Versatile Layer-By-Layer Highly Stable Multilayer Films: Study of the Loading and Release of FITC-Labeled Short Peptide in the Drug Delivery Field
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. The Preparation of HA-β-CD Gels
2.3. The Synthesis of Mesoporous Silica Nanoparticles (MSNs)
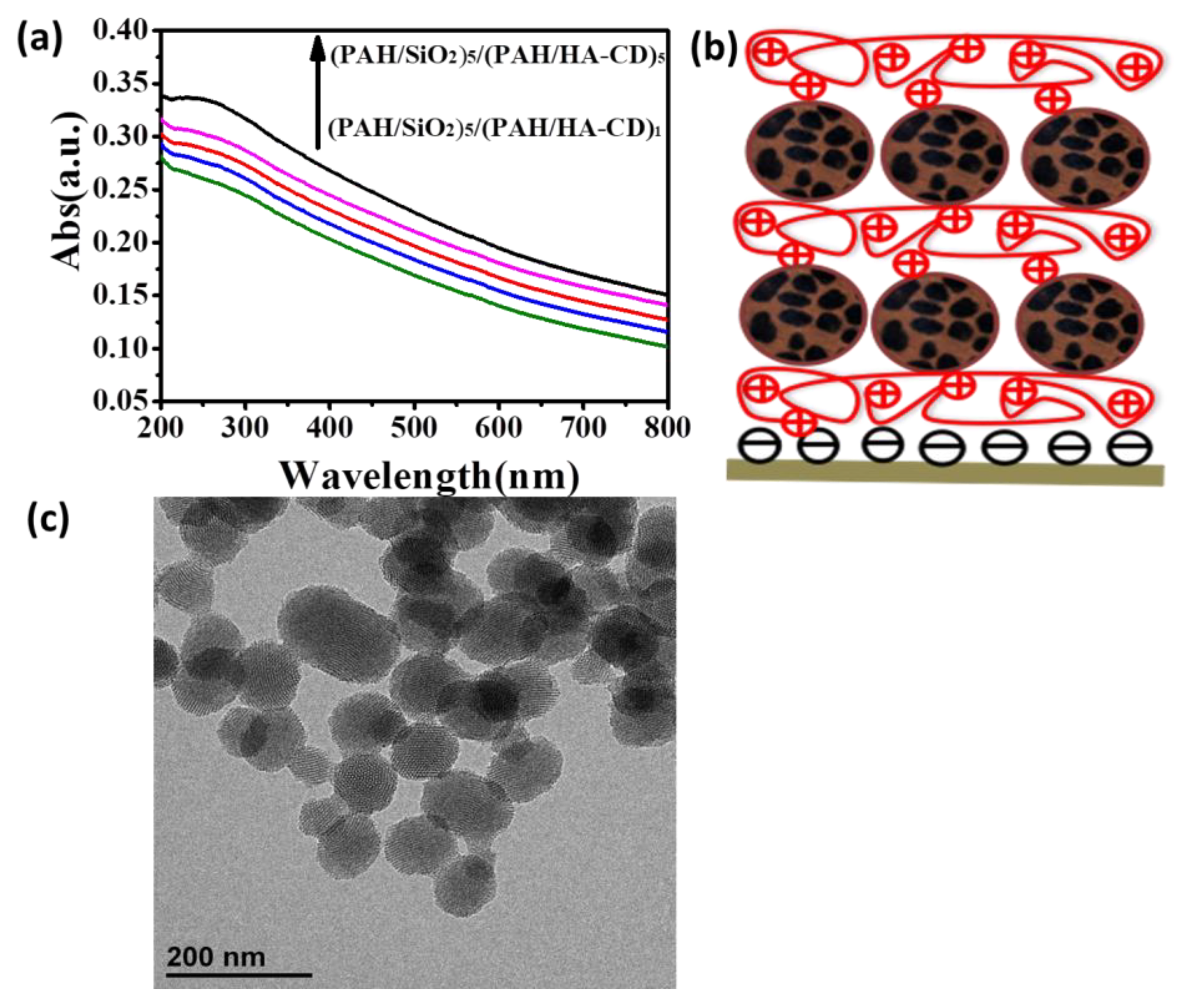
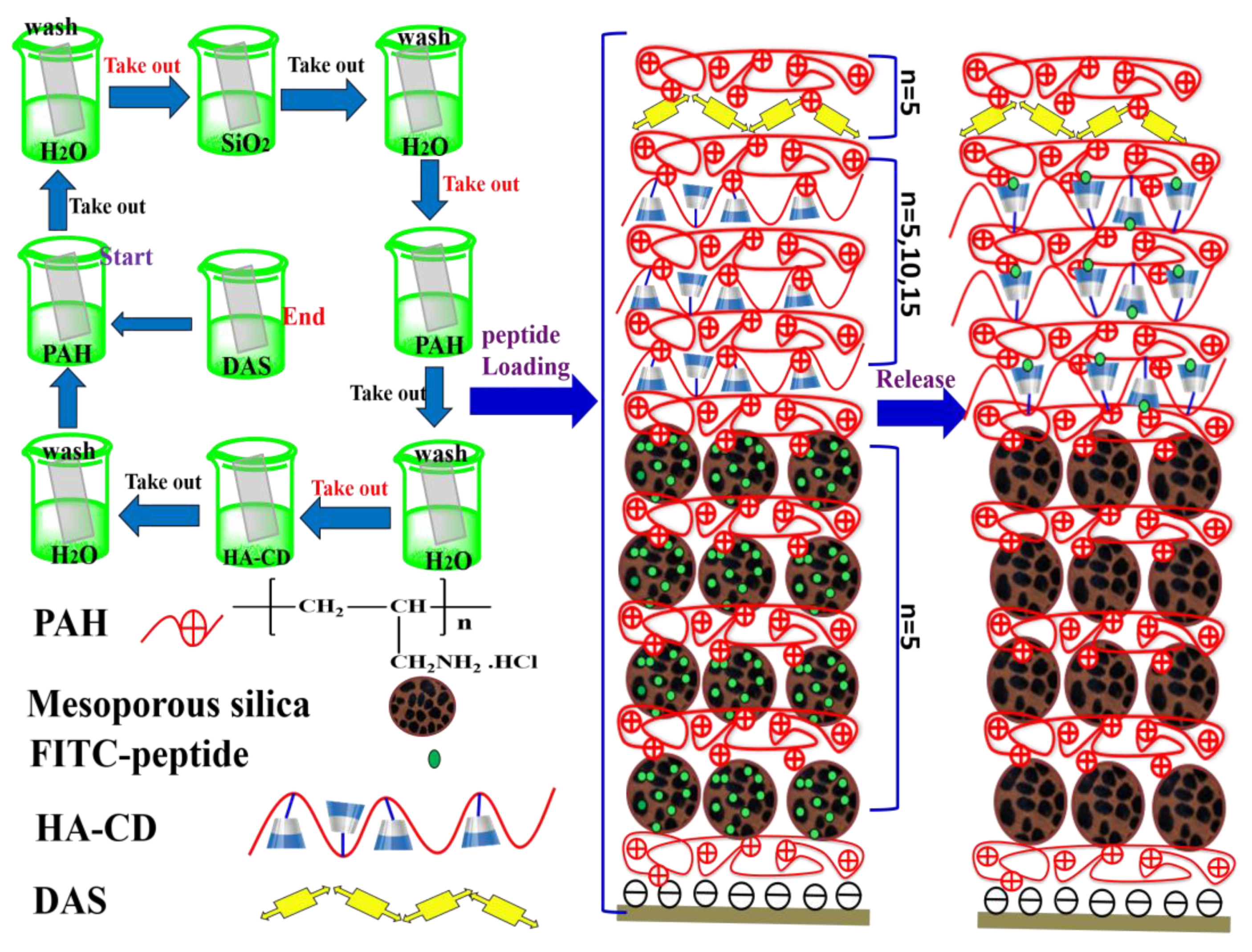
2.4. Layer-By-Layer Assembly Multilayer
2.5. Peptide Loading and Release
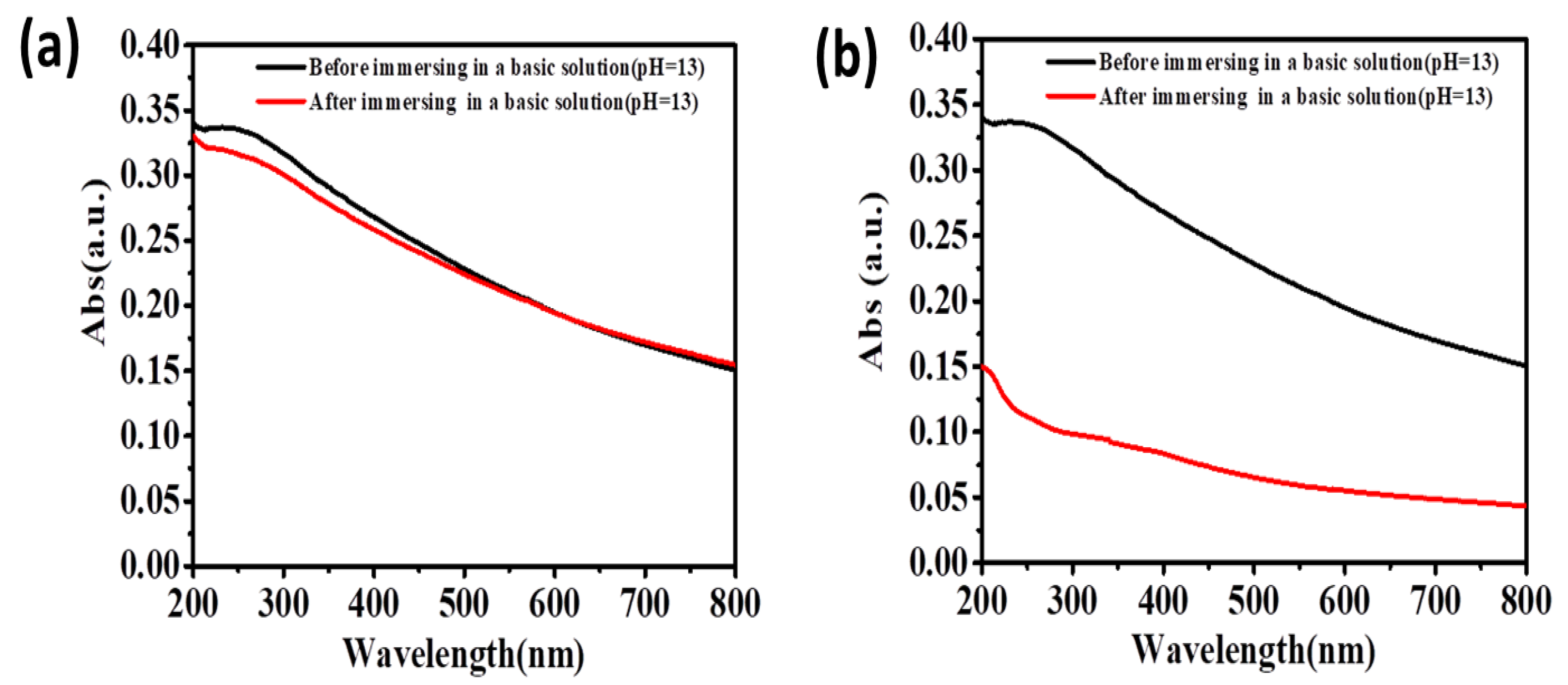
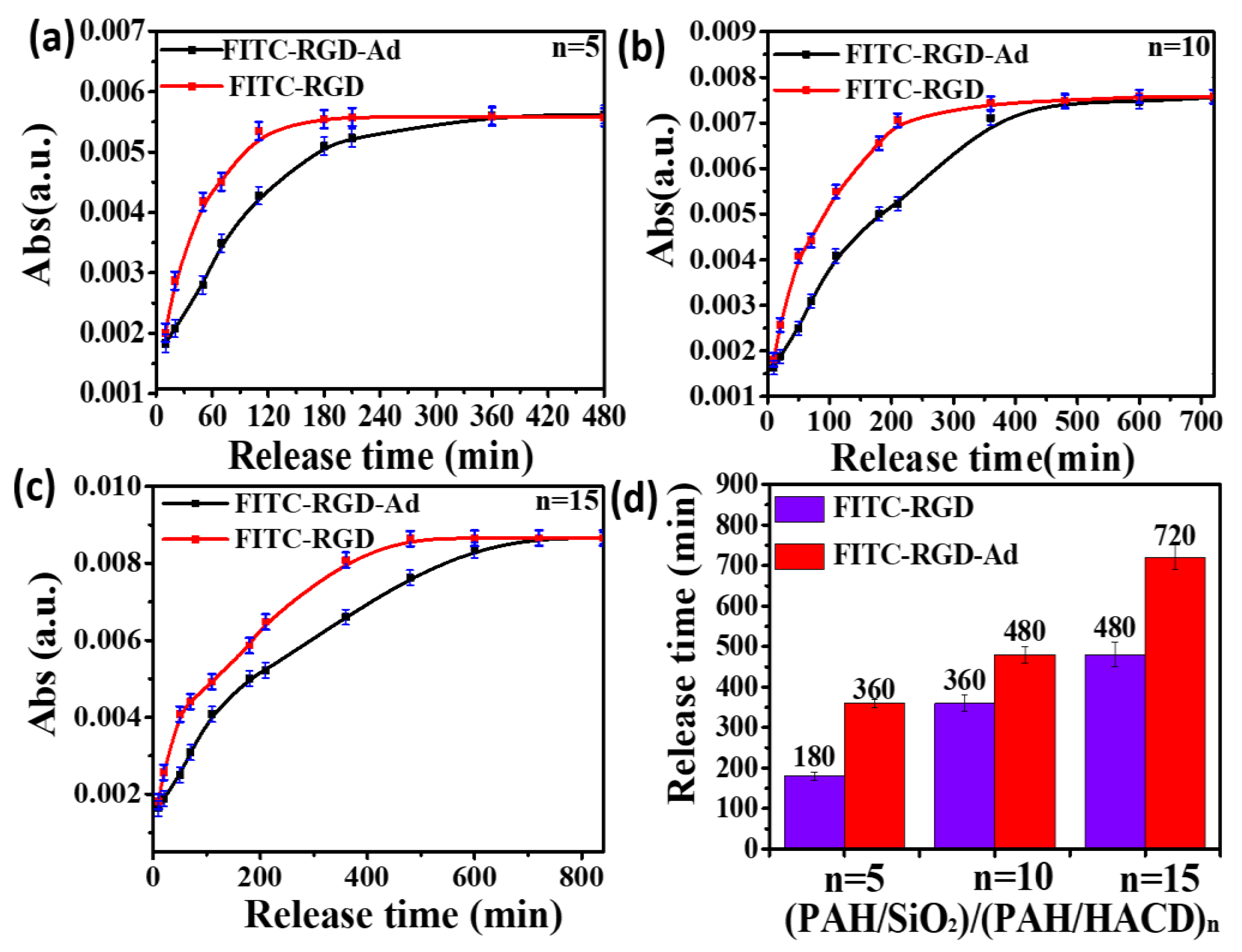
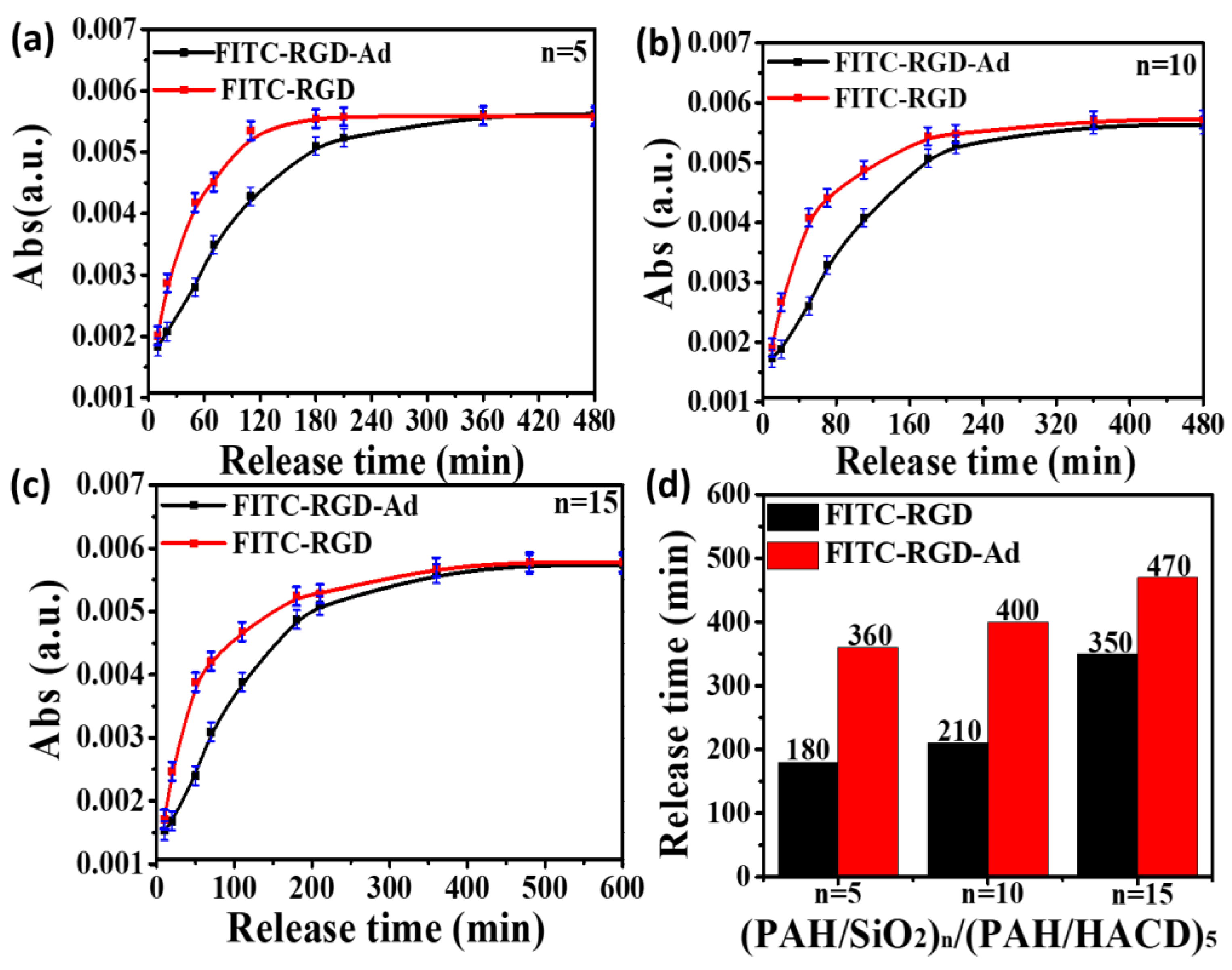
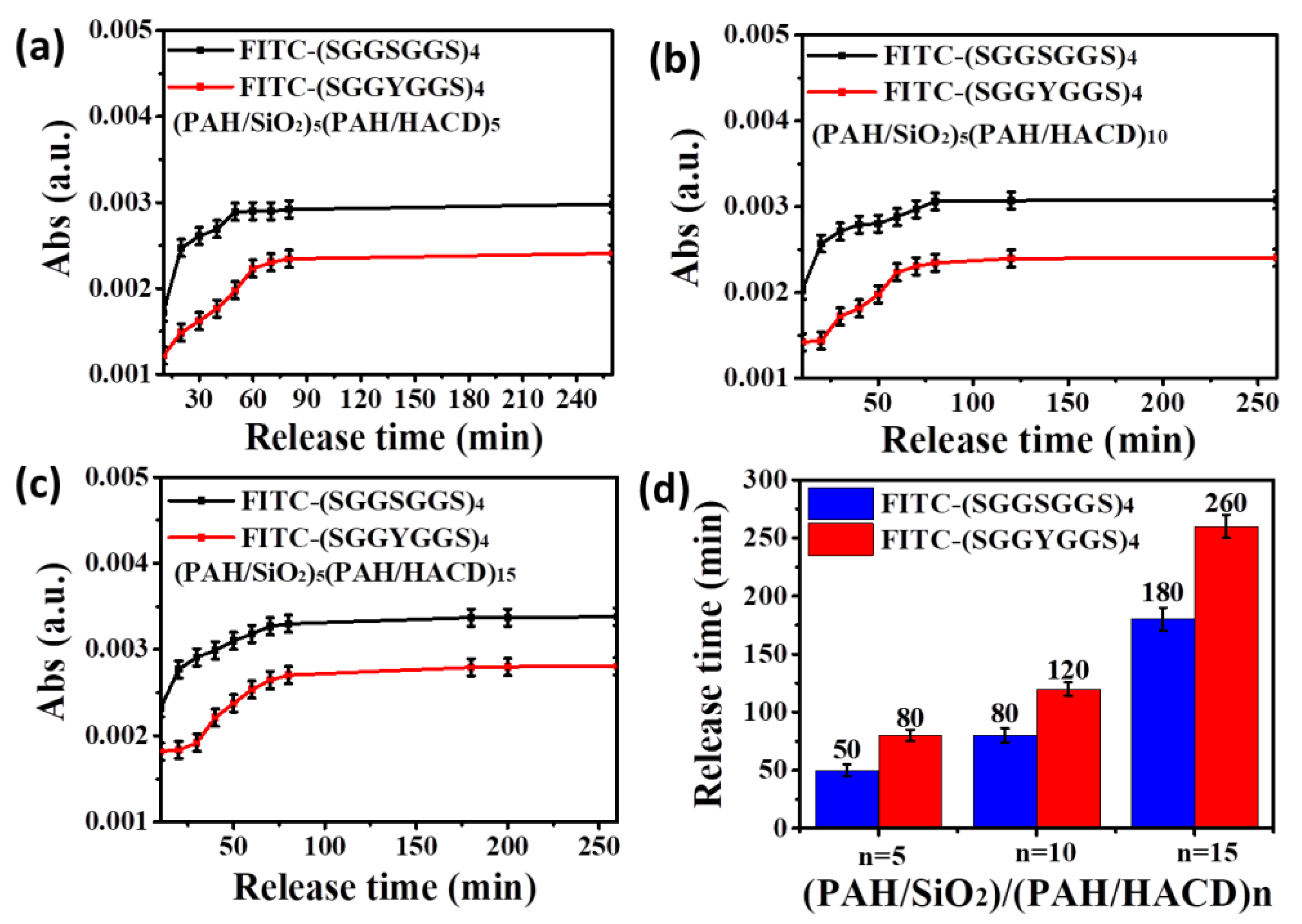
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manna, U.; Bharani, S.; Patil, S. Layer-by-Layer Self-Assembly of Modified Hyaluronic Acid/Chitosan Based on Hydrogen Bonding. Biomacromolecules 2009, 10, 2632–2639. [Google Scholar] [CrossRef]
- Campbell, M.G.; Liu, Q.; Sanders, A.; Evans, J.S.; Smalyukh, I.I. Preparation of nanocomposite plasmonic films made from cellulose nanocrystals or mesoporous silica decorated with unidirectionally aligned gold nanorods. Materials 2014, 7, 3021–3033. [Google Scholar] [CrossRef]
- Nie, K.; An, Q.; Tao, S.Y.; Zhang, Z.P.; Luan, X.L.; Zhang, Q.; Zhang, Y.H. Layer-by-layer reduced graphene oxide (rGO)/gold nanosheets (AuNSs) hybrid films: significantly enhanced photothermal transition effect compared with rGO or AuNSs films. RSC Adv. 2015, 5, 57389–57394. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Vasantha, C.S.; Sasidharan, A.V. Layer-by-layer assembly of hyaluronic acid/carboxymethylchitosan polyelectrolytes on the surface of aminated mesoporous silica for the oral delivery of 5-fluorouracil. Eur. Polym. J. 2017, 93, 572–589. [Google Scholar] [CrossRef]
