In Vitro Assessment of the Functional Dynamics of Titanium with Surface Coating of Hydroxyapatite Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Titanium Implants and Disks
2.2. Surfaces Characterization
2.3. Cell Culture Experiments
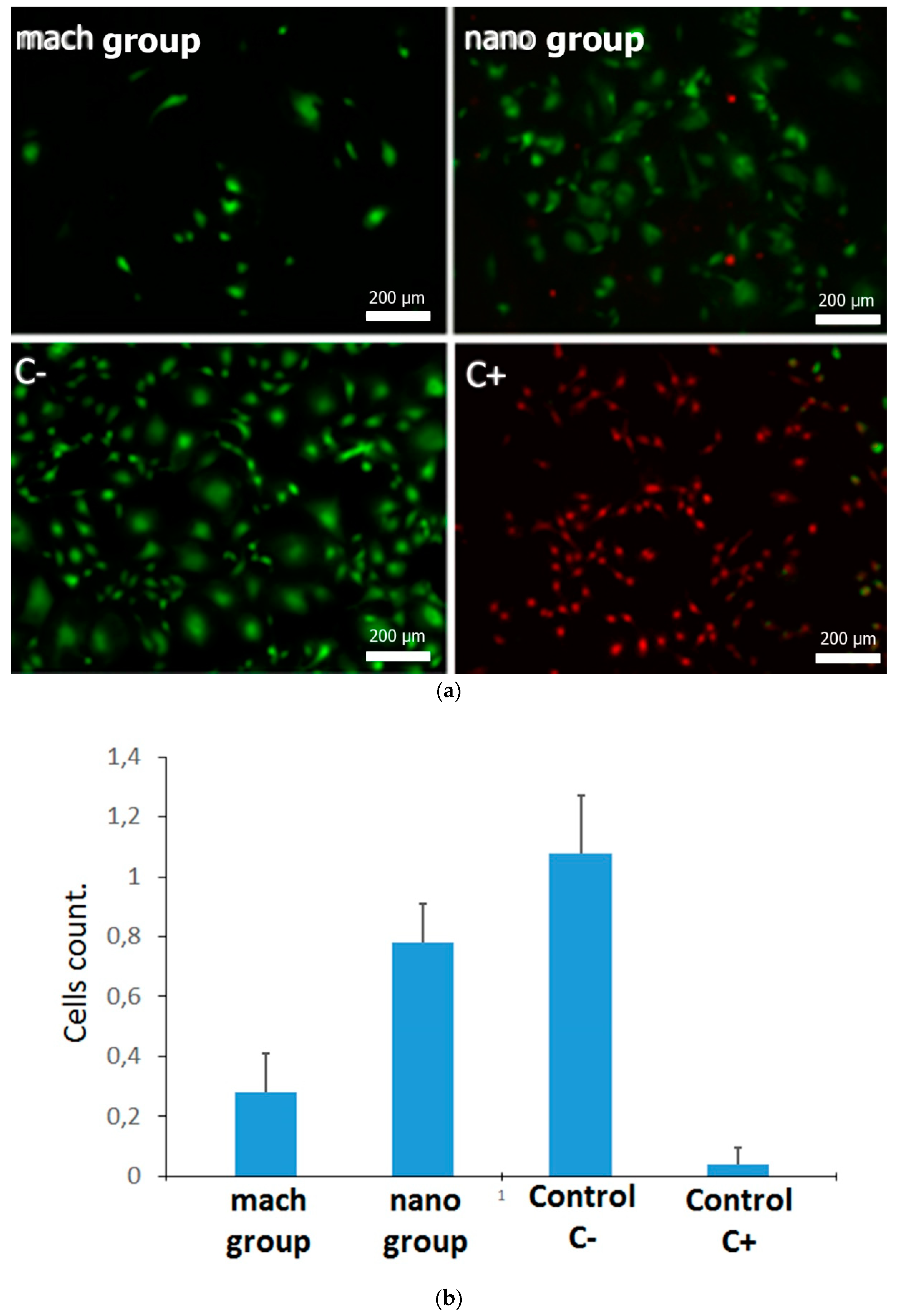
2.4. Viability Assay
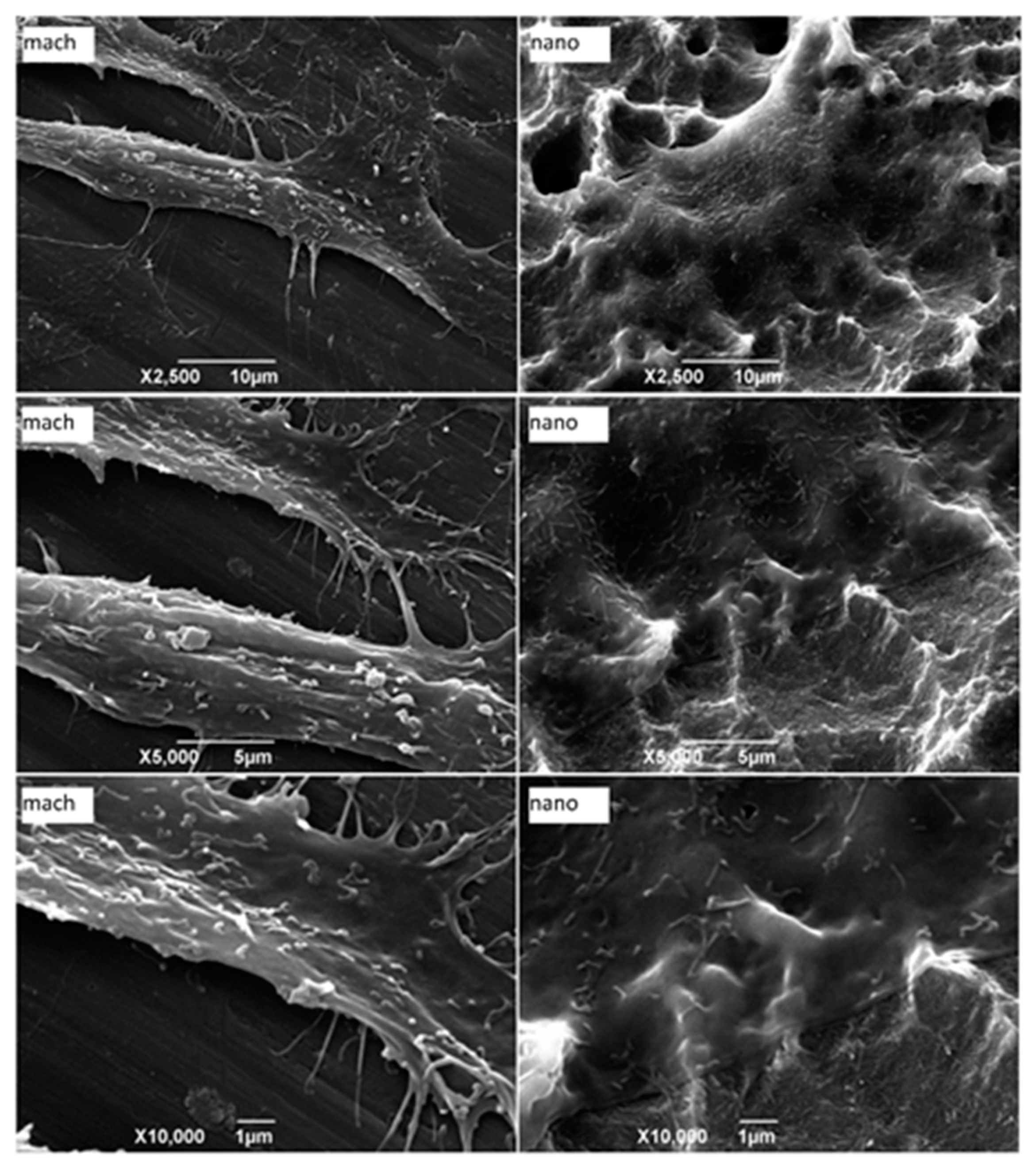
2.5. Osteoblast Cell Adhesion and Morphology
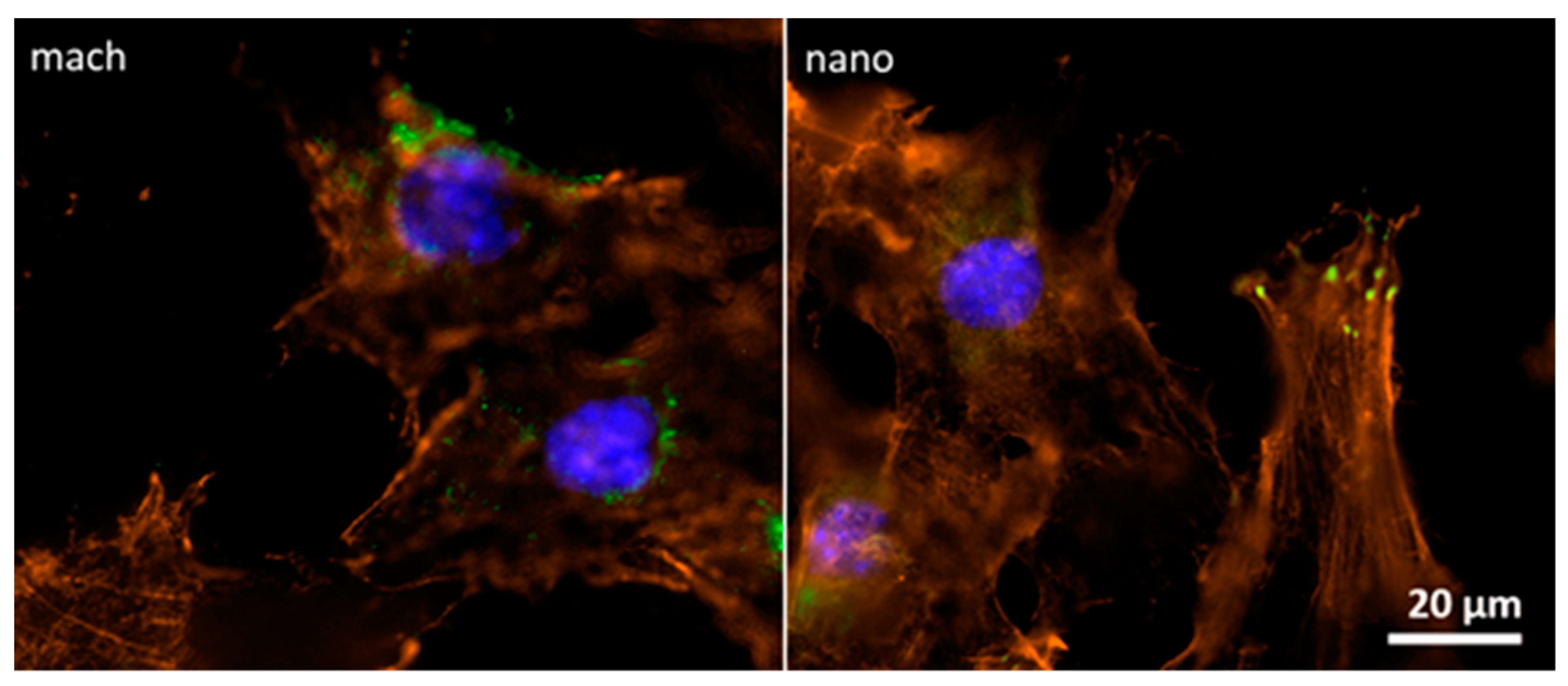
2.6. Focal Contact and Cell-Surface Interaction
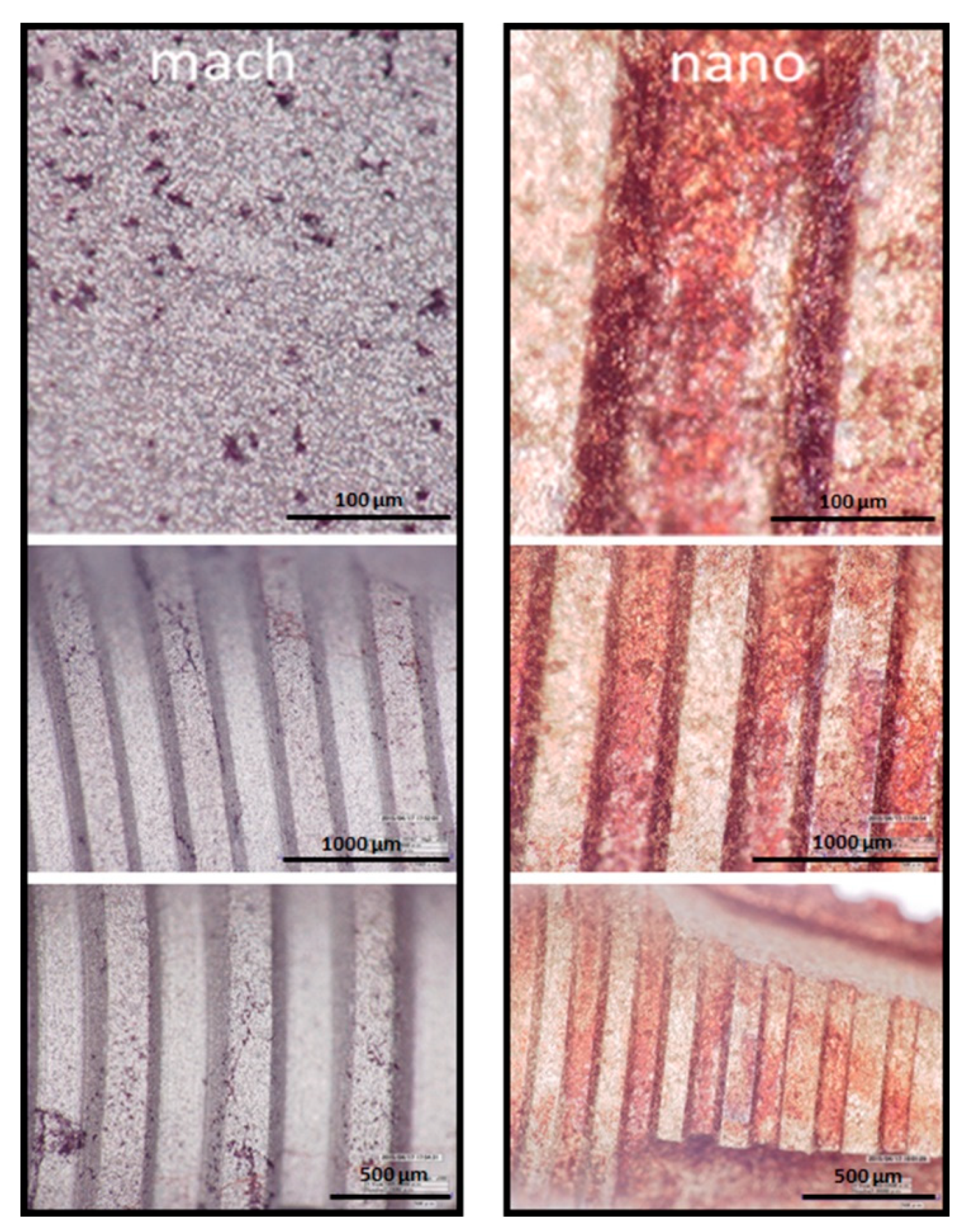
2.7. Mineralization Assay
3. Results
3.1. Surface Analysis and Characterization
3.2. Cell Viability Assay of Pre-osteoblast Growing on Different Surfaces
3.3. Morphological Characterization of Adherent Cells
3.4. Focal Contact Analysis between Pre-osteoblasts and Implant Surfaces
3.5. Osteoblast Differentiation on Nano and Mach Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Romeo, E.; Lops, D.; Margutti, E.; Ghisolfi, M.; Chiapasco, M.; Vogel, G. Long-term survival and success of oral implants in the treatment of full and partial arches: A 7-year prospective study with the ITI dental implant system. Int. J. Oral Maxillofac. Implants. 