Coplanar Donor-π-Acceptor Dyes Featuring a Furylethynyl Spacer for Dye-Sensitized Solar Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Characterization
2.3. Theoretical Calculations
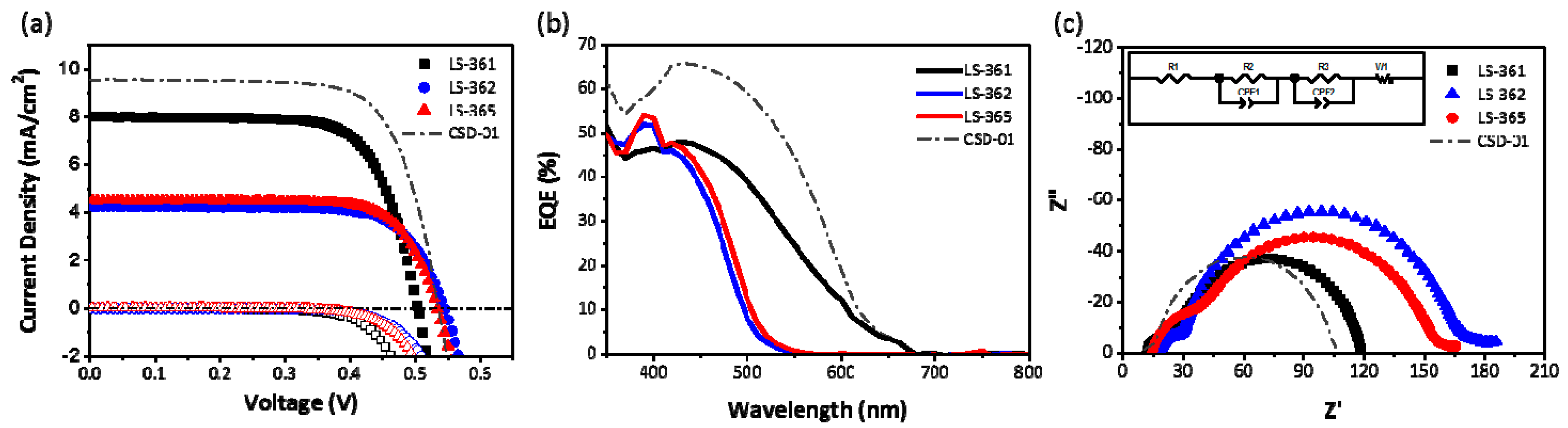
2.4. Photovoltaic Performance
3. Materials and Methods
3.1. General
3.2. Syntheses
3.3. Computational Details
3.4. DSSC Fabrication and Photovoltaic Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Petterson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Freitag, M.; Teuscher, J.; Saygil, Y.; Zhang, X.; Giordano, F.; Liska, P.; Hua, J.; Zakeeruddin, S.M.; Moser, J.-E.; Grätzel, M.; et al. Dye-sensitized solar cells for efficient power generation under ambient lightning. Nat. Photonics 2017, 11, 372–378. [Google Scholar] [CrossRef]
- Kanaparthi, R.K.; Kandhadi, J.; Giribabu, L. Metal-free organic dyes for dye-sensitized solar cells: Recent advances. Tetrahedron 2012, 68, 8383–8393. [Google Scholar] [CrossRef]
- Liang, M.; Chen, J. Arylamine organic dyes for dye-sensitized solar cells. Chem. Soc. Rev. 2013, 42, 3453–3488. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A. Triphenylamine based dyes for dye sensitized solar cells: A review. Solar Energy 2016, 123, 127–144. [Google Scholar] [CrossRef]
