Morphology and Wear Resistance of Composite Coatings Formed on a TA2 Substrate Using Hot-Dip Aluminising and Micro-Arc Oxidation Technologies
Abstract
1. Introduction
2. Materials and Methods
3. Results
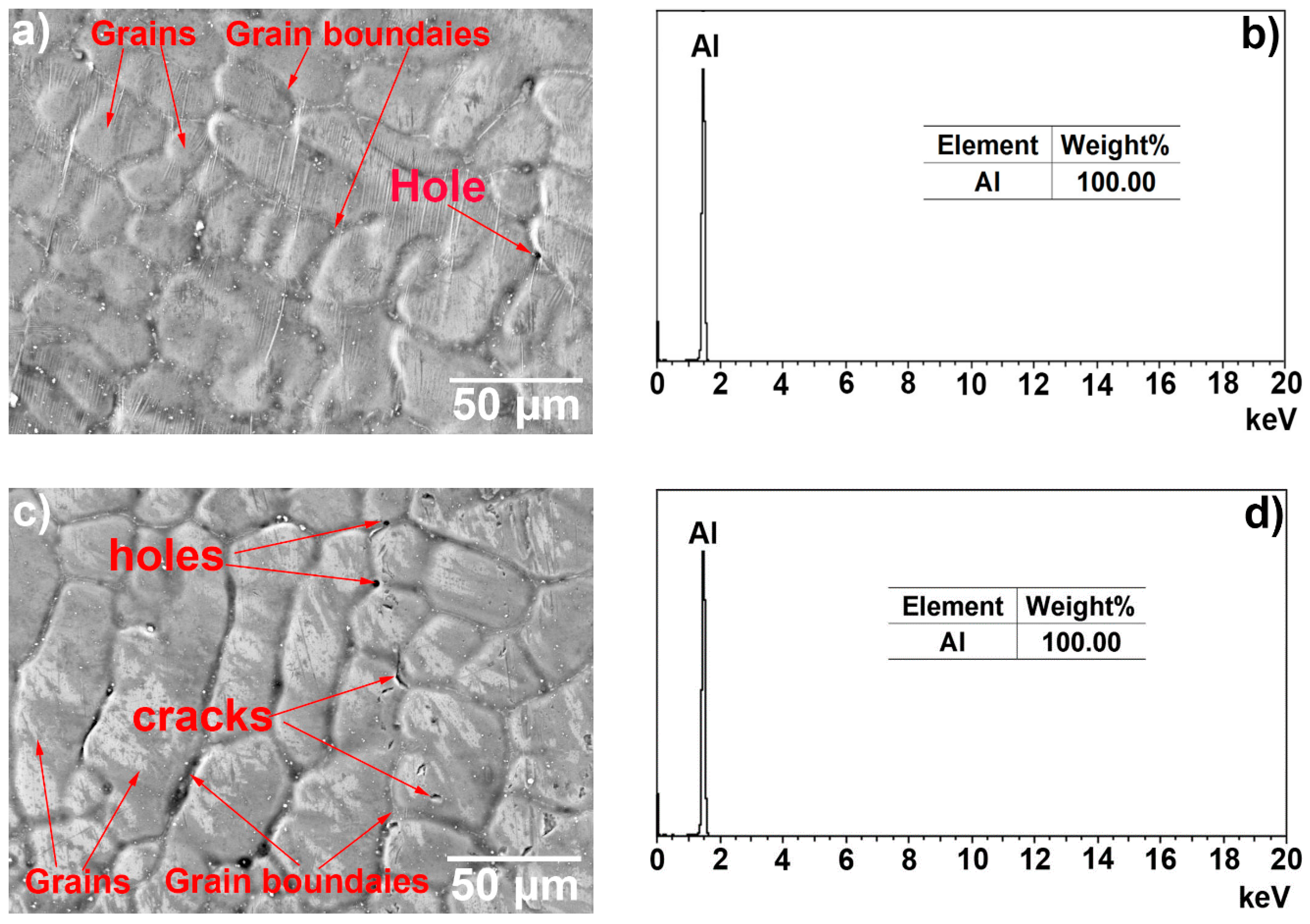
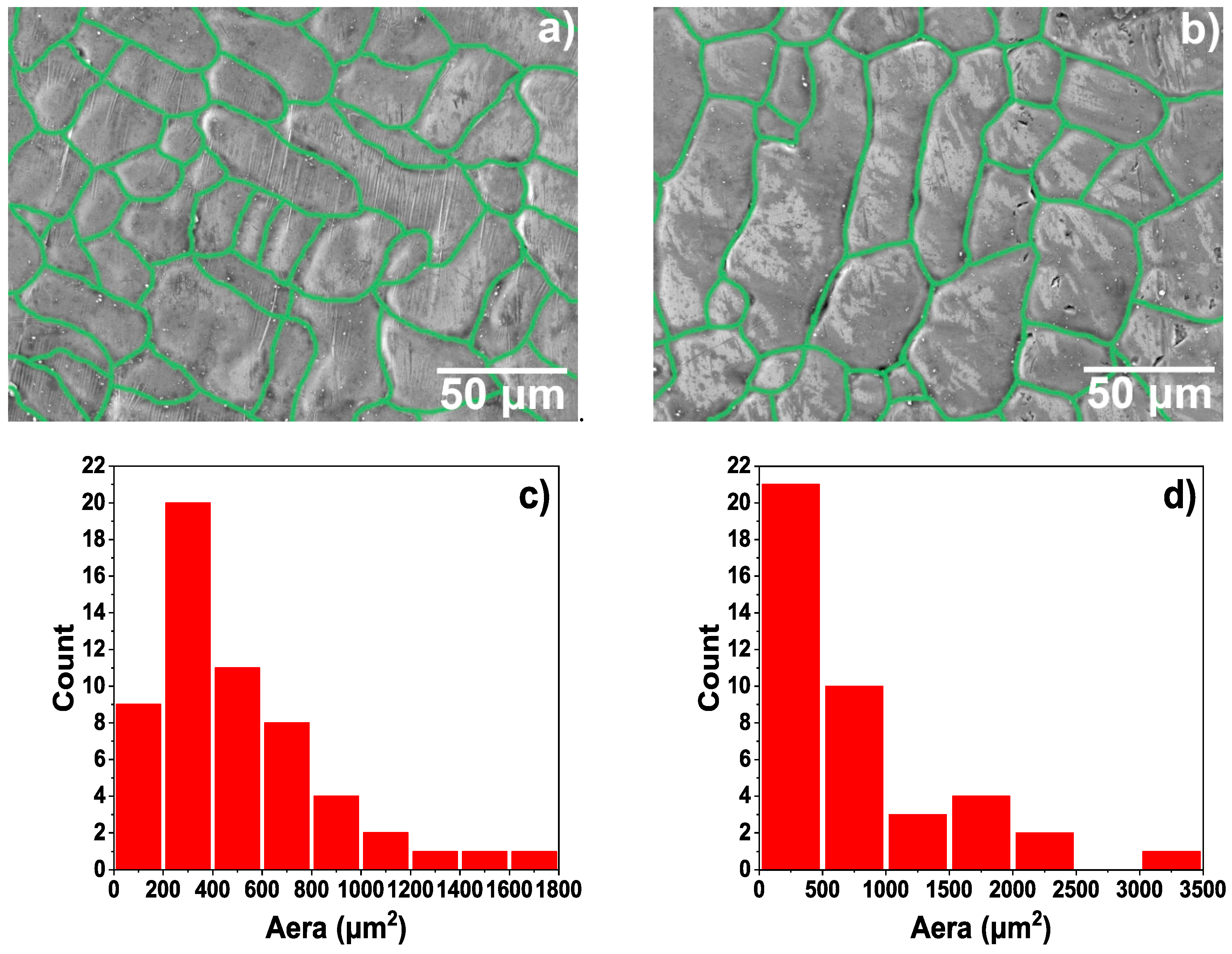
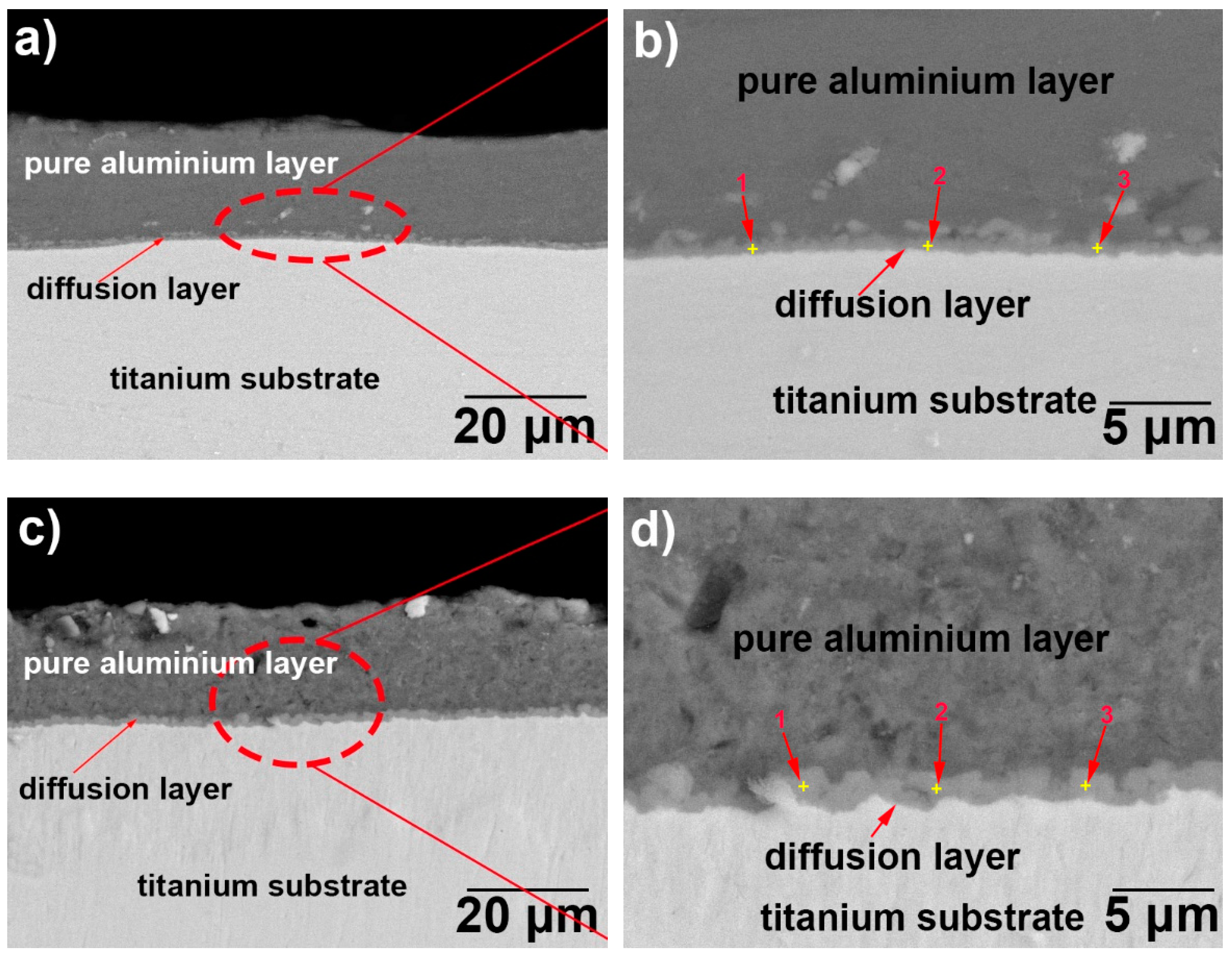
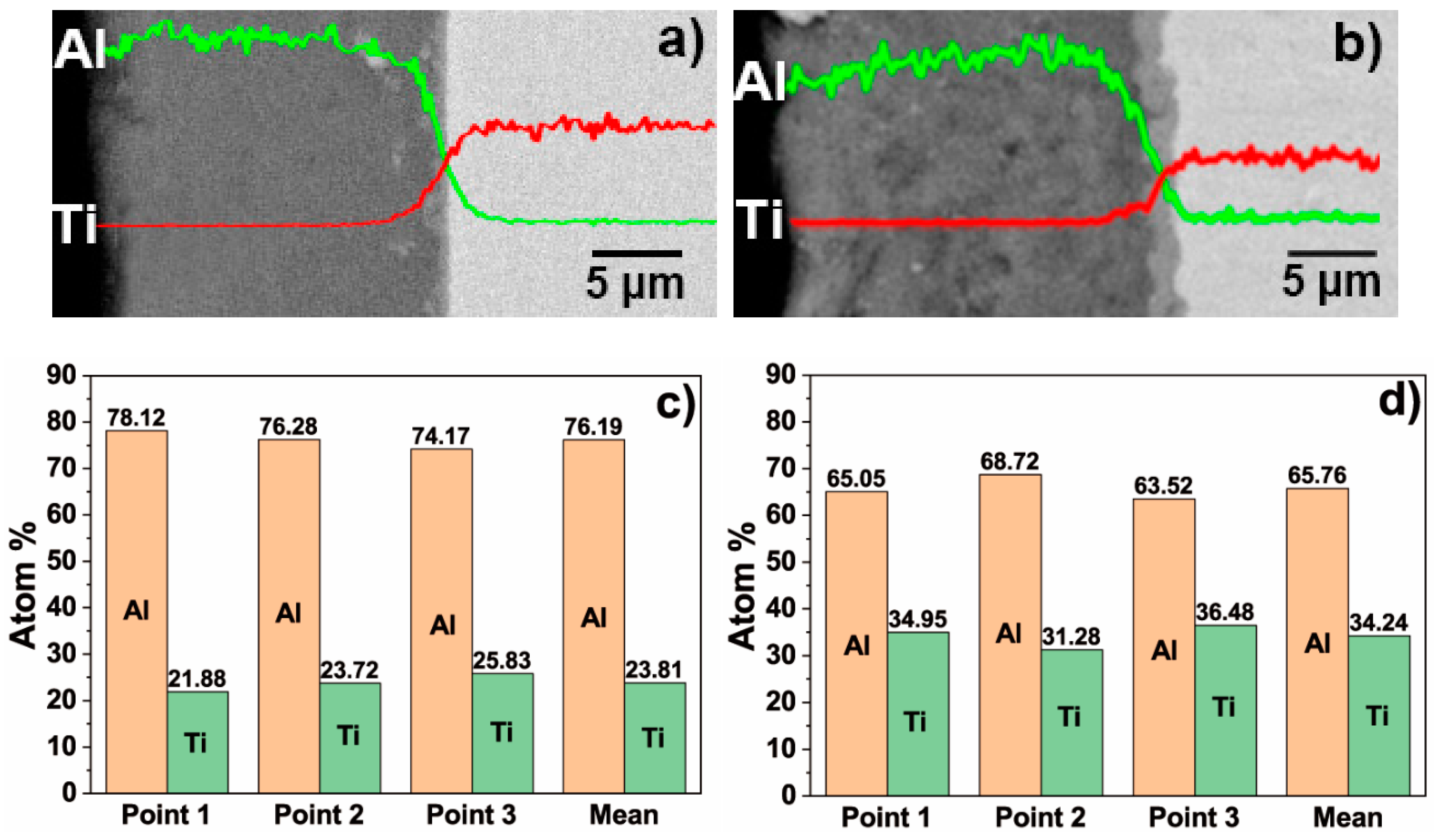
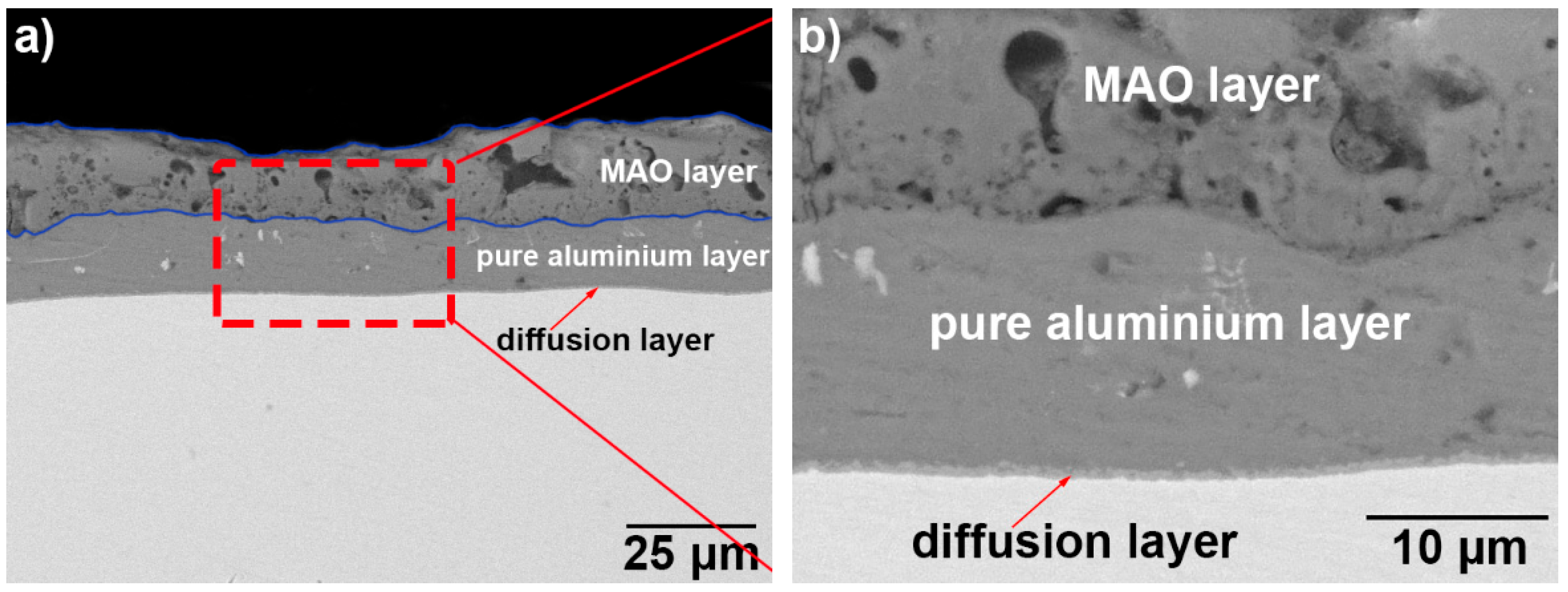
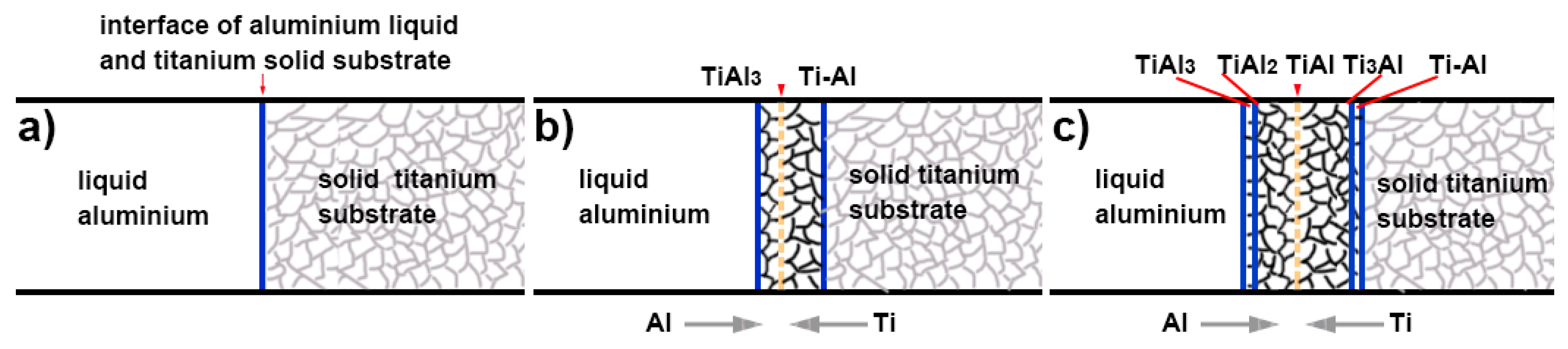
3.1. Microscopic Characteristics of Hot-Dip Aluminised Layer
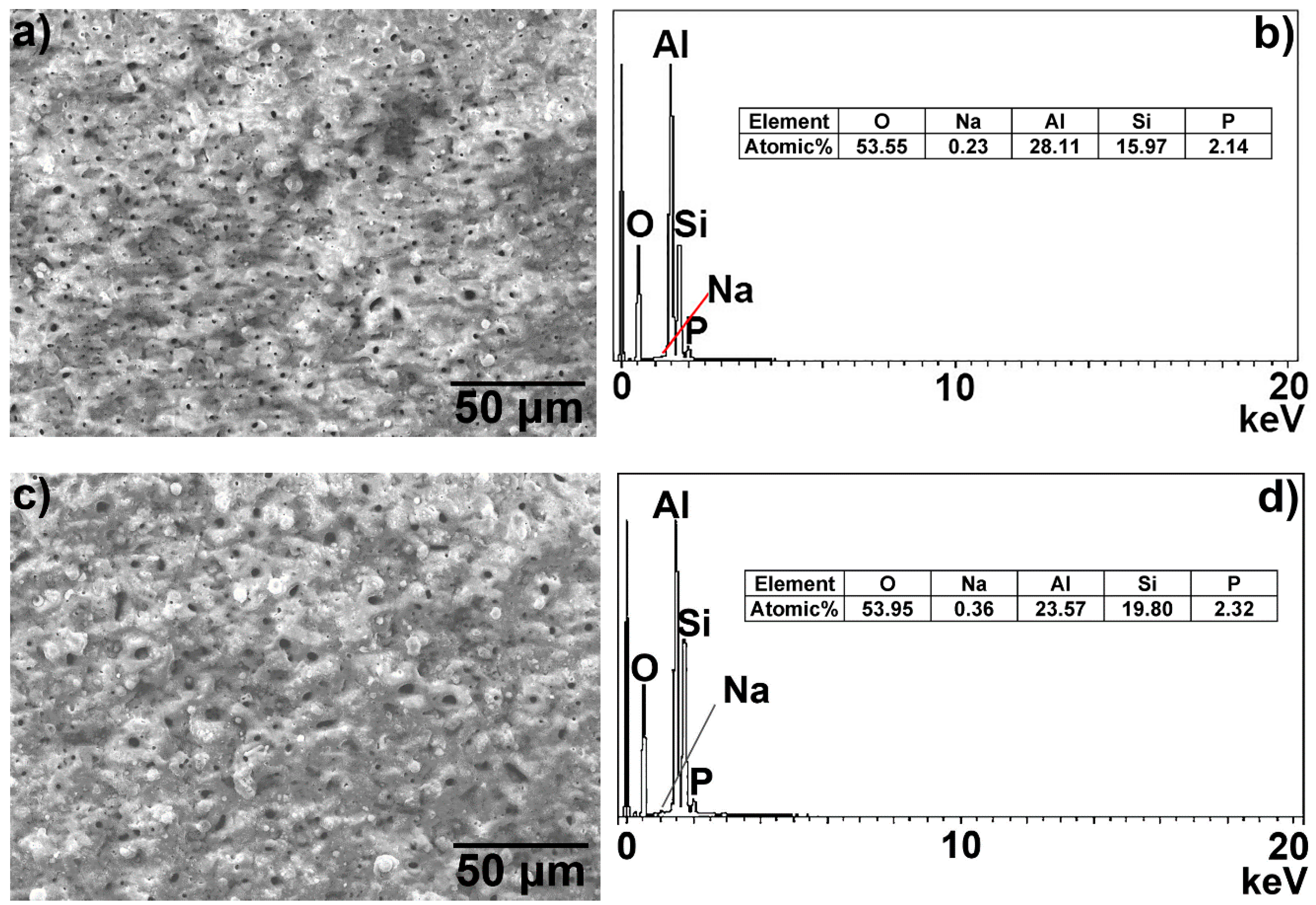
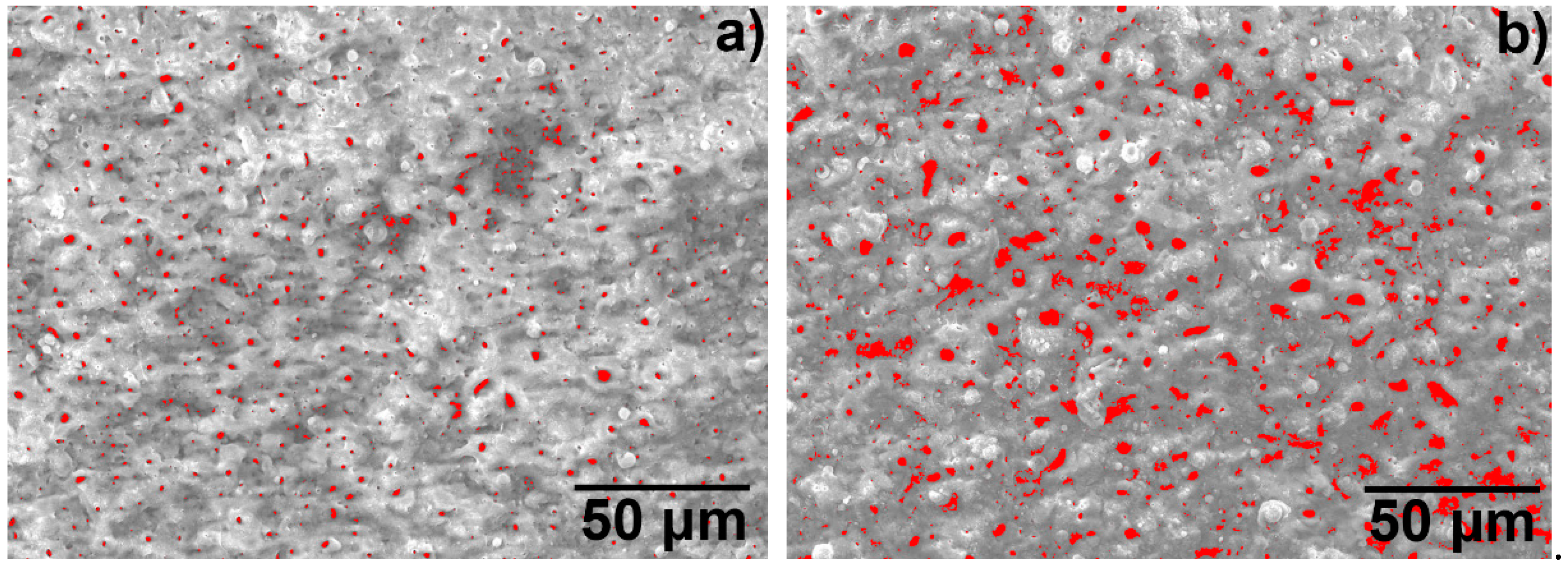
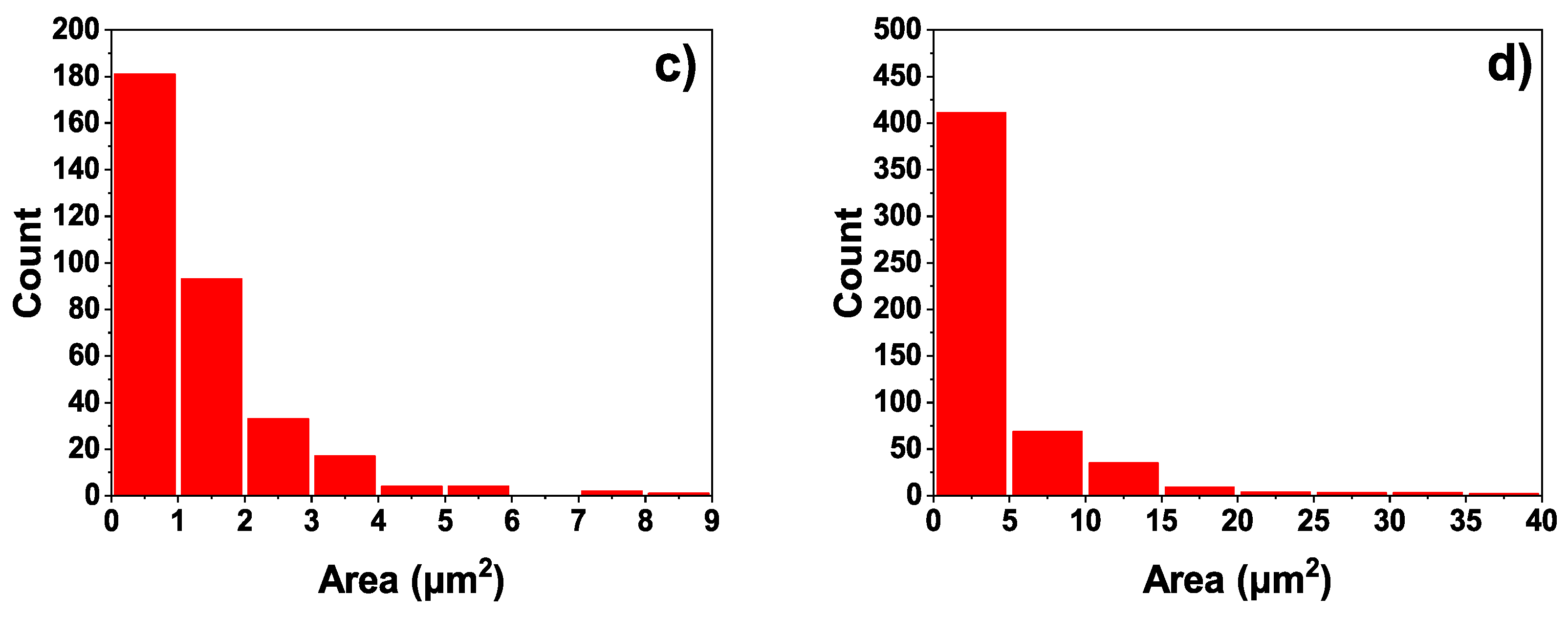
3.2. Microscopic Properties of the MAO Layer
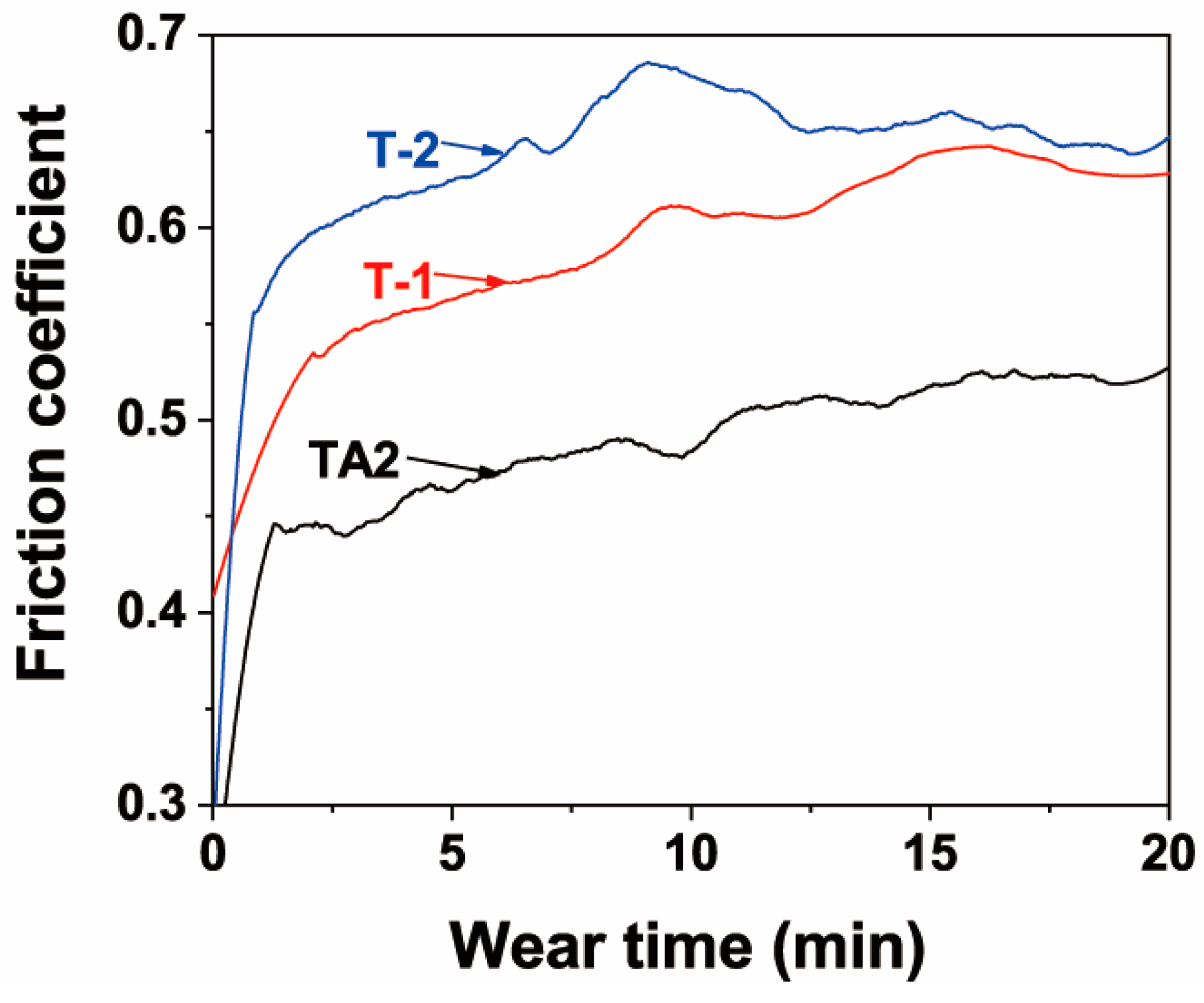
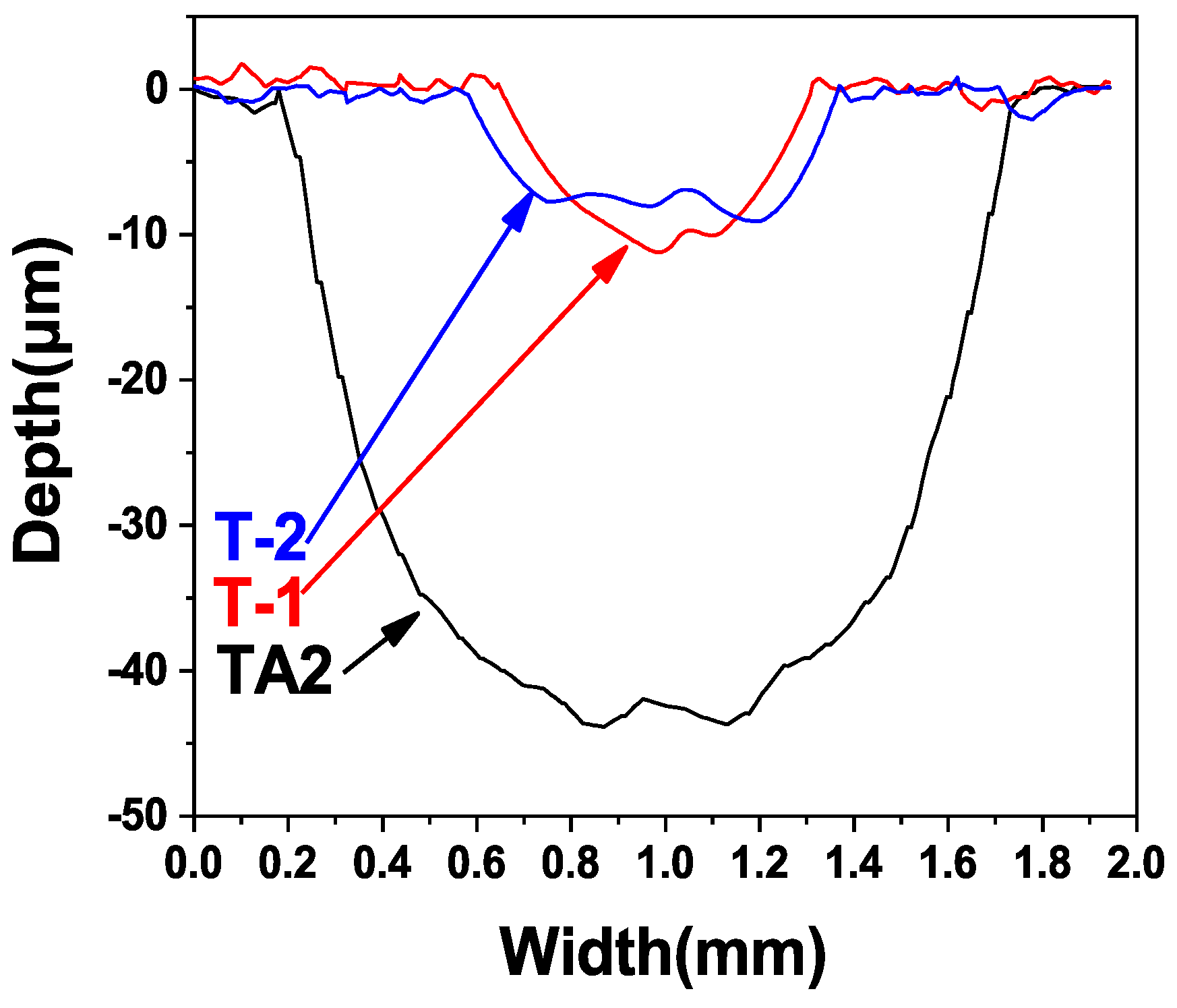
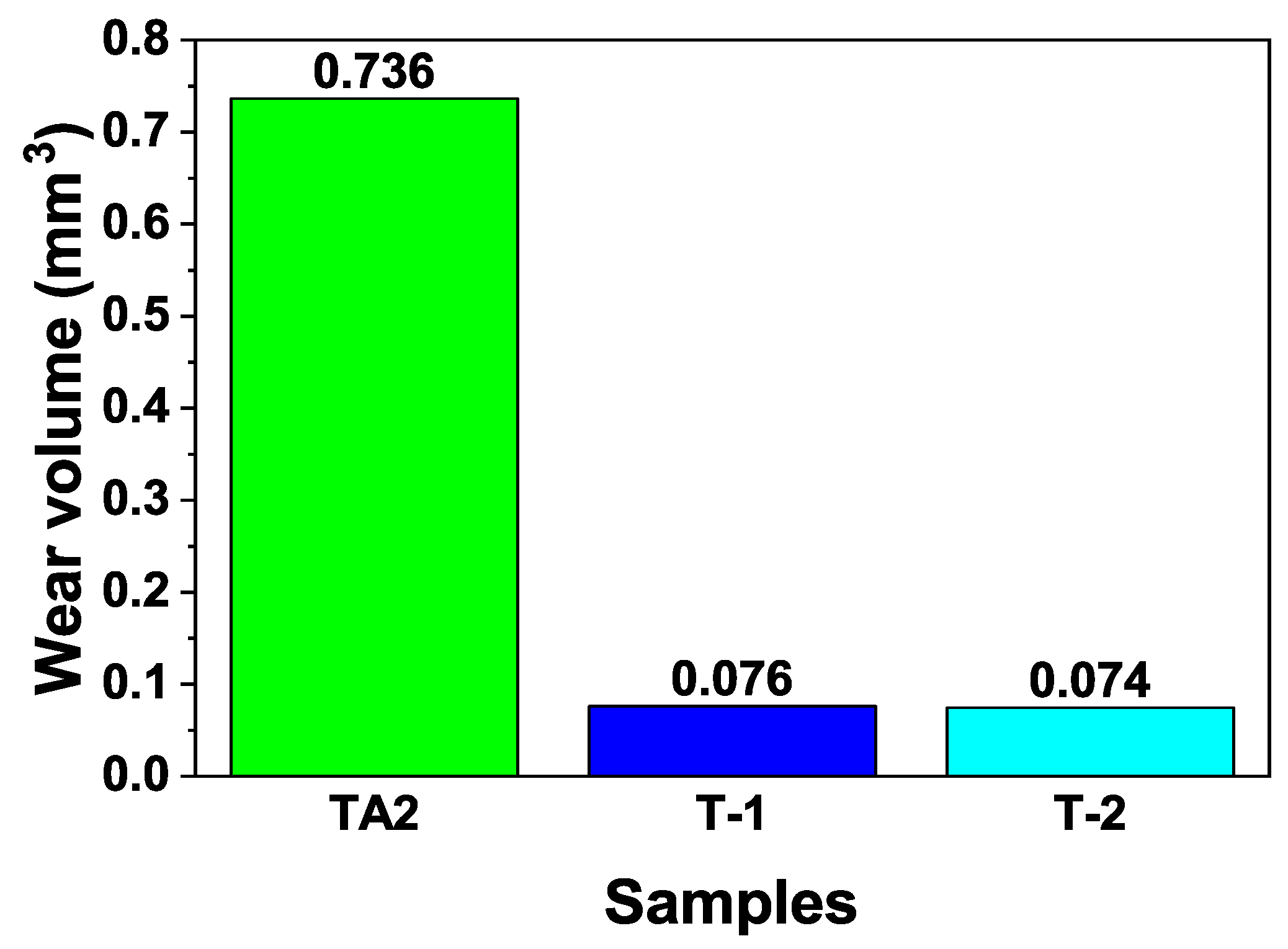
3.3. Friction and Wear Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rack, H.J.; Qazi, J.I. Titanium alloys for biomedical applications. Mater. Sci. Eng. C 2006, 26, 1269–1277. [Google Scholar] [CrossRef]
- Rajendran, R. Gas turbine coatings—An overview. Eng. Failure Anal. 2012, 26, 355–369. [Google Scholar] [CrossRef]
- Utu, I.D.; Marginean, G.; Hulka, I.; Serban, V.A.; Cristea, D. Properties of the thermally sprayed Al2O3–TiO2 coatings deposited on titanium substrate. Int. J. Refract. Met. Hard Mater. 2015, 51, 118–123. [Google Scholar] [CrossRef]
- Hatem, A.; Lin, J.; Wei, R.; Torres, R.D.; Laurindo, C.; Soares, P. Tribocorrosion behavior of DLC-coated Ti-6Al-4V alloy deposited by PIID and PEMS + PIID techniques for biomedical applications. Surf. Coat. Technol. 2017, 332, 223–232. [Google Scholar] [CrossRef]
- Utu, I.D.; Marginean, G. Effect of electron beam remelting on the characteristics of HVOF sprayed Al2O3-TiO2 coatings deposited on titanium substrate. Colloids Surf. A 2017, 526, 70–75. [Google Scholar] [CrossRef]
