An Efficient Leaching of Palladium from Spent Catalysts through Oxidation with Fe(III)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
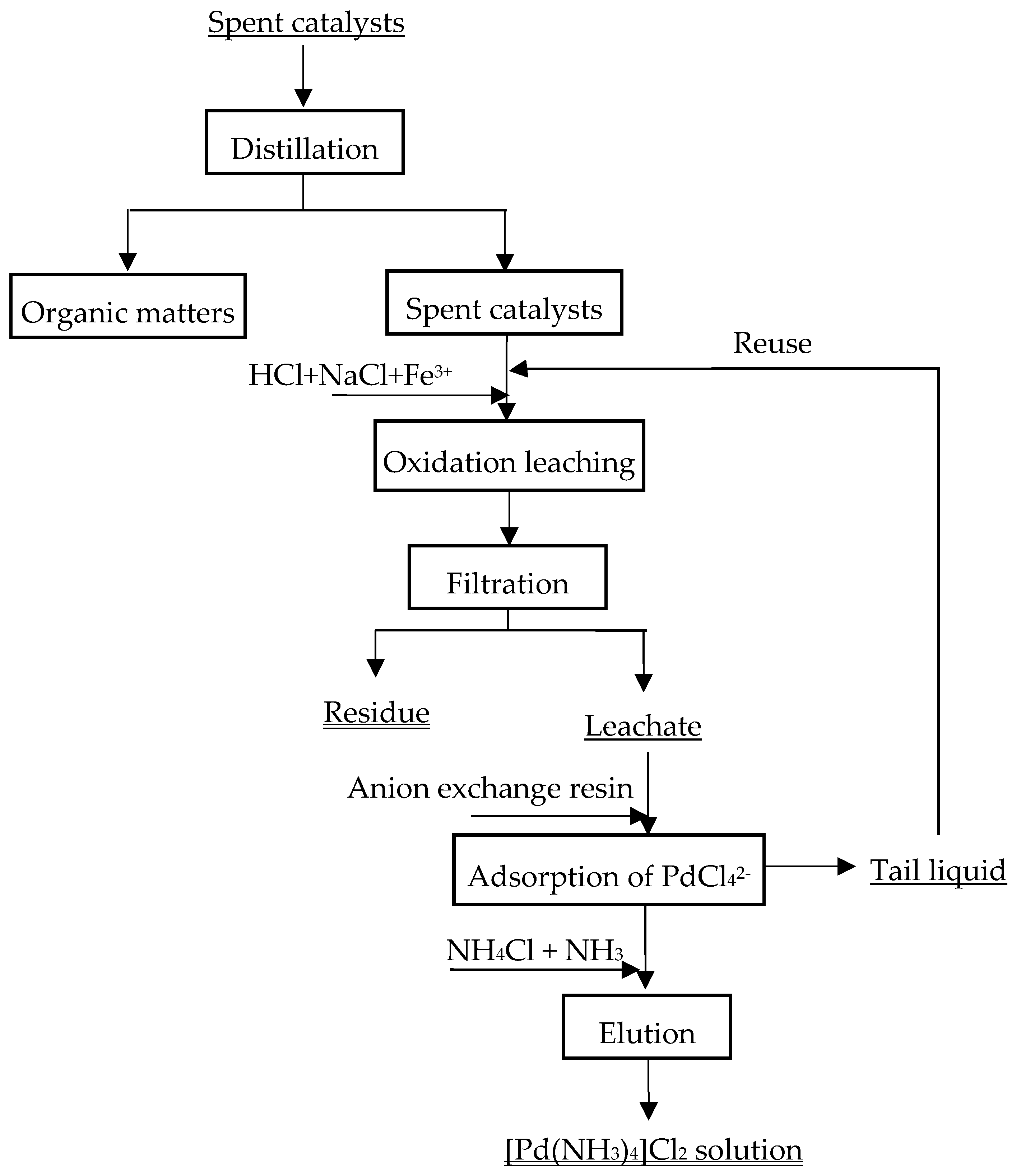
2.2. Experimental Procedures
2.3. Chemical Analysis and Characterization
3. Results and Discussion
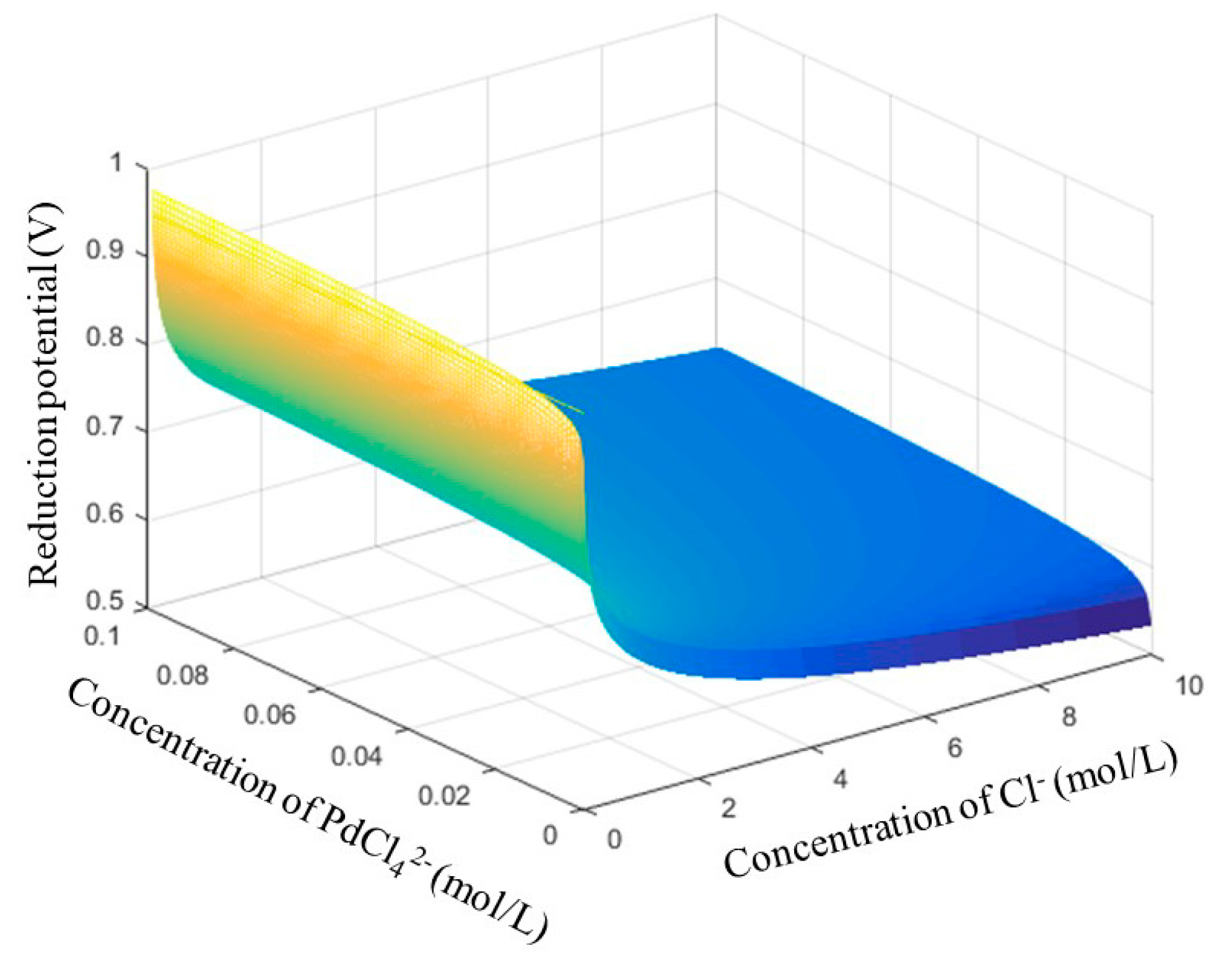
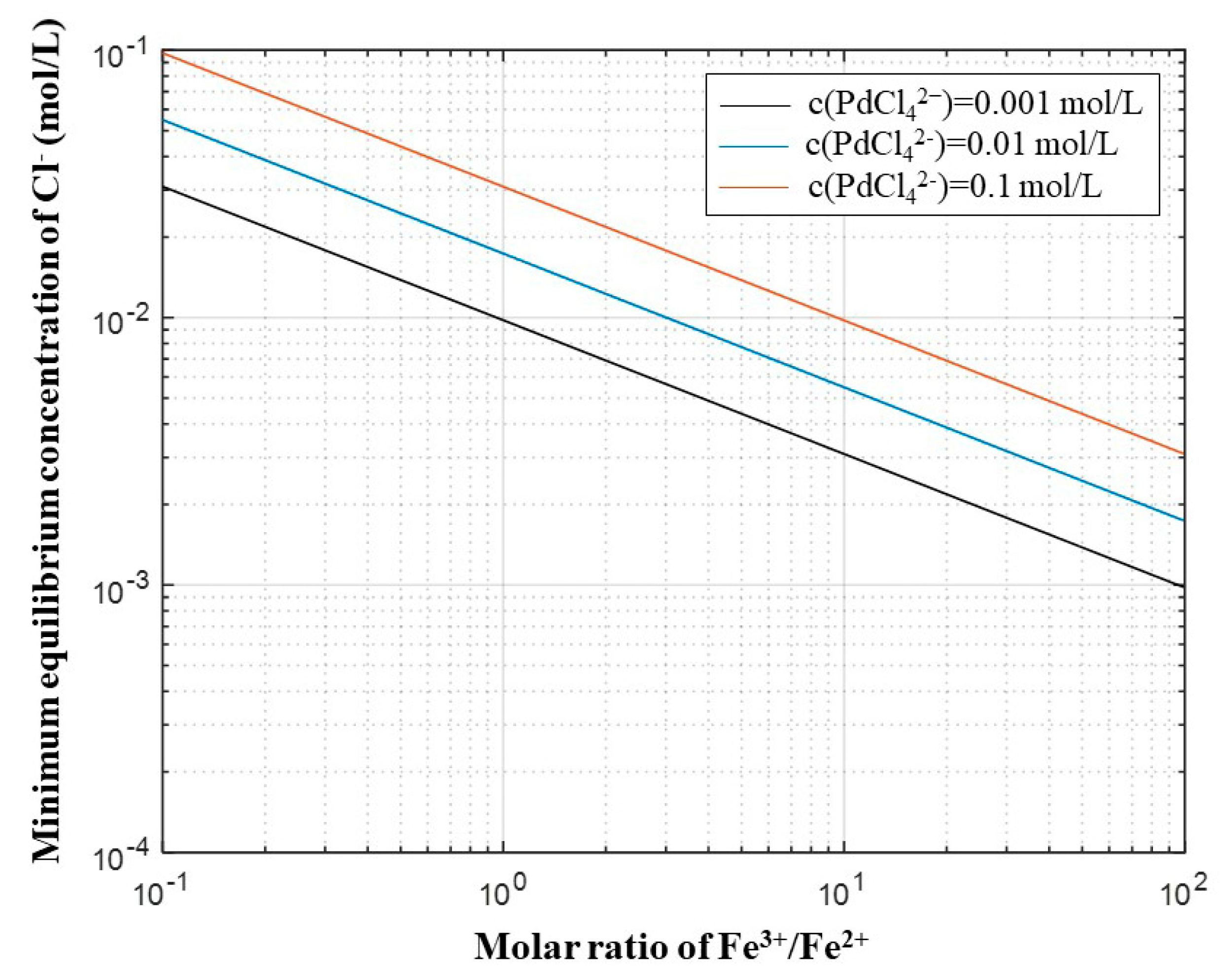
3.1. Thermodynamics of Reactions
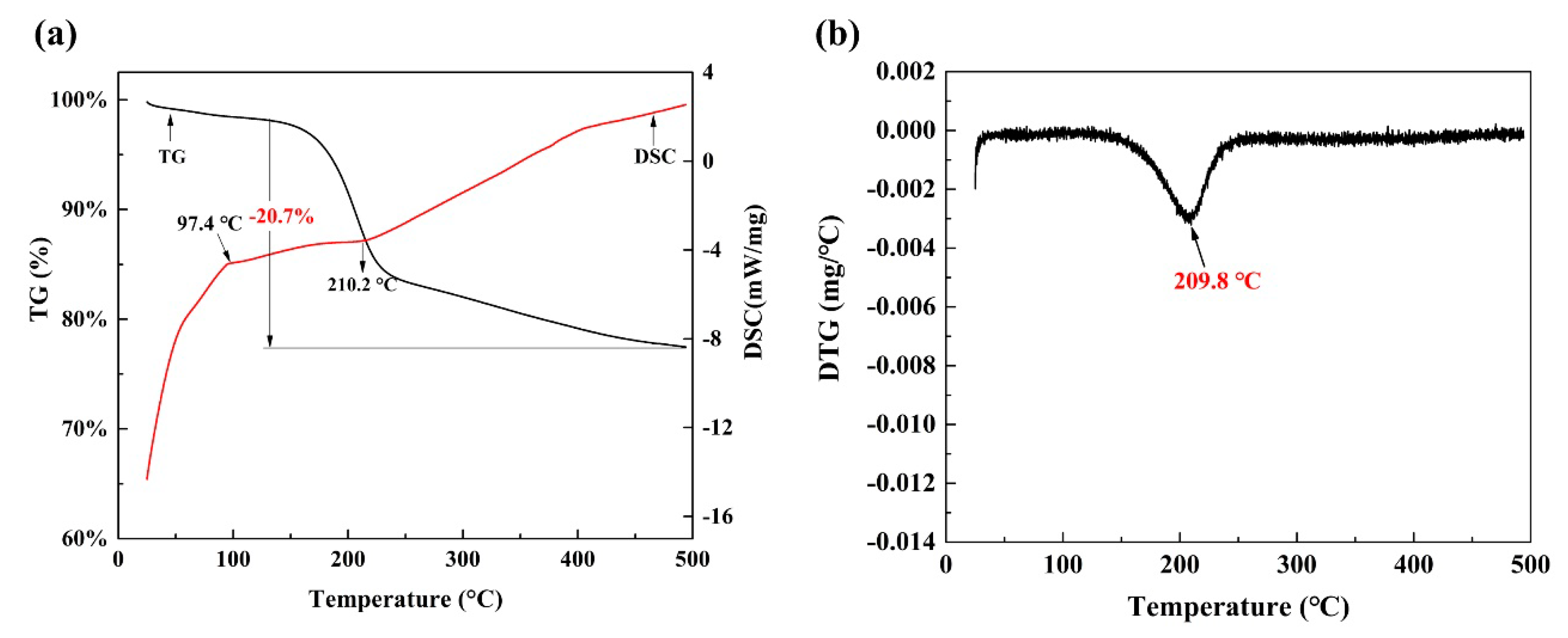
3.2. Characterization of Spent Catalysts
3.3. Fe3+ Oxidation Leaching
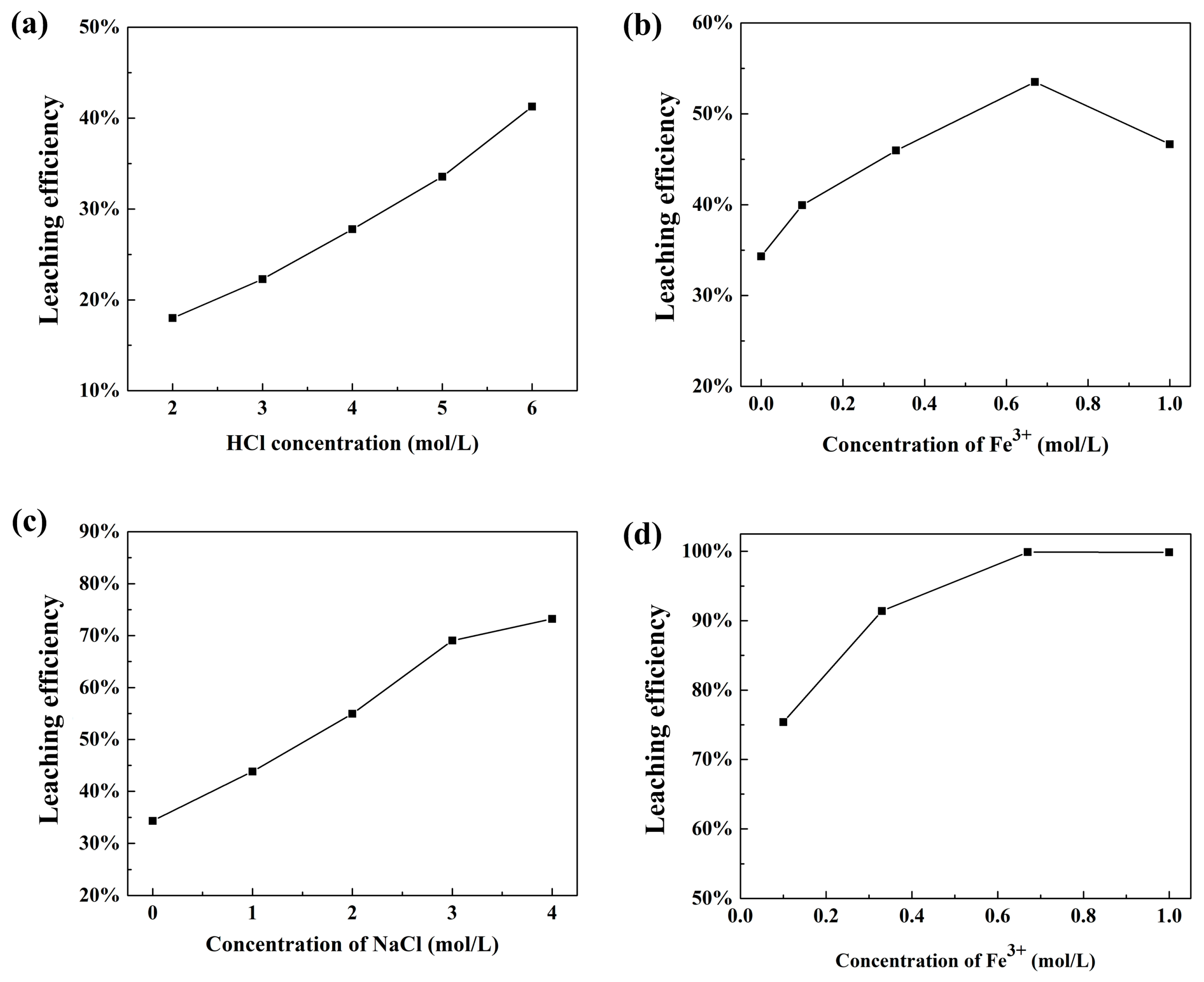
3.3.1. Effect of HCl Concentration
3.3.2. Effect of Fe3+ Concentration at 2.0 mol/L HCl
3.3.3. Effect of NaCl Concentration
3.3.4. Effect of Fe3+ Concentration at 4.0 mol/L NaCl
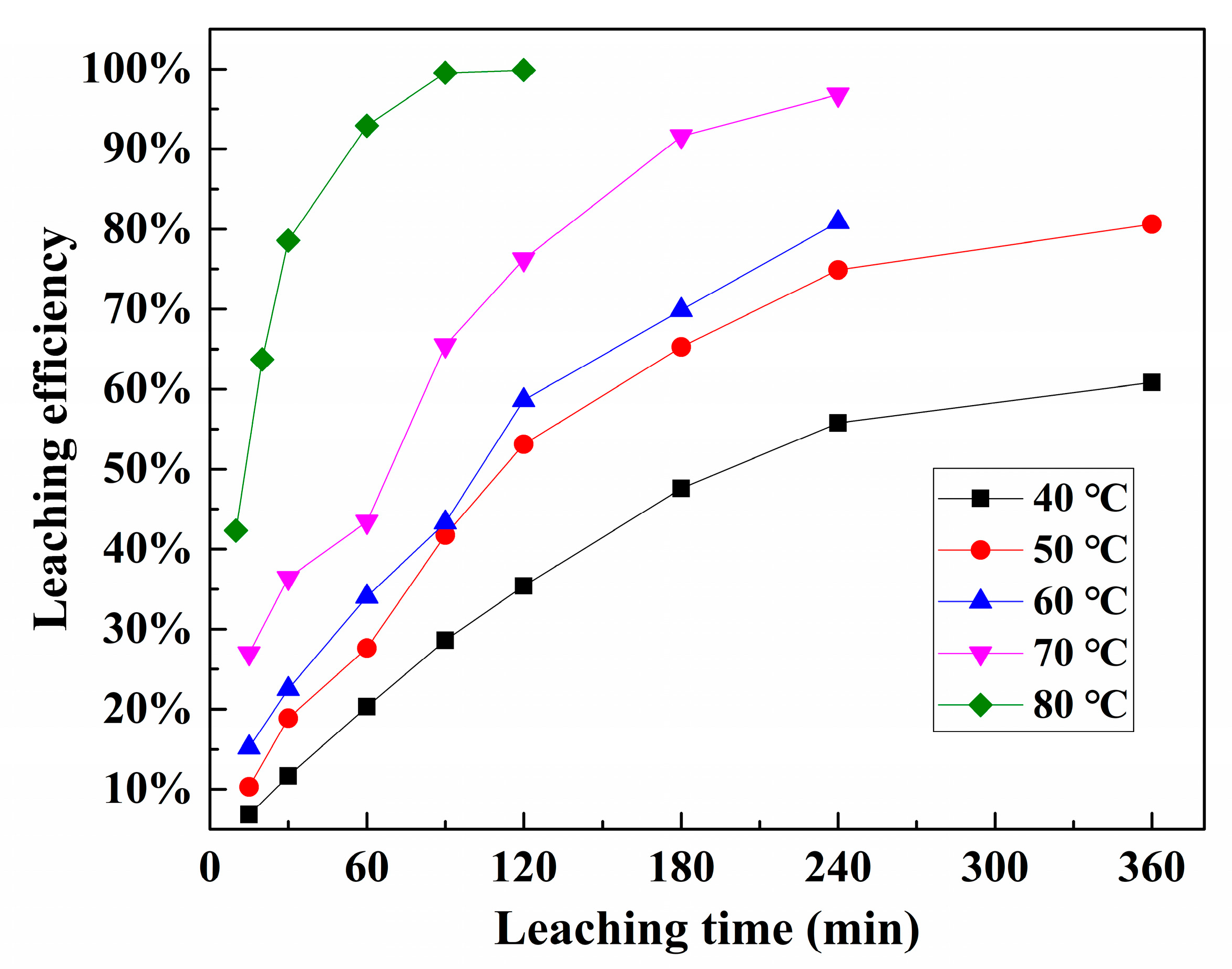
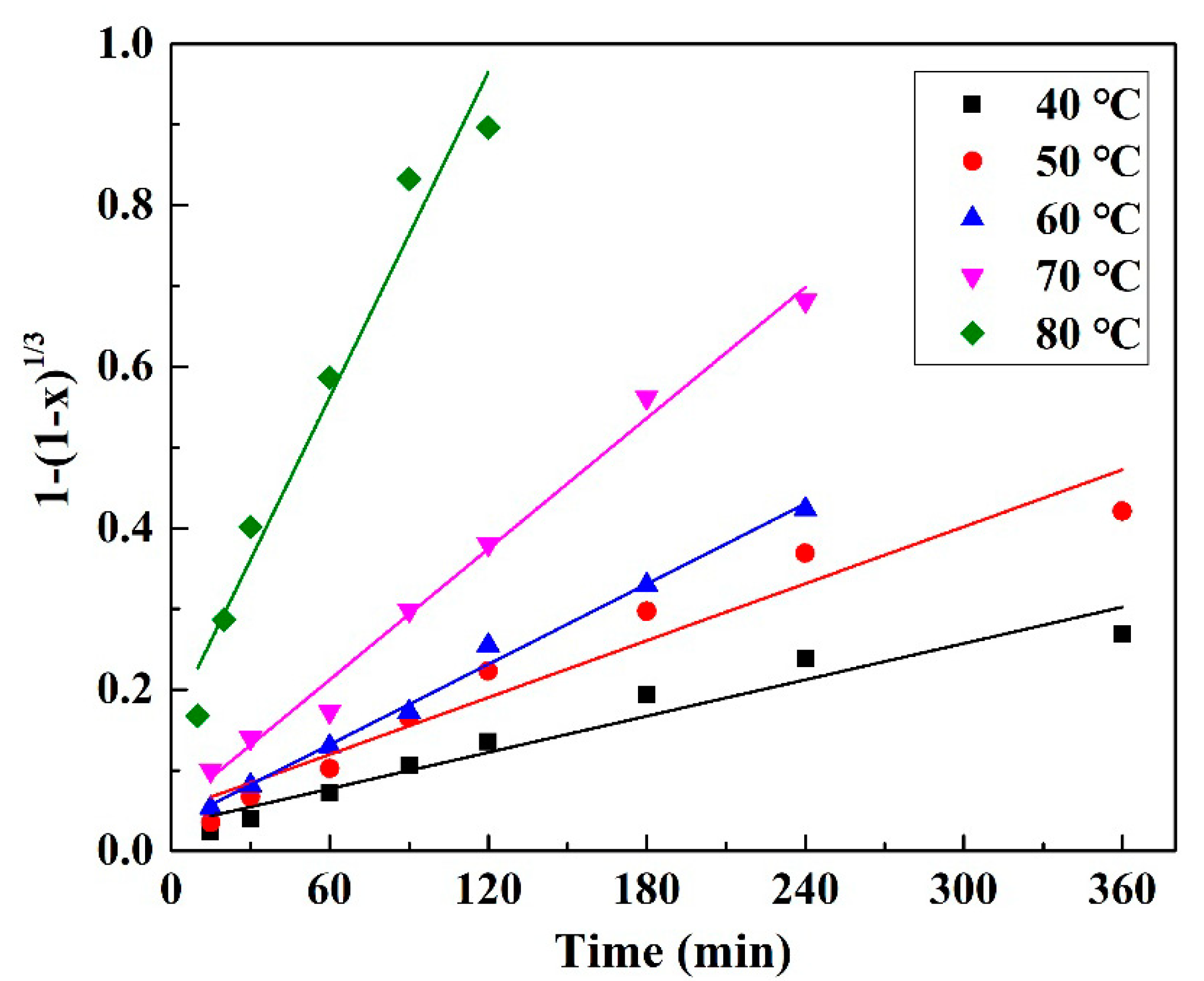
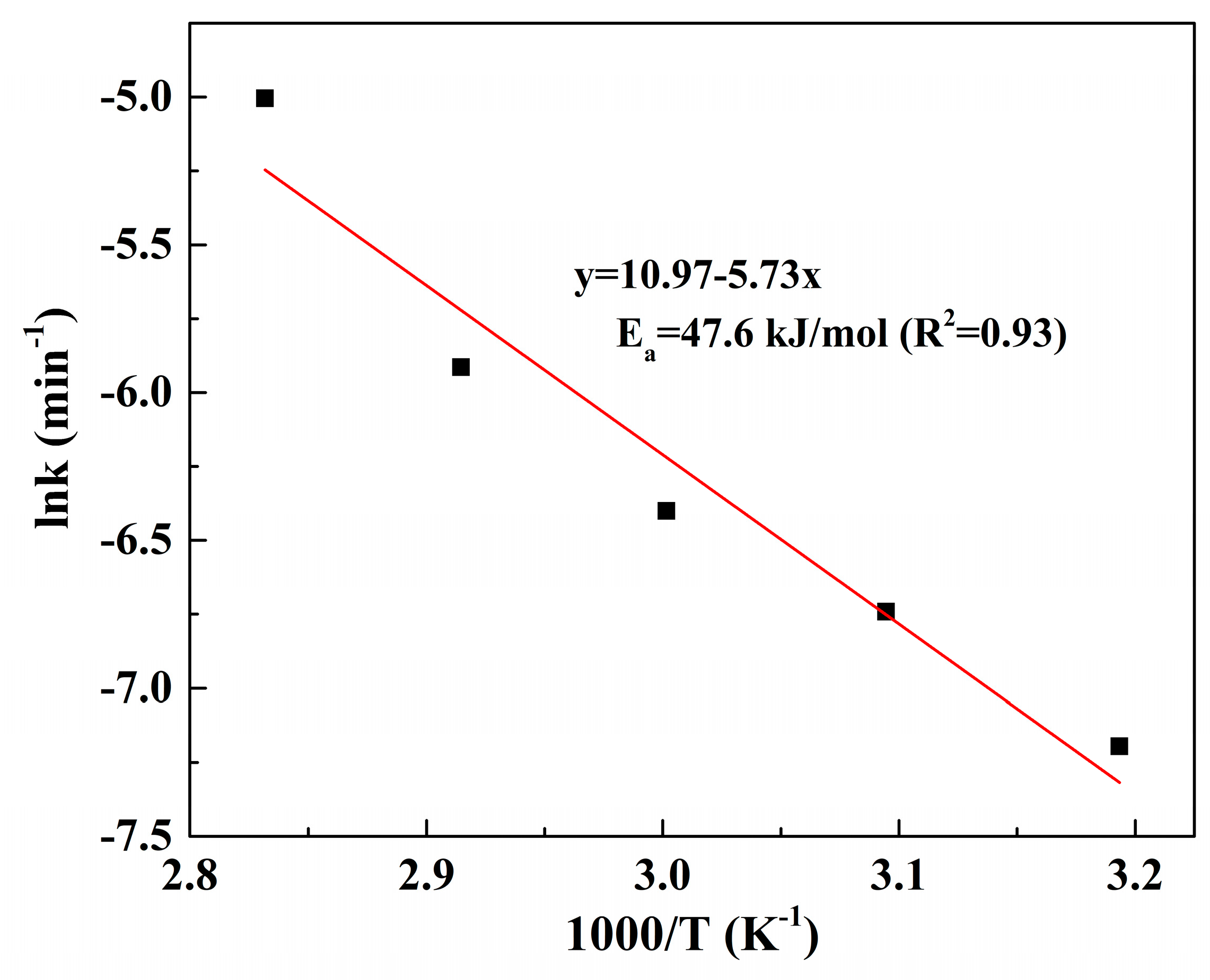
3.4. Kinetic Analysis of Pd Leaching
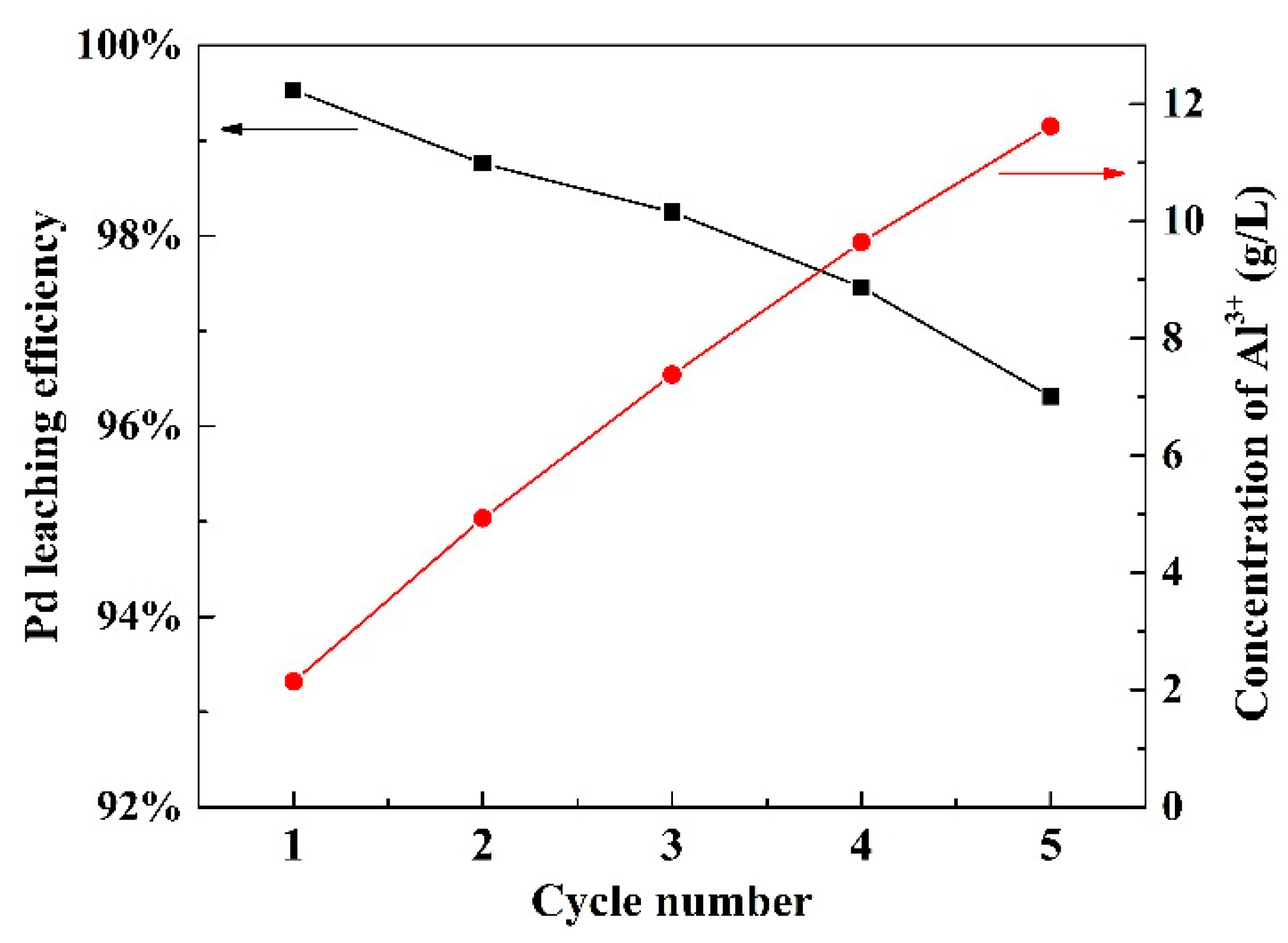
3.5. Reuse of Leaching Agent
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhogeswararao, S.; Srinivas, D. Catalytic conversion of furfural to industrial chemicals over supported Pt and Pd catalysts. J. Catal. 2015, 327, 65–77. [Google Scholar] [CrossRef]
