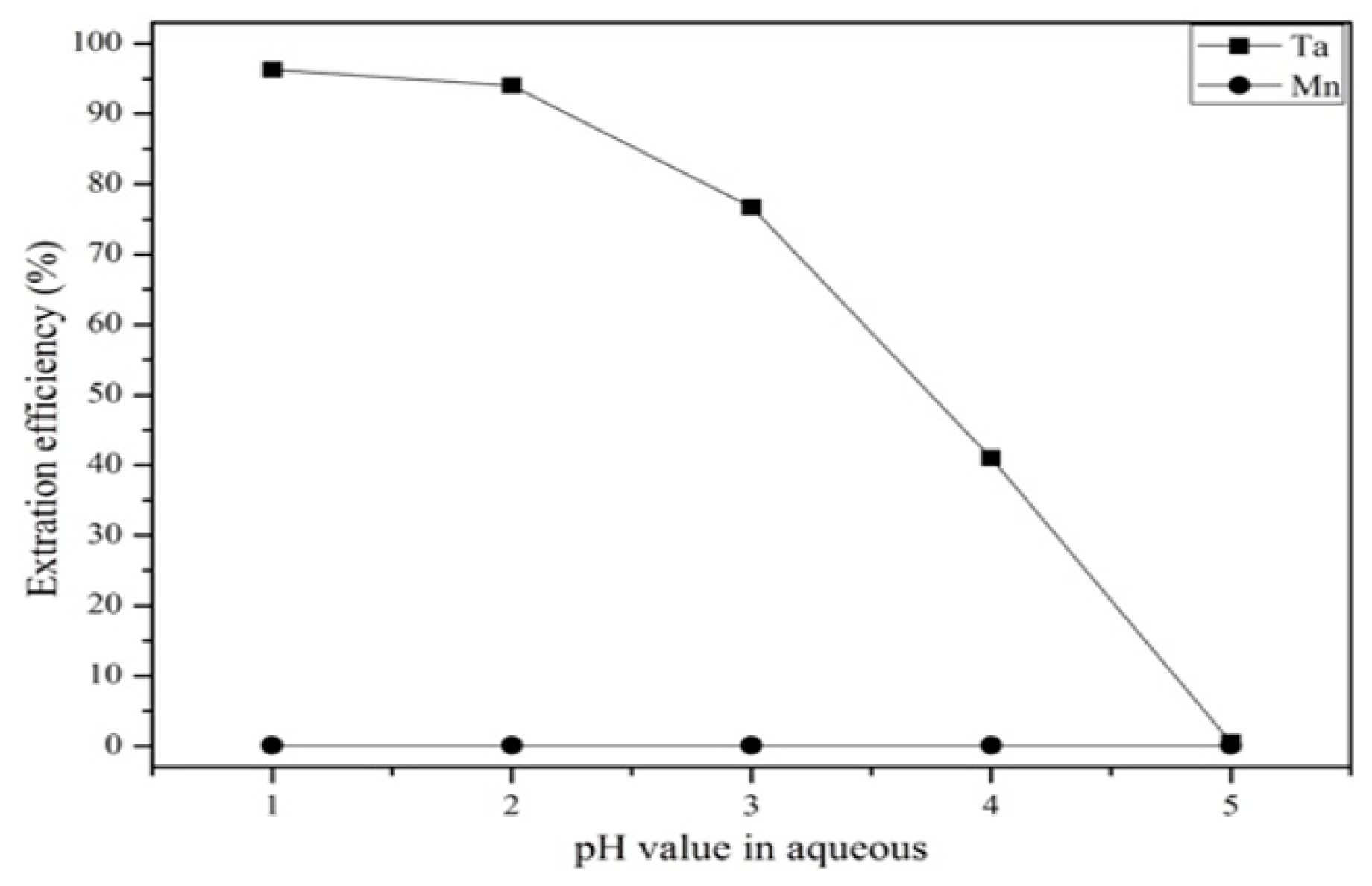
3.3.1. Comparison of Extractants and Effect of pH Value
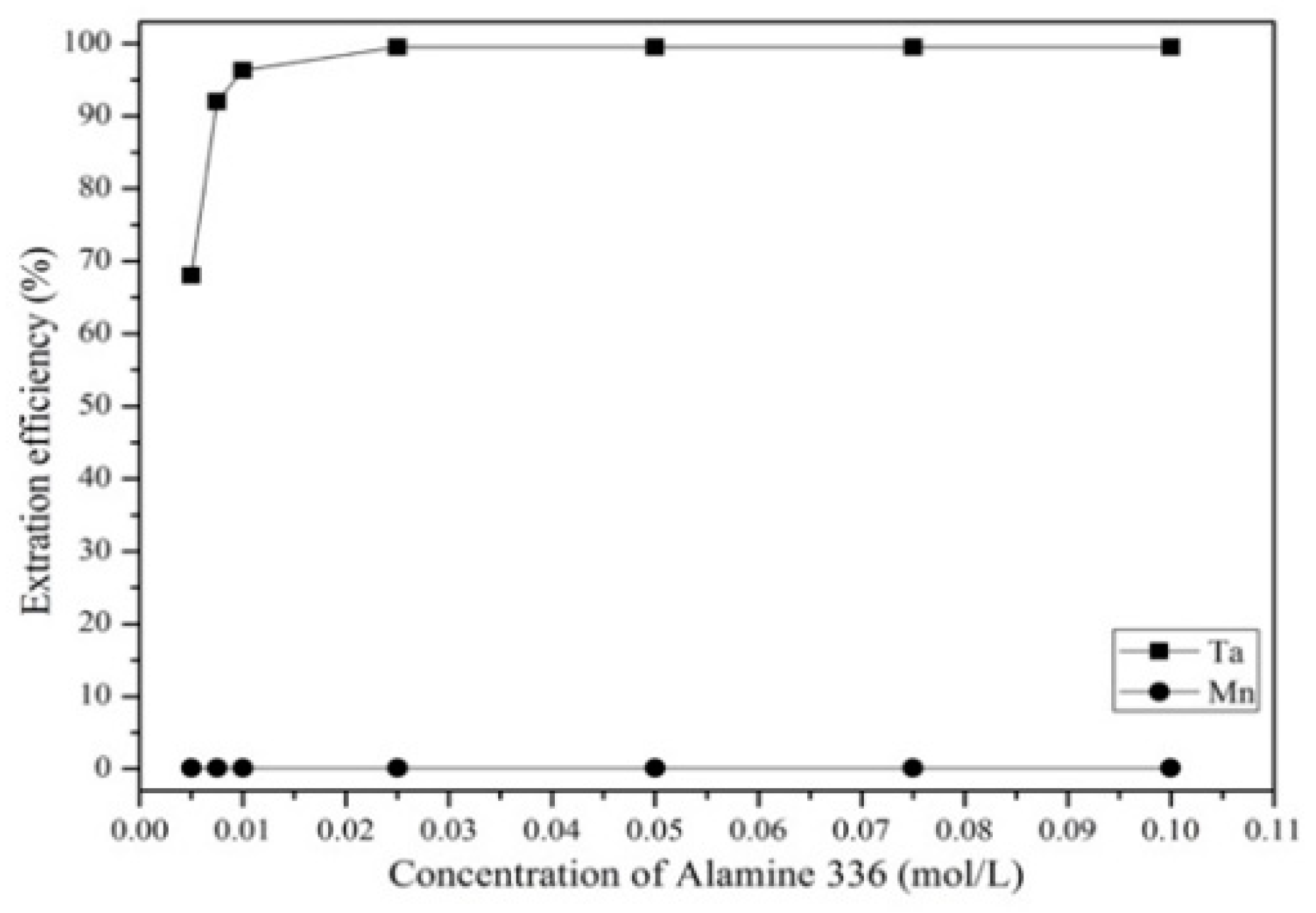