- Hatem, A.; Lin, J.; Wei, R.; Torres, R.D.; Laurindo, C.; Souza, G.B.D.; Soares, P. Tribocorrosion behavior of low friction TiSiCN nanocomposite coatings deposited on titanium alloy for biomedical applications. Surf. Coat. Technol. 2018, 347, 1–12. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Y.; Miao, L.; Ma, Y.; Zhang, X.; Cui, Y.; Dong, Y.; Chen, X.; Wang, L.; Liu, Z.; et al. Microstructure and properties of Al2O3-Y2O3 ceramic composite coatings fabricated by plasma spraying. Surf. Coat. Technol. 2018, 350, 550–559. [Google Scholar] [CrossRef]
- Alvara, F.S.; Heydari, M.; Kazemzadeh, A.; Vaezi, M.; Nikzad, L. Al2O3-TiB2 nanocomposite coating deposition on Titanium by Air Plasma Spraying. Mater. Today 2018, 5, 15739–15743. [Google Scholar] [CrossRef]
- Çomaklı, O.; Yazıcı, M.; Yetim, T.; Yetim, A.F.; Çelik, A. Effect of Ti amount on wear and corrosion properties of Ti-doped Al2O3 nanocomposite ceramic coated CP titanium implant material. Ceram. Int. 2018, 44, 7421–7428. [Google Scholar] [CrossRef]
- Datta, S.; Das, M.; Balla, V.K.; Bodhak, S.; Murugesan, V.K. Mechanical, wear, corrosion and biological properties of arc deposited titanium nitride coatings. Surf. Coat. Technol. 2018, 344, 214–222. [Google Scholar] [CrossRef]
- Gavrilov, N.V.; Kamenetskikh, A.S.; Tretnikov, P.V.; Chukin, A.V. Ion assisted deposition of α-Al2O3 coatings by anodic evaporation in the arc discharge. Surf. Coat. Technol. 2018, 337, 453–460. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, X.; Ren, L.; Wang, T.; Qi, X.; Yang, Y. Improving the Tribological Properties of Spark-Anodized Titanium by Magnetron Sputtered Diamond-Like Carbon. Coatings 2018, 8, 83. [Google Scholar] [CrossRef]