2004, 19, 247–259. [Google Scholar] [PubMed]
- Aykent, F.; Inan, O.; Ozyesil, A.G.; Alptekin, N.O. A 1- to 12-year clinical evaluation of 106 endosseous implants supporting fixed and removable prostheses. Int. J. Periodontics Restor. Dent. 2007, 27, 358–367. [Google Scholar]
- Zinger, O.; Anselme, K.; Denzer, A.; Habersetzer, P.; Wieland, M.; Jeanfils, J.; Hardouin, P.; Landolt, D. Time-dependent morphology and adhesion of osteoblastic cells on titanium model surfaces featuring scale-resolved topography. Biomaterials 2004, 25, 2695–2711. [Google Scholar] [CrossRef]
- Davidson, P.M.; Fromigué, O.; Marie, P.J.; Hasirci, V.; Reiter, G.; Anselme, K. Topographically induced self-deformation of the nuclei of cells: Dependence on cell type and proposed mechanisms. J. Mater. Sci. Mater. Med. 2010, 21, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Bloebaum, R.D.; Beeks, D.; Dorr, L.D.; Savory, C.G.; DuPont, J.A.; Hofmann, A.A. Complications with hydroxyapatite particulate separation in total hip arthroplasty. Clin. Orthop. Relat. Res. 1994, 298, 19–26. [Google Scholar]
- Schwarz, F.; Bieling, K.; Latz, T.; Nuesry, E.; Becker, J. Healing of intrabony peri-implantitis defects following application of nanocrystalline hydroxyapatite (Ostim) or a bovine-derived xenograft (Bio-Oss) in combination with a collagen membrane (Bio-Gide). A case series. J. Clin. Periodontol. 2006, 33, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Kasaj, A.; Willershausen, B.; Reichert, C.; Röhrig, B.; Smeets, R.; Schmidt, M. Ability of nanocrystalline hydroxyapatite paste to promote human periodontal ligament cell proliferation. J. Oral Sci. 2008, 50, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Kjellin, P.; Andersson, M. SE-0401524-4, Assignee. Synthetic Nano-Sized Crystalline Calcium Phosphate and Method of Production. U.S. Patent SE527610, 25 April 2006. [Google Scholar]
