Exposure of Glass Fiber Reinforced Polymer Composites in Seawater and the Effect on Their Physical Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
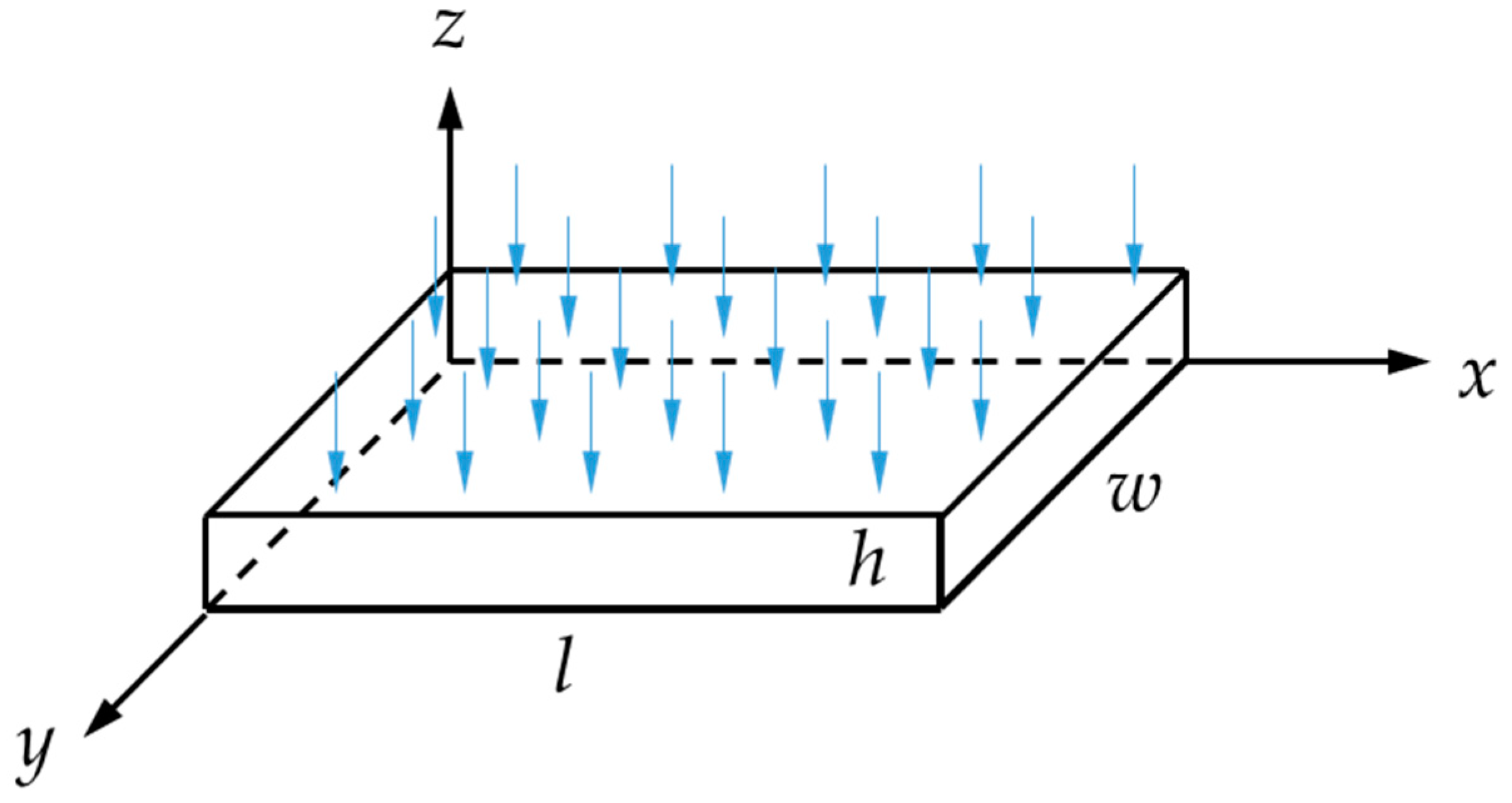
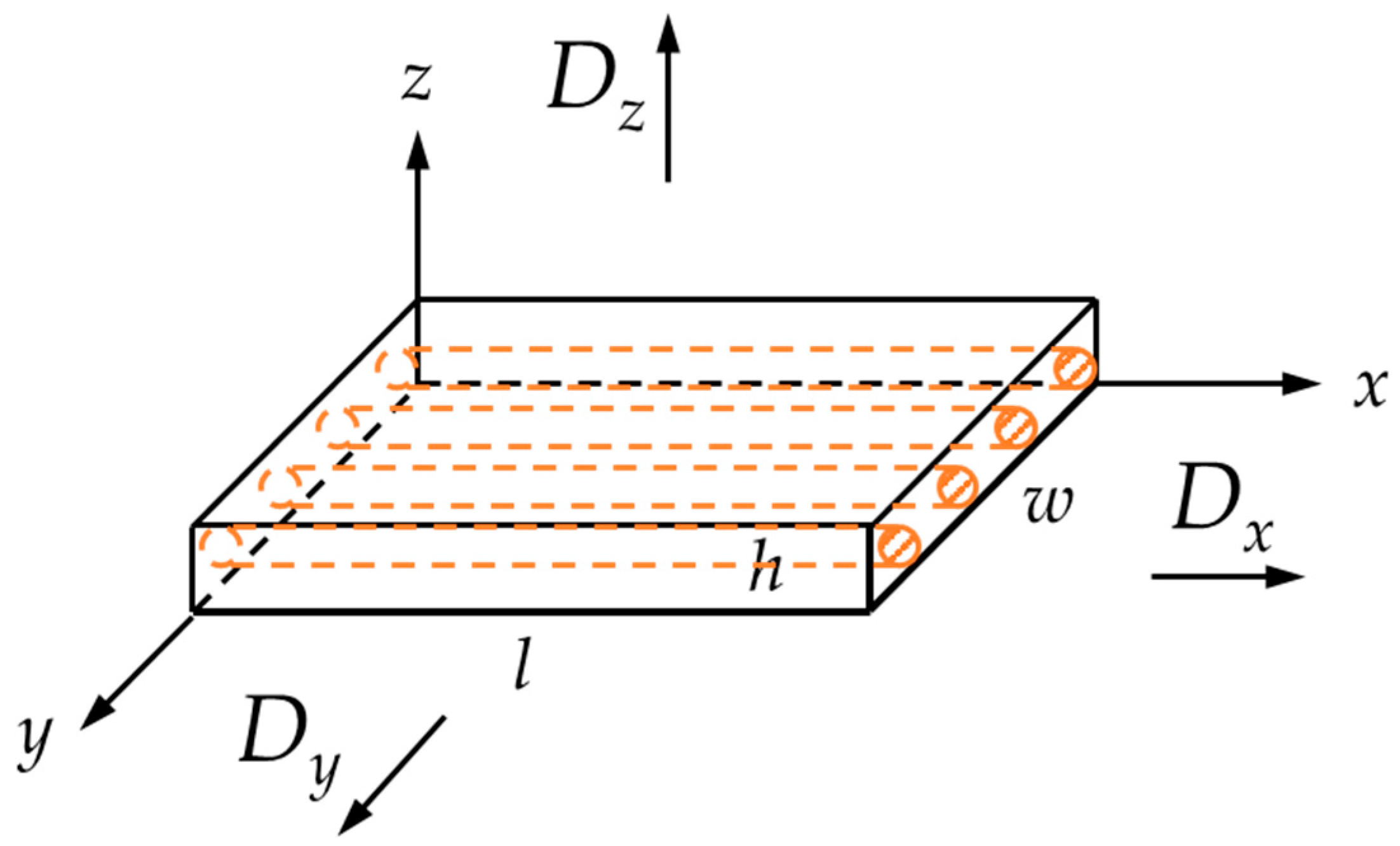
2.2.1. Conditioning in Simulated Environments
2.2.2. Dynamic Mechanical Analysis (DMA)
3. Results
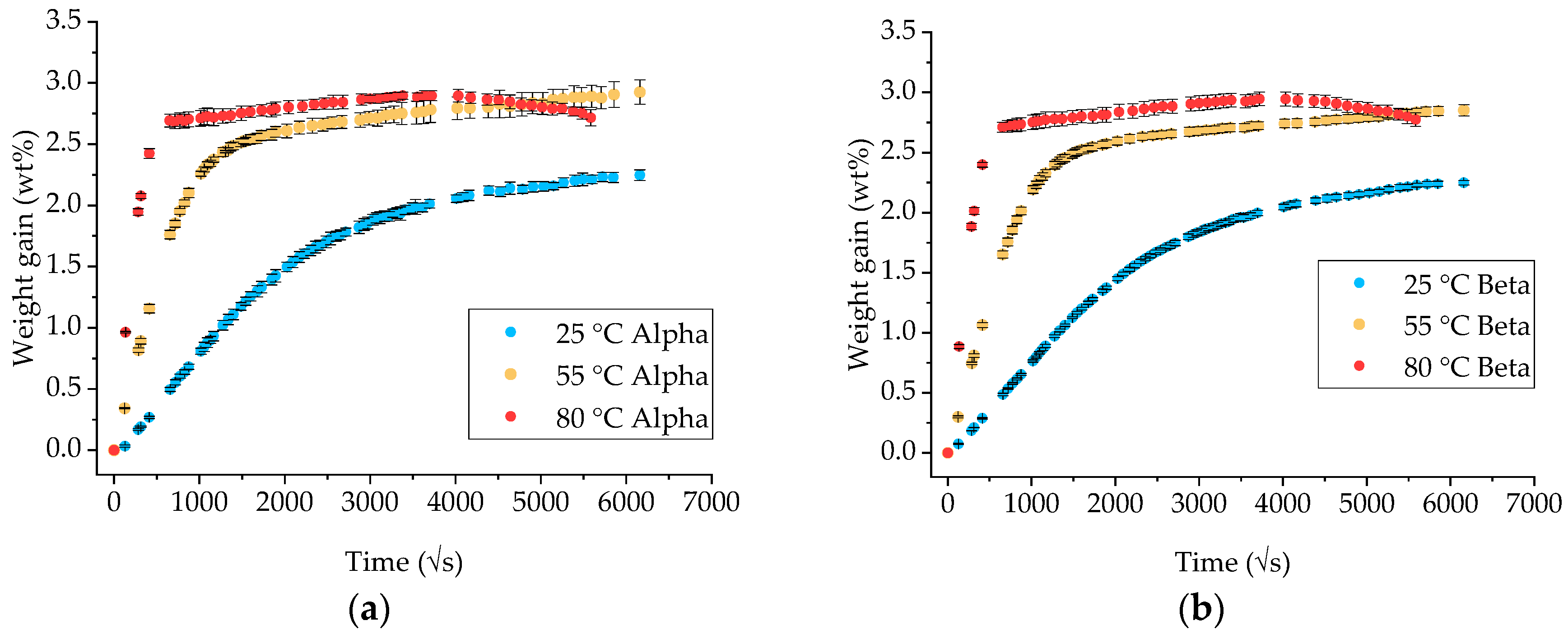
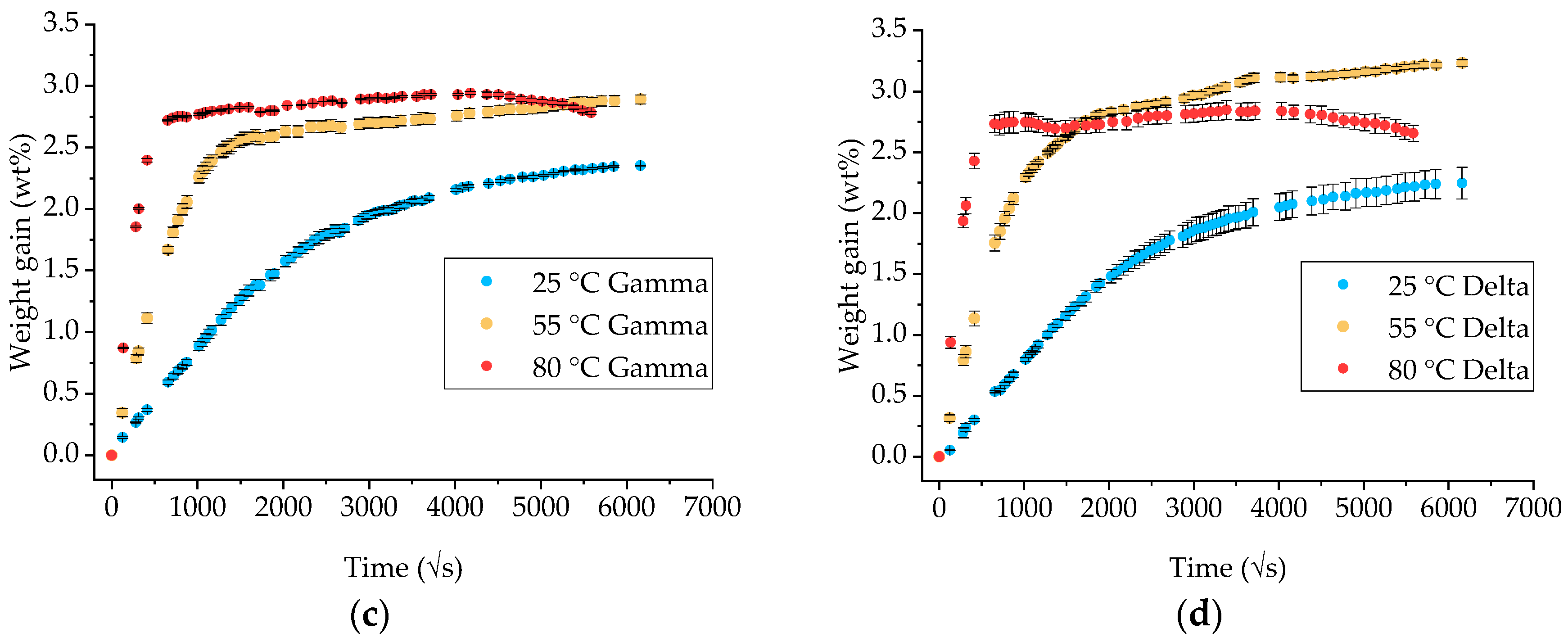