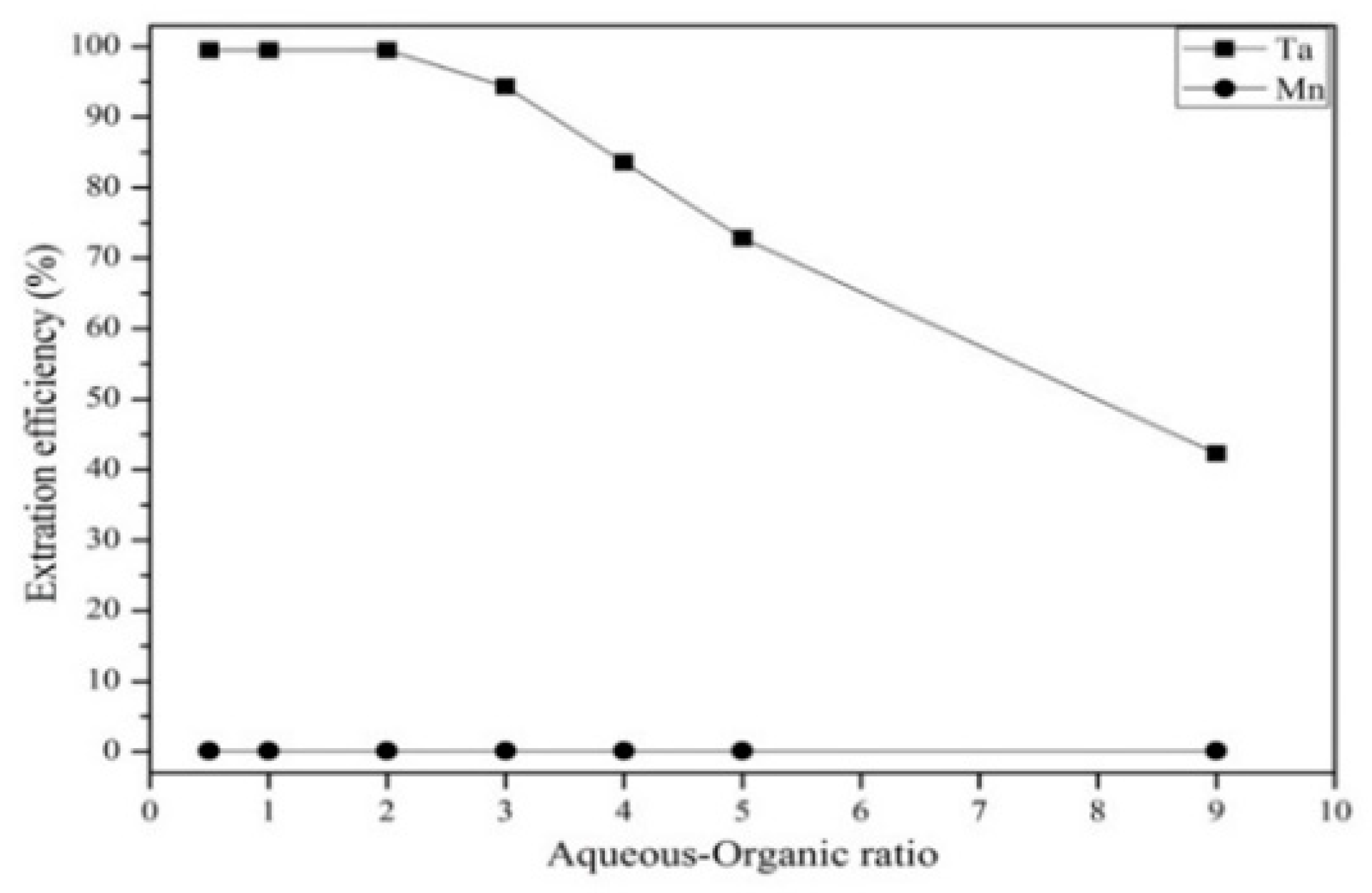
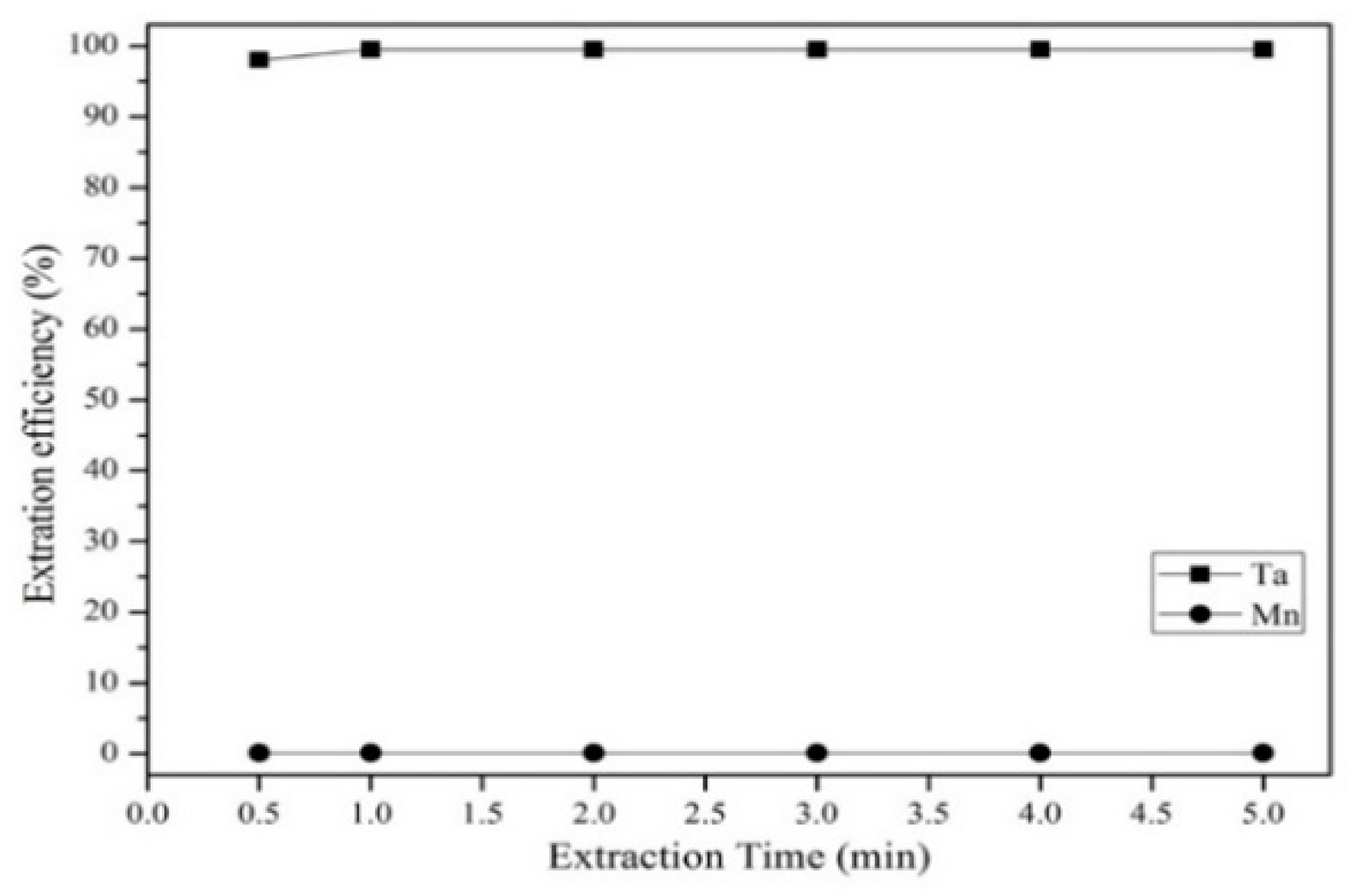
3.3.2. Effect of Extractant Concentration, Aqueous-to-Organic Ratio, and Extraction Time
