High Performance Bacteria Anchored by Nanoclay to Boost Straw Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Materials
2.2. Preparation of SRA
2.3. Straw Degradation Investigation
2.4. Pot Experiment
2.5. Pyrosequencing of 16S rRNA Gene
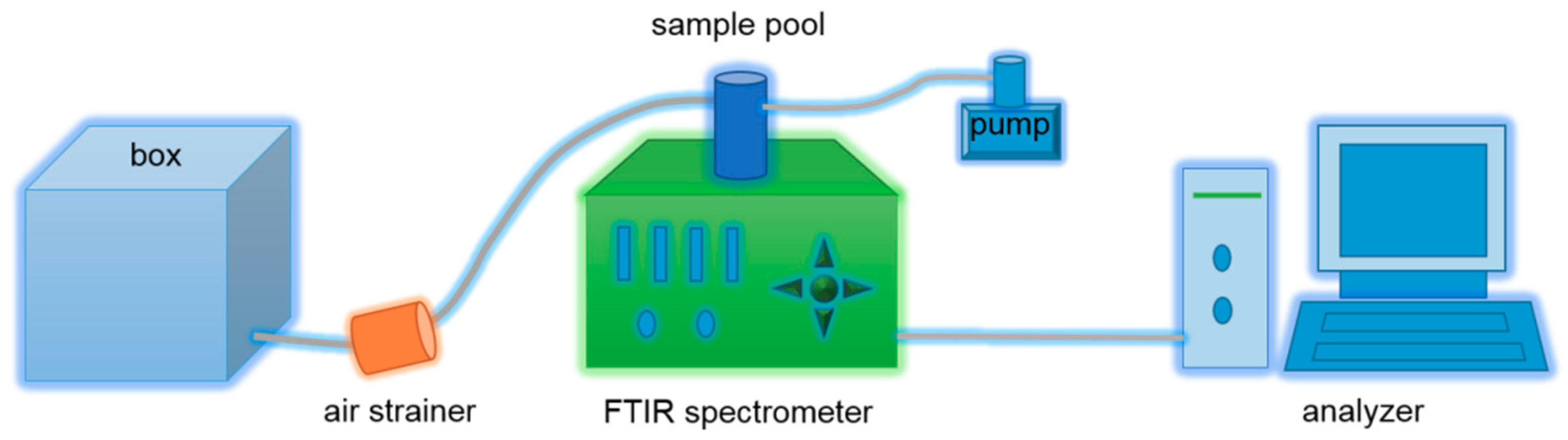
2.6. Gas Emission Investigation
2.7. Determination of Chlorophyll and Photosynthetic Efficiency
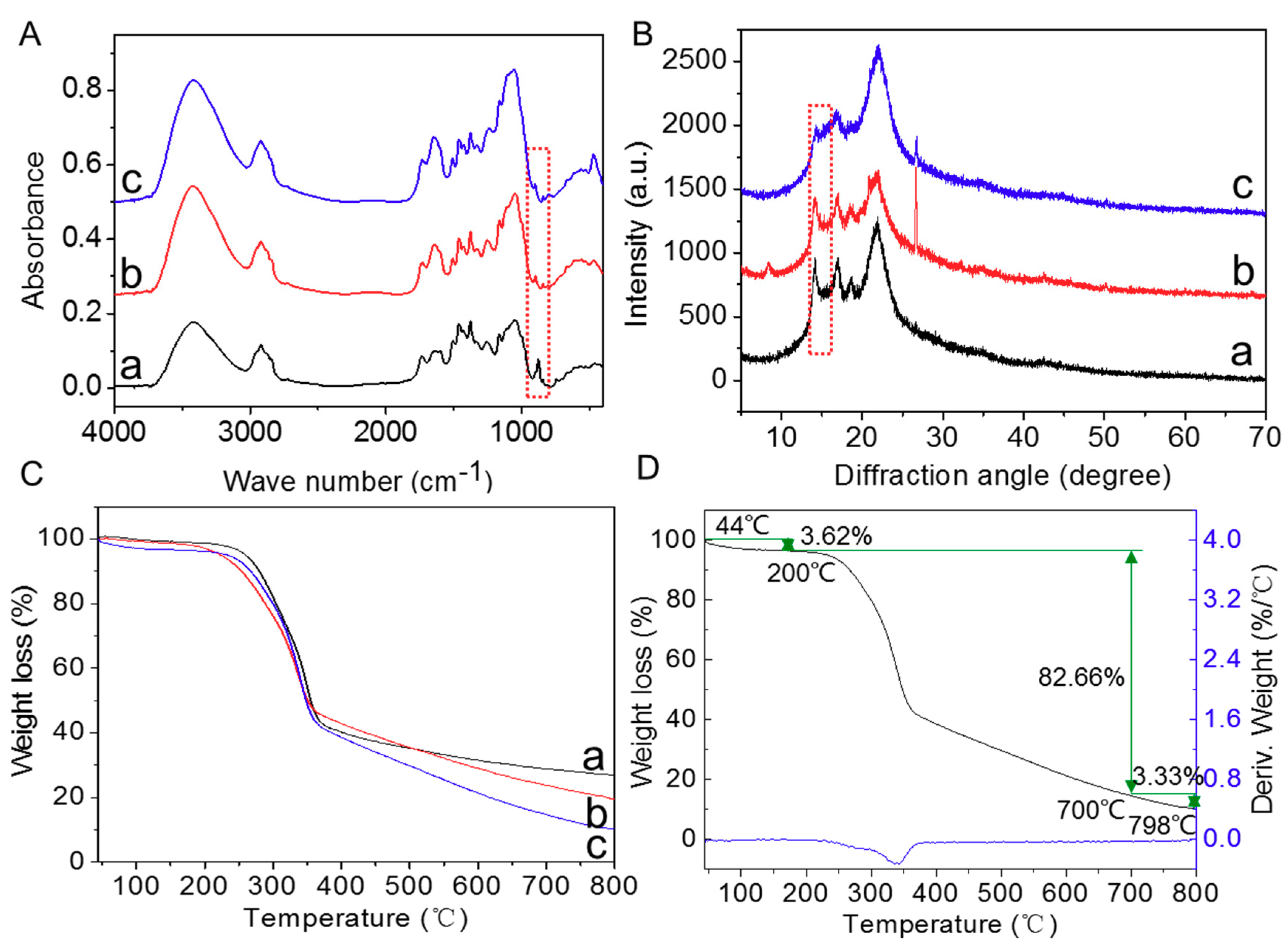
2.8. Chemical Characterization Analysis
2.9. Statistical Analyses
3. Results
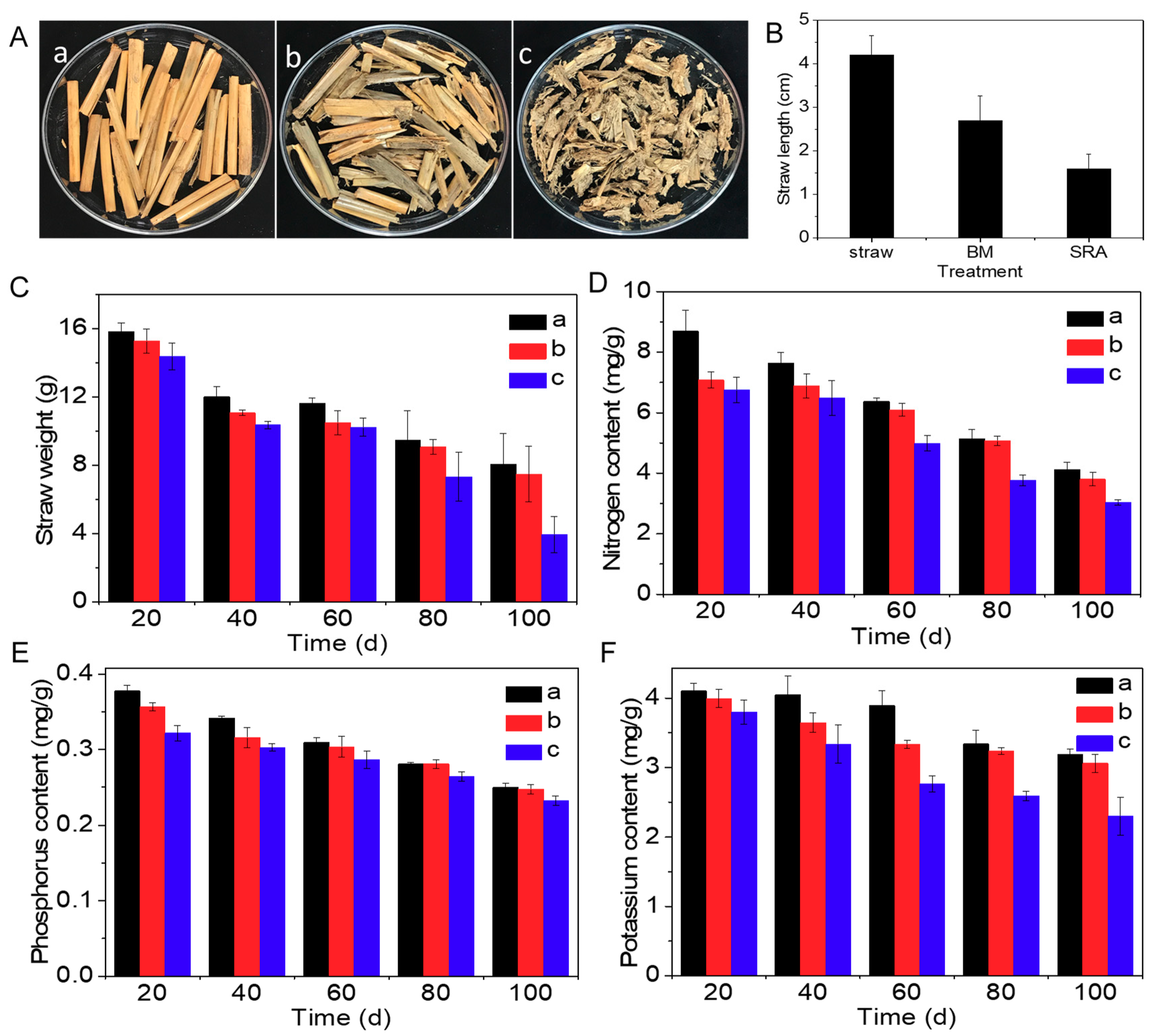
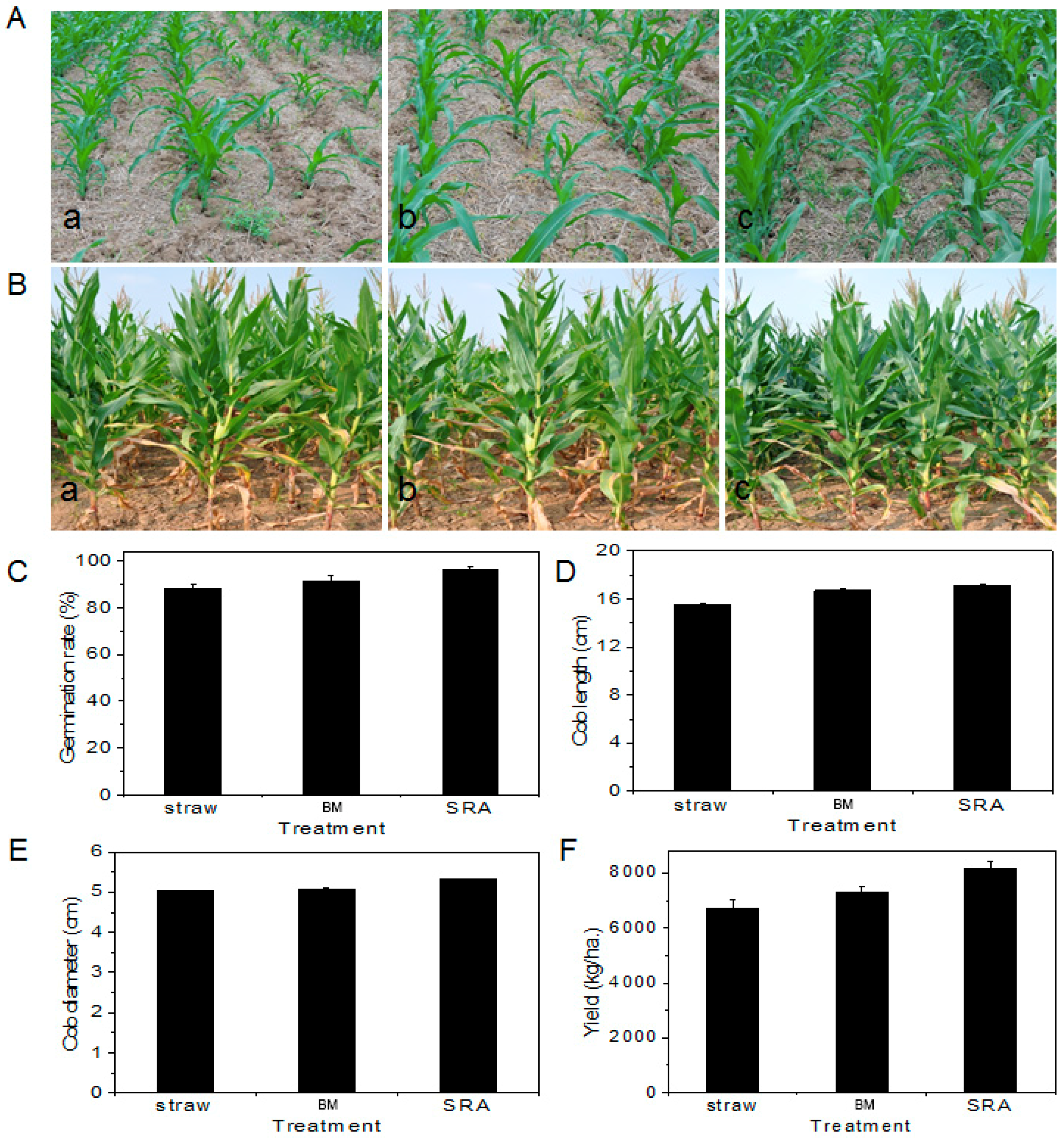
3.1. Effect of SRA on Degradation of Straw
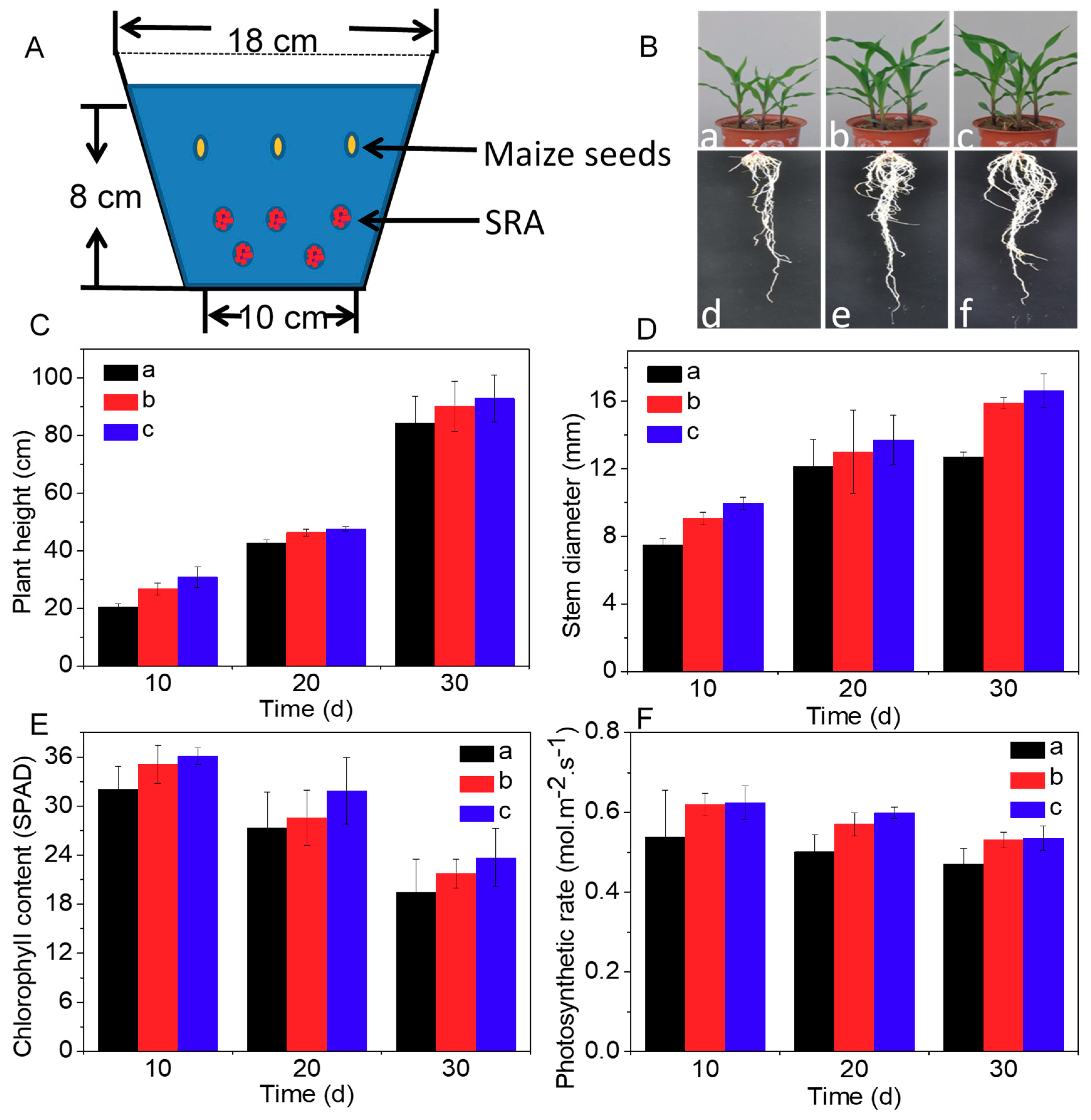
3.2. Effect of SRA on Growth of Maize
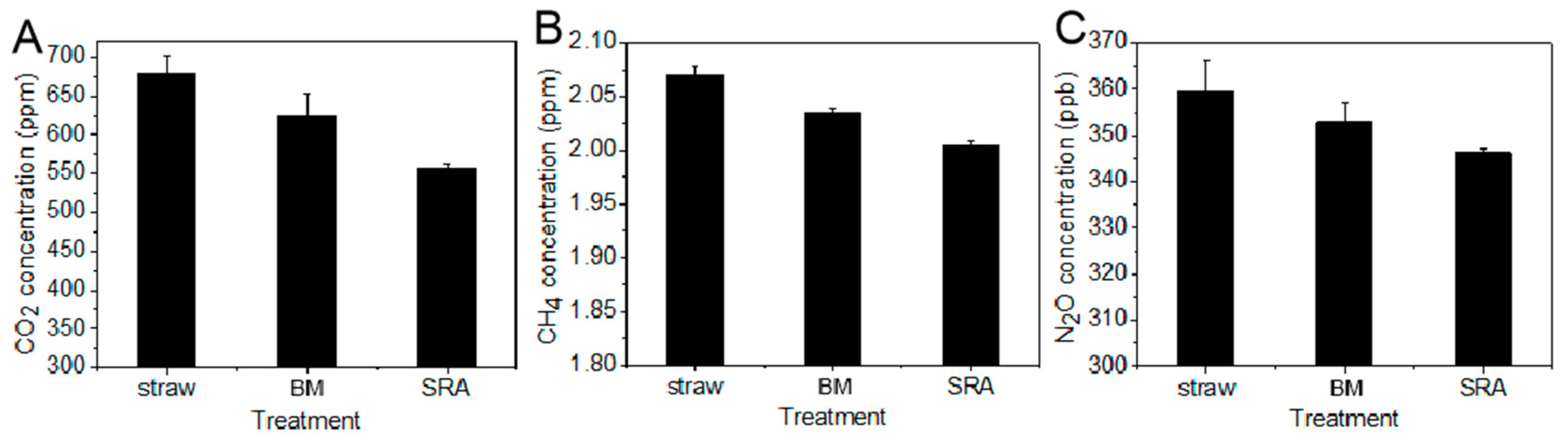
3.3. Effect of SRA on the Emission of Greenhouse Gas
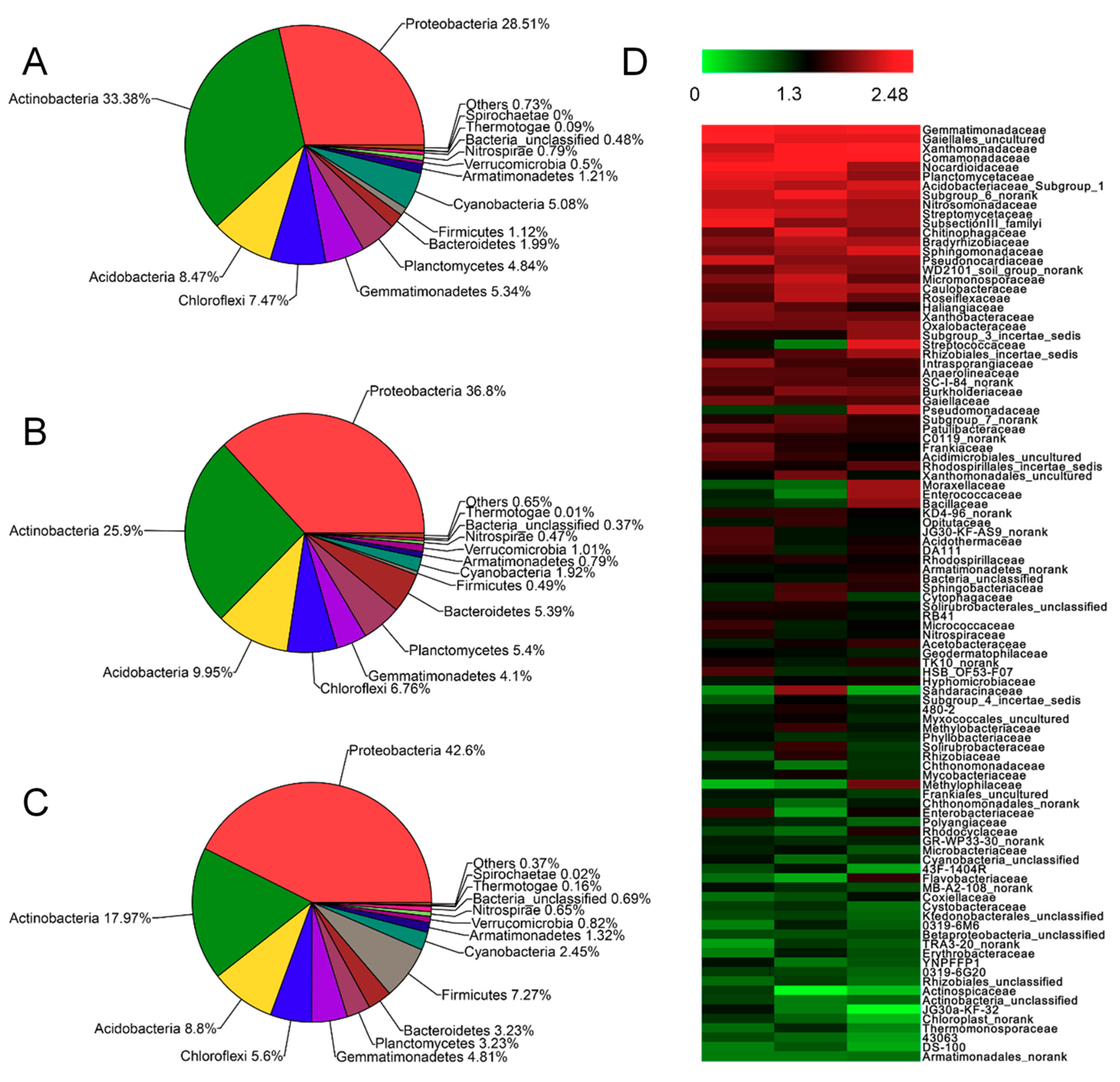
3.4. Effect of SRA on the Soil Microbial Community
3.5. Structure and Interaction Analyses
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, B.; Liu, E.K.; Tian, Q.; Yan, C.; Zhang, Y. Soil nitrogen dynamics and crop residues. A review. Agron. Sustain. Dev. 2014, 34, 429–442. [Google Scholar] [CrossRef]
- Han, W.; He, M. The application of exogenous cellulase to improve soil fertility and plant growth due to acceleration of straw decomposition. Bioresour. Technol. 2010, 101, 3724–3731. [Google Scholar] [CrossRef]