- Freakley, S.J.; He, Q.; Harrhy, J.H.; Lu, L.; Crol, A.D.; Morgan, D.; Ntainjua, E.; Edwards, J.; Carley, A.; Borisevich, A.; et al. Palladium-tin catalysts for the direct synthesis of H2O2 with high selectivity. Science 2016, 351, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Long, W.; Brunelli, N.A.; Didas, S.A.; Ping, E.W.; Jones, C.W. Aminopolymer–silica composite-supported Pd catalysts for selective hydrogenation of alkynes. ACS Catal. 2013, 3, 1700–1708. [Google Scholar] [CrossRef]
- USGS (United States Geological Survey). Platinum-Group Metals Statistics and Information. 2016 Minerals Yearbook Platinum Group Metals. Available online: https://minerals.usgs.gov/minerals/pubs/commodity/platinum/myb1-2016-plati.pdf (accessed on 17 January 2019).
- Zhang, S.G.; Ding, Y.J.; Liu, B.; Chang, C.C. Supply and demand of some critical metals and present status of their recycling in WEEE. Waste Manag. 2017, 65, 113–127. [Google Scholar] [CrossRef]
- Graedel, T.E.; Harper, E.M.; Nassar, N.T.; Nuss, P.; Reck, B.K. Criticality of metals and metalloids. Proc. Natl. Acad. Sci. USA 2015, 112, 4257–4262. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Wang, C.; Corder, G.D. Strategic evaluation of recycling high-tech metals from urban mines in China: An emerging industrial perspective. J. Clean. Prod. 2019, 208, 697–708. [Google Scholar] [CrossRef]
- Ding, Y.J.; Zhang, S.G.; Liu, B.; Zheng, H.D.; Chang, C.C.; Ekberg, C. Recovery of precious metals from electronic waste and spent catalysts: A review. Resour. Conserv. Recy. 2019, 141, 284–298. [Google Scholar] [CrossRef]
- Mudd, G.M.; Glaister, B.J. The Environmental Costs of Platinum-PGM Mining: An Excellent Case Study in Sustainable Mining. In Proceedings of the 48th Annual Conference of Metallurgists, Canadian Metallurgical Society, Sudbury, ON, Canada, 23–26 August 2009. [Google Scholar]
- Akcil, A.; Vegliò, F.; Ferella, F.; Okudan, M.D.; Tuncuk, A. A review of metal recovery from spent petroleum catalysts and ash. Waste Manag. 2015, 45, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Li, Z.; Lin, X.; Tang, H.; Ye, L.; Ma, Y.; Jiang, T. Pyrometallurgical recovery of platinum group metals from spent catalysts. JOM 2017, 69, 1553–1562. [Google Scholar] [CrossRef]