- Wang, D.G.; Sheridan, M.V.; Shan, B.; Famum, B.H.; Marquard, S.L.; Sherman, B.D.; Eberhart, M.S.; Nayak, A.; Dares, C.J.; Das, A.K.; Bullock, R.M.; Meyer, T.J. Layer-by-Layer Molecular Assemblies for Dye-Sensitized Photoelectrosynthesis Cells Prepared by Atomic Layer Deposition. J. Am. Chem. Soc. 2017, 139, 14518–14525. [Google Scholar] [CrossRef]
- Kang, E.H.; Bu, T.J.; Jin, P.C.; Sun, J.Q.; Yang, Y.Q.; Shen, J.C. Layer-by-layer deposited organic/inorganic hybrid multilayer films containing noncentrosymmetrically orientated azobenzene chromophores. Langmuir 2007, 23, 7594–7601. [Google Scholar] [CrossRef]
- Wang, H.J.; Ishihara, S.; Ariga, K.; Yamauchi, Y. All-Metal Layer-by-Layer Films: Bimetallic Alternate Layers with Accessible Mesopores for Enhanced Electrocatalysis. J. Am. Chem. Soc. 2012, 134, 10819–10821. [Google Scholar] [CrossRef]
- Nie, K.; An, Q.; Zhang, Y.H. A functional protein retention and release multilayer with high stability. Nanoscale 2016, 8, 8791–8797. [Google Scholar] [CrossRef]
- Xu, Q.W.; Li, X.; Jin, Y.Y.; Sun, L.; Ding, X.X.; Liang, L.; Wang, L.; Nan, K.H.; Ji, J.; Chen, H.; Wang, B.L. Bacterial self-defense antibiotics release from organic-inorganic hybrid multilayer films for long-term anti-adhesion and biofilm inhibition properties. Nanoscale 2017, 9, 19245–19254. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.W.; Lin, V.S.Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef]
- Lau, H.H.; Murney, R.; Yakovlev, N.L.; Novoselova, M.V.; Lim, S.H.; Roy, N.; Singh, H.; Sukhorukov, G.B.; Haigh, B.; Kiryukhin, M.V. Protein-tannic acid multilayer films: A multifunctional material for microencapsulation of food-derived bioactives. J. Colloid Interface Sci. 2017, 505, 332–340. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Belbekhouche, S.; Dubot, P.; Carbonnier, B.; Grande, D. From the functionalization of polyelectrolytes to the development of a versatile approach to the synthesis of polyelectrolyte multilayer films with enhanced stability. J. Mater. Chem. A 2017, 5, 24472–24483. [Google Scholar] [CrossRef]
- Jin, W.; Shi, X.Y.; Caruso, F. High activity enzyme microcrystal multilayer films. J. Am. Chem. Soc. 2001, 123, 8121–8122. [Google Scholar] [CrossRef]
- Kanazawa, A.; Ikeda, T.; Abe, J. Supramolecular polar thin films built by surfactant liquid crystals: Polarization-tunable multilayer self-assemblies with in-plane ferroelectric ordering of ion-based dipoles. J. Am. Chem. Soc. 2001, 123, 1748–1754. [Google Scholar] [CrossRef]
- Ma, R.Z.; Sasaki, T.; Bando, Y. Layer-by-layer assembled multilayer films of titanate nanotubes, Ag- or Au-loaded nanotubes, and nanotubes/nanosheets with polycations. J. Am. Chem. Soc. 2004, 126, 10382–10388. [Google Scholar] [CrossRef]
- Ribeiro, T.; Baleizao, C.; Farinha, J.P.S. Functional films from silica/polymer nanoparticles. Materials 2014, 7, 3881–3900. [Google Scholar] [CrossRef]
- Rest, C.; Kandanelli, R.; Fernandez, G. Strategies to create hierarchical self-assembled structures via cooperative non-covalent interactions. Chem. Soc. Rev. 2015, 44, 2543–2572. [Google Scholar] [CrossRef]
- Sakata, S.; Inoue, Y.; Ishihara, K. Molecular Interaction Forces Generated during Protein Adsorption to Well-Defined Polymer Brush Surfaces. Langmuir 2015, 31, 3108–3114. [Google Scholar] [CrossRef]
- Zhang, D.W.; Tian, J.; Chen, L.; Zhang, L.; Li, Z.T. Dimerization of Conjugated Radical Cations: An Emerging Non-Covalent Interaction for Self-Assembly. Chem. Asian J. 2015, 10, 56–68. [Google Scholar] [CrossRef]