- Li, F.Y.; Cao, X.D.; Zhao, L.; Yang, F.; Wang, J.F.; Wang, S.W. Short-term effects of raw rice straw and its derived biochar on greenhouse gas emission in five typical soils in China. J. Soil Sci. Plant Nutr. 2013, 59, 800–811. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.B.; Zhao, B.Z.; Yan, P.; Zhou, G.X.; Xin, X.L. Effects of straw amendment and moisture on microbial communities in Chinese fluvo-aquic soil. J. Soils Sediments 2014, 14, 1829–1840. [Google Scholar] [CrossRef]
- Yan, X.; Akiyam, H.; Yagi, K.; Gilkes, R.J.; Prakongkep, N. Global estimations of the inventory and mitigation potential of methane emissions from rice cultivation conducted using the 2006 IPCC guidelines. Glob. Biogeochem. Cycle 2009, 23, 627–634. [Google Scholar] [CrossRef]
- Hu, N.J.; Wang, B.J.; Gu, Z.H.; Tao, B.R.; Zhang, Z.W.; Hu, S.J.; Zhu, L.Q.; Meng, Y.L. Effects of different straw returning modes on greenhouse gas emissions and crop yields in a rice-wheat rotation system. Agric. Ecosyst. Environ. 2016, 223, 115–122. [Google Scholar] [CrossRef]
- Wu, F.P.; Jia, Z.K.; Wang, S.G.; Chang, S.; Startsev, A. Contrasting effects of wheat straw and its biochar on greenhouse gas emissions and enzyme activities in a Chernozemic soil. Biol. Fertil. Soils 2013, 49, 555–565. [Google Scholar] [CrossRef]