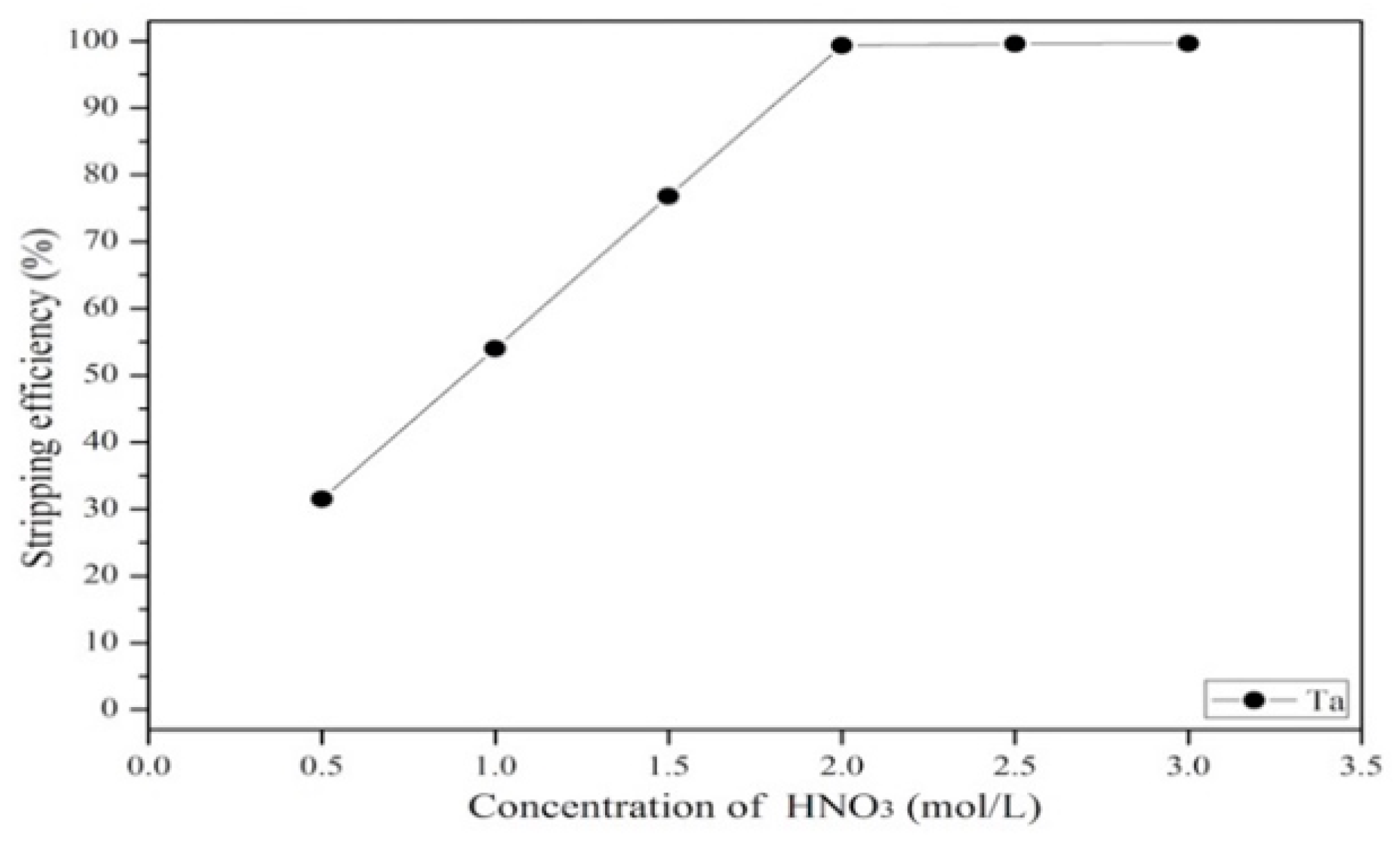
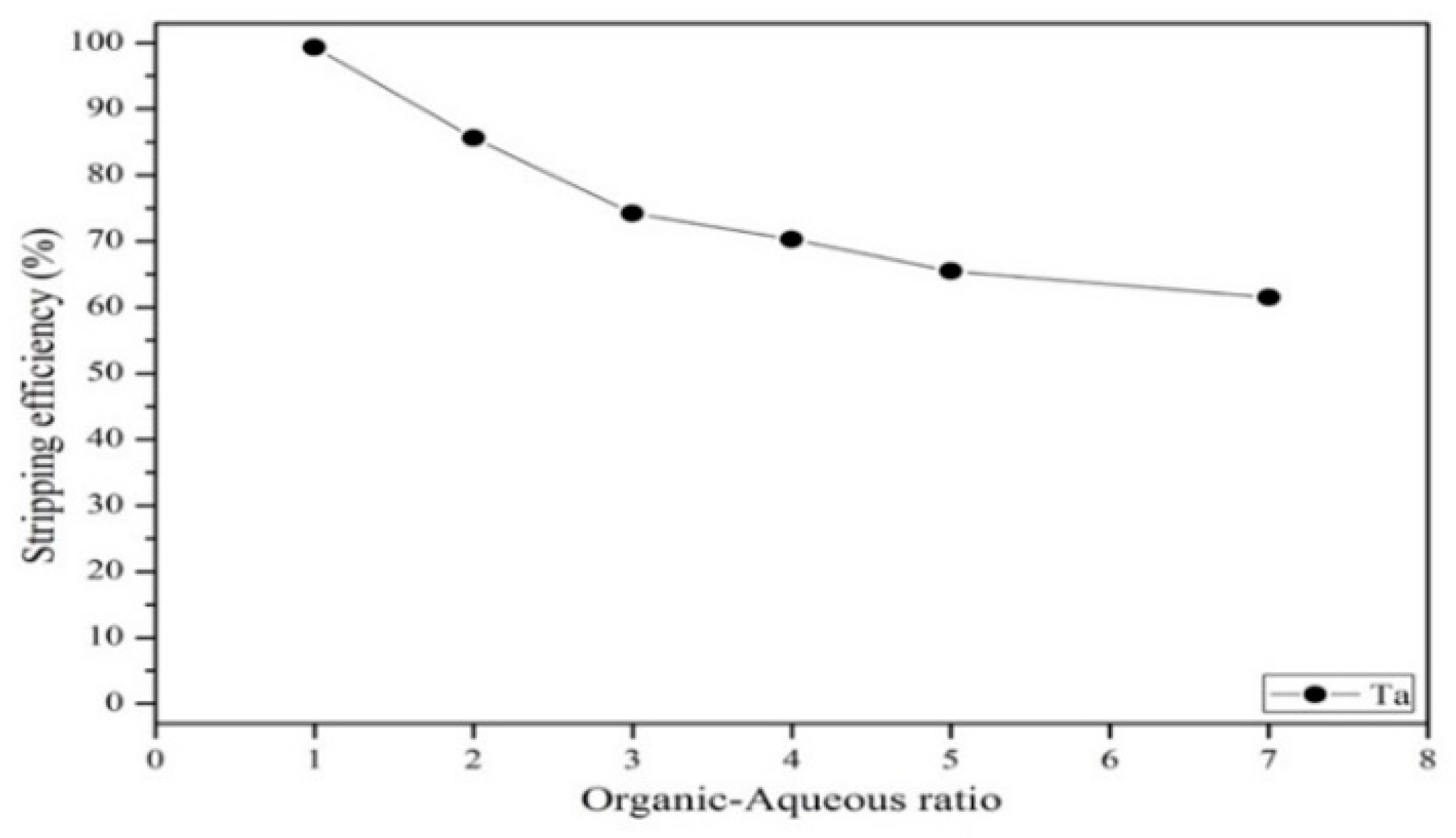
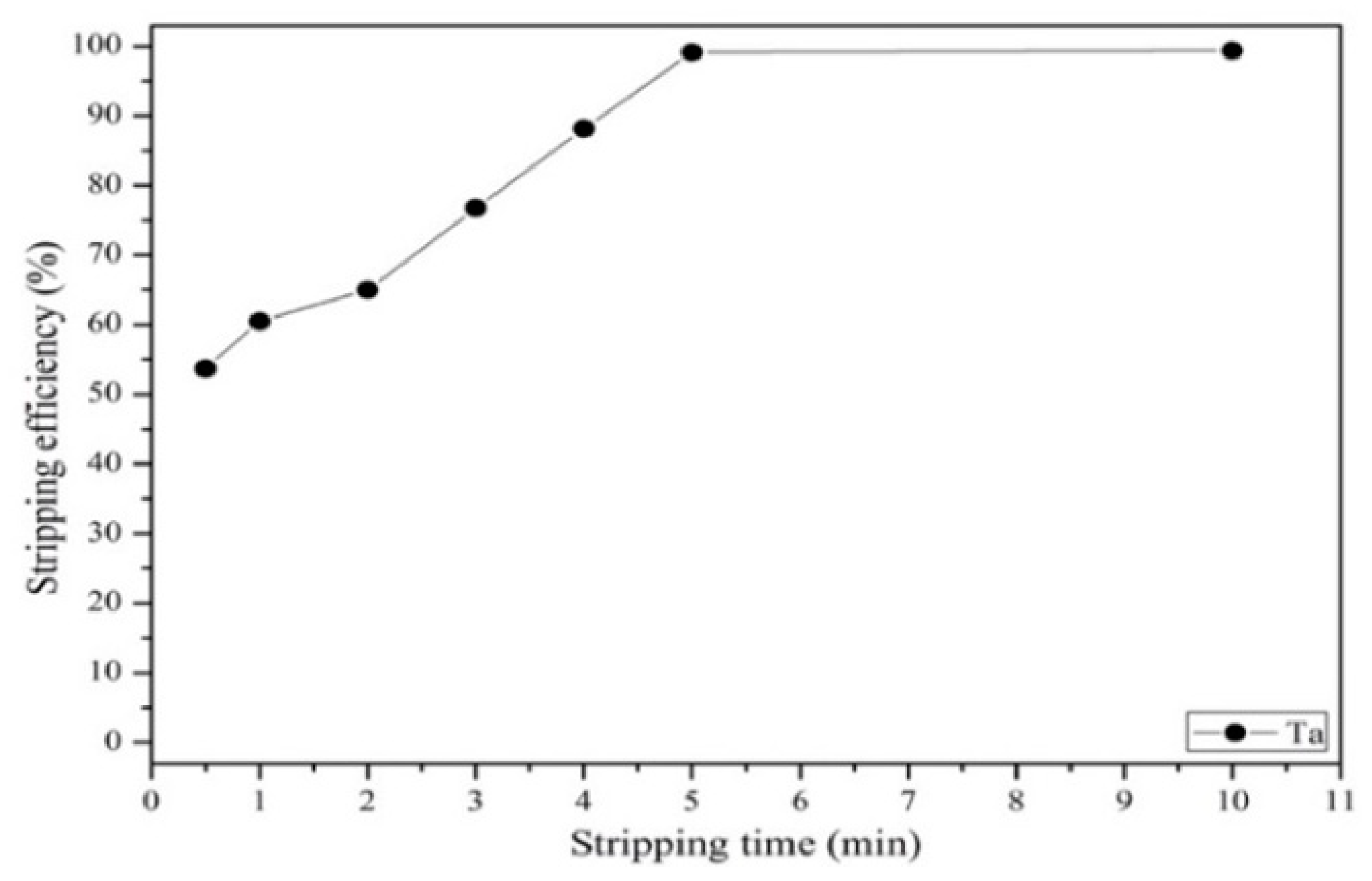