- Aliasghari, S.; Skeldon, P.; Thompson, G.E. Plasma electrolytic oxidation of titanium in a phosphate/silicate electrolyte and tribological performance of the coatings. Appl. Surf. Sci. 2014, 316, 463–476. [Google Scholar] [CrossRef]
- Jin, Q.; Xue, W.; Li, X.; Zhu, Q.; Wu, X. Al2O3 coating fabricated on titanium by cathodic microarc electrodeposition. J. Alloys Compd. 2009, 476, 356–359. [Google Scholar] [CrossRef]
- Erfanifar, E.; Aliofkhazraei, M.; Nabavi, H.F.; Sharifi, H.; Rouhaghdam, A.S. Growth kinetics and morphology of plasma electrolytic oxidation coating on aluminium. Mater. Chem. Phys. 2017, 185, 162–175. [Google Scholar] [CrossRef]
- Zhu, L.; Guo, Z.; Zhang, Y.; Li, Z.; Sui, M. A mechanism for the growth of a plasma electrolytic oxide coating on Al. Electrochim. Acta 2016, 208, 296–303. [Google Scholar] [CrossRef]
- Liu, C.; Liu, P.; Huang, Z.; Yan, Q.; Guo, R.; Li, D.; Jiang, G.; Shen, D. The correlation between the coating structure and the corrosion behavior of the plasma electrolytic oxidation coating on aluminium. Surf. Coat. Technol. 2016, 286, 223–230. [Google Scholar] [CrossRef]
- Wang, C.; Ma, F.; Liu, P.; Chen, J.; Liu, X.; Zhang, K.; Li, W.; Han, Q. The influence of alloy elements in Ti-6Al-4V and Ti-35Nb-2Ta-3Zr on the structure, morphology and properties of MAO coatings. Vacuum 2018, 157, 229–236. [Google Scholar] [CrossRef]
- Yizhou, S.; Haijun, T.; Yuebin, L.; Xiaofei, Z.; Tao, W.; Jie, T.; Lei, P. Fabrication and Wear Resistance of TiO2/Al2O3 Coatings by Micro-arc Oxidation. Rare Met. Mater. Eng. 2017, 46, 23–27. [Google Scholar] [CrossRef]
- Lu, X.; Mohedano, M.; Blawert, C.; EndzheMatykina; RaulArrabal; Kainer, K.U.; Zheludkevich, M.L. Plasma electrolytic oxidation coatings with particle additions—Areview. Surf. Coat. Technol. 2016, 307, 1165–1182. [Google Scholar] [CrossRef]