- Liu, C.; Lu, M.; Cui, J.; Li, B.; Fang, C.M. Effects of straw carbon input on carbon dynamics in agricultural soils: A meta-analysis. Glob. Chang. Biol. 2014, 20, 1366–1381. [Google Scholar] [CrossRef]
- Wyman, C.E.; Dale, B.E.; Elander, R.T.; Holtzapple, M.; Ladisch, M.R.; Lee, Y.Y. Coordinated development of leading biomass pretreatment technologies. Bioresour. Technol. 2005, 96, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.D.; Smith, T.J.; Li, N.; Zong, M.H. Novel renewable ionic liquids as highly effective solvents for pretreatment of rice straw biomass by selective removal of lignin. Biotechnol. Bioeng. 2012, 109, 2484–2493. [Google Scholar] [CrossRef]
- Zhu, S.D.; Wu, Y.X.; Yu, Z.N.; Wang, C.W.; Yu, F.Q.; Jin, S.W.; Ding, Y.G.; Chi, R.A.; Liao, J.T.; Zhang, Y. Comparison of three microwave/chemical pretreatment processes for enzymatic hydrolysis of rice straw. Biosyst. Eng. 2006, 93, 279–283. [Google Scholar] [CrossRef]
- Zhai, L.C.; Xu, P.; Zhang, Z.B.; Li, S.K.; Xie, R.Z.; Zhai, L.F.; Wei, B.H. Effects of deep vertical rotary tillage on dry matter accumulation and grain yield of summer maize in Huang-Huai-Hai Plain of China. Soil Till. Res. 2017, 170, 167–174. [Google Scholar] [CrossRef]