3.3.3. Effect of Stripping Efficiency on Agent Concentration, Organic-to-Aqueous Ratio and Stripping Time
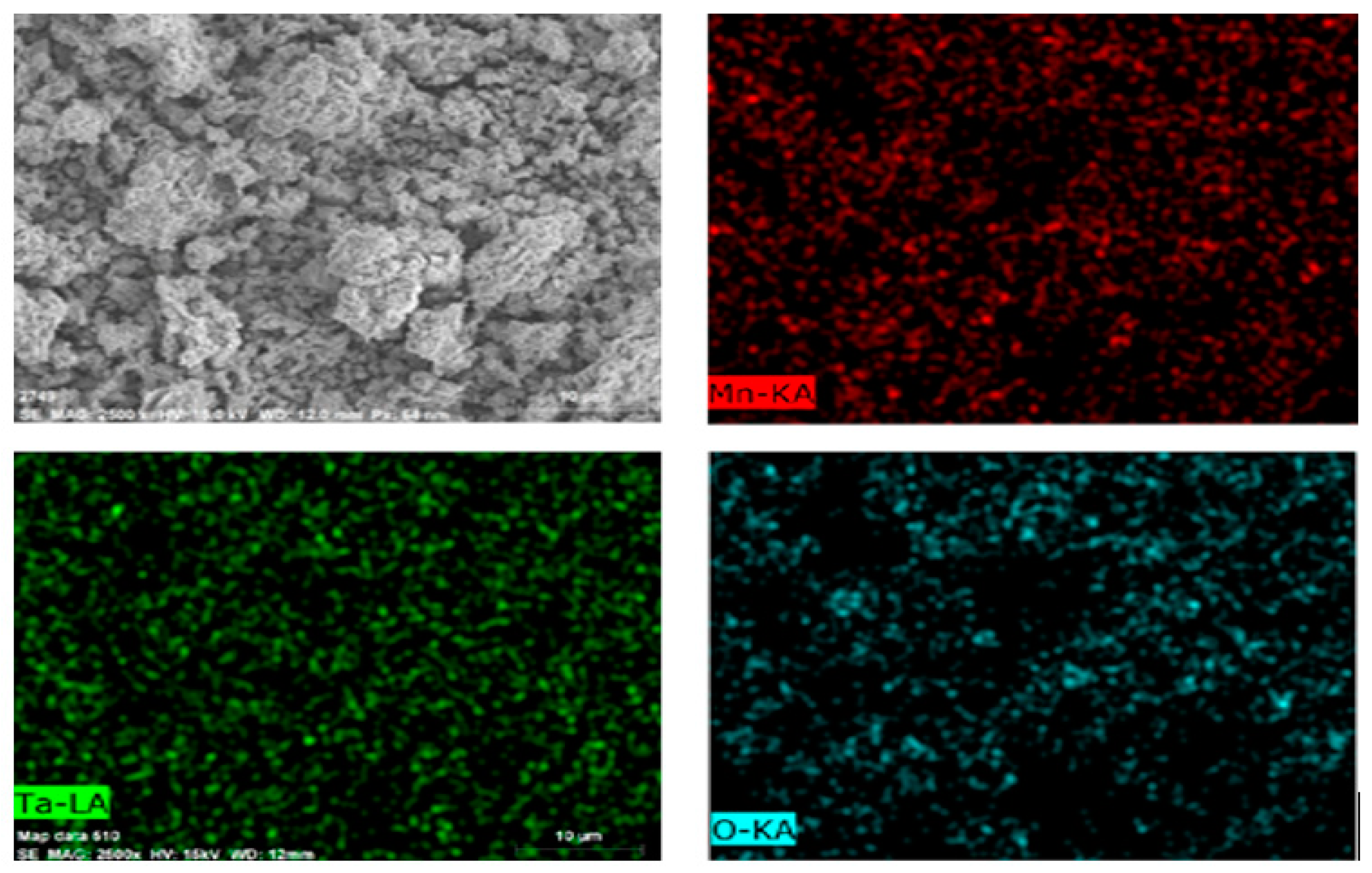