- Ma, X.X.; Mei, L.F.; Liu, H.K.; Liao, L.B.; Liu, Y.Q.; Nie, K.; Li, Z.H. Structure and fluorescent properties of Ba3Sc(PO4)(3):Sm3+ red-orange phosphor for n-UV w-LEDs. Chem. Phys. Lett. 2016, 653, 212–215. [Google Scholar] [CrossRef]
- Steiner, C.; Gebhardt, J.; Ammon, M.; Yang, Z.C.; Heidenreich, A.; Hammer, N.; Gorling, A.; Kivala, M.; Maier, S. Hierarchical on-surface synthesis and electronic structure of carbonyl-functionalized one- and two-dimensional covalent nanoarchitectures. Nat. Commun. 2017, 8, 14765. [Google Scholar] [CrossRef]
- Zhang, X.S.; Jiang, C.; Cheng, M.J.; Zhou, Y.; Zhu, X.Q.; Nie, J.; Zhang, Y.J.; An, Q.; Shi, F. Facile Method for the Fabrication of Robust Polyelectrolyte Multilayers by Post-Photo-Cross-Linking of Azido Groups. Langmuir 2012, 28, 7096–7100. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Dang, D.T.; van Dongen, J.L.J.; Brunsveld, L. Protein Dimerization Induced by Supramolecular Interactions with Cucurbit[8]uril. Angew. Chem. Int. Edit. 2010, 49, 895–898. [Google Scholar] [CrossRef]
- Boraste, D.R.; Chakraborty, G.; Ray, A.K.; Shankarling, G.S.; Pal, H. Supramolecular host-guest interaction of antibiotic drug ciprofloxacin with cucurbit[7]uril macrocycle: Modulations in photophysical properties and enhanced photostability. J. Photoch. Photobio. Chem. 2018, 358, 26–37. [Google Scholar] [CrossRef]
- Conesa-Egea, J.; Redondo, C.D.; Martinez, J.I.; Gomez-Garcia, C.J.; Castillo, O.; Zamora, F.; Amo-Ochoa, P. Supramolecular Interactions Modulating Electrical Conductivity and Nanoprocessing of Copper-Iodine Double-Chain Coordination Polymers. Inorg. Chem. 2018, 57, 7568–7577. [Google Scholar] [CrossRef]