- Kolliopoulos, G.; Balomenos, E.; Giannopoulou, I.; Yakoumis, I.; Panias, D. Behavior of platinum group metals during their pyrometallurgical recovery from spent automotive catalysts. Open Access Lib. J. 2014, 1, 1–9. [Google Scholar]
- Benson, M.; Bennett, C.R.; Harry, J.E.; Patel, M.K.; Cross, M. The recovery mechanism of platinum group metals from catalytic converters in spent automotive exhaust systems. Resour. Conserv. Recy. 2000, 31, 1–7. [Google Scholar] [CrossRef]
- Panda, R.; Jha, M.K.; Pathak, D.D. Commercial Processes for the Extraction of Platinum Group Metals (PGMs). In TMS Annual Meeting & Exhibition; Springer: Cham, Switzerland, 2018; pp. 119–130. [Google Scholar]
- Benson, M.; Bennett, C.R.; Patel, M.K.; Harry, J.E.; Cross, M. Collector-metal behaviour in the recovery of platinum-group metals from catalytic converters. Miner. Process. Ext. Met. 2000, 109, 6–10. [Google Scholar] [CrossRef]
- Mallampati, S.R.; Lee, B.H.; Mitoma, Y.; Simion, C. Sustainable recovery of precious metals from end-of-life vehicles shredder residue by a novel hybrid ball-milling and nanoparticles enabled froth flotation process. J. Clean. Prod. 2018, 171, 66–75. [Google Scholar] [CrossRef]
- Kizilaslan, E.; Aktaş, S.; Şeşen, M.K. Towards environmentally safe recovery of platinum from scrap automotive catalytic converters. Tur. J. Eng. Environ. Sci. 2009, 33, 83–90. [Google Scholar]
- Mpinga, C.N.; Eksteen, J.J.; Aldrich, C.; Dyer, L. Direct leach approaches to Platinum Group Metal (PGM) ores and concentrates: A review. Miner. Eng. 2015, 78, 93–113. [Google Scholar] [CrossRef]
- Sarioğlan, Ş. Recovery of palladium from spent activated carbon-supported palladium catalysts. Platin. Met. Rev. 2013, 57, 289–296. [Google Scholar] [CrossRef]
- Harjanto, S.; Cao, Y.; Shibayama, A.; Naitoh, I.; Nanami, T.; Kasahara, K.; Okumura, Y.; Liu, K.; Fujita, T. Leaching of Pt, Pd and Rh from automotive catalyst residue in various chloride based solutions. Mater. Trans. 2006, 47, 129–135. [Google Scholar] [CrossRef]