- Ma, J.S.; Zhang, K.K.; Huang, M.; Hector, S.; Liu, B.; Tong, C.Y.; Liu, Q.; Zeng, J.R.; Gao, Y.; Xu, T.; et al. Involvement of fenton chemistry in rice straw degradation by the lignocellulolytic bacterium Pantoea ananatis Sd-1. Biotechnol. Biofuels 2016, 9, 211. [Google Scholar] [CrossRef]
- Sun, L.; Liu, T.; Müller, B.; Schnürer, A. The microbial community structure in industrial biogas plants influences the degradation rate of straw and cellulose in batch tests. Biotechnol. Biofuels 2016, 9, 128. [Google Scholar] [CrossRef]
- Nakamura, A.; Tun, C.C.; Asakawa, S.; Kimura, M. Microbial community responsible for the decomposition of rice straw in a paddy field: Estimation by phospholipid fatty acid analysis. Biol. Fertil. Soils 2003, 38, 288–295. [Google Scholar] [CrossRef]
- Chen, H.; Wang, A. Kinetic and isothermal studies of lead ion adsorption onto palygorskite clay. J. Colloid Interface Sci. 2007, 307, 309–316. [Google Scholar] [CrossRef]
- Li, M.; Wu, Z.S.; Kao, H.T. Study on preparation, structure and thermal energy storage property of capric-palmitic acid/attapulgite composite phase change materials. Appl. Energy 2011, 88, 3125–3132. [Google Scholar] [CrossRef]