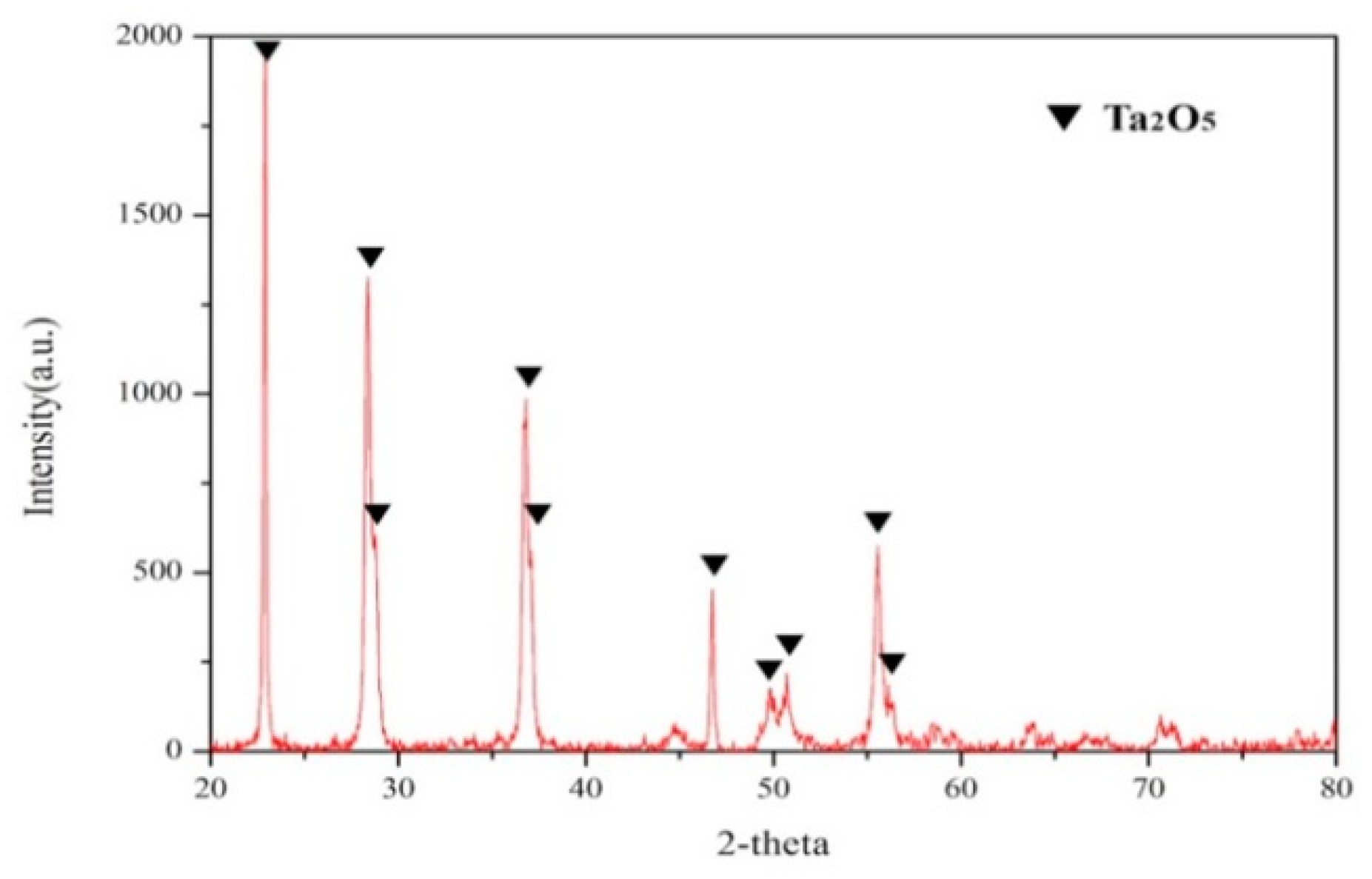
3.4. Chemical Precipitation and Calcination
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Much Ado about Tantalum-Again. Briefing Paper for TIC. Available online: http://www.tanb.org/images/Much%20ado%20about%20tantalum.pdf (accessed on 28 March 2019).
