Rapid Immobilization of Simulated Radioactive Soil Waste Using Self-Propagating Synthesized Gd2Ti2O7 Pyrochlore Matrix
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
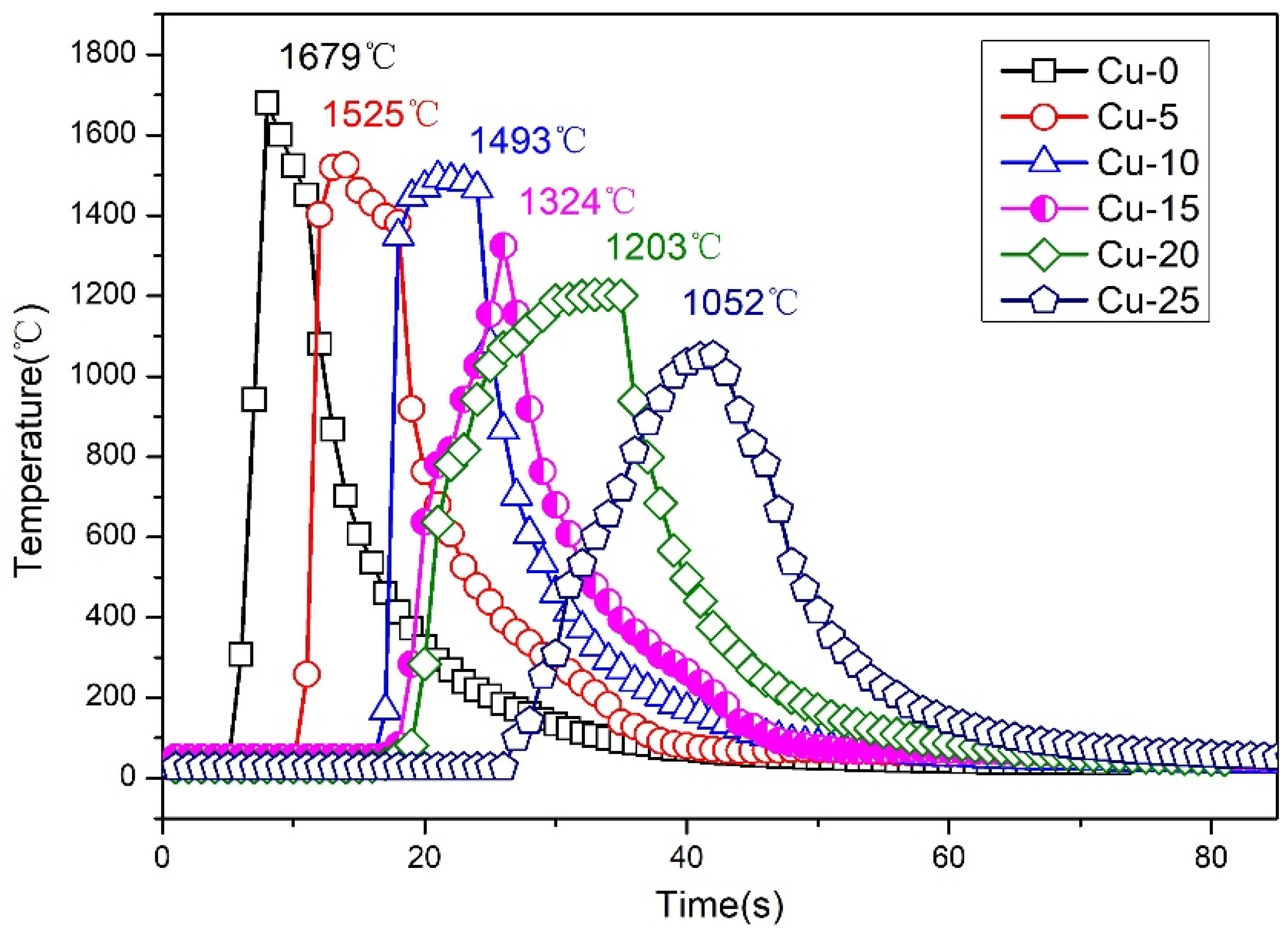
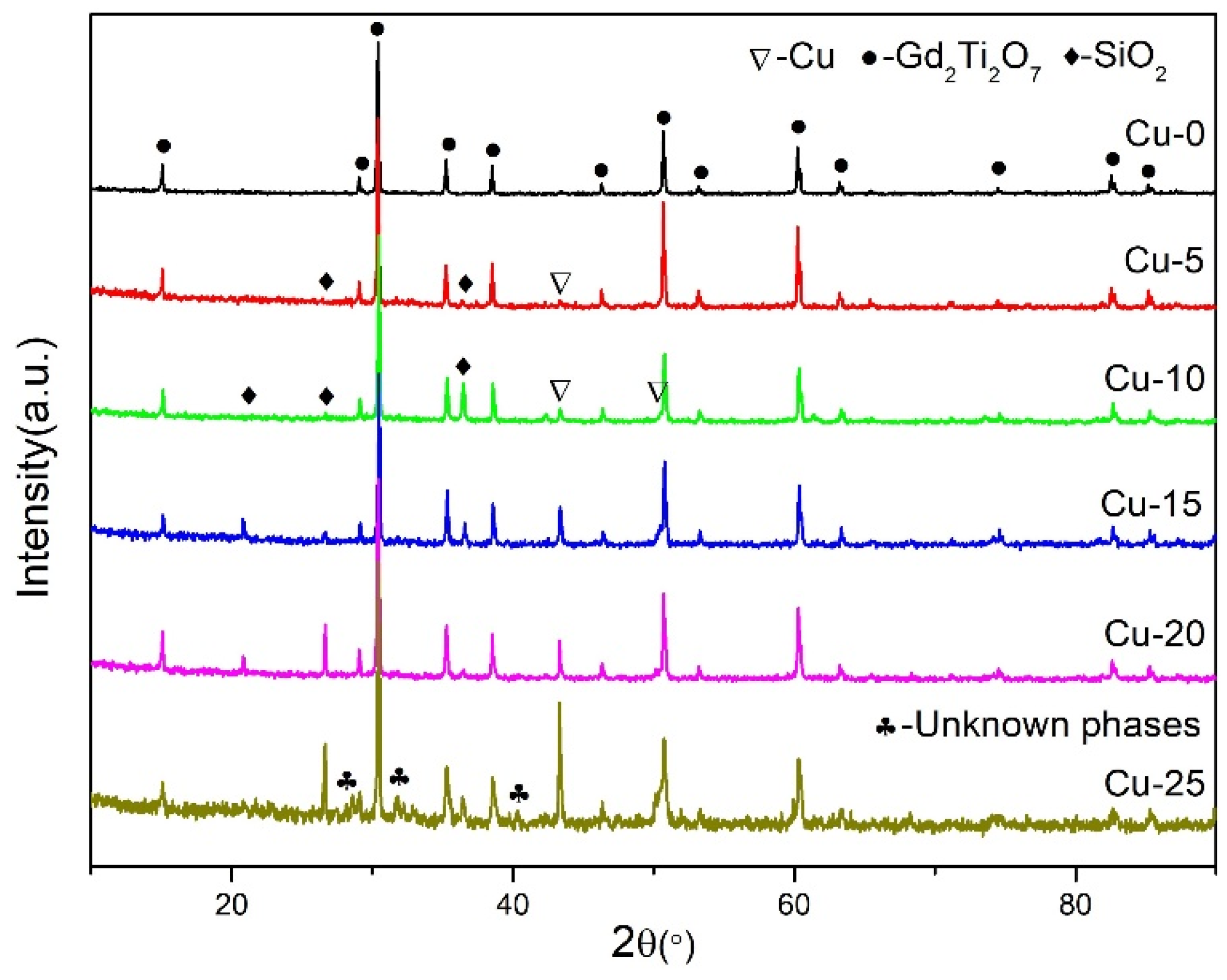
3.1. Temperature and Powder XRD Analysis
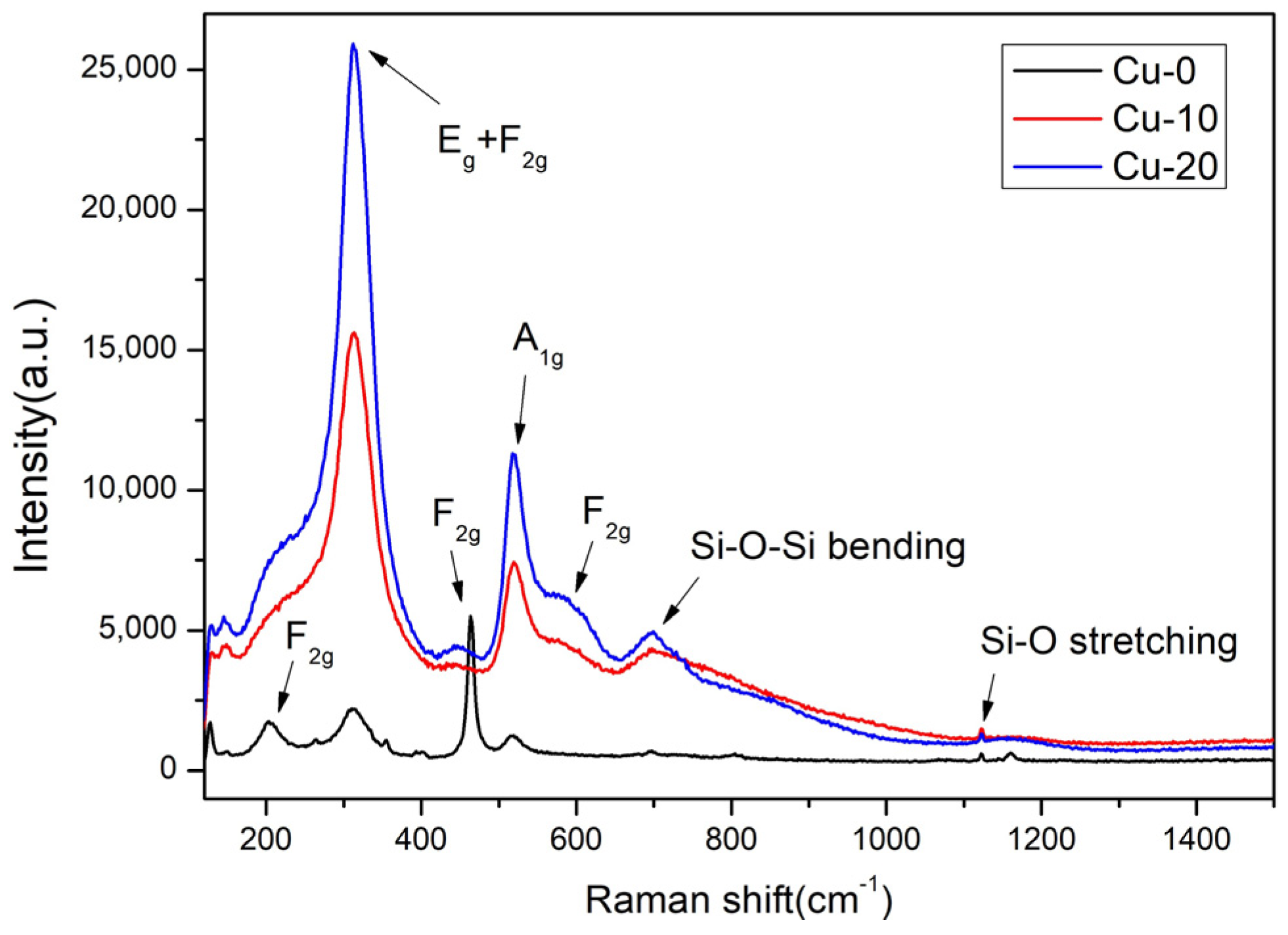
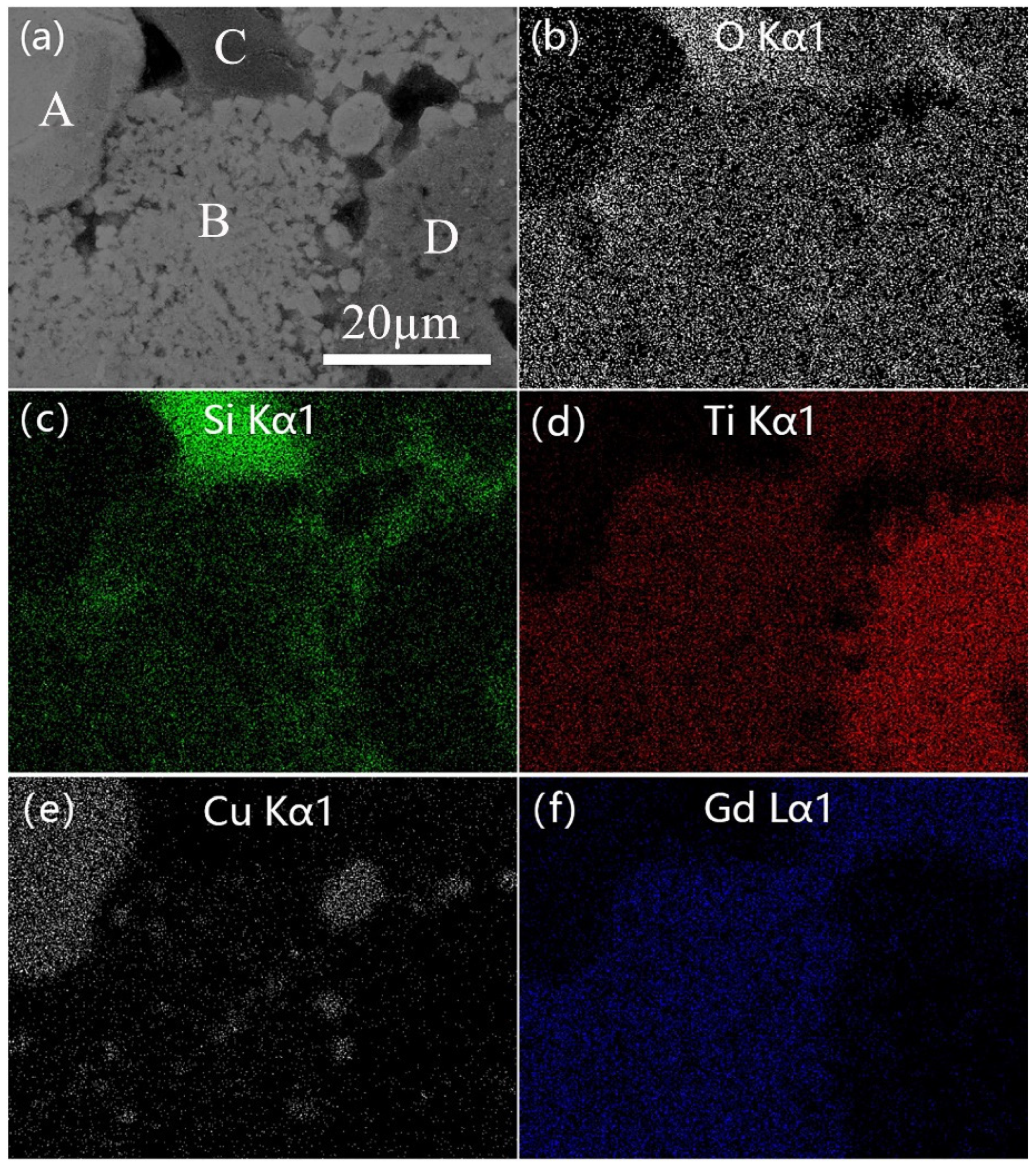
3.2. Raman Analysis and Microstructure Characterization
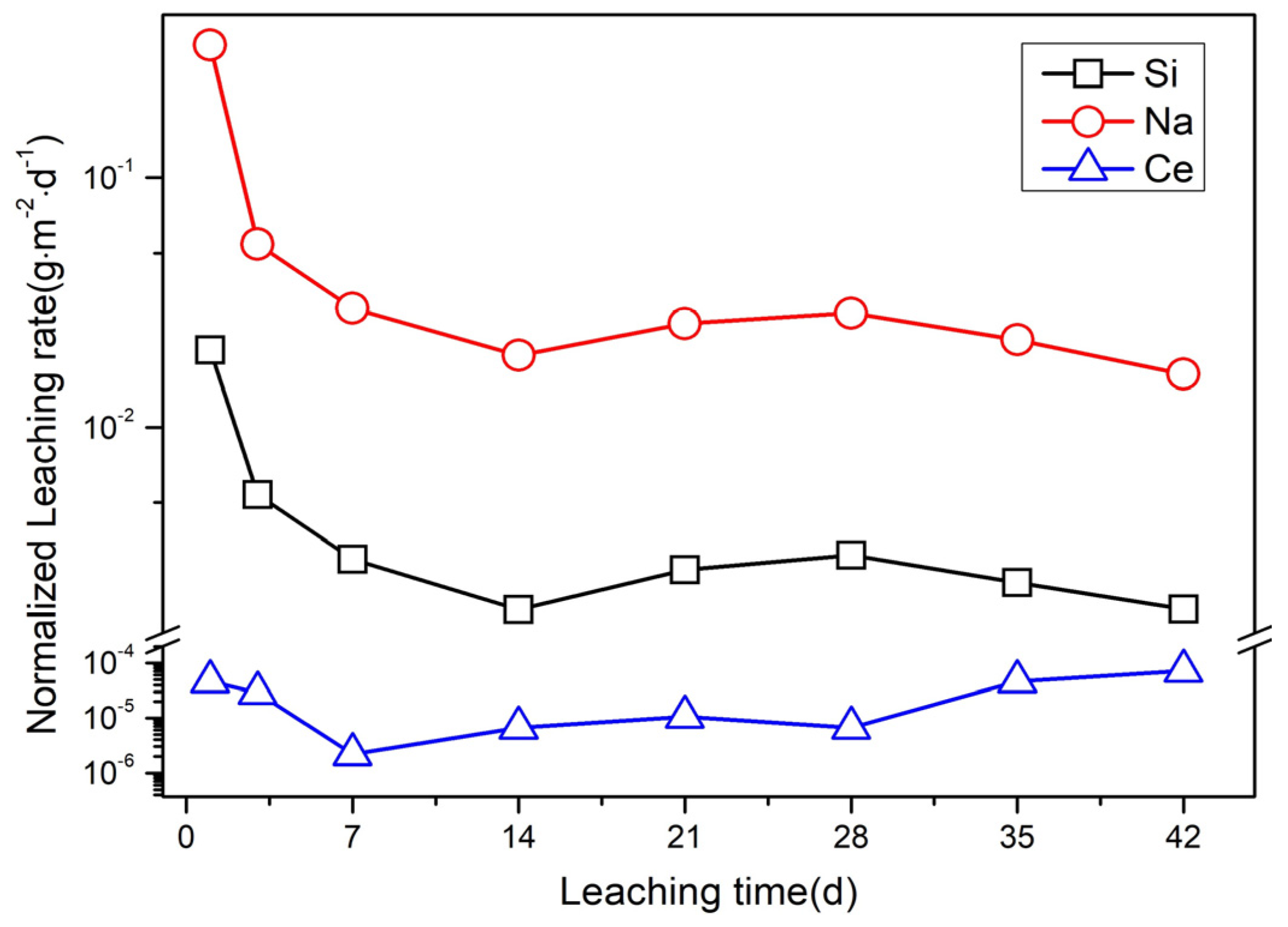
3.3. Chemical Durability Measurement by PCT Leaching Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Atomic Energy Agency. Design and Operation of High Level Waste, Vitrification and Storage Facility (Technical Report Series No. 176); IAEA: Vienna, Austria, 1977. [Google Scholar]
- Ojovan, M.I.; Lee, W.E. An Introduction to Nuclear Waste Immobilization; Elsevier Ltd.: Oxford, UK, 2005; pp. 213–267. [Google Scholar]
- Caurant, D.; Loiseau, P.; Majérus, O.; Aubin-Chevaldonnet, V.; Bardez, I.; Quintas, A. Glasses, Glass-Ceramics and Ceramics for Immobilization of Highly Radioactive Nuclear Wastes; Nova Science Publishers: New York, NY, USA, 2009. [Google Scholar]
- Zhu, Y.G.; Shaw, G. Soil contamination with radionuclides and potential remediation. Chemosphere 2000, 41, 121–128. [Google Scholar] [PubMed]