- Lee, H.Y.; Park, S.H.; Kim, J.H.; Kim, M.S. Temperature-responsive hydrogels via the electrostatic interaction of amphiphilic diblock copolymers with pendant-ion groups. Polymer Chem. 2017, 8, 6606–6616. [Google Scholar] [CrossRef]
- Derbenev, I.N.; Filippov, A.V.; Stace, A.J.; Besley, E. Electrostatic interactions between charged dielectric particles in an electrolyte solution: constant potential boundary conditions. Soft Matter 2018, 14, 5480–5487. [Google Scholar] [CrossRef]
- Mishra, A.K.; Weissman, H.; Krieg, E.; Votaw, K.A.; McCullagh, M.; Rybtchinski, B.; Lewis, F.D. Self-Assembly of Perylenediimide-Single-Strand-DNA Conjugates: Employing Hydrophobic Interactions and DNA Base-Pairing To Create a Diverse Structural Space. Chem. Eur. J. 2017, 23, 10328–10337. [Google Scholar] [CrossRef]
- Moon, S.; Park, S.O.; Ahn, Y.H.; Kim, H.; Shin, E.; Hong, S.; Lee, Y.; Kwak, S.K.; Park, Y. Distinct hydrophobic-hydrophilic dual interactions occurring in the clathrate hydrates of 3,3-dimethyl-1-butanol with help gases. Chem. Eng. J. 2018, 348, 583–591. [Google Scholar] [CrossRef]
- Shumilova, T.A.; Ruffer, T.; Lang, H.; Kataev, E.A. Straightforward Design of Fluorescent Receptors for Sulfate: Study of Non-Covalent Interactions Contributing to Host-Guest Formation. Chem. Eur. J. 2018, 24, 1500–1504. [Google Scholar] [CrossRef]
- Xue, F.C.; Wang, Y.Q.; Zhang, Q.X.; Han, S.L.; Zhang, F.Z.; Jin, T.T.; Li, C.W.; Hu, H.Y.; Zhang, J.X. Self-assembly of affinity-controlled nanoparticles via host-guest interactions for drug delivery. Nanoscale 2018, 10, 12364–12377. [Google Scholar] [CrossRef]
- Furchner, A.; Kroning, A.; Rauch, S.; Uhlmann, P.; Eichhorn, K.J.; Hinrichs, K. Molecular Interactions and Hydration States of Ultrathin Functional Films at the Solid-Liquid Interface. Anal. Chem. 2017, 89, 3240–3244. [Google Scholar] [CrossRef]