- Jiang, N.; Cai, D.Q.; He, L.L.; Zhong, N.Q.; Wen, H.; Zhang, X.; Wu, Z.Y. A facile approach to remediate the microenvironment of saline-alkali soil. ACS Sustain. Chem. Eng. 2015, 3, 374–380. [Google Scholar] [CrossRef]
- Huang, J.H.; Liu, Y.F.; Jin, Q.Z.; Wang, X.G.; Yang, J. Adsorption studies of a water soluble dye, Reactive Red MF-3B, using sonication-surfactant-modified attapulgite clay. J. Hazard Mater. 2007, 143, 541–548. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, S.D.; Yuhshan, H. Removal of fluoride ions from aqueous solution using modified attapulgite as adsorbent. J. Hazard Mater. 2009, 165, 218–222. [Google Scholar] [CrossRef]
- Wang, M.; Sun, X.; Zhong, N.Q.; Cai, D.Q.; Wu, Z.Y. Promising approach for improving adhesion capacity of foliar nitrogen fertilizer. ACS Sustain. Chem. Eng. 2015, 3, 499–506. [Google Scholar] [CrossRef]
- Frost, R.L.; Xi, Y.F.; He, H.P. Synthesis, characterization of palygorskite supported zero-valent iron and its application for methylene blue adsorption. J. Colloid Interface Sci. 2010, 341, 53–161. [Google Scholar] [CrossRef]
- Xiang, Y.B.; Wang, M.; Sun, X.; Cai, D.Q.; Wu, Z.Y. Controlling pesticide loss through nanonetworks. ACS Sustain. Chem. Eng. 2014, 2, 918–924. [Google Scholar] [CrossRef]