- Mao, X.H.; Qin, Z.G.; Yuan, X.N.; Wang, C.M.; Cai, X.N.; Zhao, W.X.; Zhao, K.; Yang, P.; Fan, X.L. Immobilization of simulated radioactive soil waste containing cerium by self-propagating high-temperature synthesis. J. Nucl. Mater. 2013, 443, 428–431. [Google Scholar] [CrossRef]
- Gavrilescu, M.; Pavel, L.V.; Cretescu, I. Characterization and remediation of soils contaminated with uranium. J. Hazard. Mater. 2009, 163, 475–510. [Google Scholar] [CrossRef] [PubMed]
- Yasunari, T.J.; Stohl, A.; Hayano, R.S.; Burkhart, J.F.; Eckhardt, S.; Yasunari, T. Cesium-137 deposition and contamination of Japanese soils due to the Fukushima nuclear accident. Proc. Natl. Acad. Sci. USA 2011, 108, 19530–19534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Wu, T.; Shi, Z.K.; Zhang, D. A very low level radioactive waste landfill soil characteristics and properties of strontium block. Chem. Res. Appl. 2013, 25, 558–562. [Google Scholar]
- Lloyd, J.R.; Renshaw, J.C. Bioremediation of radioactive waste: Radionuclide-microbe interactions in laboratory and field-scale studies. Curr. Opin. Biotech. 2005, 16, 254–260. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, Y.; Lu, X.R.; Mao, X.L.; Song, M.X. Rapid and efficient disposal of radioactive contaminated soil using microwave sintering method. Mater. Lett. 2016, 175, 165–168. [Google Scholar]