- Palao, E.; Sola-Llano, R.; Tabero, A.; Manzano, H.; Agarrabeitia, A.R.; Villanueva, A.; Lopez-Arbeloa, I.; Martinez-Martinez, V.; Ortiz, M.J. Acetylacetonate BODIPY-Biscyclometalated Iridium(III) Complexes: Effective Strategy towards Smarter Fluorescent Photosensitizer Agents. Chem. Eur. J. 2017, 23, 10139–10147. [Google Scholar] [CrossRef]
- Schwenck, J.; Maier, F.C.; Kneilling, M.; Wiehr, S.; Fuchs, K. Non-invasive In Vivo Fluorescence Optical Imaging of Inflammatory MMP Activity Using an Activatable Fluorescent Imaging Agent. Jove-J. Vis. Exp. 2017, 123, e55180. [Google Scholar] [CrossRef]
- Luan, L.; Lin, Z.J.; Wu, G.H.; Huang, X.L.; Cai, Z.M.; Chen, X. Encoding electrochemiluminescence using Ru(bpy)(3)(2+) and fluorescein isothiocyanate co-doped silica nanoparticles. Chem. Commun. 2011, 47, 3963–3965. [Google Scholar] [CrossRef]
- Tang, A.M.; Mei, B.; Wang, W.J.; Hu, W.L.; Li, F.; Zhou, J.; Yang, Q.; Cui, H.; Wu, M.; Liang, G.L. FITC-quencher based caspase 3-activatable nanoprobes for effectively sensing caspase 3 in vitro and in cells. Nanoscale 2013, 5, 8963–8967. [Google Scholar] [CrossRef]
- Tay, A.; Sohrabi, A.; Poole, K.; Seidlits, S.; Di Carlo, D. A 3D Magnetic Hyaluronic Acid Hydrogel for Magnetomechanical Neuromodulation of Primary Dorsal Root Ganglion Neurons. Adv. Mater. 2018, 30, 1800927. [Google Scholar] [CrossRef]
- Souchek, J.J.; Wojtynek, N.E.; Payne, W.M.; Holmes, M.B.; Dutta, S.; Qi, B.W.; Datta, K.; LaGrange, C.A.; Mohs, A.M. Hyaluronic acid formulation of near infrared fluorophores optimizes surgical imaging in a prostate tumor xenograft. Acta Biomater. 2018, 75, 323–333. [Google Scholar] [CrossRef]
- Hunt, J.A.; Joshi, H.N.; Stella, V.J.; Topp, E.M. Diffusion and Drug Release in Polymer-Films Prepared from Ester Derivatives of Hyaluronic-Acid. J. Control. Release 1990, 12, 159–169. [Google Scholar] [CrossRef]