- Lemmens, B.; Springer, H.; Graeve, I.D.; Strycker, J.D.; Raabe, D.; Verbeken, K. Effect of silicon on the microstructure and growth kinetics of intermetallic phases formed during hot-dip aluminizing of ferritic steel. Surf. Coat. Technol. 2017, 319, 104–109. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Zhou, Y.; Liu, J.Q.; Chen, K.M.; Mo, J.G.; Cui, X.H.; Wang, S.Q. Comparative research on dry sliding wear of hot-dip aluminized and uncoated AISI H13 steel. Wear 2015, 344-345, 22–31. [Google Scholar] [CrossRef]
- Cheng, W.-J.; Wang, C.-J. High-temperature oxidation behavior of hot-dipped aluminide mild steel with various silicon contents. Appl. Surf. Sci. 2013, 274, 258–265. [Google Scholar] [CrossRef]
- Danzo, I.I.; Verbeken, K.; Houbaert, Y. Microstructure of hot dip coated Fe–Si steels. Thin Solid Films 2011, 520, 1638–1644. [Google Scholar] [CrossRef]
- Chen, C.; Li, D.Y.; Shang, C.J. Nanocrystallization of aluminized surface of carbon steel for enhanced resistances to corrosion and corrosive wear. Electrochim. Acta 2009, 55, 118–124. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Cheng, W.-J.; Wang, C.-J. Growth and surface morphology of hot-dip Al–Si on 9Cr-1Mo steel. Mater. Charact. 2009, 60, 144–149. [Google Scholar] [CrossRef]
- Wang, D.; Shi, Z. Aluminizing and oxidation treatment of 1Cr18Ni9 stainless steel. Appl. Surf. Sci. 2004, 227, 255–260. [Google Scholar] [CrossRef]
- Lihong, L.; Jingwu, Z.; Dejiu, S.; Lailei, W.; Guirong, J.; Liang, L. TEM analysis and wear resistance of the ceramic coatings on Q235 steel prepared by hybrid method of hot-dipping aluminium and plasma electrolytic oxidation. J. Alloys Compd. 2012, 512, 57–62. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, Y.; Zhao, X.; Ma, R.; Du, A.; Cao, X. Influence of duty cycle on the growth behavior and wear resistance of microarc oxidation coatings on hot dip aluminized cast iron. Surf. Coat. Technol. 2018, 337, 141–149. [Google Scholar] [CrossRef]