- Lin, J.T.; Chen, P.-C.; Yen, Y.-S.; Hsu, Y.-C.; Chou, H.-H.; Yeh, M.-C.P. Organic dyes containing furan moiety for high-performance dye-sensitized solar cells. Org. Lett. 2009, 11, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lv, X.; Shi, D.; Zhou, D.; Cheng, Y.; Zhang, G.; Wang, P. Dye-sensitized solar cells based on organic sensitizers with different conjugated linkers: Furan, bifuran, thiophene, bithiophene, selenophene, and biselenophene. J. Phys. Chem. C 2009, 113, 7469–7479. [Google Scholar] [CrossRef]
- Qu, S.; Wang, B.; Guo, F.; Li, J.; Wu, W.; Kong, C.; Long, Y.; Hua, J. New diketo-pyrrolo-pyrrole (DPP) sensitizer containing a furan moiety for efficient and stable dye-sensitized solar cells. Dyes Pigm. 2012, 92, 1384–1393. [Google Scholar] [CrossRef]
- Cariello, M.; Abdalhadi, S.M.; Yadav, P.; Decoppet, J.-D.; Zakeeruddin, S.M.; Gräzel, M.; Hagfeldt, A.; Cooke, G. An investigation of the roles furan versus thiophene π-bridge play in donor-π-acceptor porphyrin based DSSCs. Dalton Trans. 2018, 47, 6549–6556. [Google Scholar] [CrossRef]