- Zhang, S.; Shu, X.Y.; Chen, S.Z.; Yang, H.M.; Hou, C.X.; Mao, X.L.; Chi, F.T.; Song, M.X.; Lu, X.R. Rapid immobilization of simulated radioactive soil waste by microwave sintering. J. Hazard. Mater. 2017, 337, 20–26. [Google Scholar] [PubMed]
- Gin, S.; Abdelouas, A.; Criscenti, L.J.; Ebert, W.L. An international initiative on long-term behavior of high-level nuclear waste glass. Mater. Today 2013, 16, 243–248. [Google Scholar] [CrossRef]
- Mccarthy, G.J.; Ebert, W.L.; Roy, R.; Scheetz, B.E. Interactions between nuclear waste and surrounding rock. Nature 1978, 273, 216–217. [Google Scholar] [CrossRef]
- Loiseau, P.; Caurant, D.; Majerus, O.; Baffier, N.; Fillet, C. Crystallization study of (TiO2, ZrO2)-rich SiO2-Al2O3-CaO glasses. Part II. Surface and internal crystallization processes investigated by differential thermal analysis (DTA). J. Mater. Sci. 2003, 38, 843–852. [Google Scholar]
- Caurant, D.; Majérus, O.; Loiseau, P.; Bardez, I.; Baffier, N.; Dussossoy, J.L. Crystallization of neodymium-rich phases in silicate glasses developed for nuclear waste immobilization. J. Nucl. Mater. 2006, 354, 143–162. [Google Scholar]
- Ringwood, A.E.; Kesson, S.E.; Ware, N.G.; Hibberson, W.; Major, A. Immobilization of high level nuclear reactor wastes in SYNROC. Nature 1979, 278, 219–223. [Google Scholar] [CrossRef]