- Pitarresi, G.; Palumbo, F.S.; Albanese, A.; Fiorica, C.; Picone, P.; Giammona, G. Self-assembled amphiphilic hyaluronic acid graft copolymers for targeted release of antitumoral drug. J. Drug Target 2010, 18, 264–276. [Google Scholar] [CrossRef]
- Ramamurthy, V.; Jockusch, S.; Pore, M. Supramolecular Photochemistry in Solution and on Surfaces: Encapsulation and Dynamics of Guest Molecules and Communication between Encapsulated and Free Molecules. Langmuir 2015, 31, 5554–5570. [Google Scholar] [CrossRef]
- Mondal, P.; Rath, S.P. Efficient Host-Guest Complexation of a Bisporphyrin Host with Electron Deficient Guests: Synthesis, Structure, and Photoinduced Electron Transfer. Isr. J. Chem. 2016, 56, 144–155. [Google Scholar] [CrossRef]
- Kanagaraj, K.; Pitchumani, K. The Aminocyclodextrin/Pd(OAc)(2) Complex as an Efficient Catalyst for the Mizoroki-Heck Cross-Coupling Reaction. Chem. Eur. J. 2013, 19, 14425–14431. [Google Scholar] [CrossRef]
- Kaur, N.; Garg, T.; Goyal, A.K.; Rath, G. Formulation, optimization and evaluation of curcumin-β-cyclodextrin-loaded sponge for effective drug delivery in thermal burns chemotherapy. Drug Deliv. 2016, 23, 2245–2254. [Google Scholar] [CrossRef]
- Nakahata, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Redox-responsive self-healing materials formed from host-guest polymers. Nat. Commun. 2011, 2, 511. [Google Scholar] [CrossRef]
- Wei, S.J.; Chu, H.M.; Xu, L.S.; Wang, Z.Z.; Huang, Q. Macrocyclic drug conjugates of metronidazole-cyclodextrin for colon-targeted delivery. J. Control. Release 2017, 259, E120–E121. [Google Scholar] [CrossRef]