3.1. Gravimetric Measurements on Neat Ampreg 26 Epoxy
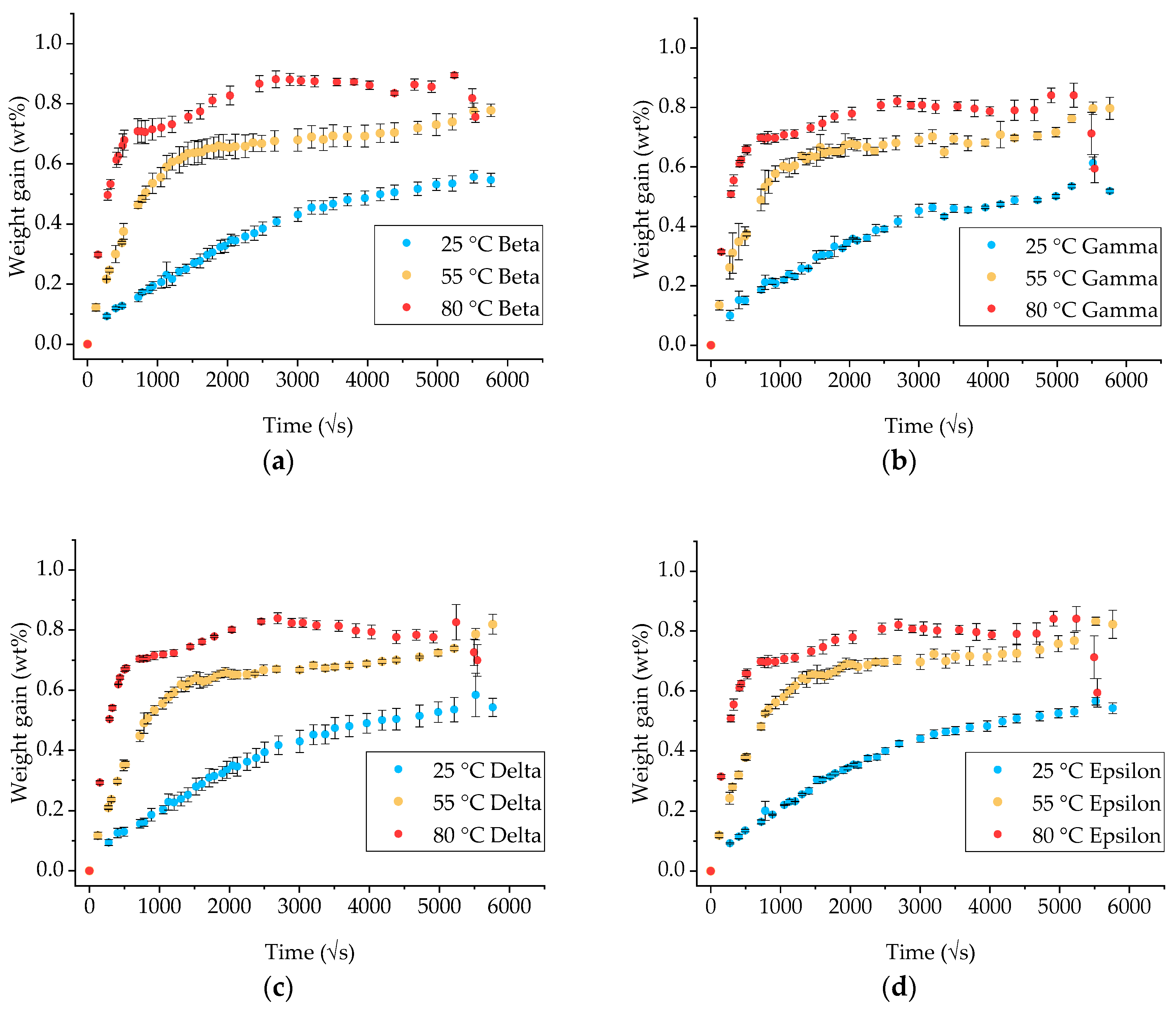
3.2. Gravimetric Measurements on GFRP Composite
3.3. Mechanical Performance
4. Discussion
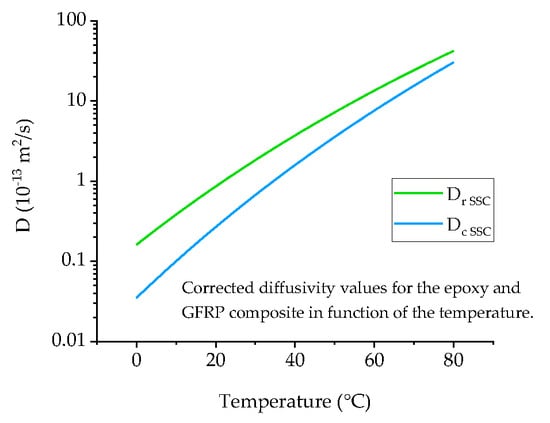
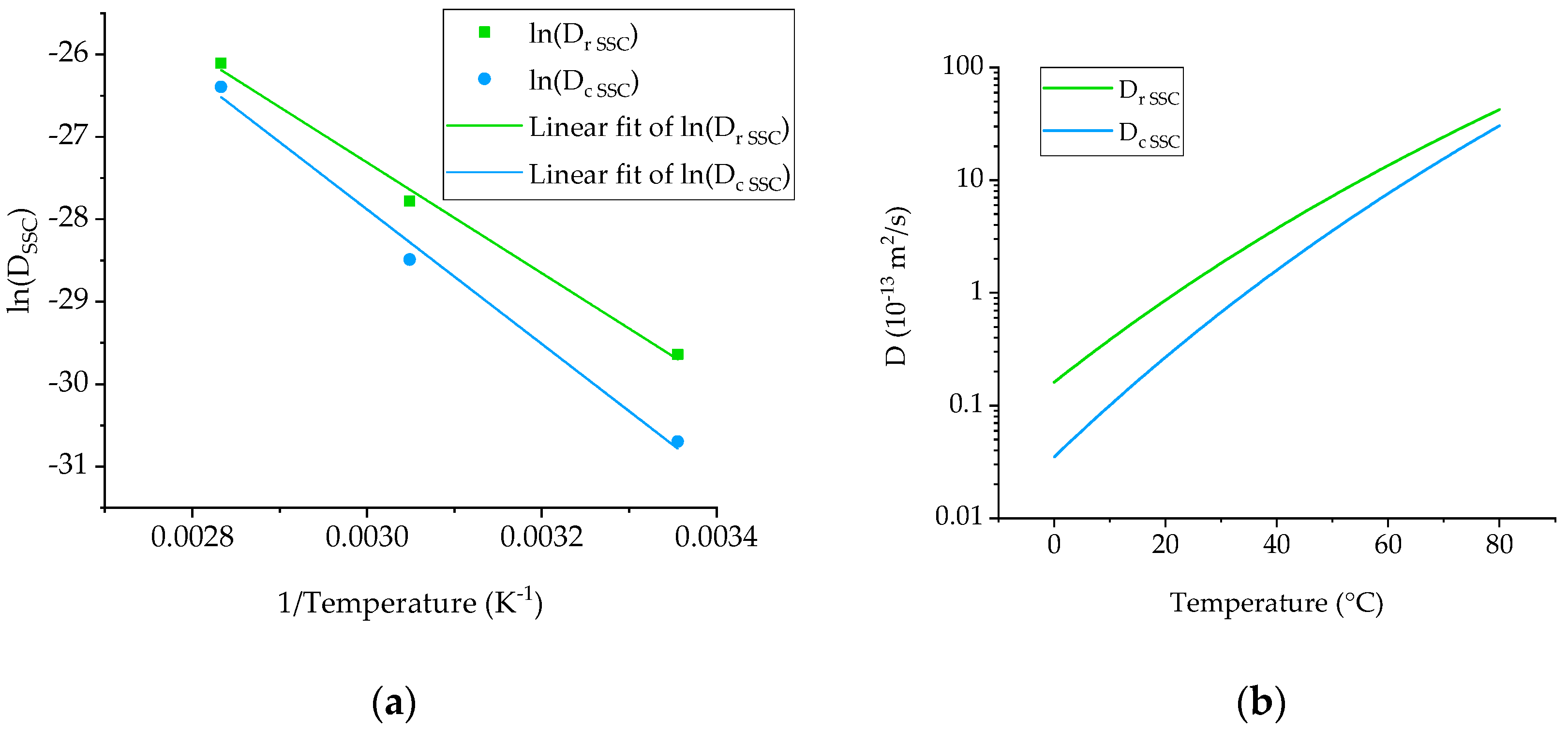
4.1. Diffusivity Calculation for Neat Ampreg 26 Epoxy
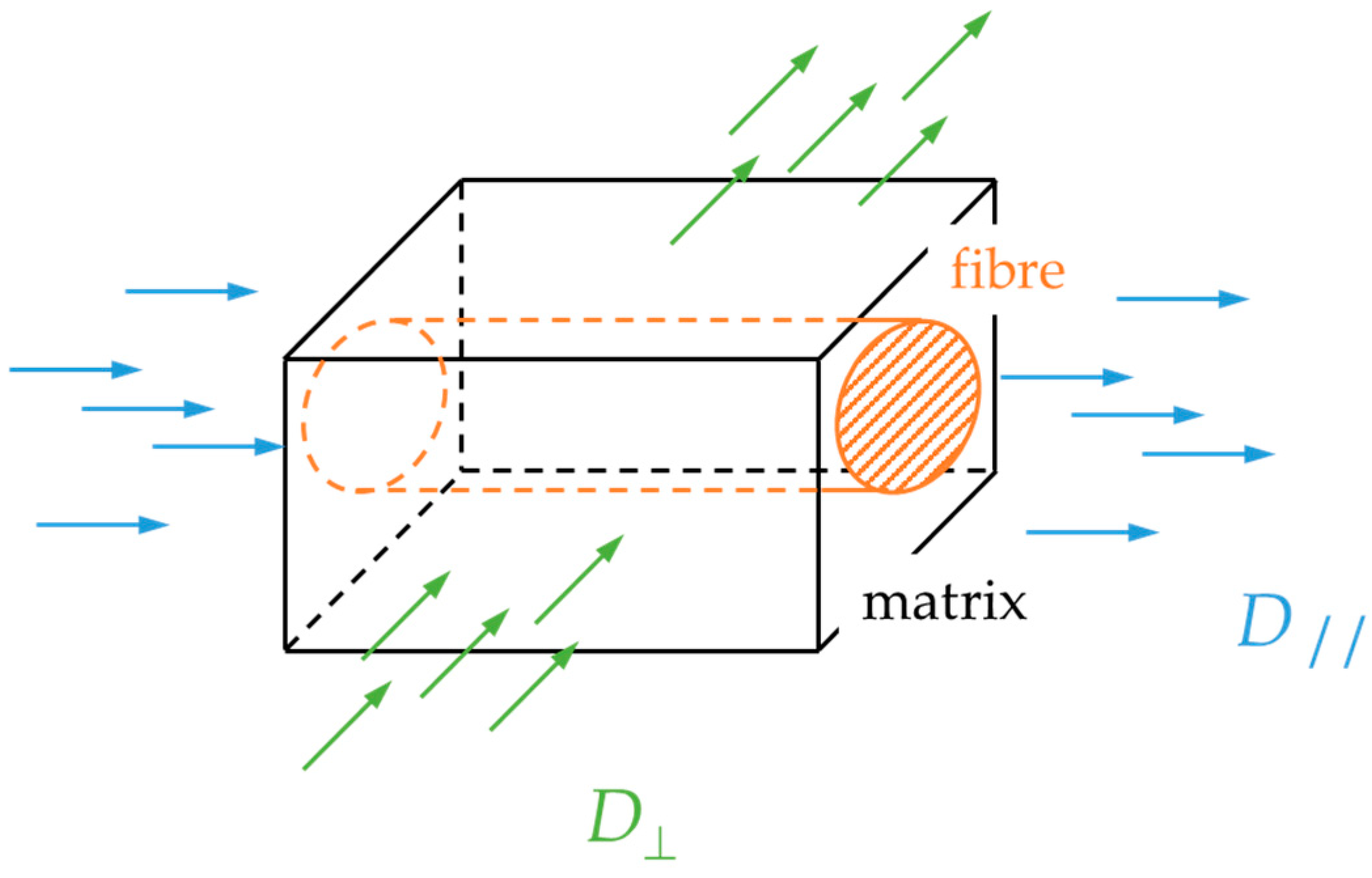
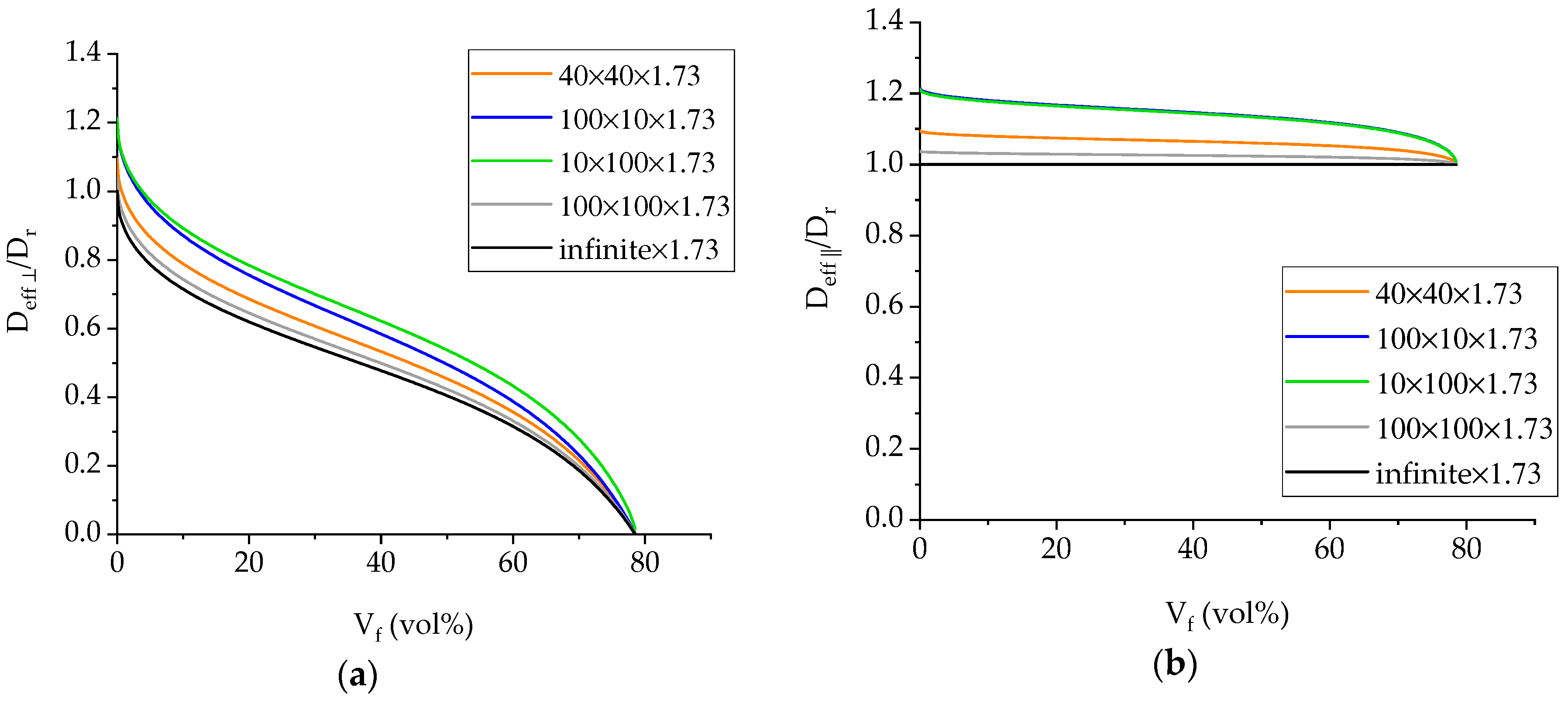
4.2. Diffusivity Calculation for the GFRP Composite
- The effective diffusivity transverse to the fiber direction (Deff,⊥) is approximately half than of the resin’s, while the diffusivity along the fibers (Deff,∥) is slightly higher than that of the resin;
- There is satisfactory agreement for the values of the GFRP diffusivity at 25 °C to Deff,⊥ but it progressively deviates at higher temperatures towards Deff,∥. This is an indication that while at ambient temperature, diffusion is occurring mainly through the thickness of the specimen, and as the temperature increases, there is significantly more seawater travelling through the edges of the specimens and along the fibers, which in turn suggests possible weakening of the fiber-matrix interface, providing an easy pathway for the diffusing liquid.