- Franck, P.; John, M.H.; Urs, S. The current state and future of accessory mineral research. Chem. Geol. 2002, 191, 3–24. [Google Scholar]
- Vance, E.R. Development of ceramic waste forms for high-level nuclear waste over the last 30 years. Mater. Res. Soc. Symp. Proc. 2006, 985, 135–140. [Google Scholar] [CrossRef]
- Weber, W.J.; Navrotsky, A.; Stefanovsky, S.; Vance, E.R.; Vernaz, E. Materials science of high-level nuclear waste immobilization. MRS Bull. 2009, 34, 46–53. [Google Scholar] [CrossRef]
- Zhang, K.B.; Yin, D.; Han, P.W.; Zhang, H.B. Two-step synthesis of zirconolite-rich ceramic waste matrice and its physicochemical properties. Int. J. Appl. Ceram. Technol. 2018, 15, 171–178. [Google Scholar] [CrossRef]
- Muthuraman, M.; Dhas, N.A.; Patil, K.C. Combustion synthesis of oxide materials for nuclear waste immobilization. Bull. Mater. Sci. 1994, 17, 977–987. [Google Scholar] [CrossRef]
- Muthuraman, M.; Patil, K.C.; Senbagaraman, S.; Umarji, A.M. Sintering, microstructure and dilatometric studies of combustion synthesized Synroc phases. Mater. Res. Bull. 1996, 31, 1375–1381. [Google Scholar] [CrossRef]
- He, Z.S.; Zhang, K.B.; Xue, J.L.; Zhao, W.W.; Zhang, H.B. Self-propagating chemical furnace synthesis of nanograin Gd2Zr2O7 ceramic and its aqueous durability. J. Nucl. Mater. 2018, 512, 385–390. [Google Scholar] [CrossRef]