- Huang, S.W.; Sheng, P.; Zhang, H.Y. Isolation and identification of cellulolytic bacteria from the gut of Holotrichia parallela larvae (Coleoptera: Scarabaeidae). Int. J. Mol. Sci. 2012, 13, 2563–2577. [Google Scholar] [CrossRef]
- Huang, S.W.; Deng, G.J.; Yang, Y.; Wu, Z.Y.; Wu, L.F. Optimization of Endoglucanase Production from a Novel Bacterial Isolate, Arthrobacter sp. HPG166 and Characterization of Its Properties. Braz. Arch. Biol. Technol. 2015, 58, 692–701. [Google Scholar] [CrossRef]
- Wang, W.J.; Smith, C.; Chalk, P.M.; Chen, D.L. Evaluating chemical and physical indices of nitrogen mineralization capacity with an unequivocal reference. Soil Sci. Soc. Am. J. 2001, 65, 368–376. [Google Scholar] [CrossRef]
- Aleksandra, S.S.; Zoran, D.; Dobrivoj, P.; Dubravka, S.; Renata, I.; Dragana, J.; Radmila, P. Levels of macro and trace elements in vegetable crops as influenced by metallurgical slag addition to marginal soil. Fresenius Environ. Bull. 2017, 26, 1017–1025. [Google Scholar]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothor: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Akhtar, T.; Zia-ur-Rehman, M.; Naeem, A.; Nawaz, R.; Ali, S.; Murtaza, G.; Maqsood, M.A.; Azhar, M.; Khalid, H.; Rizwan, M. Photosynthesis and growth response of maize (Zea mays L.) hybrids exposed to cadmium stress. Environ. Sci. Pollut. Res. 2016, 24, 1–9. [Google Scholar] [CrossRef]