- Trinh, H.B.; Lee, J.; Srivastava, R.R.; Kim, S.; Ilyas, S. Eco-threat minimization in HCl leaching of PGMs from spent automobile catalysts by formic acid prereduction. ACS Sustain. Chem. Eng. 2017, 5, 7302–7309. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.; Park, S.; Jeong, J.; Kumar, V. Dissolution behavior of platinum by electro-generated chlorine in hydrochloric acid solution. J. Chem. Technol. Biot. 2013, 88, 1212–1219. [Google Scholar] [CrossRef]
- Nogueira, C.A.; Paiva, A.P.; Oliveira, P.C.; Costa, M.C.; Rosa da Costa, A.M. Oxidative leaching process with cupric ion in hydrochloric acid media for recovery of Pd and Rh from spent catalytic converters. J. Hazard. Mater. 2014, 278, 82–90. [Google Scholar] [CrossRef]
- Jun, M.; Srivastava, R.R.; Jeong, J.; Lee, J.C.; Kim, M.S. Simple recycling of copper by the synergistic exploitation of industrial wastes: A step towards sustainability. Green Chem. 2016, 18, 3823–3834. [Google Scholar] [CrossRef]
- Ikeda, S.; Mori, T.; Ikeda, Y.; Takao, K. Microwave-Assisted Solvent Extraction of Inert Platinum Group Metals from HNO3(aq) to Betainium-Based Thermomorphic Ionic Liquid. ACS Sustain. Chem. Eng. 2016, 4, 2459–2463. [Google Scholar] [CrossRef]