4.3. Arrhenius Plot and Prediction of Low Temperature Diffusivity
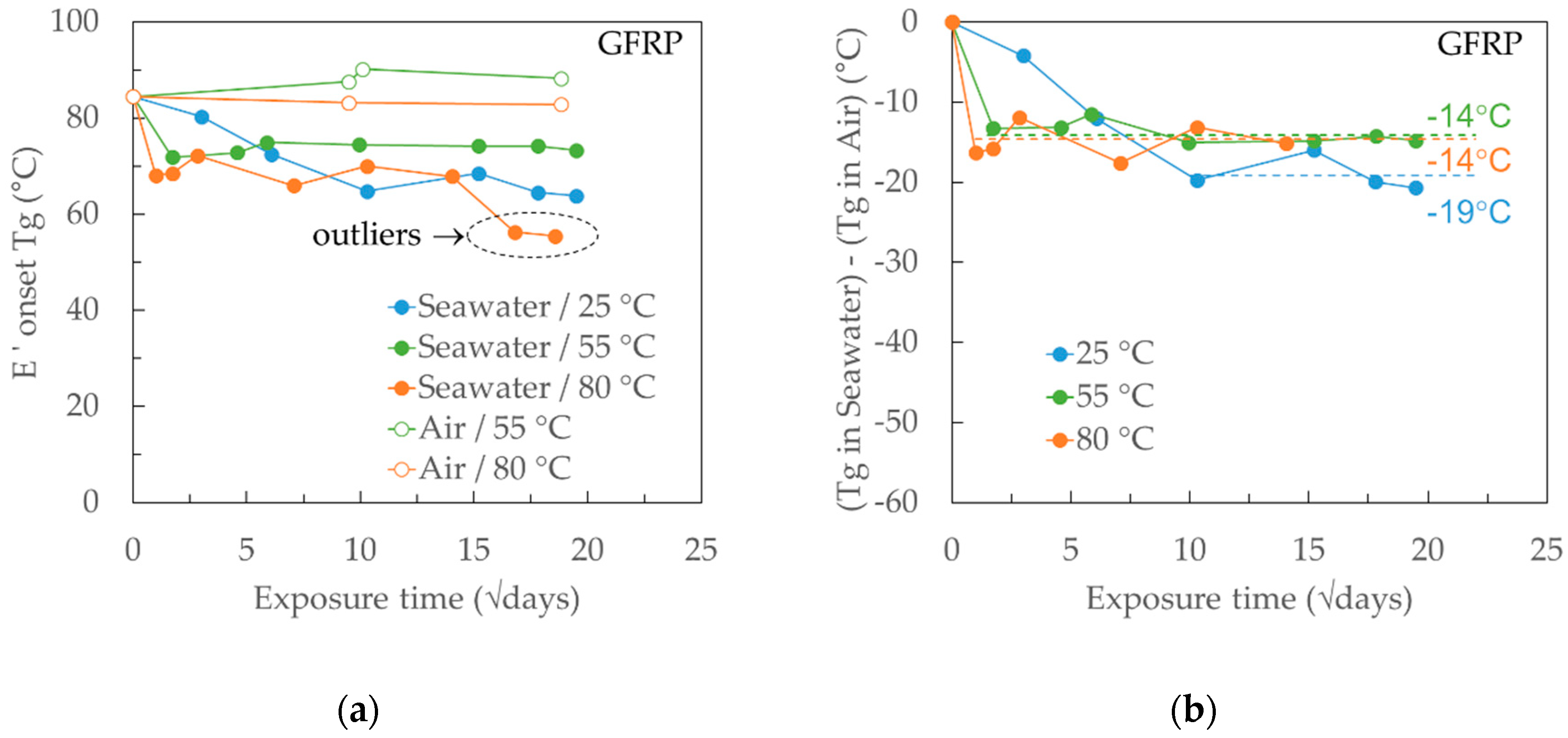
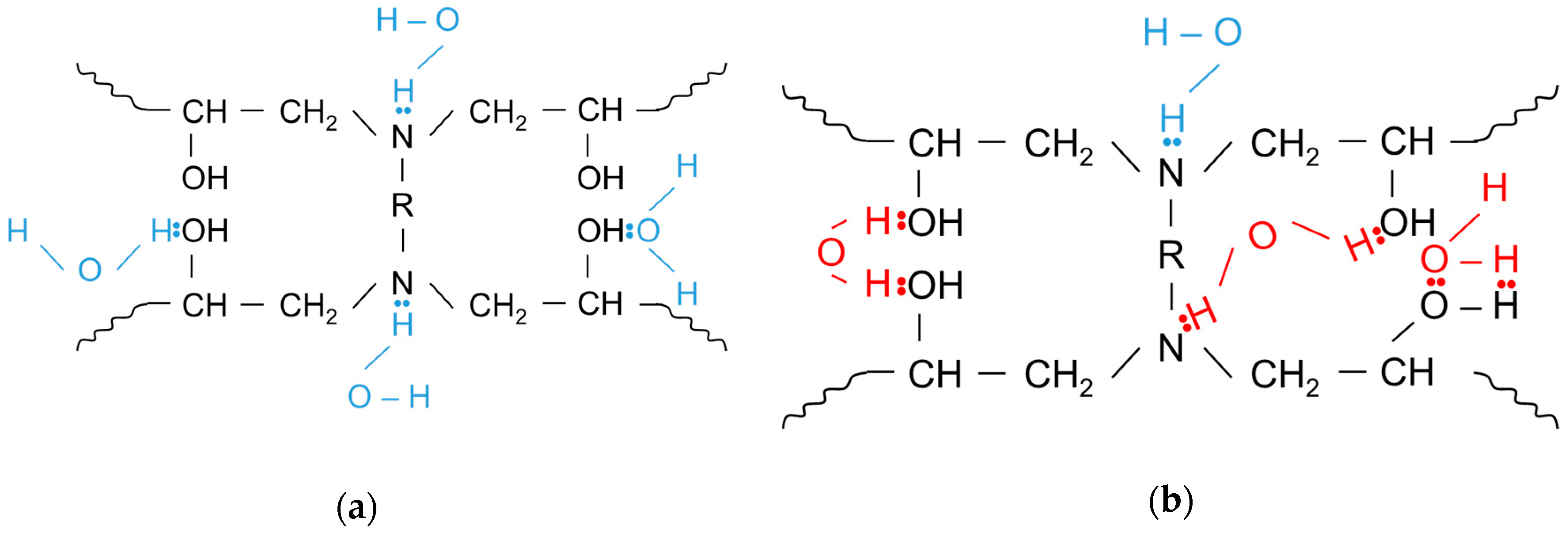
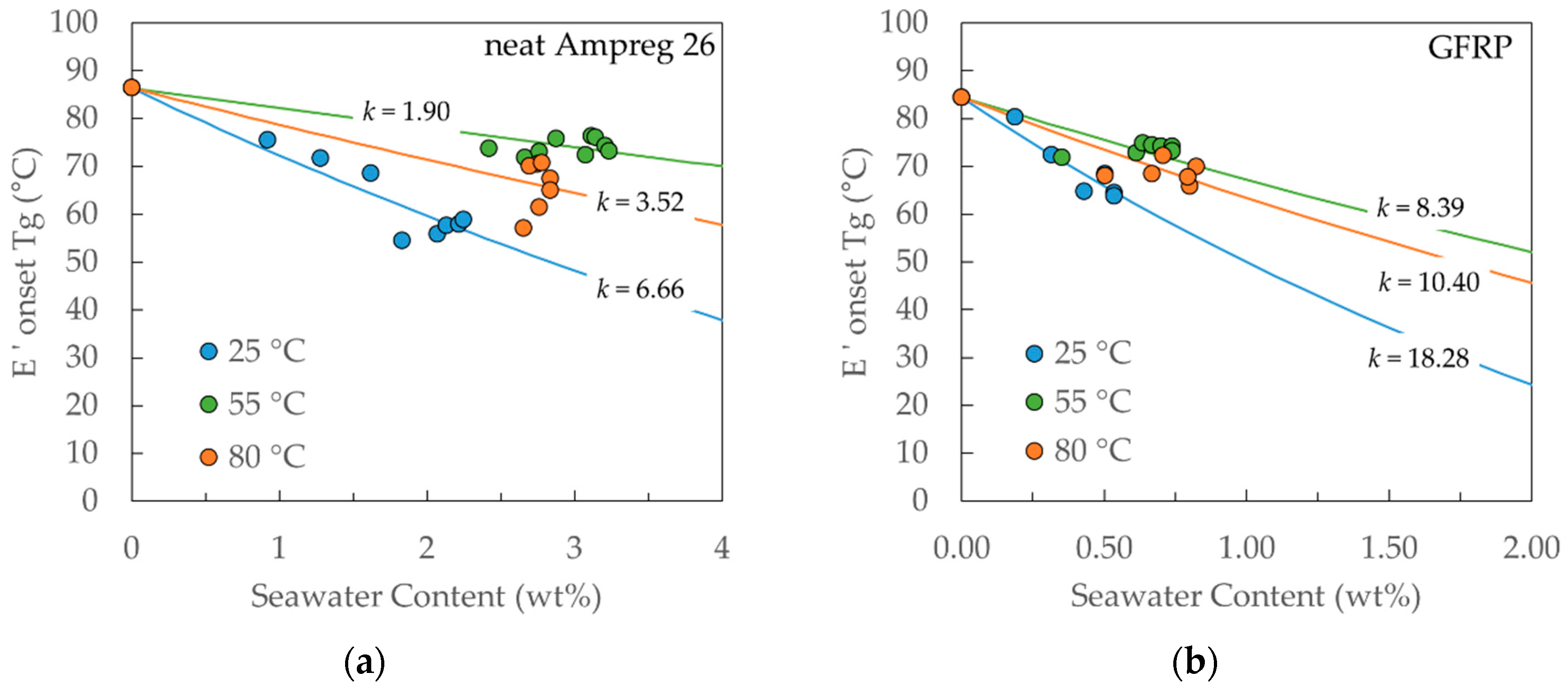
4.4. Tg Measurements on Neat Ampreg 26 and GFRP Composite
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Effective Equilibrium Criterion | Temperature | 25 °C | 55 °C | 80 °C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Specimen | alpha | beta | gamma | delta | alpha | beta | gamma | delta | alpha | beta | gamma | delta | ||
| ASTM D5229M Equation (1) | Effective equilibrium 1 | (days) | 131 | 145 | 117 | 113 | 70 | 63 | 76 | 55 | 40 | 22 | 35 | 9 |
| Moisture content | (%) | 1.93 | 1.96 | 1.99 | 1.88 | 2.66 | 2.63 | 2.68 | 2.85 | 2.78 | 2.78 | 2.79 | 2.75 | |
| Uptake rate 2 | (10−8/s) | 1.48 | 0.65 | 2.88 | 1.59 | 1.29 | 2.57 | 1.70 | 3.00 | 0.90 | 0.35 | 8.60 | 3.30 | |
| Criterion A as Equation (2) (0.02, n = 2) | Effective equilibrium | (days) | 113 | 107 | 110 | 107 | 55 | 33 | 33 | 48 | 16 | 9 | 9 | 9 |
| Moisture content | (%) | 1.94 | 1.90 | 2.01 | 1.91 | 2.69 | 2.60 | 2.63 | 2.91 | 2.77 | 2.77 | 2.79 | 2.73 | |
| Uptake rate | (10−8/s) | 0.16 | 2.00 | 2.90 | 3.00 | 1.70 | 1.67 | 2.95 | 1.12 | 1.60 | 2.60 | 2.60 | 1.20 | |
| Criterion B as Equation (2) (0.05, n = 3) | Effective equilibrium | (days) | 62 | 62 | 65 | 62 | 27 | 26 | 28 | 35 | 8 | 8 | 9 | 8 |
| Moisture content | (%) | 1.63 | 1.59 | 1.75 | 1.62 | 2.52 | 2.49 | 2.57 | 2.76 | 2.69 | 2.73 | 2.75 | 2.75 | |
| Uptake rate | (10−8/s) | 4.87 | 6.56 | 8.01 | 6.34 | 4.90 | 5.12 | 6.40 | 6.00 | 4.80 | 1.00 | 2.60 | 1.10 | |
References
- Davies, P.; Rajapakse, Y.D.S. Durability of Composites in a Marine Environment; Springer: Berlin, Germany, 2014. [Google Scholar]
- Weitsman, J.Y. Fluid Effects in Polymers and Polymeric Composites; Springer: Berlin, Germany, 2012. [Google Scholar]
- Grammatikos, S.A.; Jones, R.G.; Evernden, M.; Correia, J.R. Thermal cycling effects on the durability of a pultruded GFRP material for off-shore civil engineering structures. Comp. Struct. 2016, 153, 297–310. [Google Scholar] [CrossRef]
- Grammatikos, S.A.; Evernden, M.; Mitchels, J.; Zafari, B.; Mottram, J.T.; Papanicolaou, G.C. On the response to hygrothermal aging of pultruded FRPs used in the civil engineering sector. Mater. Des. 2016, 96, 283–295. [Google Scholar] [CrossRef]