- Ma, J.; Li, X.L.; Xu, H.; Han, Y.; Cai, Z.C.; Yagi, K. Effects of nitrogen fertilizer and wheat straw application on CH4 and N2O emissions from a paddy rice field. Aust. J. Soil Res. 2007, 45, 359–367. [Google Scholar] [CrossRef]
- Xia, L.L.; Wang, S.W.; Yan, X.Y. Effects of long-term straw incorporation on the net global warming potential and the net economic benefit in a rice–wheat cropping system in China. Agric. Ecosyst. Environ. 2014, 197, 118–127. [Google Scholar] [CrossRef]
- Khosa, M.K.; Sidhu, B.S.; Benbi, D.K. Effect of organic materials and rice cultivars on methane emission from rice field. J. Environ. Biol. 2010, 31, 281–285. [Google Scholar]
- Ma, E.D.; Zhang, G.B.; Ma, J.; Xu, H.; Cai, Z.C.; Yagi, K. Effects of rice straw returning methods on N2O emission during wheat-growing season. Nutr. Cycl. Agroecosyst. 2010, 88, 463–469. [Google Scholar] [CrossRef]
- Reddy, A.P.; Simmons, C.W.; Haeseleer, P.D.; Khudyakov, J.; Burd, H.; Hadi, M.; Simmons, B.A.; Singer, S.W.; Thelen, M.P.; Vandergheynst, J.S. Discovery of microorganisms and enzymes involved in high-solids decomposition of rice strawusing metagenomic analyses. PLoS ONE 2013, 8, e77985. [Google Scholar] [CrossRef]