- Startton, P.; Henderson, D. Outlook for the Global Tantalum Market. In Proceedings of the 2nd International Tin & Tantalum Seminar, New York, NY, USA, 11 December 2013. [Google Scholar]
- European Commission. Critical Raw Materials for the EU: Report of the Ad-Hoc Working Group on Defining Critical Raw Materials. Available online: https://ec.europa.eu/growth/tools-databases/eip-raw-materials/en/system/files/ged/79%20report-b_en.pdf (accessed on 28 March 2019).
- Yen, F.C.; Chang, T.C.; Xu, W.H. Substance Flow Analysis of Tantalum in Taiwan. Mater. Trans. 2016, 57, 613–617. [Google Scholar] [Green Version]
- Okabe, T.H.; Sato, N.; Mitsuda, Y.; Ono, S. Production of tantalum powder by magnesiothermic reduction of feed preform. Mater. Trans. 2003, 44, 2646–2653. [Google Scholar] [CrossRef]
- Mineta, K.; Okabe, T.H. Development of a recycling process for tantalum from capacitor scraps. J. Phys. Chem. Solids. 2005, 66, 318–321. [Google Scholar] [CrossRef]
- Sasaki, M.; Ishikawa, S.; Nasu, K.; Quitain, A.T.; Goto, M. Recovery of tantalum from capacitor with solvothermal treatment. In Proceedings of the 6th International Symposium on Feedstock Recycling of Polymeric Materials, Toledo, Spain, 5–7 October 2011. [Google Scholar]
- Niu, B.; Chen, Z.; Xu, Z. Recovery of Tantalum from Waste Tantalum Capacitors by Supercritical Water Treatment. ACS Sustain. Chem. Eng. 2017, 5, 4421–4428. [Google Scholar] [CrossRef]
- Katano, S.; Wajima, T.; Nakagome, H. Recovery of Tantalum Sintered Compact from Used Tantalum Condenser Using Steam Gasification with Sodium Hydroxide. APCBEE Proc. 2014, 10, 182–186. [Google Scholar] [CrossRef] [Green Version]
- Hussaini, O.M.E.; Mahdy, M.A. Sulfuric acid leaching of Kab Amiri niobium–tantalum bearing minerals, Central Eastern Desert, Egypt. Hydrometallurgy 2002, 64, 219–229. [Google Scholar] [CrossRef]
- Wu, B.; Shang, H.; Wen, J.K. Sulfuric acid leaching of low-grade refractory tantalum–niobium and associated rare earths minerals in Panxi area of China. J. Rar. Met. 2015, 34, 202–206. [Google Scholar] [CrossRef]
- Awakura, Y.; Mashima, M.; Hirato, T. Dissolution of columbite and tantalite in acidic fluoride media. Metall. Trans. 1988, 19, 355–363. [Google Scholar]
- Li, S.; Zhou, Y.; Du, H.; Qian, G.; Goto, M. Development and Applying Technology of Niobium Resource; Metallurgical Industry Press: Beijing, China, 1992; 120p. (In Chinese) [Google Scholar]
- Krismer, B.; Hoppe, U.S. Process for Recovering Niobium and/or Tantalum Compounds from Such Ores Further Containing Complexes of Uranium, Thorium, Titanium and/or Rare Earth Metals. U.S. Patent No. 4,446,116, 1 May 1984. [Google Scholar]
- Wang, X.H.; Zheng, S.L.; Xu, H.B.; Zhang, Y. Leaching of niobium and tantalum from a low-grade ore using a KOH roast–water leach system. Hydrometallurgy 2009, 98, 219–223. [Google Scholar] [CrossRef]
- Rodriguez, M.H.; Rosales, G.D.; Pinna, E.G.; Suarez, D.S. Extraction of niobium and tantalum from ferrocolumbite by hydrofluoric acid pressure leaching. Hydrometallurgy 2015, 156, 17–20. [Google Scholar] [CrossRef]
- Roethe, G. Processing of Tantalum and Niobium Ores. In Lanthanides, Tantalum and Niobium; Springer: Berlin, Germany, 1989; pp. 331–341. [Google Scholar]
- Xie, J. A study on the mechanism of extraction of tantalum and niobium with MIBK in HF–H2SO4 system. Rare Met. 1988, 7, 213–214. [Google Scholar]
- Gupta, C.K.; Suri, A.K. Niobium and Tantalum Separation Process, Extractive Metallurgy of Niobium; CRC Press Inc.: Boca Raton, FL, USA, 1994. [Google Scholar]
- Salles, J.J.C. Production of niobium and tantalum from the Pitinga hard rock tin mine. TIC Bull. 2000, 101, 4–7. [Google Scholar]