- Xiang, X.; Wang, X.; Zhang, G.; Tang, T.; Lai, X. Preparation technique and alloying effect of aluminide coatings as tritium permeation barriers: A review. Int. J. Hydrogen Energy 2015, 40, 3697–3707. [Google Scholar] [CrossRef]
- Wu, Z.; Xia, Y.; Li, G.; Xu, F. Structure and mechanical properties of ceramic coatings fabricated by plasma electrolytic oxidation on aluminized steel. Appl. Surf. Sci. 2007, 253, 8398–8403. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, Y.; Li, S.; Huang, Y.; Hao, K.; Peng, P. Friction Stir Spot Welding-Brazing of Al and Hot-Dip Aluminized Ti Alloy with Zn Interlayer. Metals 2018, 8, 922. [Google Scholar] [CrossRef]
- Sadeq, F.O.; Sharifitabar, M.; Afarani, M.S. Synthesis of Ti–Si–Al coatings on the surface of Ti–6Al–4V alloy via hot dip siliconizing route. Surf. Coat. Technol. 2018, 337, 349–356. [Google Scholar] [CrossRef]
- Jeng, S.-C. Oxidation behavior and microstructural evolution ofhot-dipped aluminium coating on Ti-6Al-4V alloy at 800 °C. Surf. Coat. Technol. 2013, 235, 867–874. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Peng, Y.P.; Mao, Y.L.; Pang, C.J.; Lu, L.Y. Effect of hot-dip aluminizing on the oxidation resistance of Ti–6Al–4V alloy at high temperatures. Corros. Sci. 2012, 55, 187–193. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Wang, Y.J.; Xiao, L.J.; Zhang, L.Q.; Su, Y.; Lin, J.S. High-temperature oxidation of hot-dip aluminizing coatings on a Ti3Al–Nb alloy and the effects of element additions. Corros. Sci. 2012, 64, 137–144. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, J.; Yan, J.; Fan, H.; Wang, J. Oxidation resistance and corrosion behavior of hot-dip aluminized coatings on commercial-purity titanium. Surf. Coat. Technol. 2011, 206, 1277–1282. [Google Scholar] [CrossRef]