- Meirelles, L.; Arvidsson, A.; Andersson, M.; Kjellin, P.; Albrektsson, T.; Wennerberg, A. Nano hydroxyapatite structures influence early bone formation. J. Biomed. Mater. Res. A 2008, 87, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, L.; Currie, F.; Jacobsson, M.; Albrektsson, T.; Wennerberg, A. The effect of chemical and nanotopographical modifications on the early stages of osseointegration. Int. J. Oral Maxillofac. Implants. 2008, 23, 641–647. [Google Scholar]
- Zaidel-Bar, R.; Cohen, M.; Addadi, L.; Geiger, B. Hierarchical assembly of cell-matrix adhesion complexes. Biochem. Soc. Trans. 2004, 32, 416–420. [Google Scholar] [CrossRef]
- Nikolopoulos, S.N.; Turner, C.E. Actopaxin, a new focal adhesion protein that binds paxillin LD motifs and actin and regulates cell adhesion. J. Cell Biol. 2000, 151, 1435–1448. [Google Scholar] [CrossRef] [PubMed]
- Bays, J.L.; DeMali, K.A. Vinculin in cell-cell and cell-matrix adhesions. Cell. Mol. Life Sci. 2017, 74, 2999–3009. [Google Scholar] [CrossRef] [PubMed]