- Bakhshandeh, E.; Jannesari, A.; Ranjbar, Z.; Sobhani, S.; Sobhani, S.; Saeb, M. Anti-corrosion hybrid coatings based on epoxy silica nano-composites: Toward relationship between the morphology and EIS data. Prog. Org. Coat. 2014, 2014, 1169–1183. [Google Scholar] [CrossRef]
- Kalpakjian, S.; Schmid, S.R. Surface Treatments, Coatings, and Cleaning. In Manufacturing Engineering and Technology; Paerson: London, UK, 2013; p. 985. [Google Scholar]
- Jukes, P.; Eltaher, A.; Sun, J.; Harrison, G. Extra High-Pressure High-Temperature (XHPHT) Flowlines: Design Considerations and Challenges. In Proceedings of the ASME 2009 28th International Conference on Ocean, Offshore and Arctic Engineering, Honolulu, HI, USA, 31 May–5 June 2009. [Google Scholar]
- Jha, V.; Latto, J.; Dodds, N.; Anderson, T.A.; Finch, D.; Vermilyea, M. Qualification of Flexible Fiber-Reinforced Pipe for 10,000-Foot Water Depths. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 6–9 May 2013. [Google Scholar]
- Rafie, R. On the mechanical performance of glass-fibre-reinforced thermosetting-resin pipes: A review. Compos. Struct. J. 2016, 143, 151–164. [Google Scholar] [CrossRef]
- Roseman, M.; Martin, R.; Morgan, G. Composites in offshore oil and gas applications. In Marine Applications of Advanced Fibre-Reinforced Composites; Elsevier: Amsterdam, The Netherlands, 2016; pp. 233–257. [Google Scholar]
- Martin, R. Composite Materials: An Enabling Material for Offshore Piping Systems. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 6–9 May 2013. [Google Scholar]
- le Gac, P.Y.; Davies, P.; Choqueuse, D. Evaluation of Long Term Behaviour of Polymers for Offshore Oil and Gas Applications. Oil Gas Sci. Technol. 2015, 70, 279–289. [Google Scholar] [CrossRef]
- Gurit, “Ampreg 26—Epoxy Laminating System” 2015. Available online: http://www.gurit.com/sitecore/content/Old-Product-Pages/Other-Products/Laminating-Infusion-Systems/Ampreg-26 (accessed on 5 October 2016).
- ASTM. D5229—Standard Test Method for Moisture Absorption Properties and Equilibrium Conditioning of Polymer Matrix Composite Materials. 2014. Available online: https://www.astm.org/Standards/D5229.htm (accessed on 5 October 2016).
- ASTM. D1141-98 Standard Practice for the Preparation of Substitute Ocean Water. 2013. Available online: https://www.astm.org/Standards/D1141.htm (accessed on 5 October 2016).
