Flame-Retardant Mechanism of Layered Double Hydroxides in Asphalt Binder
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Test Methods
3. Results and Discussion
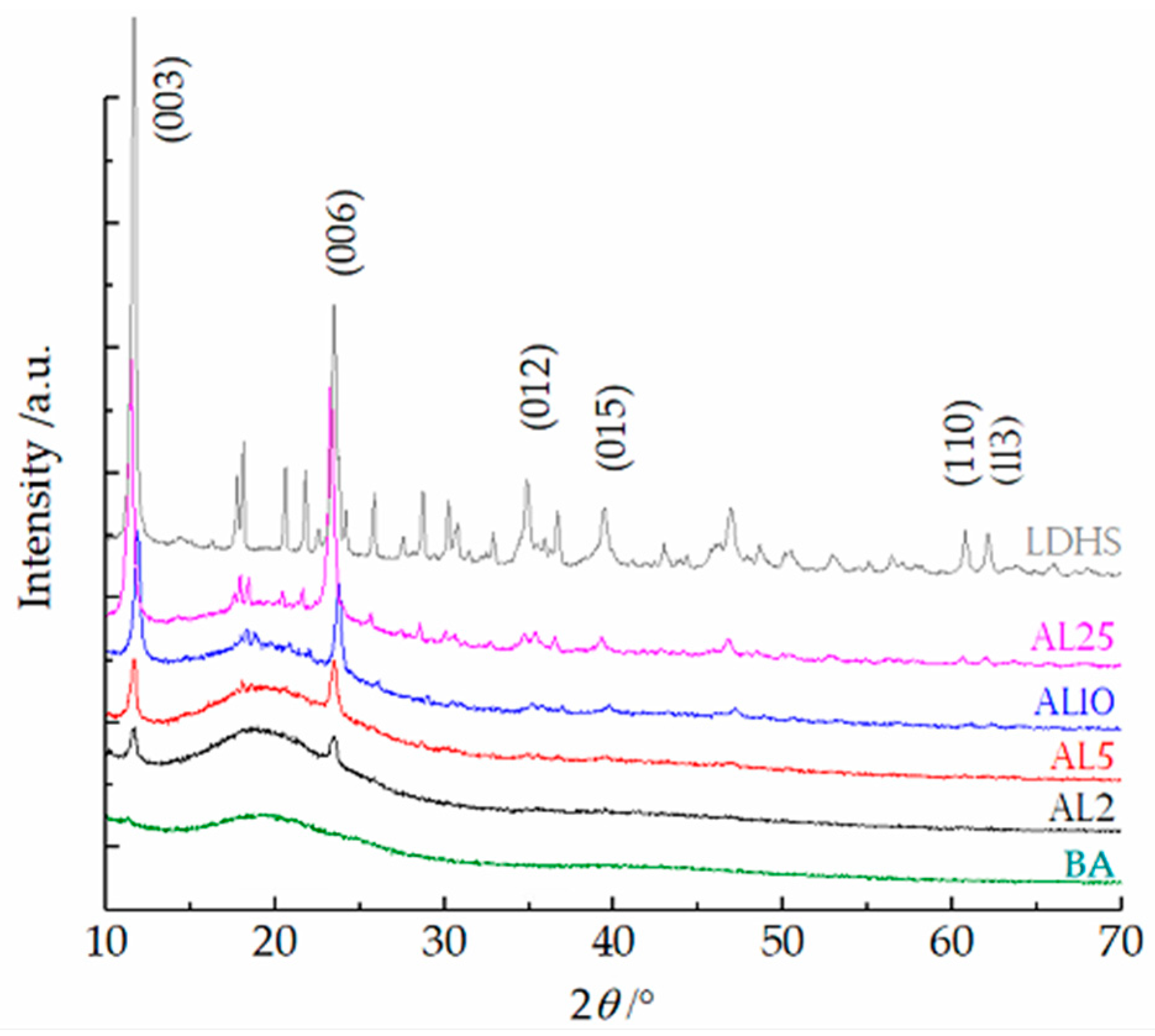
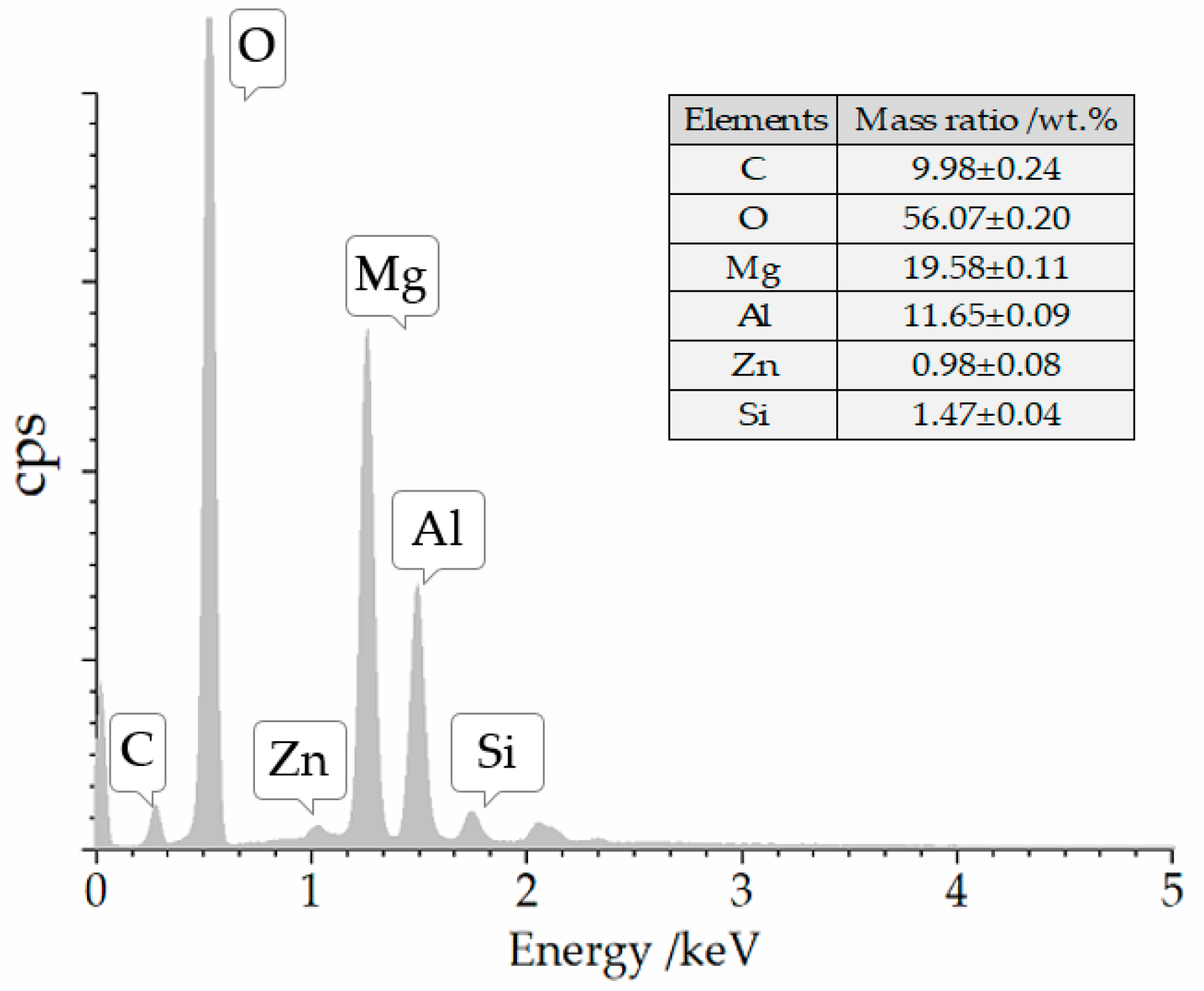
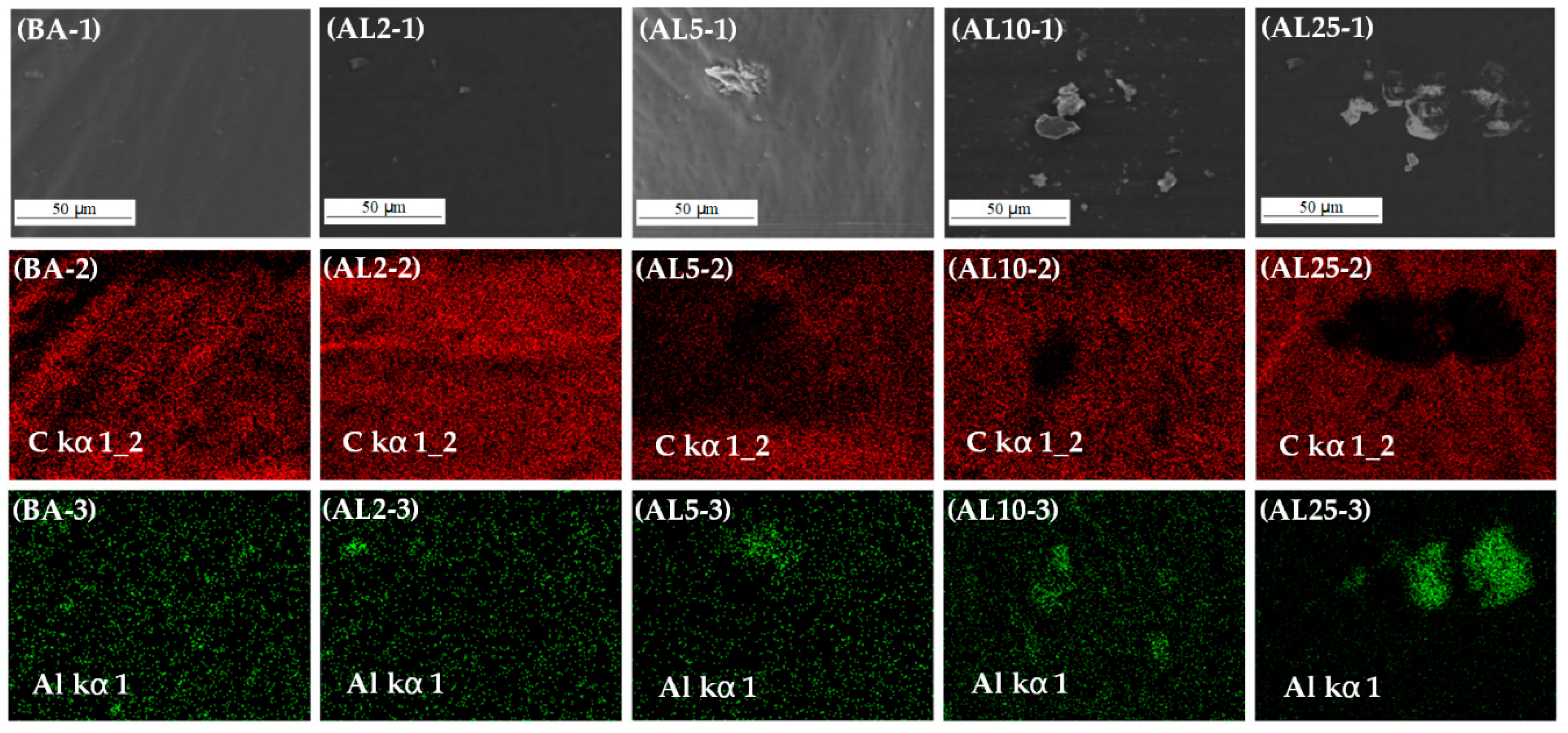