- Zhang, K.B.; Wen, G.J.; Zhang, H.B.; Teng, Y.C. Self-propagating high-temperature synthesis of Y2Ti2O7 pyrochlore and its aqueous durability. J. Nucl. Mater. 2015, 465, 1–5. [Google Scholar]
- Zhang, K.B.; He, Z.S.; Peng, L.; Zhang, H.B.; Lu, X.R. Self-propagating synthesis of Y2-xNdxTi2O7 pyrochlore and its aqueous durability as nuclear waste form. Scripta. Mater. 2018, 146, 300–303. [Google Scholar]
- Zhang, K.B.; He, Z.S.; Xue, J.L.; Zhao, W.W.; Zhang, H.B. Self-propagating synthesis of Y2-xNdxTi2O7 pyrochlores using CuO as the oxidant and its characterizations as waste form. J. Nucl. Mater. 2018, 507, 93–100. [Google Scholar] [CrossRef]
- Zhang, K.B.; Yin, D.; Peng, L.; Wu, J.J. Self-propagating synthesis and CeO2 immobilization of zirconolite-rich composites using CuO as the oxidant. Ceram. Int. 2017, 43, 1415–1423. [Google Scholar] [CrossRef]
- Peng, L.; Zhang, K.B.; Yin, D.; Wu, J.J.; He, S.H.; He, H.M. Self-propagating synthesis, mechanical property and aqueous durability of Gd2Ti2O7 pyrochlore. Ceram. Int. 2016, 42, 18907–18913. [Google Scholar] [CrossRef]
- Peng, L.; Zhang, K.B.; He, Z.S.; Yin, D.; Xue, J.L.; Xu, C.; Zhang, H.B. Self-propagating high-temperature synthesis of ZrO2 incorporated Gd2Ti2O7 pyrochlore. J. Adv. Ceram. 2018, 7, 41–49. [Google Scholar] [CrossRef]