- Al-Eid, M.; Lim, S.H.; Park, K.-W.; Fitzpatrick, B.; Han, C.-H.; Kwak, K.; Hong, J.; Cooke, G. Facile synthesis of metal-free organic dyes featuring a thienylethynyl spacer for dye sensitized solar cells. Dyes Pigm. 2014, 104, 197–203. [Google Scholar] [CrossRef]
- Park, K.-W.; Ahn, S.; Baek, M.H.; Lim, D.-S.; Wiles, A.A.; Kim, M.G.; Hong, J. Coplanar D-π-A organic sensitizers featuring a thienylethynyl spacer for efficient dye-sensitized solar cells. Mater. Express 2017, 7, 43–50. [Google Scholar] [CrossRef]
- Bredas, J.-L. Mind the gap. Mater. Horiz. 2014, 1, 17–19. [Google Scholar] [CrossRef]
- Seo, D.; Park, K.-W.; Kim, J.; Hong, J.; Kwak, K. DFT computational investigation of tuning the electron donating ability in metal-free organic dyes featuring a thienylethynyl spacer for dye sensitized solar cells. Comput. Theor. Chem. 2016, 1081, 30–37. [Google Scholar] [CrossRef]
- Song, J.; Xu, J. Density functional theory study on D-π-A-type organic dyes containing different electron-donors for dye-sensitized solar cells. Bull. Korean Chem. Soc. 2013, 34, 3211–3217. [Google Scholar] [CrossRef]