- Grace, L.R.; Altan, M.C. Characterization of anisotropic moisture absorption in polymeric composites using hindered diffusion model. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1187–1196. [Google Scholar] [CrossRef]
- Grace, L.R. Projecting long-term non-Fickian diffusion behavior in polymeric composites based on short-term data: A 5-year validation study. J. Mater. Sci. 2016, 51, 845–853. [Google Scholar] [CrossRef]
- Bao, L.R.; Yee, A.F. Moisture diffusion and hygrothermal aging in bismaleimide matrix carbon fiber composites: Part II-woven and hybrid composites. Compos. Sci. Technol. 2002, 62, 2002. [Google Scholar] [CrossRef]
- Shen, C.H.; Springer, G.S. Moisture Absorption and Desorption of Composite Materials. J. Compos. Mater. 1976, 10, 2–20. [Google Scholar] [CrossRef]
- Starink, M.J.; Starink, L.M.P.; Chambers, A.R. Moisture uptake in monolithic and composite materials: Edge correction for rectanguloid samples. J. Mateir. Sci. 2002, 37, 287–294. [Google Scholar] [CrossRef]
- Arnold, J.C.; Alston, S.M.; Korkees, F. An assessment of methods to determine the directional moisture diffusion coefficients of composite materials. Compos. Part A Appl. Sci. Manuf. 2013, 55, 120–128. [Google Scholar] [CrossRef]
- Laidler, K.J. The Development of the Arrhenius Equation. J. Chem. Educ. 1984, 61, 494–498. [Google Scholar] [CrossRef]
- Masaro, L.; Zhu, X.X. Physical models of diffusion for polymer solutions, gels and solids. Prog. Polym. Sci. 1999, 24, 731–775. [Google Scholar] [CrossRef]
- Bellot, C.M.; Olivero, M.; Sangermano, M.; Salvo, M. Towards self-diagnosis composites: Detection of moisture diffusion through epoxy by embedded evanescent wave optical fibre sensors. Polym. Test. 2018, 71, 248–254. [Google Scholar] [CrossRef]
- Zhou, J.; Lucas, J.P. Hygrothermal effects of epoxy resin. Part I: The nature of water in epoxy. Polymer 1999, 40, 5505–5512. [Google Scholar] [CrossRef]
- Popineau, S.; Rondeau-Mouro, C.; Sulpice-Gaillet, C.; Shanahan, M.E.R. Free/bound water absorption in an epoxy adhesive. Polymer 2006, 46, 10733–10740. [Google Scholar] [CrossRef]
- Carter, H.; Kibler, K. Langmuir-Type Model for Anomalous Moisture Diffusion In Composite Resins. J. Compos. Mater. 1978, 12, 118–131. [Google Scholar] [CrossRef]
- Choi, S.; Douglas, E. Complex hygrothermal effects on the glass transition of an epoxy-amine thermoset. Acs Appl. Mater. Interfaces 2010, 2, 934–941. [Google Scholar] [CrossRef]
- Starkova, S.T. Buschhorn, Mannov, Schulte, and Aniskevich. Water transport in epoxy/MWCNT composites. Eur. Polym. J. 2013, 49, 2138–2148. [Google Scholar] [CrossRef]
- Yagoubi, E.J.; Lubineau, G.; Roger, F.; Verdu, J. A fully coupled diffusion-reaction scheme for moisture sorption-desorption in an an epoxy. Polymers 2012, 53, 5582–5595. [Google Scholar] [CrossRef]
- Papanicolaou, T.; Kosmidou, V.; Vatalis, D. Water absorption mechanism and some anomalous effects on the mechanical and viscoelastic behavior of an epoxy system. J. Appl. Polym. Sci. 2006, 99, 1328–1339. [Google Scholar] [CrossRef]
- Gordon, M.; Taylor, J.S. Ideal copolymers and the second-order transitions of synthetic rubbers. i. non-crystalline copolymers. J. Appl. Chem. 1952, 2, 493–500. [Google Scholar] [CrossRef]
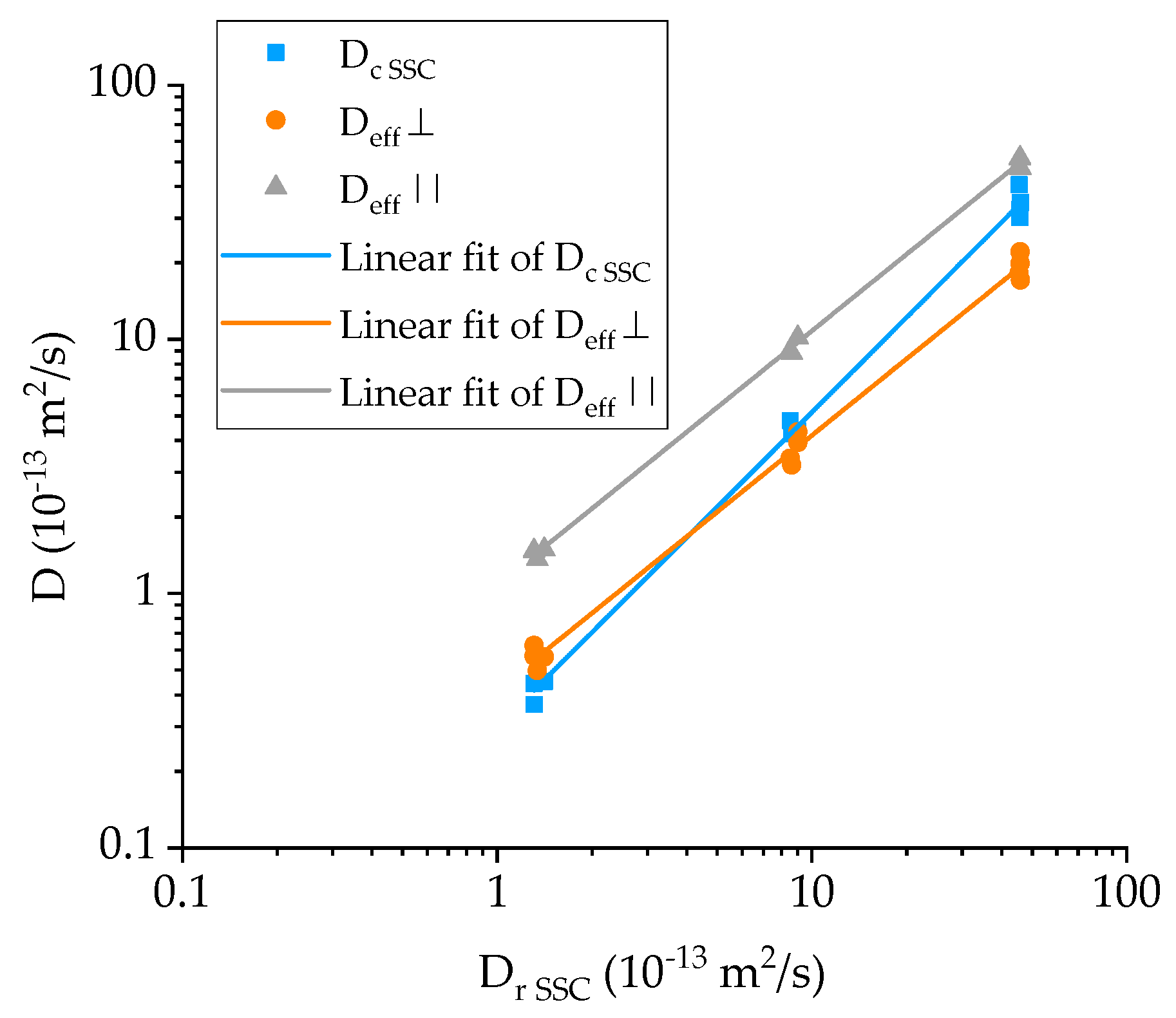

| Compound | Concentration |
|---|---|
| (g/L) | |
| NaCl | 24.53 |
| MgCl2 | 5.20 |
| Na2SO4 | 4.09 |
| CaCl2 | 1.16 |
| KCl | 0.695 |
| NaHCO3 | 0.201 |
| KBr | 0.101 |
| H3BO3 | 0.027 |
| SrCl2 | 0.025 |
| NaF | 0.003 |
| Other metal nitrates/nitrites | <0.1 mg/L |
| H2O | balance |
| Code | h × w × l |
|---|---|
| (mm × mm × mm) | |
| α | 2 × 19 × 19 |
| β | 2 × 40 × 40 |
| γ—quasi-infinite | 2 × 100 × 100 |
| δ 1—fibers along l direction | 2 × 10 × 100 |
| ε 1—fibers along w direction | 2 × 100 × 10 |
| Temperature | Specimen | h × w × l | Slope | M∞ | t∞ |
|---|---|---|---|---|---|
| (°C) | (mm × mm × mm) | (%) | (days) | ||
| 25 | alpha | 2.09 × 18.86 × 19.15 | 8.8 | 1.94 | 113 |
| beta | 2.18 × 40.00 × 40.08 | 7.9 | 1.90 | 107 | |
| gamma | 2.04 × 100.04 × 100.07 | 8.4 | 2.01 | 110 | |
| delta | 2.15 × 10.08 × 100.03 | 8.2 | 1.91 | 107 | |
| 55 | alpha | 2.11 × 18.96 × 19.05 | 28.4 | 2.69 | 55 |
| beta | 2.16 × 40.03 × 39.99 | 26.6 | 2.60 | 33 | |
| gamma | 2.12 × 100.05 × 100.05 | 26.6 | 2.63 | 33 | |
| delta | 2.07 × 10.06 × 100.05 | 28.4 | 2.91 | 48 | |