- Xiong, H.-P.; Mao, W.; Ma, W.-L.; Xie, Y.-H.; Chen, Y.-F.; Yuan, H.; Li, X.-H. Liquid-phase aluminizing and siliconizing at the surface of a Ti60 alloy and improvement in oxidation resistance. Mater. Sci. Eng. A 2006, 433, 108–113. [Google Scholar] [CrossRef]
- Cammarota, G.P.; Casagrande, A.; Sambogna, G. Effect of Ni, Si and Cr in the structural formation of diffusion aluminide coatings on commercial-purity titanium. Surf. Coat. Technol. 2006, 201, 230–242. [Google Scholar] [CrossRef]
- Deqing, W.; Ziyuan, S.; Yingli, T. Microstructure and oxidation of hot-dip aluminized titanium at high temperature. Appl. Surf. Sci. 2005, 250, 238–246. [Google Scholar] [CrossRef]
- Lin, Q.; Li, F.; Jin, P.; Zhong, W. Reactive wetting of TA2 pure Ti and TC4 alloy by molten Al 4043 alloy at 873-973 K. Vacuum 2017, 145, 95–102. [Google Scholar] [CrossRef]
- Shen, P.; Fujii, H.; Nogi, K. Wettability of polycrystalline rutile TiO2 by molten Al in different atmospheres. Acta Mater. 2006, 54, 1559–1569. [Google Scholar] [CrossRef]
- Yu, H.; Lu, C.; KietTieu, A.; Li, H.; Godbole, A.; Kong, C. Annealing effect on microstructure and mechanical properties of Al/Ti/Al laminate sheets. Mater. Sci. Eng. A 2016, 660, 195–204. [Google Scholar] [CrossRef]
- Xu, L.; Cui, Y.Y.; Hao, Y.L.; Yang, R. Growth of intermetallic layer in multi-laminated Ti/Al diffusion couples. Mater. Sci. Eng. A 2006, 435, 638–647. [Google Scholar] [CrossRef]
- Gupta, S.P. Intermetallic compounds in diffusion couples of Ti with an Al–Si eutectic alloy. Mater. Charact. 2003, 49, 321–330. [Google Scholar] [CrossRef]