- He, Z.S.; Zhang, K.B.; Peng, L.; Zhao, W.W.; Xue, J.L.; Zhang, H.B. Self-propagating plus quick pressing synthesis and characterizations of Gd2-xNdxTi1.3Zr0.7O7 (0 ≤ x ≤ 1.4) pyrochlores. J. Nucl. Mater. 2018, 504, 61–67. [Google Scholar] [CrossRef]
- Kim, H.S.; Joung, C.Y.; Lee, B.H.; Oh, J.Y.; Koo, Y.H.; Heimgartner, P. Applicability of CeO2 as a surrogate for PuO2 in a MOX fuel development. J. Nucl. Mater. 2008, 378, 98–104. [Google Scholar] [CrossRef]
- ASTM Committee. Standard Test Methods for Determining Chemical Durability of Nuclear, Hazardous, and Mixed Waste Glasses and Multiphase Glass Ceramics: The Product Consistency Test (PCT); ASTM International: West Conshohocken, PA, USA, 2002. [Google Scholar]
- Ma̧czka, M.; Hanuza, J.; Hermanowicz, K.; Fuentes, A.F.; Matsuhira, K.; Hiroi, Z. Temperature-dependent Raman scattering studies of the geometrically frustrated pyrochlores Dy2Ti2O7, Gd2Ti2O7 and Er2Ti2O7. J. Raman Spectrosc. 2008, 39, 537–544. [Google Scholar] [CrossRef]
- Ma̧czka, M.; Sanjuán, M.L.; Fuentes, A.F.; Macalik, L.; Hanuza, J.; Matsuhira, K.; Hiroi, Z. Temperature-dependent studies of the geometrically frustrated pyrochlores Ho2Ti2O7 and Dy2Ti2O7. Phys. Rev. B 2009, 79, 214437. [Google Scholar] [CrossRef]
- Zhang, F.X.; Manoun, B.; Saxena, S.K. Pressure-induced order-disorder transitions in pyrochlore RE2Ti2O7 (RE = Y, Gd). Mater. Lett. 2006, 60, 2773–2776. [Google Scholar] [CrossRef]