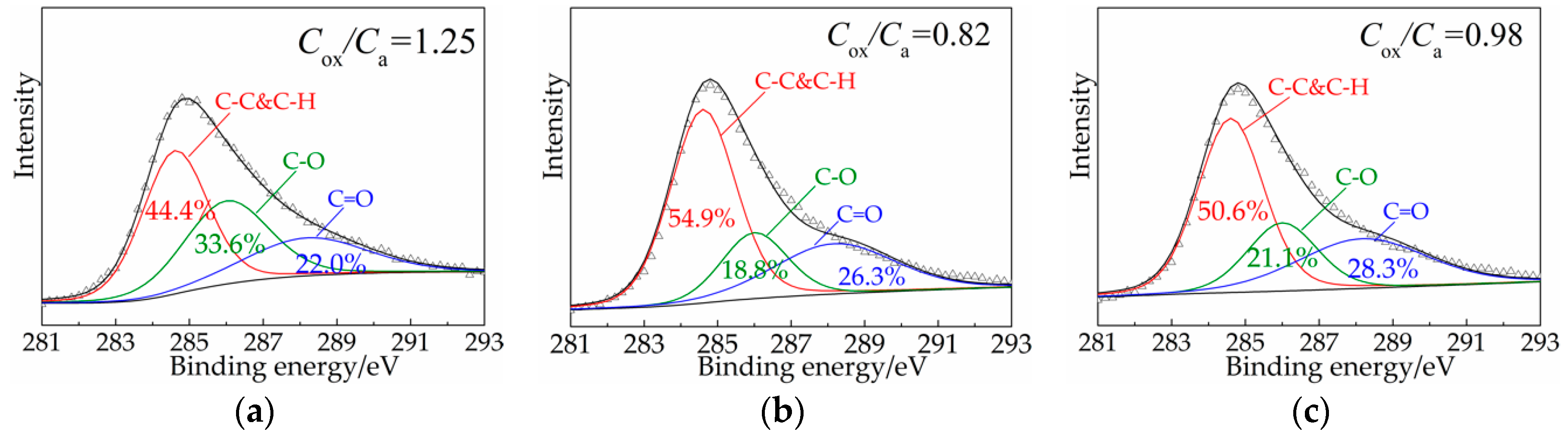
3.1. Structure and Morphology Characterization
3.2. Flammability Characterization of Asphalt Binders
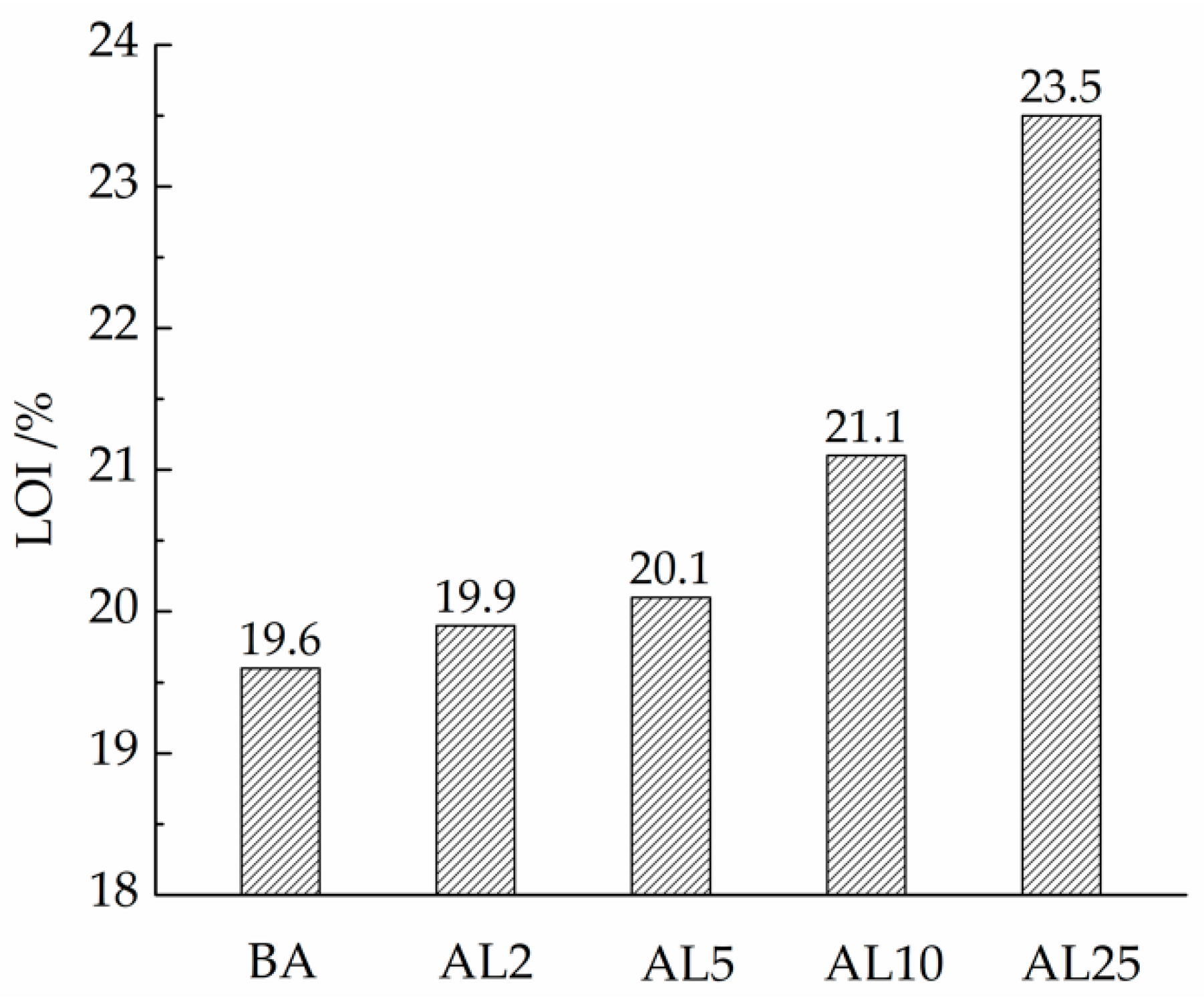
3.2.1. LOI Tests
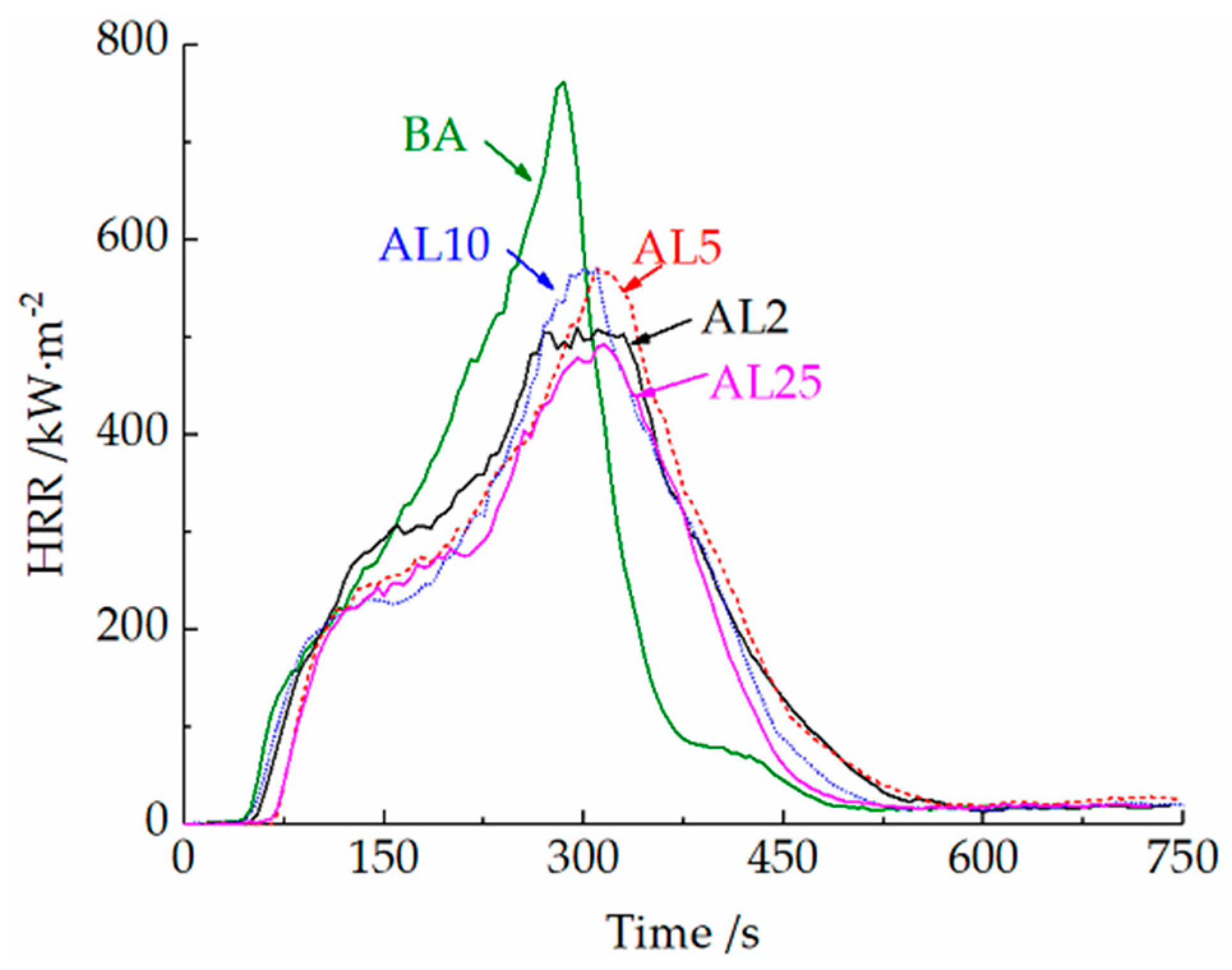
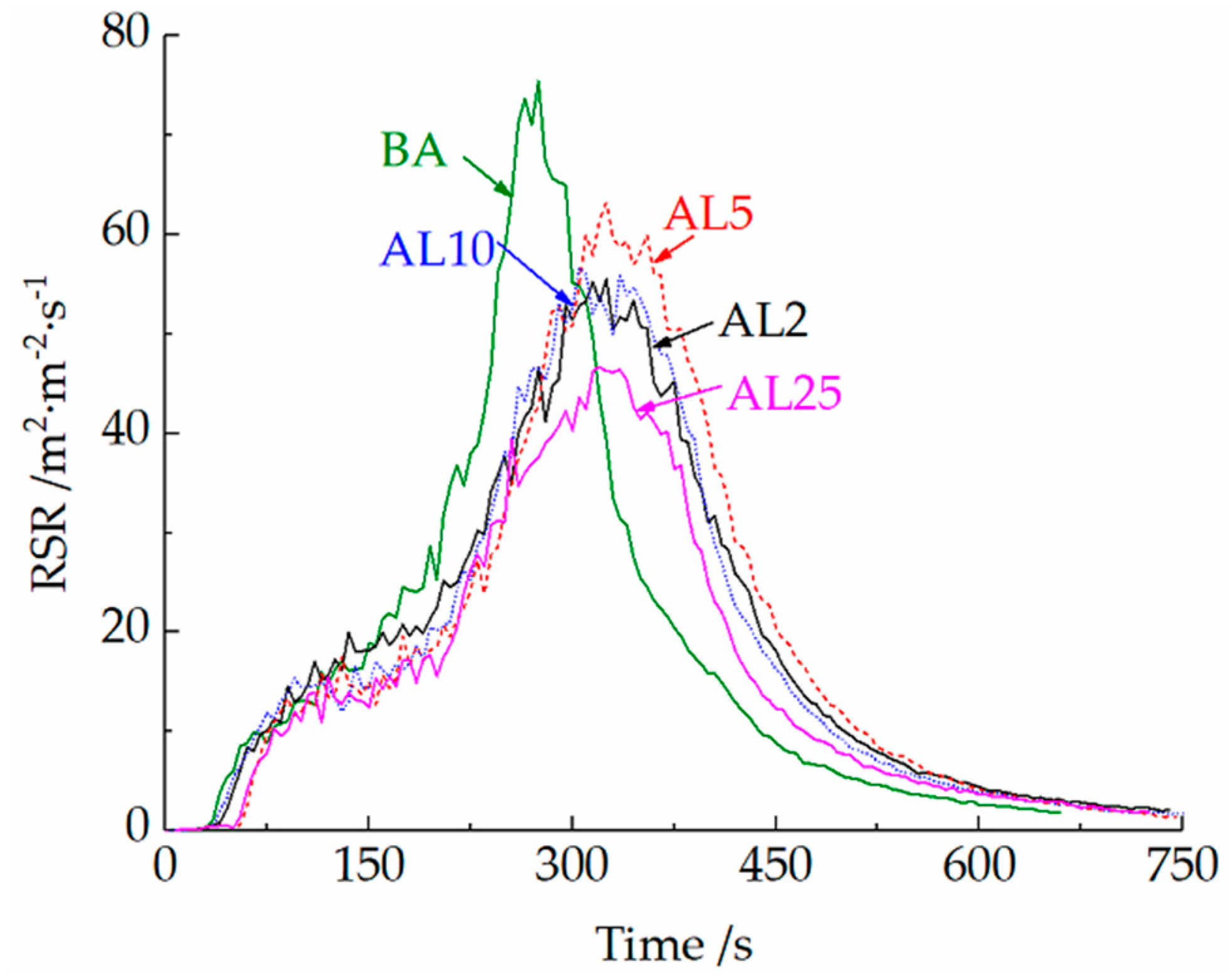
3.2.2. Cone Calorimeter Tests