- Hussaini, O.M.E.; Mahdy, M.A.; El, H. Extraction of niobium and tantalum from Nitrate and Sulphate media by using MIBK. Min. Process. Extract. Metall. 2001, 22, 633–650. [Google Scholar] [CrossRef]
- Okada, T. Manufacturing of Special Niobium Oxides for Optical and Ceramic Applications. In Proceedings of the International Symposium on Niobium, Orlando, FL, USA, 2–5 December 2001. [Google Scholar]
- Nikolaev, A.I.; Maiorov, V.G. New approaches to niobium and tantalum extraction technology. Dokl. Chem. 2007, 415, 167–169. [Google Scholar] [CrossRef]
- Nete, M.; Purcell, W.; Nel, J.T. Separation and isolation of tantalum and niobium from tantalite using solvent extraction and ion exchange. Hydrometallurgy 2014, 149, 31–40. [Google Scholar] [CrossRef]
- Sanda, O.; Taiwo, E.A. Solvent extraction of tantalum(V) from aqueous sulphate/fluoride solution using trioctyl phosphine oxide in MIBK. Hydrometallurgy 2012, 127–128, 168–171. [Google Scholar] [CrossRef]
- Nishimura, S.; Moriyama, J.; Kushima, I. Extraction and separation of tantalum and niobium by liquid–liquid extraction in the HF–H2SO4–TBP system. Trans. Jpn. Inst. Met. 1964, 5, 39–42. [Google Scholar] [CrossRef]
- Bhattacharyya, S.N.; Ganguly, B. Solvent extraction separation of niobium and tantalum. Solvent Extr. Ion Exch. 1984, 2, 699–740. [Google Scholar] [CrossRef]
- Han, J.; Zhou, Y. Development of Ta & Nb extraction and stripping technology and equipment. Rare Met. Cem. Carb. 2004, 32, 15–20. [Google Scholar]
- Markland, S.A. Separation of Niobium and Tantalum by Solvent Extraction with Tertiary Amines from Sulphuric Acid Solutions. Proc. Int. Solvent Extr. Conf. 1974, 3, 2185–2196. [Google Scholar]
- Feather, A.; Clark, P.; Boshoff, G. Production of High Purity Tantalum Oxide by Solvent Extraction. Proc. Int. Solvent Extr. Conf. 1999, 1, 789–794. [Google Scholar]
- Hussaini, O.M.E.; Rice, N.M. Liquid–liquid extraction of niobium and tantalum from aqueous sulphate/fluoride solutions by a tertiary amine. Hydrometallurgy 2004, 72, 259–267. [Google Scholar] [CrossRef]
- Zhu, Z.W.; Chu, Y.C. Solvent extraction technology for the separation and purification of niobium and tantalum: A review. Hydrometallurgy 2011, 107, 1–12. [Google Scholar] [CrossRef]
| Tantalum Electrode | Epoxy Resin | Wire |
|---|---|---|
| 54.8% | 38.2% | 7% |
| Element | Ta | Si | Mn | Fe | Ni |
|---|---|---|---|---|---|
| Wt.% | 40–42% | 6–7% | 6–8% | 3% | 4% |
| Table 3 | Si | Ta |
|---|---|---|
| Before | 400 mg/kg | 4500 mg/kg |
| After | 0.2 mg/kg | 4500 mg/kg |
| Remove percentage | 99.9% | 0% |
| Table 4 | Ni | Fe | Ta |
|---|---|---|---|
| Mag. | 99.5% | 99.5% | 0% |
| Non-Mag. | 0.5% | 0.5% | 100% |
| Removed percentage | 99.5% | 99.5% | 0% |
| Table 5 | Leaching Efficiency of Ta (%) |
|---|---|
| Hydrofluoric acid (HF) | 17.57% |
| Hydrochloric acid (HCl) | 0.13% |
| Sulfuric acid (H2SO4) | 0.01% |
| Nitric acid (HNO3) | 0.15% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-S.; Ho, H.-J.; Lin, K.-Y. Hydrometallurgical Process for Tantalum Recovery from Epoxy-Coated Solid Electrolyte Tantalum Capacitors. Materials 2019, 12, 1220. https://doi.org/10.3390/ma12081220
Chen W-S, Ho H-J, Lin K-Y. Hydrometallurgical Process for Tantalum Recovery from Epoxy-Coated Solid Electrolyte Tantalum Capacitors. Materials. 2019; 12(8):1220. https://doi.org/10.3390/ma12081220
Chicago/Turabian StyleChen, Wei-Sheng, Hsing-Jung Ho, and Kuan-Yan Lin. 2019. "Hydrometallurgical Process for Tantalum Recovery from Epoxy-Coated Solid Electrolyte Tantalum Capacitors" Materials 12, no. 8: 1220. https://doi.org/10.3390/ma12081220
APA StyleChen, W.-S., Ho, H.-J., & Lin, K.-Y. (2019). Hydrometallurgical Process for Tantalum Recovery from Epoxy-Coated Solid Electrolyte Tantalum Capacitors. Materials, 12(8), 1220. https://doi.org/10.3390/ma12081220