- Parlati, S.; Gobetto, R.; Barolo, C.; Arrais, A.; Buscaino, R.; Medana, C.; Savarino, P. Preparation and application of a beta-cyclodextrin-disperse/reactive dye complex. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 463–470. [Google Scholar] [CrossRef]
- Nie, K.; An, Q.; Zink, J.I.; Yu, X.; Zhang, Y.H. Layer by Layer Mesoporous Silica-Hyaluronic Acid-Cyclodextrin Bifunctional “Lamination”: Study of the Application of Fluorescent Probe and Host-Guest Interactions in the Drug Delivery Field. Materials 2018, 11, 1745. [Google Scholar] [CrossRef]
- Trebosc, J.; Wiench, J.W.; Huh, S.; Lin, V.S.Y.; Pruski, M. Solid-state NMR study of MCM-41-type mesoporous silica nanoparticles. J. Am. Chem. Soc. 2005, 127, 3057–3068. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, K.; Yu, X.; Kumar, N.; Zhang, Y. Versatile Layer-By-Layer Highly Stable Multilayer Films: Study of the Loading and Release of FITC-Labeled Short Peptide in the Drug Delivery Field. Materials 2019, 12, 1206. https://doi.org/10.3390/ma12081206
Nie K, Yu X, Kumar N, Zhang Y. Versatile Layer-By-Layer Highly Stable Multilayer Films: Study of the Loading and Release of FITC-Labeled Short Peptide in the Drug Delivery Field. Materials. 2019; 12(8):1206. https://doi.org/10.3390/ma12081206
Chicago/Turabian StyleNie, Kun, Xiang Yu, Navnita Kumar, and Yihe Zhang. 2019. "Versatile Layer-By-Layer Highly Stable Multilayer Films: Study of the Loading and Release of FITC-Labeled Short Peptide in the Drug Delivery Field" Materials 12, no. 8: 1206. https://doi.org/10.3390/ma12081206
APA StyleNie, K., Yu, X., Kumar, N., & Zhang, Y. (2019). Versatile Layer-By-Layer Highly Stable Multilayer Films: Study of the Loading and Release of FITC-Labeled Short Peptide in the Drug Delivery Field. Materials, 12(8), 1206. https://doi.org/10.3390/ma12081206