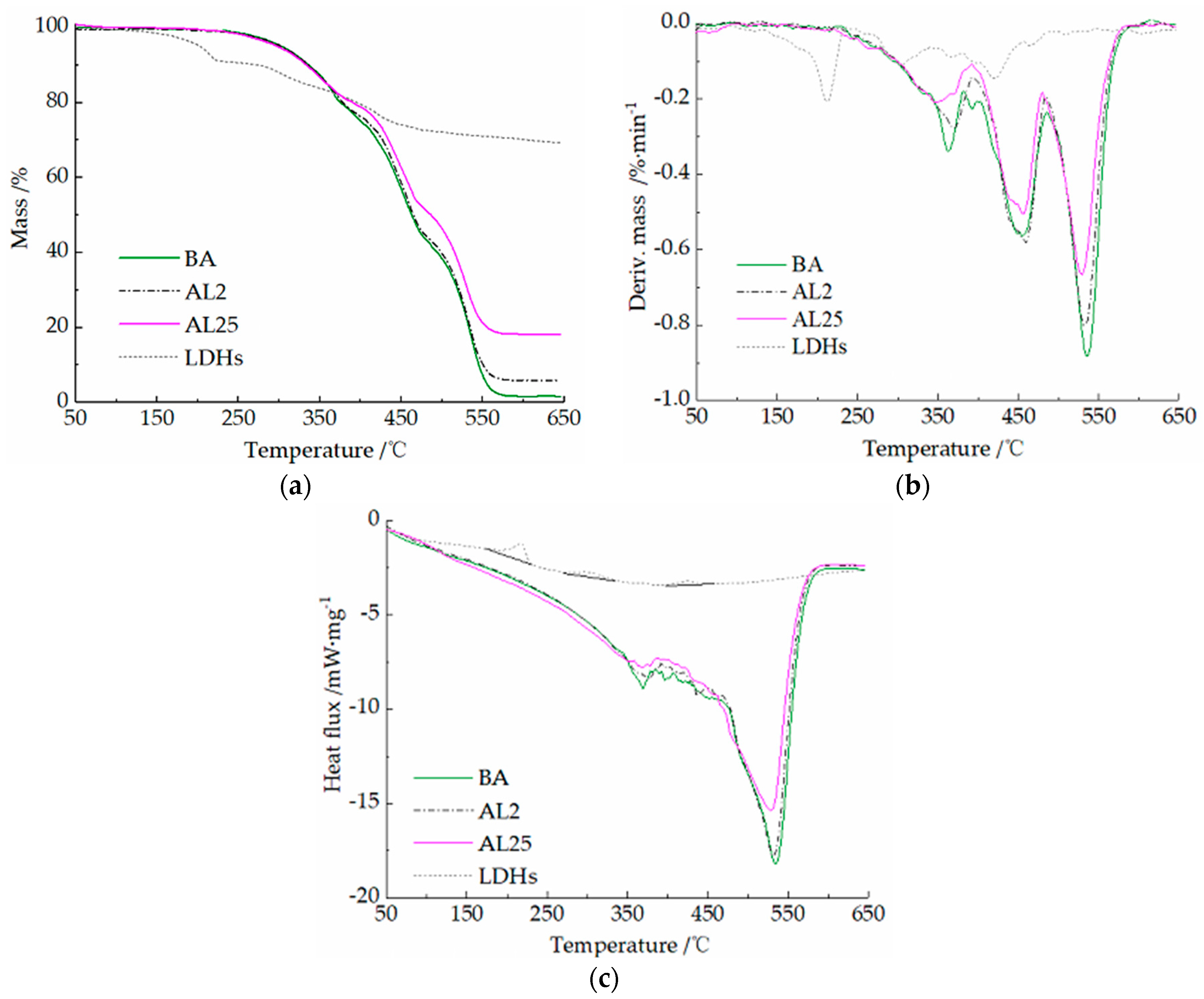
3.3. Thermal Behaviors Analysis
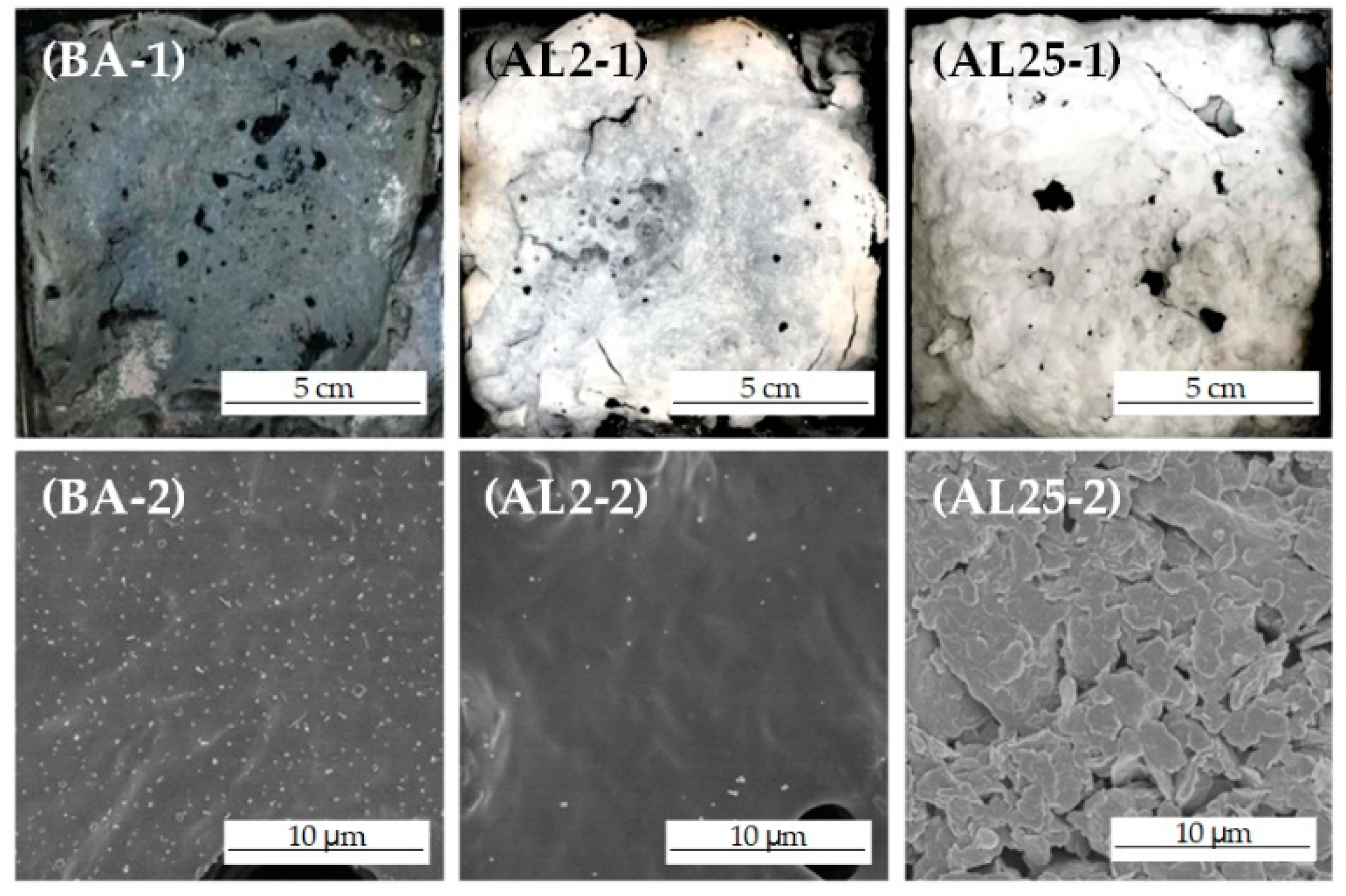
3.4. Combustion Residues Analysis
4. Conclusions
- A small amount of LDHs (2 wt.%) can decrease the peak heat and smoke release rate of LDHs in cone calorimeter tests. The layered structures can suppress the release of light components and favor the formation of polyaromatic structures during combustion, thus improving the oxidation resistance and compactness of the char layer. Well-dispersed LDHs can also prevent the collapse of the char layer, thus improving the integrity of the residue.
- A content of 25 wt.% LDHs can further increase the LOI of asphalt binder and decrease the heat and smoke release during combustion. The decomposition of LDHs can absorb the heat release of the initial two stages of asphalt combustion and reduce the burning rate of asphalt. Due to the loss of loosely bound water from the LDHs during the blending process and the decrease of dispersibility at a high LDH dose, the improvement of thermal stability was limited.
Author Contributions
Funding
Conflicts of Interest
References
- Asphalt Pavement in Tunnel; European Asphalt Pavement Association: Brussels, Belgium, 2008.