- Puvvada, G.V.K.; Sridhar, R.; Lakshmanan, V.I. Chloride metallurgy: PGM recovery and titanium dioxide production. JOM 2003, 55, 38–41. [Google Scholar] [CrossRef]
- Grumett, P. Precious metal recovery from spent catalysts. Platin. Met. Rev. 2003, 47, 163–166. [Google Scholar]
- Haynes, W.M. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- He, L.P.; Sun, S.Y.; Mu, Y.Y.; Song, X.F.; Yu, J.G. Recovery of lithium, nickel, cobalt, and manganese from spent lithium-ion batteries using L-tartaric acid as a leachant. ACS Sustain. Chem. Eng. 2016, 5, 714–721. [Google Scholar] [CrossRef]
| Elements | Al2O3 | P2O5 | CaO | K2O | SiO2 | Fe2O3 | Na2O | TiO2 | Others |
|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 92.85 | 4.38 | 0.98 | 0.88 | 0.42 | 0.13 | 0.097 | 0.059 | 0.204 |
| HCl(aq)/mol/L | 2.0 | 3.0 | 4.0 | 5.0 | 6.0 |
|---|---|---|---|---|---|
| Mass (g) | 49.20 | 48.63 | 47.48 | 44.79 | 42.56 |
| Weight loss (g) | 0.8 | 1.37 | 2.53 | 5.21 | 7.44 |
| Weight loss (%) | 1.6% | 2.7% | 5.1% | 10.4% | 14.9% |