- Wang, Z.-S.; Cui, Y.; Dan-oh, Y.; Kasada, C.; Shinpo, A.; Hara, K. Thiophene-functionalized courmarin dye for efficient dye-sensitized solar cells: Electron lifetime improved by coadsorption of deoxycholic acid. J. Phys. Chem. C 2007, 111, 7224–7230. [Google Scholar] [CrossRef]
- Wang, Q.; Moser, J.-E.; Gräzel, M. Electrochemical impedance spectroscopic analysis of dye-sensitized solar cells. J. Phys. Chem. B 2005, 109, 14945–14953. [Google Scholar] [CrossRef] [PubMed]
- Hoshikawa, T.; Yamada, M.; Kikuchi, R.; Eguchi, K. Impedance analysis of internal resistance affecting the photoelectrochemical performance of dye-sensitized solar cells. J. Electrochem. Soc. 2015, 152, E68–E73. [Google Scholar] [CrossRef]
- Park, K.-W.; Serrano, L.A.; Ahn, S.; Baek, M.H.; Wiles, A.A.; Cooke, G.; Hong, J. An investigation of the role the donor moiety plays in modulating the efficiency of ‘donor-π-acceptor-π-acceptor’ organic DSSCs. Tetrahedron 2017, 73, 1098–1104. [Google Scholar] [CrossRef]
- Gaussian, R.A.; Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; et al. Gaussian. Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Cho, T.-Y.; Han, C.-W.; Jun, Y.; Yoon, S.-G. Formation of artificial pores in nano-TiO2 photo-electrode films using acetylene-black for high-efficiency dye-sensitized solar cells. Sci. Rep. 2013, 3, 1496. [Google Scholar] [CrossRef] [PubMed]