| 80 | alpha | 2.15 × 19.31 × 19.14 | 71.7 | 2.77 | 16 |
| beta | 2.15 × 39.96 × 40.03 | 65.9 | 2.77 | 9 | |
| gamma | 2.13 × 100.03 × 100.05 | 64.9 | 2.79 | 9 | |
| delta | 2.12 × 10.05 × 99.97 | 69.9 | 2.73 | 9 |
| Temperature | Specimen | h × w × l | Slope | M∞ | t∞ |
|---|---|---|---|---|---|
| (°C) | (mm × mm × mm) | (%) | (days) | ||
| 25 | beta | 1.72 × 39.99 × 40.00 | 1.4 | 0.48 | 143 |
| gamma | 1.70 × 99.84 × 99.73 | 1.3 | 0.46 | 132 | |
| delta | 1.72 × 9.99 × 99.96 | 1.4 | 0.51 | 182 | |
| epsilon | 1.70 × 99.90 × 10.03 | 1.5 | 0.47 | 119 | |
| 55 | beta | 1.73 × 40.00 × 40.04 | 6.2 | 0.65 | 29 |
| gamma | 1.70 × 99.91 × 99.98 | 5.9 | 0.67 | 41 | |
| delta | 1.73 × 9.95 × 99.98 | 6.1 | 0.64 | 31 | |
| epsilon | 1.72 × 99.93 × 9.99 | 6.6 | 0.66 | 29 | |
| 80 | beta | 1.73 × 39.99 × 39.99 | 20.0 | 0.72 | 8 |
| gamma | 1.76 × 99.12 × 99.75 | 20.0 | 0.82 | 24 | |
| delta | 1.71 × 10.00 × 100.03 | 19.6 | 0.75 | 17 | |
| epsilon | 1.74 × 99.93 × 10.01 | 21.1 | 0.80 | 70 |
| Temperature | Specimen | h × w × l | fS&S | fSSC | Dr | Dr,S&S | Dr,SSC |
|---|---|---|---|---|---|---|---|
| (°C) | (mm × mm × mm) | (× 10−13 m2/s) | |||||
| 25 | alpha | 2.09 × 18.86 × 19.15 | 1.22 | 1.12 | 1.74 | 1.17 | 1.38 |
| beta | 2.18 × 40.00 × 40.08 | 1.11 | 1.06 | 1.59 | 1.29 | 1.41 | |
| gamma | 2.04 × 100.04 × 100.07 | 1.04 | 1.02 | 1.40 | 1.29 | 1.34 | |
| delta | 2.15 × 10.08 × 100.03 | 1.24 | 1.13 | 1.67 | 1.09 | 1.31 | |
| 55 | alpha | 2.09 × 18.86 × 19.15 | 1.22 | 1.12 | 9.57 | 6.43 | 7.59 |
| beta | 2.16 × 40.03 × 39.99 | 1.11 | 1.06 | 9.57 | 7.80 | 8.53 | |
| gamma | 2.12 × 100.05 × 100.05 | 1.04 | 1.02 | 9.03 | 8.31 | 8.63 | |
| delta | 2.07 × 10.06 × 100.05 | 1.23 | 1.12 | 8.02 | 5.33 | 9.01 | |
| 80 | alpha | 2.15 × 19.31 × 19.14 | 1.22 | 1.13 | 61.09 | 40.80 | 48.27 |
| beta | 2.15 × 39.96 × 40.03 | 1.11 | 1.06 | 51.01 | 41.60 | 45.49 | |
| gamma | 2.13 × 100.03 × 100.05 | 1.04 | 1.02 | 48.13 | 44.28 | 45.98 | |
| delta | 2.12 × 10.05 × 99.97 | 1.23 | 1.13 | 58.21 | 38.32 | 45.83 | |
| Temp. | Specimen | h × w × l | fS&S | fortho,⊥ | fortho,∥ | Dc | Dc,S&S | Dc,SSC | Deff,⊥ | Deff,∥ |
|---|---|---|---|---|---|---|---|---|---|---|
| (°C) | (mm × mm × mm) | (× 10−13 m2/s) | ||||||||
| 25 | beta | 1.72 × 39.99 × 40.00 | 1.09 | 1.06 | 1.03 | 0.51 | 0.43 | 0.45 | 0.56 | 1.49 |
| gamma | 1.70 × 99.84 × 99.73 | 1.03 | 1.02 | 1.01 | 0.49 | 0.46 | 0.47 | 0.50 | 1.37 | |
| delta | 1.72 × 9.99 × 99.96 | 1.19 | 1.11 | 1.06 | 0.45 | 0.31 | 0.37 | 0.57 | 1.47 | |
| epsilon | 1.70 × 99.90 × 10.03 | 1.19 | 1.16 | 1.06 | 0.60 | 0.43 | 0.44 | 0.63 | 1.47 | |
| 55 | beta | 1.73 × 40.00 × 40.04 | 1.09 | 1.06 | 1.03 | 5.40 | 4.58 | 4.78 | 3.40 | 9.01 |
| gamma | 1.70 × 99.91 × 99.98 | 1.03 | 1.02 | 1.01 | 4.46 | 4.17 | 4.25 | 3.20 | 8.82 | |
| delta | 1.73 × 9.95 × 99.98 | 1.19 | 1.11 | 1.06 | 5.37 | 3.78 | 4.36 | 3.92 | 10.15 | |
| epsilon | 1.72 × 99.93 × 9.99 | 1.19 | 1.17 | 1.06 | 5.75 | 4.06 | 4.23 | 4.33 | 10.14 | |
| 80 | beta | 1.73 × 39.99 × 39.99 | 1.09 | 1.06 | 1.03 | 45.81 | 38.80 | 40.57 | 18.17 | 48.06 |
| gamma | 1.76 × 99.12 × 99.75 | 1.04 | 1.03 | 1.01 | 36.29 | 33.85 | 34.50 | 17.10 | 47.03 | |
| delta | 1.71 × 10.00 × 100.03 | 1.19 | 1.11 | 1.06 | 39.91 | 28.27 | 32.52 | 19.89 | 51.53 | |
| epsilon | 1.74 × 99.93 × 10.01 | 1.19 | 1.17 | 1.06 | 40.94 | 28.84 | 30.05 | 22.08 | 51.63 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavasin, M.; Sangermano, M.; Thomson, B.; Giannis, S. Exposure of Glass Fiber Reinforced Polymer Composites in Seawater and the Effect on Their Physical Performance. Materials 2019, 12, 807. https://doi.org/10.3390/ma12050807
Cavasin M, Sangermano M, Thomson B, Giannis S. Exposure of Glass Fiber Reinforced Polymer Composites in Seawater and the Effect on Their Physical Performance. Materials. 2019; 12(5):807. https://doi.org/10.3390/ma12050807
Chicago/Turabian StyleCavasin, Matteo, Marco Sangermano, Barry Thomson, and Stefanos Giannis. 2019. "Exposure of Glass Fiber Reinforced Polymer Composites in Seawater and the Effect on Their Physical Performance" Materials 12, no. 5: 807. https://doi.org/10.3390/ma12050807
APA StyleCavasin, M., Sangermano, M., Thomson, B., & Giannis, S. (2019). Exposure of Glass Fiber Reinforced Polymer Composites in Seawater and the Effect on Their Physical Performance. Materials, 12(5), 807. https://doi.org/10.3390/ma12050807