- Jian, Y.; Huang, Z.; Xing, J.; Sun, L.; Liu, Y.; Gao, P. Phase stability, mechanical properties and electronic structures of Ti-Al binary compounds by first principles calculations. Mater. Chem. Phys. 2019, 221, 311–321. [Google Scholar] [CrossRef]
- Demchishin, A.V.; Gnilitskyi, I.; Orazi, L.; Ascari, A. Structure, phase composition and microhardness of vacuum-arcmultilayered Ti/Al, Ti/Cu, Ti/Fe, Ti/Zr nano-structures with differentperiods. Appl. Surf. Sci. 2015, 342, 127–135. [Google Scholar] [CrossRef]
- Liang, G.; Ali, Y.; You, G.; Zhang, M.-X. Effect of cooling rate on grain refinement of cast aluminium alloys. Materialia 2018, 3, 113–121. [Google Scholar] [CrossRef]
- Wang, R.Z.; Qi, J.G.; Wang, B.; Zhang, W.; Wang, J.Z. Solidification behavior and crystal growth mechanism of aluminumunder electric pulse. J. Mater. Process. Technol. 2016, 237, 235–242. [Google Scholar] [CrossRef]
- Tang, F.L.; Che, X.X.; Lu, W.J.; Chen, G.B.; Xie, Y.; Yu, W.Y. Surface structure and solidification morphology of aluminium nanoclusters. Physica B 2009, 404, 2489–2494. [Google Scholar] [CrossRef]
- Çelik, İ.; Alsaran, A.; Purcek, G. Effect of different surface oxidation treatments on structural, mechanical and tribological properties of ultrafine-grained titanium. Surf. Coat. Technol. 2014, 258, 842–848. [Google Scholar] [CrossRef]
- Sharifi, H.; Aliofkhazraei, M.; Darband, G.B.; Rouhaghdam, A.S. Characterization of PEO nanocomposite coatings on titanium formed in electrolyte containing atenolol. Surf. Coat. Technol. 2016, 304, 438–449. [Google Scholar] [CrossRef]
- Kang, S.-H.; Tu, W.-B.; Han, J.-X.; Li, Z.; Cheng, Y.-L. A significant improvement of the wear resistance of Ti6Al4V alloy by a combined method of magnetron sputtering and plasma electrolytic oxidation (PEO). Surf. Coat. Technol. 2019, 358, 879–890. [Google Scholar] [CrossRef]