- Qin, S.; Jiao, K.; Lyu, D.; Shi, L.; Liu, L. Effects of maize residue and cellulose-decomposing bacteria inocula on soil microbial community, functional diversity, organic fractions, and growth of Malus hupehensis Rehd. Arch. Agron. Soil Sci. 2015, 61, 173–184. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Tang, C.; Chen, X.; Huang, S.; Zhao, W.; Cai, D.; Wu, Z.; Wu, L. High Performance Bacteria Anchored by Nanoclay to Boost Straw Degradation. Materials 2019, 12, 1148. https://doi.org/10.3390/ma12071148
Li M, Tang C, Chen X, Huang S, Zhao W, Cai D, Wu Z, Wu L. High Performance Bacteria Anchored by Nanoclay to Boost Straw Degradation. Materials. 2019; 12(7):1148. https://doi.org/10.3390/ma12071148
Chicago/Turabian StyleLi, Minghao, Caiguo Tang, Xue Chen, Shengwei Huang, Weiwei Zhao, Dongqing Cai, Zhengyan Wu, and Lifang Wu. 2019. "High Performance Bacteria Anchored by Nanoclay to Boost Straw Degradation" Materials 12, no. 7: 1148. https://doi.org/10.3390/ma12071148
APA StyleLi, M., Tang, C., Chen, X., Huang, S., Zhao, W., Cai, D., Wu, Z., & Wu, L. (2019). High Performance Bacteria Anchored by Nanoclay to Boost Straw Degradation. Materials, 12(7), 1148. https://doi.org/10.3390/ma12071148