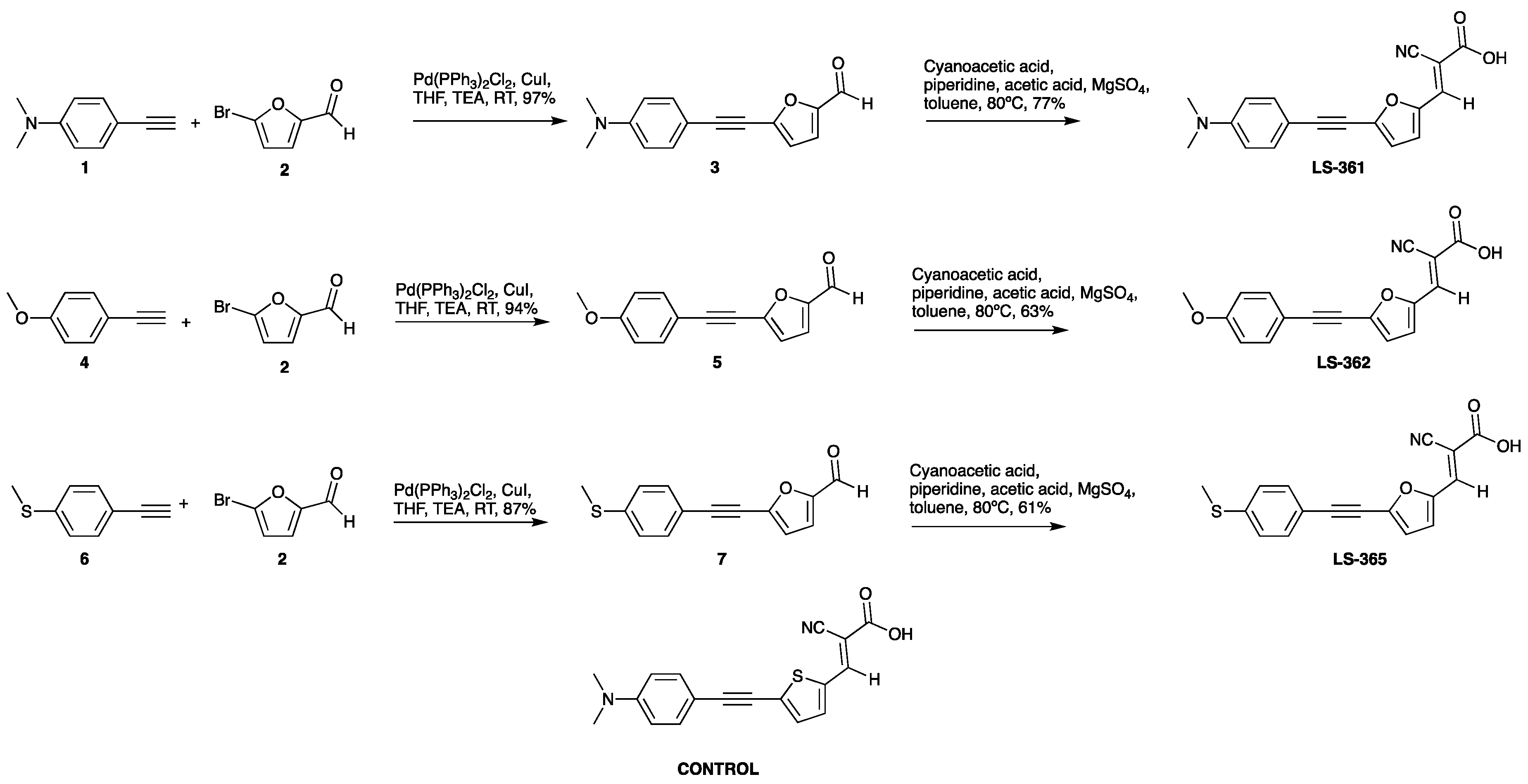
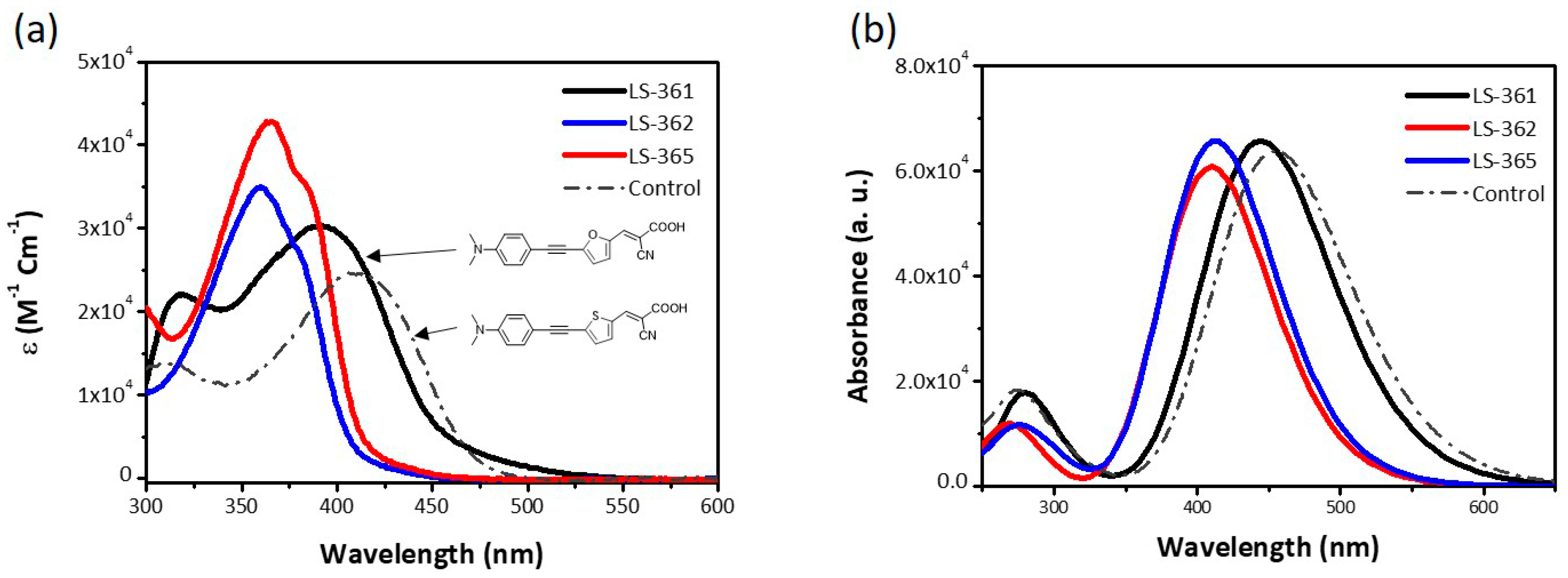
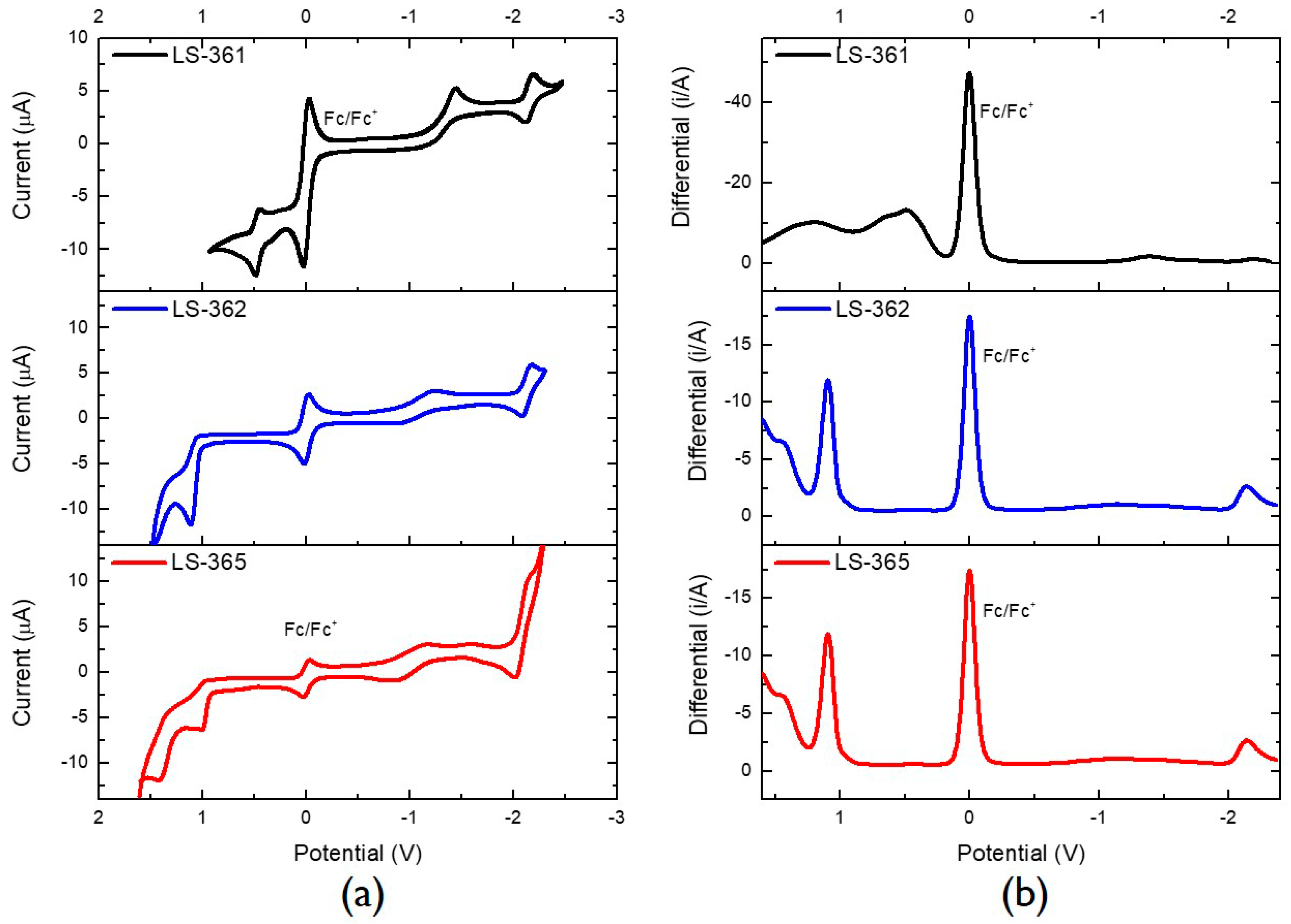
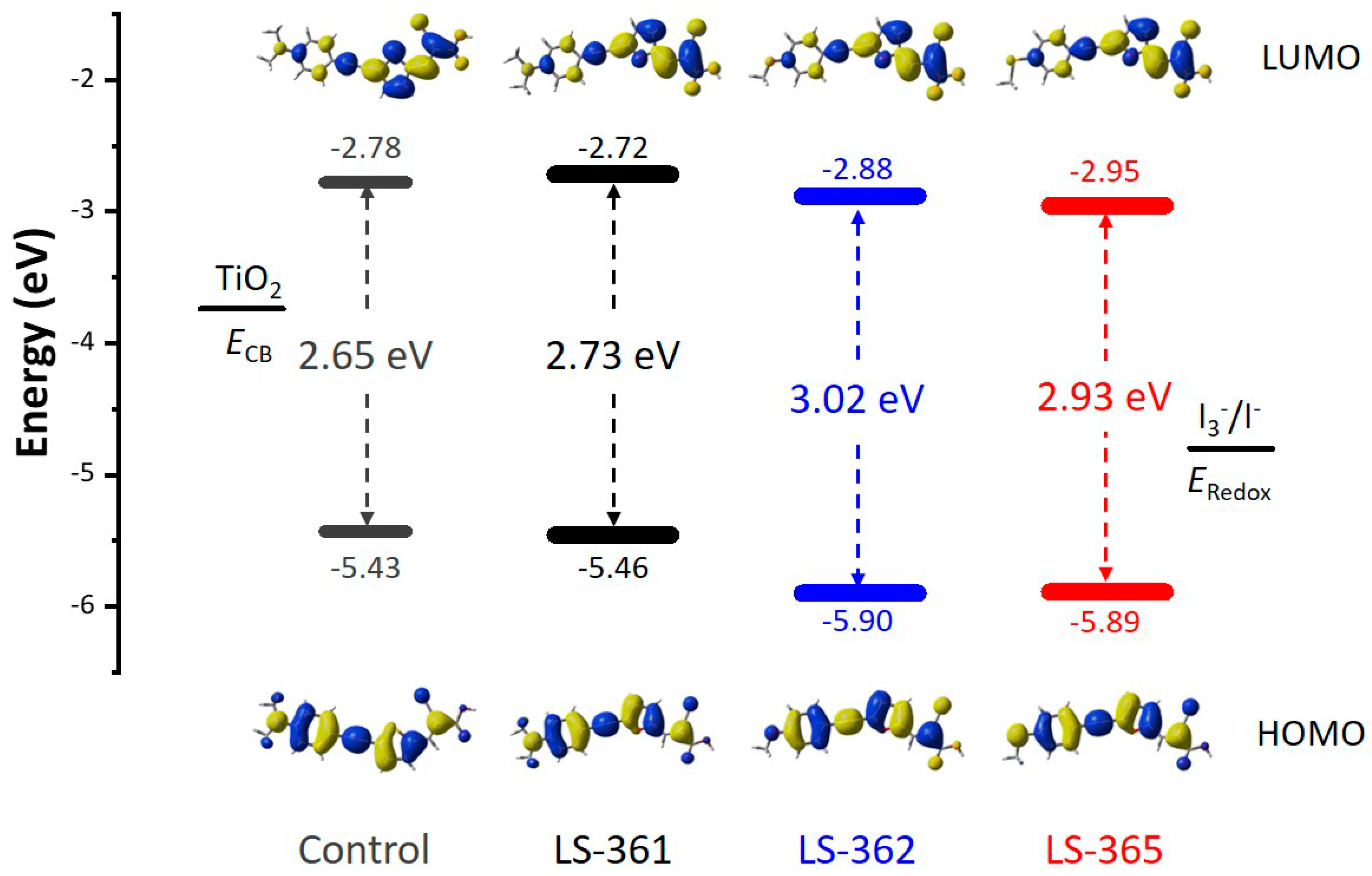
| Dyes | λmax (nm) | E0-0 (eV) | Ered (V) | Eox (V) | IP (eV) | EA (eV) | Egap (eV) |
|---|---|---|---|---|---|---|---|
| LS-361 | 317, 392 | 2.27 | −2.19 | 0.49 | −5.29 | −2.61 | 2.68 |
| LS-362 | 360 | 2.73 | −2.13 | 1.12 | −5.92 | −2.67 | 3.25 |
| LS-365 | 366 | 2.66 | −2.08 | 1.04 | −5.84 | −2.72 | 3.12 |
| Control | 312, 408 | 2.52 | −2.08 | 0.47 | −5.27 | −2.72 | 2.55 |
| Dyes | λmax (Eex) | f | LHE | Transition Assignment |
|---|---|---|---|---|
| LS-361 | 444.38(2.79) | 1.6246 | 0.976 | H − 1→L + 0(12%) H − 0→L + 0(84%) H − 0→L + 1(3%) |
| LS-362 | 409.71(3.03) | 1.5021 | 0.969 | H − 1→L + 0(6%) H − 0→L + 0(90%) |
| LS-365 | 412.50(3.01) | 1.6247 | 0.976 | H − 1→L + 0(12%) H − 0→L + 0(83%) |
| Control | 454.59(2.73) | 1.5768 | 0.974 | H − 1→L + 0(10%) H − 0→L + 0(85%) H − 0→L + 1(2%) |
| Dyes | Voc (V) | Jsc (mA/cm2) | FF | η (%) |
|---|---|---|---|---|
| LS-361 | 0.505 ± 0.001 | 7.97 ± 0.04 | 71.46 ± 0.19 | 2.88 ± 0.01 |