- Sanjuán, M.L.; Guglieri, C.; DíazMoreno, S.; Aquilanti, G.; Fuentes, A.F.; Olivi, L.; Chaboy, J. Raman and X-ray absorption spectroscopy study of the phase evolution induced by mechanical milling and thermal treatments in R2Ti2O7 pyrochlores. Phys. Rev. B 2011, 84, 104207. [Google Scholar] [CrossRef]
- Zhang, Y.; Stewart, M.W.A.; Li, H.; Carter, M.L.; Vance, E.R.; Moricca, S. Zirconolit-rich titanate ceramics for immobilisation of actinides-Waste form/HIP can interactions and chemical durability. J. Nucl. Mater. 2009, 395, 69–74. [Google Scholar] [CrossRef]
- Xue, J.L.; Zhang, K.B.; He, Z.H.; Zhao, W.W.; Li, W.W.; Xie, D.Y.; Zhang, H.B. Rapid disposal of simulated Ce-bearing radioactive soil waste using self-propagating synthesized zirconolite-rich waste matrice. Ceram. Int. 2018, 44, 14534–14540. [Google Scholar]
- Wu, L.; Li, Y.X.; Teng, Y.C.; Meng, G.L. Preparation and characterization of borosilicate glass-ceramics containing zirconolite and titanite crystalline phases. J. Non-Cryst. Solids 2013, 380, 123–127. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Lee, W.E. Glassy Wasteforms for Nuclear Waste Immobilization. Metall. Mater. Trans. A 2011, 42, 837–851. [Google Scholar] [CrossRef]