- Stein, G.S.; Lian, J.B. Molecular mechanisms mediating developmental and hormone-regulated expression of genes in osteoblasts: An integrated relationship of cell growth and differentiation. In Cellular and Molecular Biology of Bone; Noda, M., Ed.; Academic Press: Tokyo, Japan, 2002; Volume 199, pp. 47–95. [Google Scholar]
- Lindgren, M.; Åstrand, M.; Wiklund, U.; Engqvist, H. Investigation of boundary conditions for biomimetic HA deposition on titanium oxide surfaces. J. Mater. Sci. Mater. Med. 2009, 20, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.; Wilkinson, C. New depths in cell behavior: Reactions of cells to nanotopography. Biochem. Soc. Symp. 1999, 65, 15–26. [Google Scholar] [PubMed]
- Wennerberg, A. The importance of surface roughness for implant incorporation. Int. J. Mach. Tools Manuf. 1998, 38, 657–662. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. BioMed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Dedavid, B.A.; Aramburú, J.S.; Júnior Pérez-Díaz, L.; Calvo Guirado, J.L.; Canales, P.M.; De Aza, P.N. Effect of Different Morphology of Titanium Surface on the Bone Healing in Defects Filled Only with Blood Clot: A New Animal Study Design. BioMed Res. Int. 2018, 2018, 4265474. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.; Nishiwaki, N.; Ueda, K.; Narushima, T.; Kawamura, H.; Takahashi, T. Evaluation of thin amorphous calcium phosphate coatings on titanium dental implants deposited using magnetron sputtering. Implant Dent. 2014, 23, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, J.; Xu, Y. Study of the in vitro cytotoxicity testing of medical devices. Biomed. Rep. 2015, 3, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.O.; Etheridge, J.M.; Thompson, J.A.; Vorwald, C.E.; Dean, D.; Fisher, J.P. Evaluation of the in vitro cytotoxicity of cross-linked biomaterials. Biomacromolecules 2013, 14, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Wieslander, A.P.; Nordin, M.K.; Hansson, B.; Baldetorp, B.; Kjellstrand, P.T. In vitro toxicity of biomaterials determined with cell density, total protein, cell cycle distribution, and adenine nucleotides. Biomater. Artif. Cells Immobil. Biotechnol. 1993, 21, 63–70. [Google Scholar] [CrossRef]
- Van Tienhoven, E.A.; Korbee, D.; Schipper, L.; Verharen, H.W.; De Jong, W.H. In vitro and in vivo (cyto)toxicity assays using PVC and LDPE as model materials. J. Biomed. Mater. Res. A 2006, 78, 175–182. [Google Scholar] [CrossRef] [PubMed]
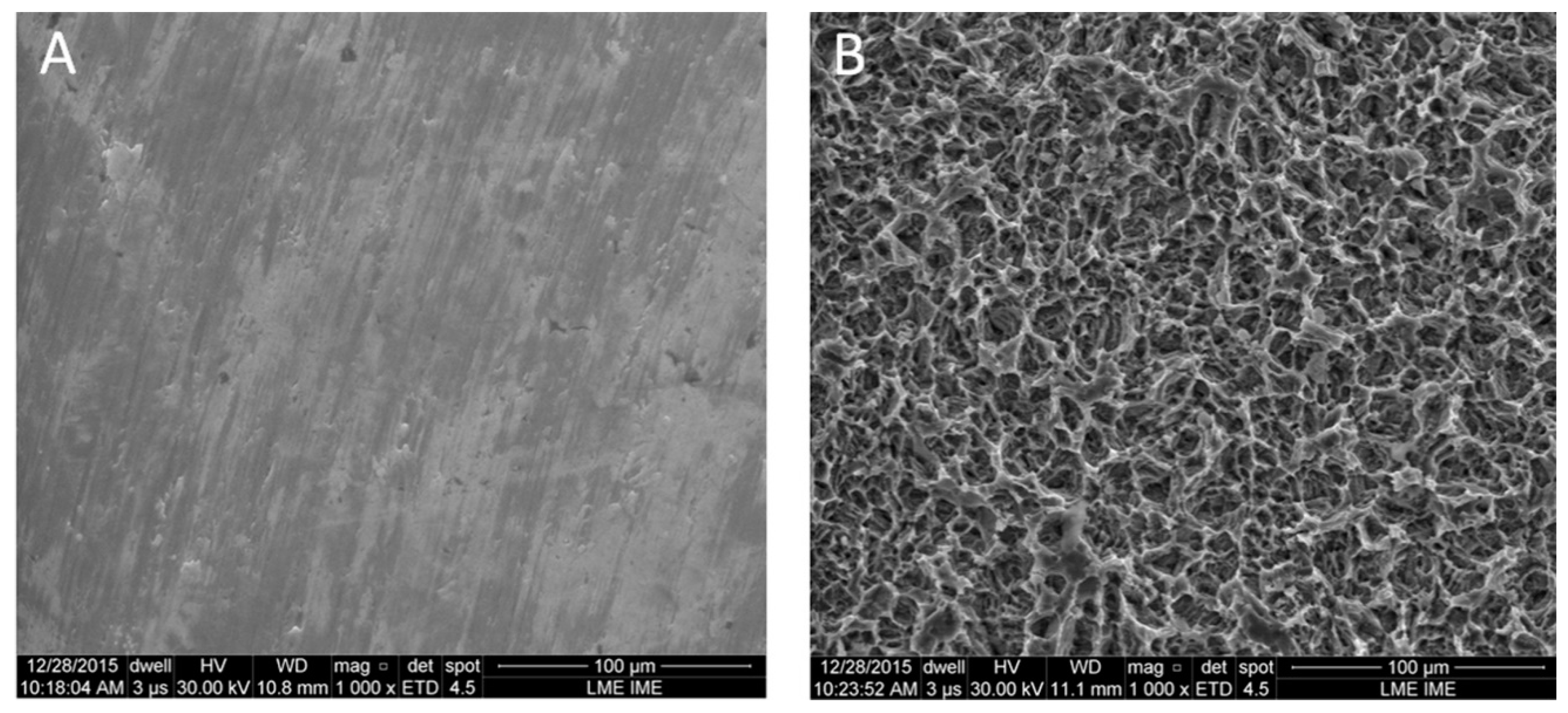
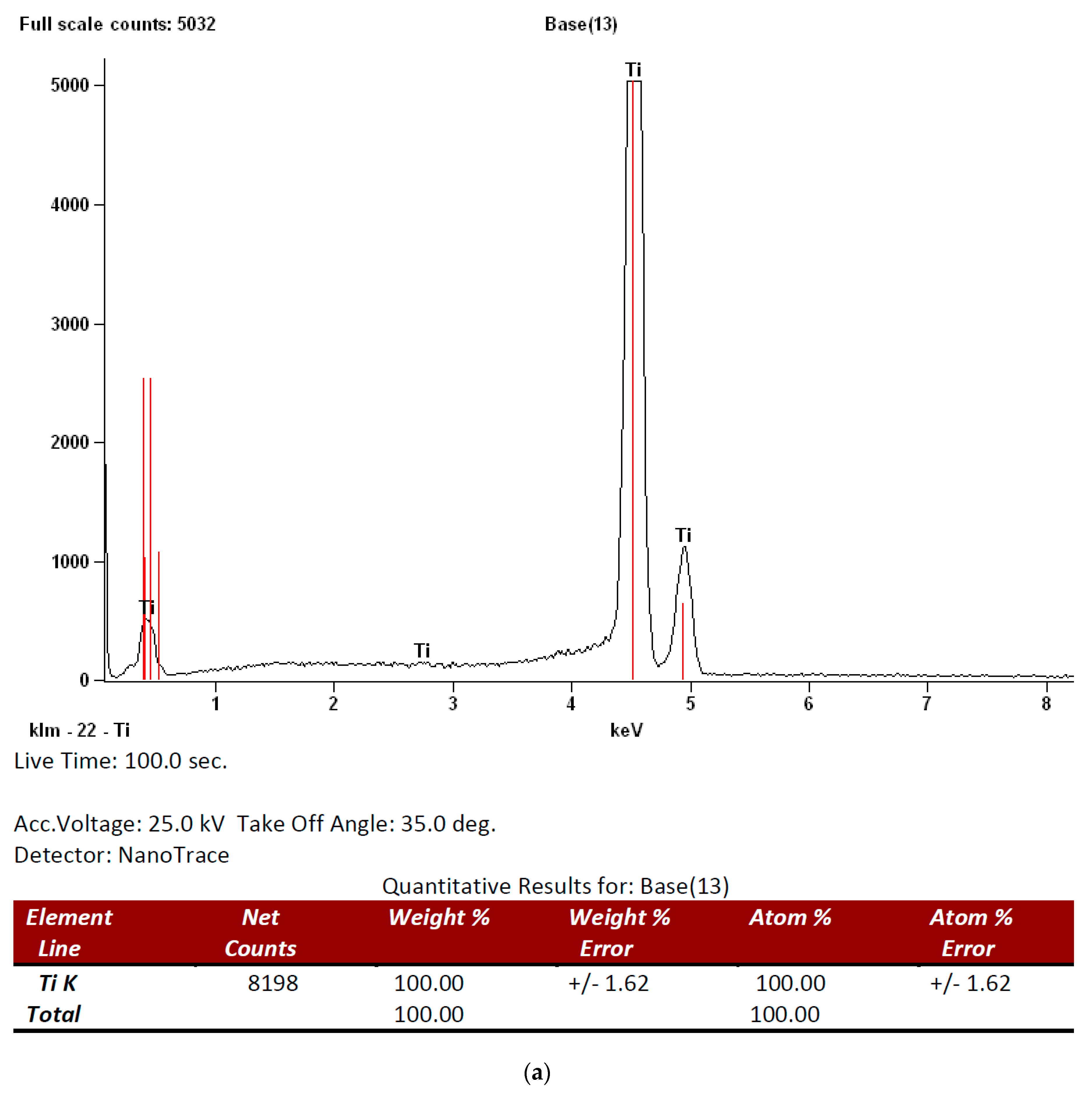
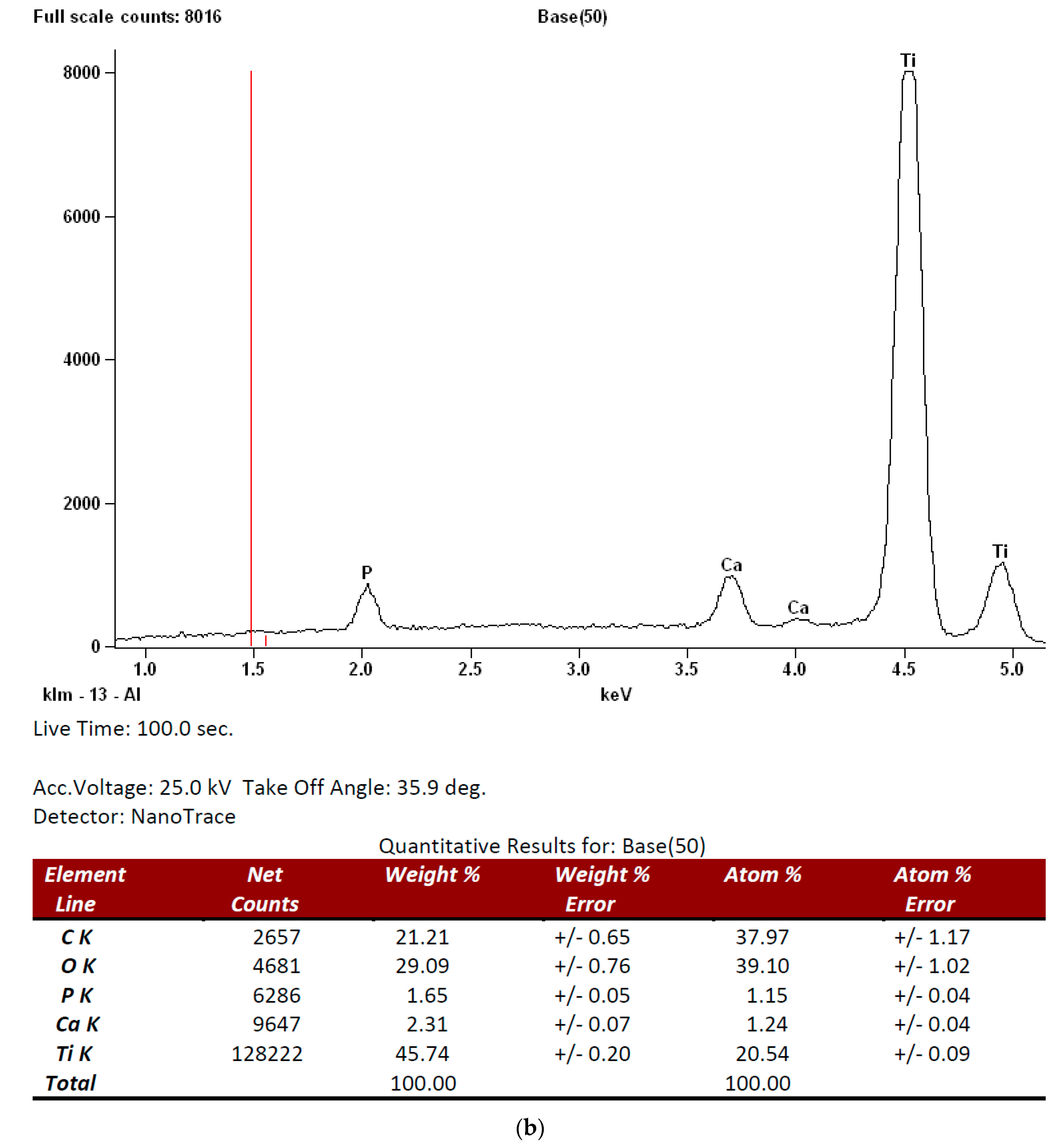
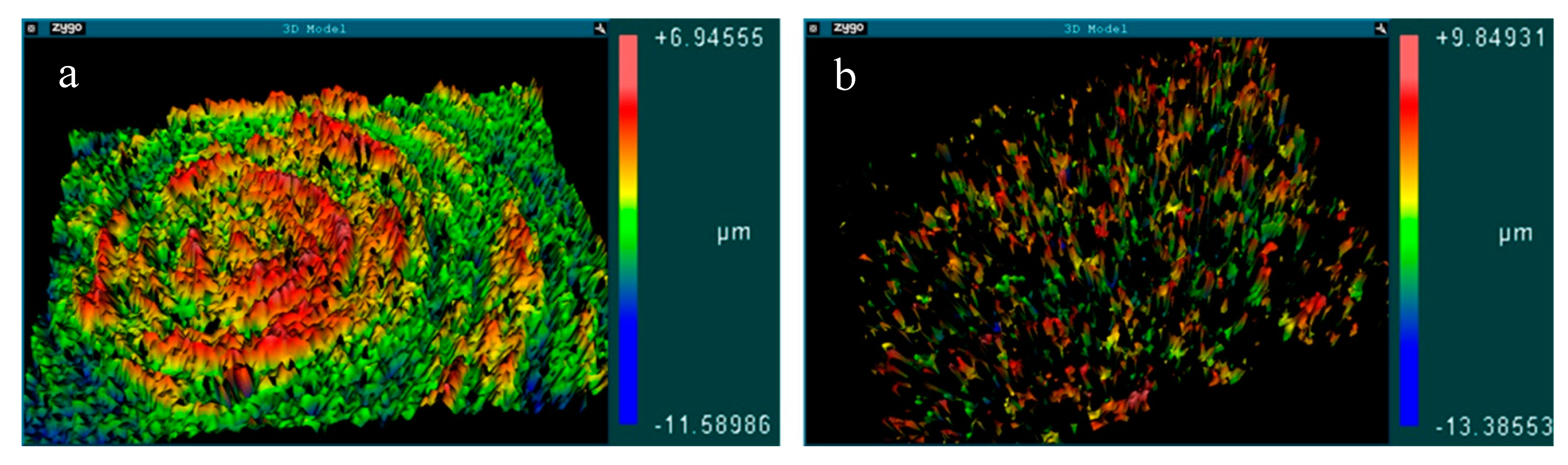
| Roughness Parameters | Z | Rq | Ra | Rz |
|---|---|---|---|---|
| Mach group | 0.89 ± 0.12 | 0.14 ± 0.06 | 0.11 ± 0.04 | 0.96 ± 0.12 |
| Nano group | 2.03 ± 0.15 | 0.54 ± 0.11 | 0.36 ± 0.09 | 2.37 ± 0.67 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Lima Cavalcanti, J.H.; Matos, P.C.; Depes de Gouvêa, C.V.; Carvalho, W.; Calvo-Guirado, J.L.; Aragoneses, J.M.; Pérez-Díaz, L.; Gehrke, S.A. In Vitro Assessment of the Functional Dynamics of Titanium with Surface Coating of Hydroxyapatite Nanoparticles. Materials 2019, 12, 840. https://doi.org/10.3390/ma12050840