| LS-362 | 0.544 ± 0.001 | 4.26 ± 0.01 | 73.18 ± 0.46 | 1.70 ± 0.01 |
| LS-365 | 0.535 ± 0.001 | 4.48 ± 0.02 | 74.26 ± 0.21 | 1.78 ± 0.01 |
| Control | 0.541 ± 0.001 | 9.72 ± 0.02 | 71.52 ± 0.17 | 3.78 ± 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, L.A.; Park, K.-W.; Ahn, S.; Wiles, A.A.; Hong, J.; Cooke, G. Coplanar Donor-π-Acceptor Dyes Featuring a Furylethynyl Spacer for Dye-Sensitized Solar Cells. Materials 2019, 12, 839. https://doi.org/10.3390/ma12050839
Serrano LA, Park K-W, Ahn S, Wiles AA, Hong J, Cooke G. Coplanar Donor-π-Acceptor Dyes Featuring a Furylethynyl Spacer for Dye-Sensitized Solar Cells. Materials. 2019; 12(5):839. https://doi.org/10.3390/ma12050839
Chicago/Turabian StyleSerrano, Luis A., Kwang-Won Park, Sungwoo Ahn, Alan A. Wiles, Jongin Hong, and Graeme Cooke. 2019. "Coplanar Donor-π-Acceptor Dyes Featuring a Furylethynyl Spacer for Dye-Sensitized Solar Cells" Materials 12, no. 5: 839. https://doi.org/10.3390/ma12050839
APA StyleSerrano, L. A., Park, K.-W., Ahn, S., Wiles, A. A., Hong, J., & Cooke, G. (2019). Coplanar Donor-π-Acceptor Dyes Featuring a Furylethynyl Spacer for Dye-Sensitized Solar Cells. Materials, 12(5), 839. https://doi.org/10.3390/ma12050839