- Bonati, A.; Merusi, F.; Bochicchio, G.; Tessadri, B.; Polacco, G.; Filippi, S.; Giuliani, F. Effect of nanoclay and conventional flame retardants on asphalt mixtures fire reaction. Constr. Build. Mater. 2013, 47, 990–1000. [Google Scholar] [CrossRef]
- Carvel, R.; Beard, A. The Handbook of Tunnel Fire Safety, 1st ed.; ICE Publishing: London, UK, 2005; pp. 274–276. [Google Scholar]
- Bourbigot, S.; Cerin, O.; Duquesne, S.; Clavel, N. Flame retardancy of bitumen: A calorimetry study. J. Fire Sci. 2013, 31, 112–130. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Y.; Xia, W.J.; Hu, Z.H. Effects of flame retardants on thermal decomposition of SARA fractions separated from asphalt binder. Constr. Build. Mater. 2018, 173, 209–219. [Google Scholar] [CrossRef]
- Yu, J.Y.; Cong, P.L.; Wu, S.P. Investigation of the properties of asphalt and its mixtures containing flame retardant modifier. Constr. Build. Mater. 2009, 23, 2277–2282. [Google Scholar]
- Pei, J.M.; Wen, Y.; Li, Y.W.; Shi, X.; Zhang, J.P.; Li, R.; Du, Q.L. Flame-retarding effects and combustion properties of asphalt binder blended with organo montmorillonite and alumina trihydrate. Constr. Build. Mater. 2014, 72, 41–47. [Google Scholar] [CrossRef]
- Qin, X.T.; Zhu, S.Y.; Chen, S.; Li, Z.; Dou, H. Flame retardancy of asphalt mixtures and mortars containing composite flame-retardant materials. Road Mater. Pavement Des. 2014, 15, 66–77. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, Y.H.; Zhou, Q.; Tang, D.Q.; Gu, L.Z.; Wu, K. Investigation on Smoke Suppression Mechanism of Hydrated Lime in Asphalt Combustion. J. Chem. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Wu, K.; Zhu, K.; Kang, C.; Wu, B.; Huang, Z.Y. An experimental investigation of flame retardant mechanism of hydrated lime in asphalt mastics. Mater. Des. 2016, 103, 223–229. [Google Scholar] [CrossRef]
- Xu, T.; Huang, X.M. Combustion properties of asphalt binder containing flame retardant. Fire Mater. 2012, 36, 97–106. [Google Scholar] [CrossRef]
- Altarawneh, M.; Saeed, A.; Al-Harahsheh, M.; Dlugogorski, B.Z. Thermal decomposition of brominated flame retardants (BFRs): Products and mechanisms. Prog. Energy Combust. Sci. 2019, 70, 212–259. [Google Scholar] [CrossRef]
- Altarawneh, M.; Ahmed, O.H.; Jiang, Z.T.; Dlugogorski, B.Z. Thermal Recycling of Brominated Flame Retardants with Fe2O3. J. Phys. Chem. A 2016, 120, 6039–6047. [Google Scholar] [CrossRef] [PubMed]
- Altarawneh, M.; Dlugogorski, B.Z. Formation of polybrominated dibenzofurans from polybrominated biphenyls. Chemosphere 2015, 119, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Huang, X.M. A TG-FTIR investigation into smoke suppression mechanism of magnesium hydroxide in asphalt combustion process. J. Anal. Appl. Pyrolysis 2010, 87, 217–223. [Google Scholar] [CrossRef]
- Xu, T.; Wang, H.C.; Huang, X.M.; Li, G.F. Inhibitory action of flame retardant on the dynamic evolution of asphalt pyrolysis volatiles. Fuel 2013, 105, 757–763. [Google Scholar] [CrossRef]
- Barral, M.; Garmendia, P.; Munoz, M.E.; Palmillas, Z.; Romera, R. Novel bituminous mastics for pavements with improved fire performance. Constr. Build. Mater. 2012, 30, 650–656. [Google Scholar] [CrossRef]
- Bonati, A.; Merusi, F.; Polacco, G.; Filippi, S.; Felice, G. Ignitability and thermal stability of asphalt binders and mastics for flexible pavements in highway tunnels. Constr. Build. Mater. 2012, 37, 660–668. [Google Scholar] [CrossRef]
- Zhu, K.; Wu, K.; Wu, B.; Huang, Z.Y. Investigations of the montmorillonite and aluminium trihydrate addition effects on the ignitability and thermal stability of asphalt. J. Chem. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Wu, S.P.; Han, J.; Pang, L.; Yu, M.; Wang, T. Rheological properties for aged bitumen containing ultraviolate light resistant materials. Constr. Build. Mater. 2012, 33, 133–138. [Google Scholar] [CrossRef]
- Liu, X.; Wu, S.; Liu, G.; Li, L. Effect of Ultraviolet Aging on Rheology and Chemistry of LDH-Modified Bitumen. Materials 2015, 8, 5238–5249. [Google Scholar] [CrossRef]
- Xu, S.; Yu, J.Y.; Wu, W.F.; Xue, L.H.; Sun, Y.B. Synthesis and characterization of layered double hydroxides intercalated by UV absorbents and their application in improving UV aging resistance of bitumen. Appl. Clay Sci. 2015, 114, 112–119. [Google Scholar] [CrossRef]
- Wang, G.R.; Rao, D.M.; Li, K.T.; Lin, Y. UV Blocking by Mg-Zn-Al Layered Double Hydroxides for the Protection of Asphalt Road Surfaces. Ind. Eng. Chem. Res. 2014, 53, 4165–4172. [Google Scholar] [CrossRef]
- Huang, Y.F.; Feng, Z.G.; Zhang, H.G. Effect of Layered Double Hydroxides (LDHs) on Aging Properties of Bitumen. J. Test. Eval. 2012, 40, 734–739. [Google Scholar] [CrossRef]
- Costa, F.R.; Saphiannikova, M.; Wagenknecht, U.; Heinrich, G. Layered Double Hydroxide Based Polymer Nanocomposites. In Wax Crystal Control Nanocomposites Stimuli-Responsive Polymers; Springer: Berlin/Heidelberg, Germany, 2008; Volume 210, pp. 101–168. [Google Scholar]
- Manzi-Nshuti, C.; Songtipya, P.; Manias, E.; Jimenez-Gasco, M.M.; Hossenlopp, J.M.; Wilkie, C.A. Polymer nanocomposites using zinc aluminum and magnesium aluminum oleate layered double hydroxides: Effects of LDH divalent metals on dispersion, thermal, mechanical and fire performance in various polymers. Polymer 2009, 50, 3564–3574. [Google Scholar] [CrossRef]