de Lima Cavalcanti JH, Matos PC, Depes de Gouvêa CV, Carvalho W, Calvo-Guirado JL, Aragoneses JM, Pérez-Díaz L, Gehrke SA. In Vitro Assessment of the Functional Dynamics of Titanium with Surface Coating of Hydroxyapatite Nanoparticles. Materials. 2019; 12(5):840. https://doi.org/10.3390/ma12050840
Chicago/Turabian Stylede Lima Cavalcanti, José Henrique, Patrícia C. Matos, Cresus Vinícius Depes de Gouvêa, Waldimir Carvalho, José Luis Calvo-Guirado, Juan Manuel Aragoneses, Letícia Pérez-Díaz, and Sergio Alexandre Gehrke. 2019. "In Vitro Assessment of the Functional Dynamics of Titanium with Surface Coating of Hydroxyapatite Nanoparticles" Materials 12, no. 5: 840. https://doi.org/10.3390/ma12050840
APA Stylede Lima Cavalcanti, J. H., Matos, P. C., Depes de Gouvêa, C. V., Carvalho, W., Calvo-Guirado, J. L., Aragoneses, J. M., Pérez-Díaz, L., & Gehrke, S. A. (2019). In Vitro Assessment of the Functional Dynamics of Titanium with Surface Coating of Hydroxyapatite Nanoparticles. Materials, 12(5), 840. https://doi.org/10.3390/ma12050840