| Composition | SiO2 | Al2O3 | Fe2O3 | CaO | K2O | MgO | Na2O | TiO2 |
|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | 66.32 | 16.57 | 5.87 | 4.67 | 2.86 | 1.66 | 0.81 | 0.74 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, J.; Zhang, K.; He, Z.; Zhao, W.; Li, W.; Xie, D.; Luo, B.; Xu, K.; Zhang, H. Rapid Immobilization of Simulated Radioactive Soil Waste Using Self-Propagating Synthesized Gd2Ti2O7 Pyrochlore Matrix. Materials 2019, 12, 1163. https://doi.org/10.3390/ma12071163
Xue J, Zhang K, He Z, Zhao W, Li W, Xie D, Luo B, Xu K, Zhang H. Rapid Immobilization of Simulated Radioactive Soil Waste Using Self-Propagating Synthesized Gd2Ti2O7 Pyrochlore Matrix. Materials. 2019; 12(7):1163. https://doi.org/10.3390/ma12071163
Chicago/Turabian StyleXue, Jiali, Kuibao Zhang, Zongsheng He, Wenwen Zhao, Weiwei Li, Dayan Xie, Baozhu Luo, Kai Xu, and Haibin Zhang. 2019. "Rapid Immobilization of Simulated Radioactive Soil Waste Using Self-Propagating Synthesized Gd2Ti2O7 Pyrochlore Matrix" Materials 12, no. 7: 1163. https://doi.org/10.3390/ma12071163
APA StyleXue, J., Zhang, K., He, Z., Zhao, W., Li, W., Xie, D., Luo, B., Xu, K., & Zhang, H. (2019). Rapid Immobilization of Simulated Radioactive Soil Waste Using Self-Propagating Synthesized Gd2Ti2O7 Pyrochlore Matrix. Materials, 12(7), 1163. https://doi.org/10.3390/ma12071163