- Matusinovic, Z.; Feng, J.; Wilkie, C.A. The role of dispersion of LDH in fire retardancy: The effect of different divalent metals in benzoic acid modified LDH on dispersion and fire retardant properties of polystyrene– and poly(methyl-methacrylate)–LDH–B nanocomposites. Polym. Degrad. Stab. 2013, 98, 1515–1525. [Google Scholar] [CrossRef]
- Loeber, L.; Muller, G.; Morel, J.; Sutton, O. Bitumen in colloid science: A chemical, structural and rheological approach. Fuel 1998, 77, 1443–1450. [Google Scholar] [CrossRef]
- Li, M.L.; Pang, L.; Chen, M.Z.; Xie, J.; Liu, Q.T. Effects of Aluminum Hydroxide and Layered Double Hydroxide on Asphalt Fire Resistance. Materials 2018, 11, 1939. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Chen, Y.H. Preparation of polypropylene/Mg-Al layered double hydroxides nanocomposites through wet pan-milling: Formation of a second-staging structure in LDHs intercalates. RSC Adv. 2017, 7, 152–153. [Google Scholar] [CrossRef]
- Mazumder, M.; Ahmed, R.; Wajahat Ali, A.; Lee, S. SEM and ESEM techniques used for analysis of asphalt binder and mixture: A state of the art review. Constr. Build. Mater. 2018, 186, 313–329. [Google Scholar] [CrossRef]
- Morgan, A.B.; Wilkie, C.A. Flame Retardant Polymer Nanocomposites; Wiley-Interscience: New York, NY, USA, 2007; pp. 113–118. [Google Scholar]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials—Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Wang, P.J.; Hu, X.P.; Liao, D.J.; Wen, Y.; Hull, T.R.; Miao, F.; Zhang, Q.T. Dual fire retardant action: The combined gas and condensed phase effects of azo-modified NiZnAl layered double hydroxide on intumescent polypropylene. Ind. Eng. Chem. Res. 2017, 56, 920–932. [Google Scholar] [CrossRef]
- Thomson, M.; Mitra, T. A radical approach to soot formation. Science 2018, 361, 978–979. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Li, S.; Chen, X. Preparation of mesoporous silica-LDHs system and its coordinated flame-retardant effect on EVA. J. Therm. Anal. Calorim. 2017, 130, 2055–2067. [Google Scholar] [CrossRef]
- Wang, Z.; Han, E.; Ke, W. Influence of nano-LDHs on char formation and fire-resistant properties of flame-retardant coating. Prog. Org. Coat. 2005, 53, 29–37. [Google Scholar] [CrossRef]
- Liu, W.; Chen, D.Q.; Wang, Y.Z.; Qu, M.H. Char-forming mechanism of a novel polymeric flame retardant with char agent. Polym. Degrad. Stab. 2007, 92, 1046–1052. [Google Scholar] [CrossRef]

| Properties | Standard | Test Results | |
|---|---|---|---|
| Penetration at 25 °C/0.1 mm | ASTM D5-06 | 65.8 | |
| Ductility at 10 °C/cm | ASTM D113-07 | 42.0 | |
| Softening point/°C | ASTM D36-06 | 47.8 | |
| Flash point/°C | ASTM D92-05 | 340 | |
| Viscosity at 60 °C/Pa·s | ASTM D4402-06 | 193 | |
| Element content/wt.% | C | ASTM D5373 | 84.4 |
| H | ASTM D5373 | 10.9 | |
| N | ASTM D5373 | 0.5 | |
| S | ASTM D5373 | 2.7 | |
| O | ASTM D5373 | 0.9 | |
| SARA fraction/wt.% | Saturates | ASTM D4124-09 | 21.1 |
| Aromatics | ASTM D4124-09 | 50.2 | |
| Resin | ASTM D4124-09 | 20.7 | |
| Asphaltenes | ASTM D4124-09 | 7.9 | |
| Samples | TTI | HRR (kW·m−2) | FIGRA | THR | PRSR | SEA | TSR | |
|---|---|---|---|---|---|---|---|---|
| (s) | Peak | t (s) | (kW·m−2·s−1) | (MJ·m−2) | (m2·m−2·s−1) | (m2·kg−1) | (m2·m−2) | |
| BA | 29 | 761.7 | 285 | 2.67 | 146.1 | 75.8 | 3004 | 14750 |
| AL2 | 33 | 510.4 | 295 | 1.73 | 135.3 | 55.4 | 2825 | 13744 |
| AL5 | 40 | 570.2 | 310 | 1.84 | 133.1 | 63.3 | 2823 | 14171 |
| AL10 | 36 | 570.7 | 300 | 1.90 | 127.1 | 56.6 | 2702 | 13479 |
| AL25 | 47 | 493.2 | 315 | 1.57 | 115.5 | 46.4 | 2636 | 11265 |
| Samples | Stage I | Stage II | Stage III | Residue Ratio /% | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TR /°C | MMLR /%·min−1 | PT /°C | TR /°C | MMLR /%·min−1 | PT /°C | TR /°C | MMLR /%·min−1 | PT /°C | ||
| BA | 264–380 | −0.339 | 363 | 380–486 | −0.564 | 454 | 486–600 | −0.883 | 534 | 3.6 |
| L2 | 260–393 | −0.278 | 369 | 393–485 | −0.581 | 459 | 485–600 | −0.800 | 532 | 7.9 |
| L25 | 256–391 | −0.209 | 346 | 391–480 | −0.505 | 456 | 480–587 | −0.666 | 529 | 20.3 |
| LDHs | 133–232 | −0.206 | 212 | 232–343 | −0.110 | 305 | 343–493 | −0.145 | 420 | 73.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, K.; Wang, Y.; Tang, D.; Wang, Q.; Li, H.; Huang, Y.; Huang, Z.; Wu, K. Flame-Retardant Mechanism of Layered Double Hydroxides in Asphalt Binder. Materials 2019, 12, 801. https://doi.org/10.3390/ma12050801
Zhu K, Wang Y, Tang D, Wang Q, Li H, Huang Y, Huang Z, Wu K. Flame-Retardant Mechanism of Layered Double Hydroxides in Asphalt Binder. Materials. 2019; 12(5):801. https://doi.org/10.3390/ma12050801
Chicago/Turabian StyleZhu, Kai, Yunhe Wang, Daquan Tang, Qiang Wang, Haihang Li, Yadong Huang, Zhiyi Huang, and Ke Wu. 2019. "Flame-Retardant Mechanism of Layered Double Hydroxides in Asphalt Binder" Materials 12, no. 5: 801. https://doi.org/10.3390/ma12050801
APA StyleZhu, K., Wang, Y., Tang, D., Wang, Q., Li, H., Huang, Y., Huang, Z., & Wu, K. (2019). Flame-Retardant Mechanism of Layered Double Hydroxides in Asphalt Binder. Materials, 12(5), 801. https://doi.org/10.3390/ma12050801