| T | 40 °C | 50 °C | 60 °C | 70 °C | 80° C |
|---|---|---|---|---|---|
| k | 0.00075 | 0.0012 | 0.0017 | 0.0027 | 0.0067 |
| R2 | 0.9322 | 0.9334 | 0.9924 | 0.9890 | 0.9565 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Zheng, H.; Li, J.; Zhang, S.; Liu, B.; Ekberg, C. An Efficient Leaching of Palladium from Spent Catalysts through Oxidation with Fe(III). Materials 2019, 12, 1205. https://doi.org/10.3390/ma12081205
Ding Y, Zheng H, Li J, Zhang S, Liu B, Ekberg C. An Efficient Leaching of Palladium from Spent Catalysts through Oxidation with Fe(III). Materials. 2019; 12(8):1205. https://doi.org/10.3390/ma12081205
Chicago/Turabian StyleDing, Yunji, Huandong Zheng, Jiayi Li, Shengen Zhang, Bo Liu, and Christian Ekberg. 2019. "An Efficient Leaching of Palladium from Spent Catalysts through Oxidation with Fe(III)" Materials 12, no. 8: 1205. https://doi.org/10.3390/ma12081205
APA StyleDing, Y., Zheng, H., Li, J., Zhang, S., Liu, B., & Ekberg, C. (2019). An Efficient Leaching of Palladium from Spent Catalysts through Oxidation with Fe(III). Materials, 12(8), 1205. https://doi.org/10.3390/ma12081205