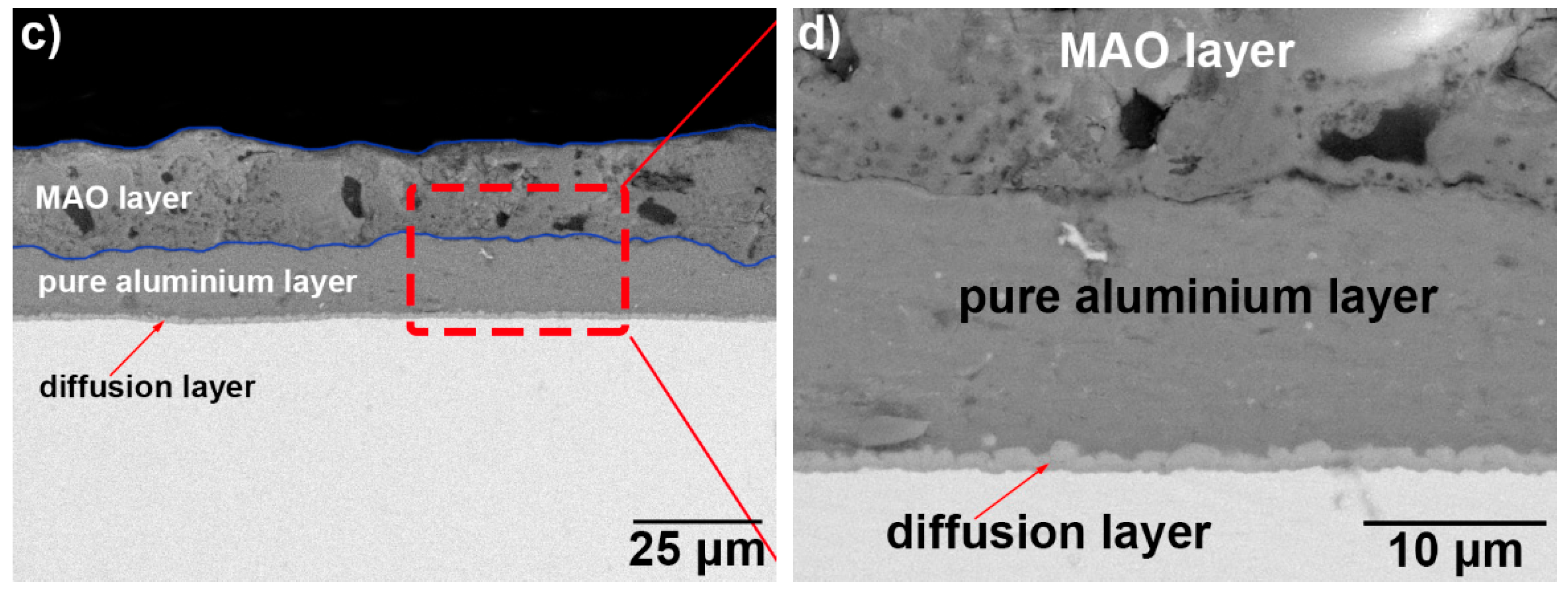
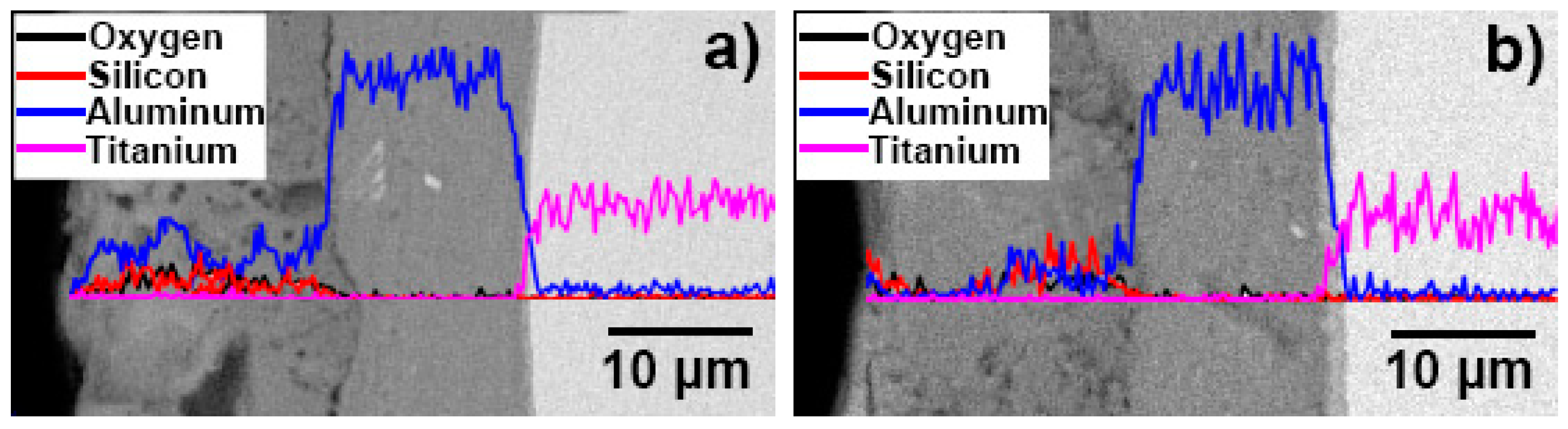
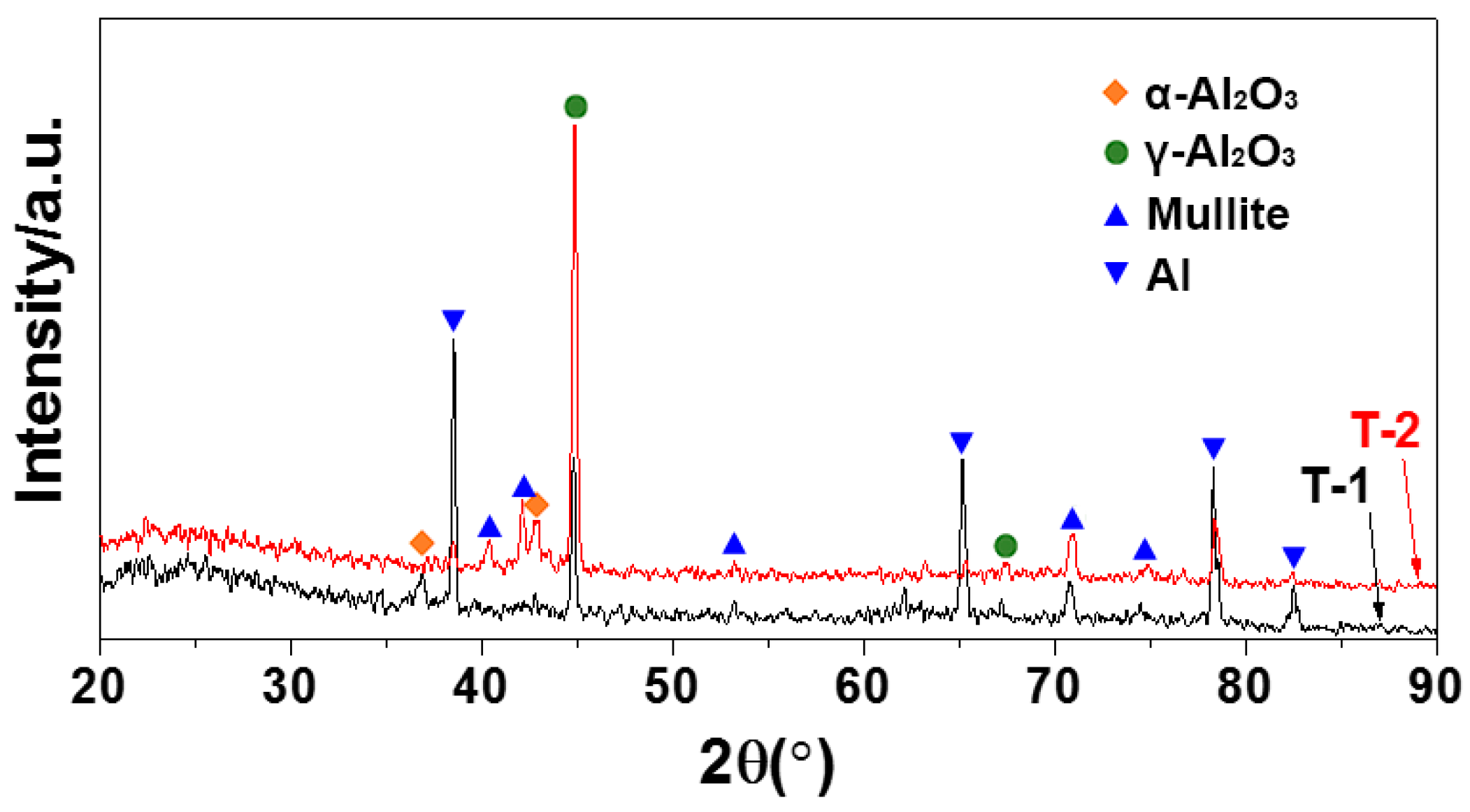
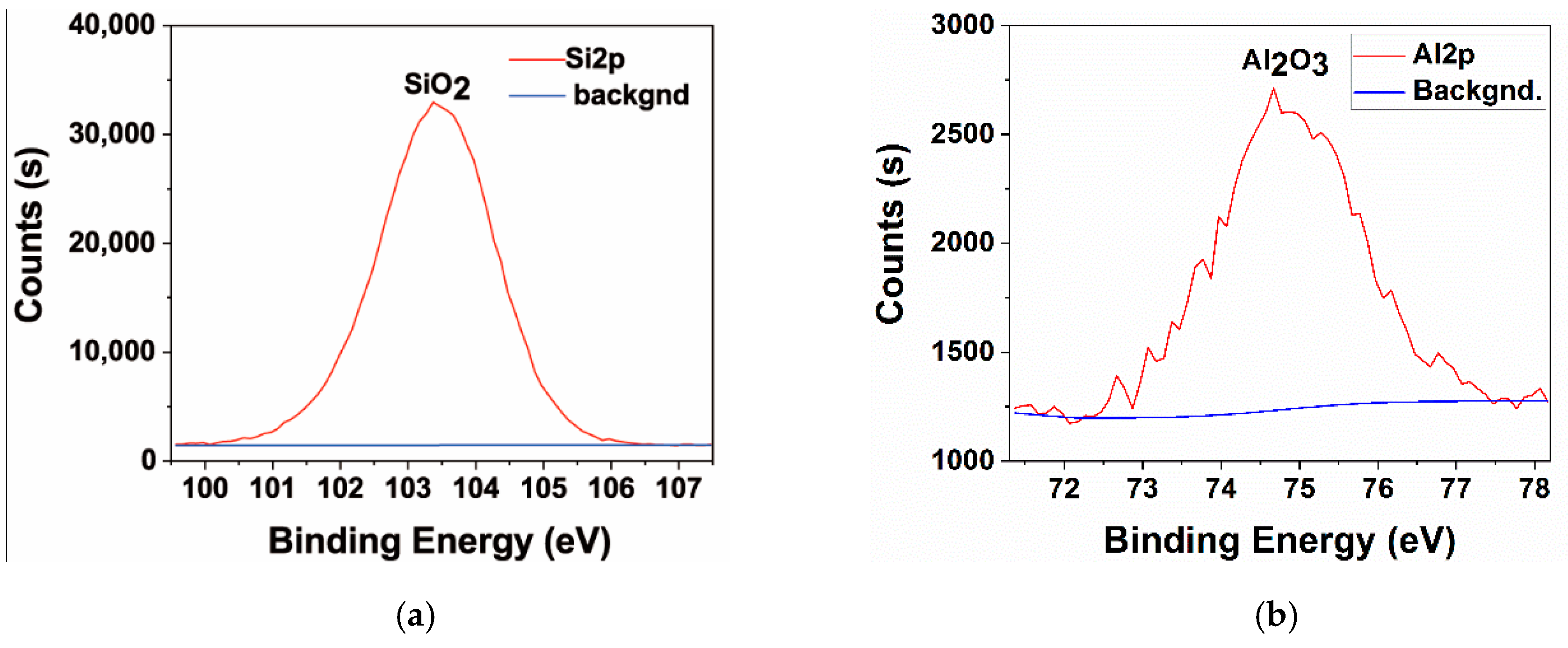
| Sample No. | Hot-Dip Aluminising | MAO | |||||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Time (min) | Lifting Rate (cm·s−1) | Duty Cycle (%) | Pulse Frequency (Hz) | Voltage (V) | Time (min) | |
| T-1 | 710 | 3 | 5 | 20 | 100 | 400–450 | 30 |
| T-2 | 750 | 3 | 5 | 20 | 100 | 400–450 | 30 |
| Sample | Wear Scar Depth (mm) | Wear Scar Width (mm) | Wear Volume (mm3) |
|---|---|---|---|
| TA2 | 0.044 | 1.597 | 0.736 |
| T-1 | 0.011 | 0.662 | 0.076 |
| T-2 | 0.009 | 0.787 | 0.074 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhou, L.; Li, C.; Li, Z.; Li, H. Morphology and Wear Resistance of Composite Coatings Formed on a TA2 Substrate Using Hot-Dip Aluminising and Micro-Arc Oxidation Technologies. Materials 2019, 12, 799. https://doi.org/10.3390/ma12050799
Wang S, Zhou L, Li C, Li Z, Li H. Morphology and Wear Resistance of Composite Coatings Formed on a TA2 Substrate Using Hot-Dip Aluminising and Micro-Arc Oxidation Technologies. Materials. 2019; 12(5):799. https://doi.org/10.3390/ma12050799
Chicago/Turabian StyleWang, Shaopeng, Lian Zhou, Changjiu Li, Zhengxian Li, and Hongzhan Li. 2019. "Morphology and Wear Resistance of Composite Coatings Formed on a TA2 Substrate Using Hot-Dip Aluminising and Micro-Arc Oxidation Technologies" Materials 12, no. 5: 799. https://doi.org/10.3390/ma12050799
APA StyleWang, S., Zhou, L., Li, C., Li, Z., & Li, H. (2019). Morphology and Wear Resistance of Composite Coatings Formed on a TA2 Substrate Using Hot-Dip Aluminising and Micro-Arc Oxidation Technologies. Materials, 12(5), 799. https://doi.org/10.3390/ma12050799




