Nanocellular Polymers: The Challenge of Creating Cells in the Nanoscale
Abstract
1. Introduction
2. Production of Nanocellular Polymers
2.1. Phase-Separation Mechanisms
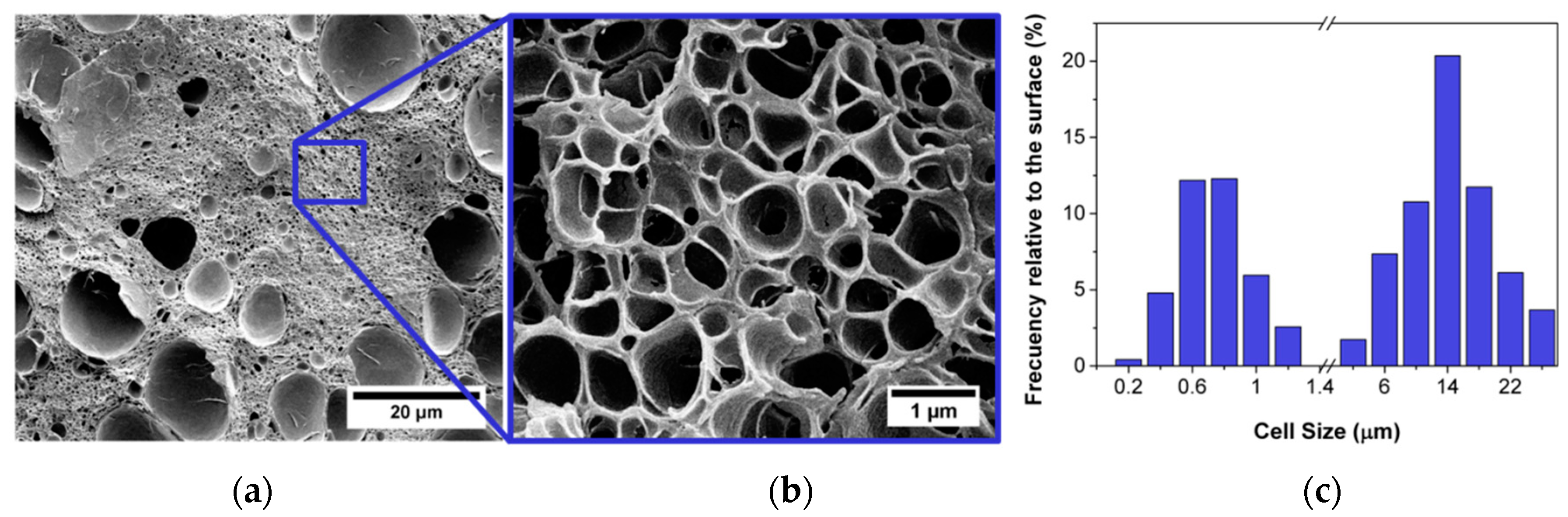
2.1.1. Spinodal Decomposition
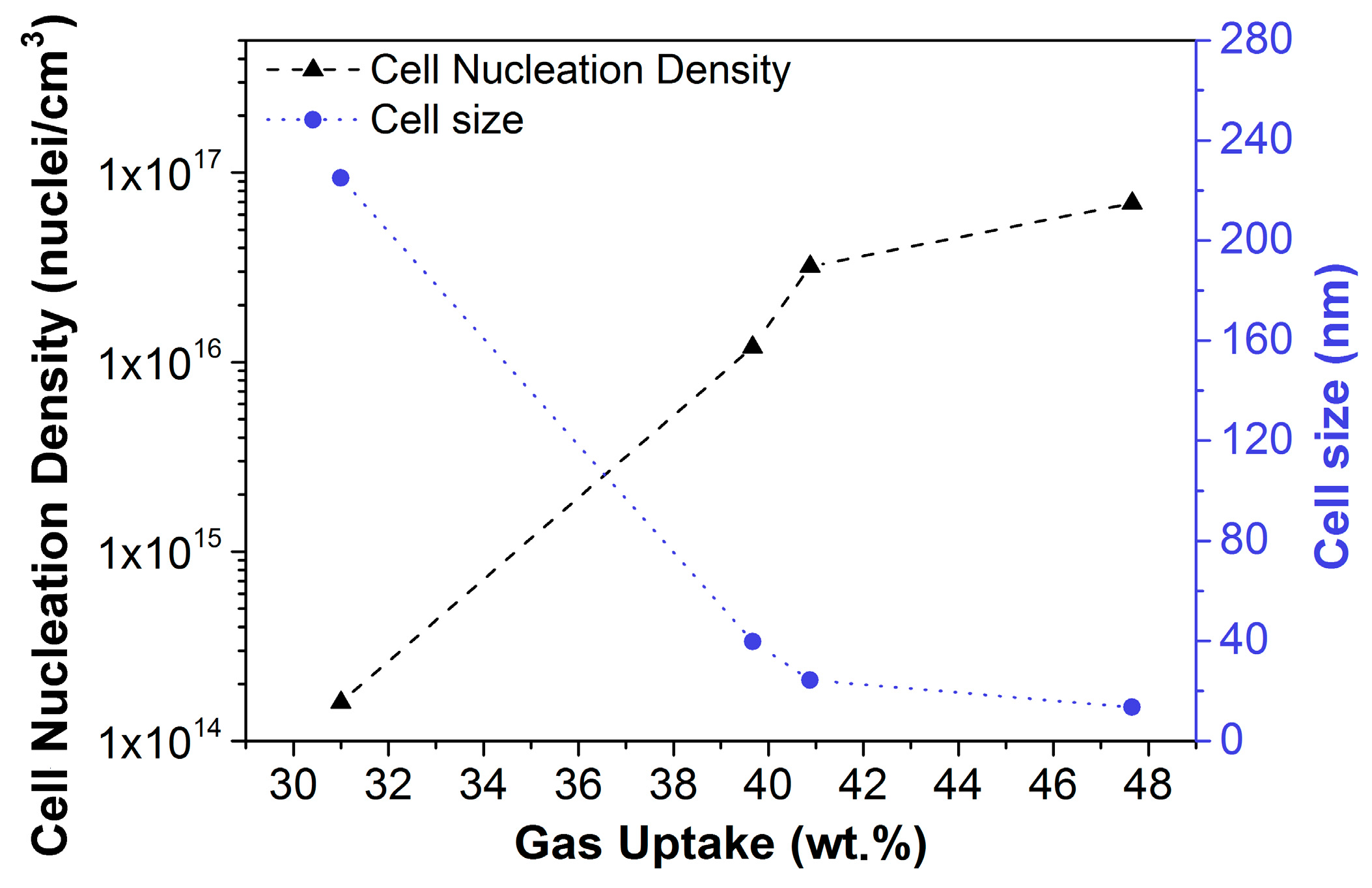
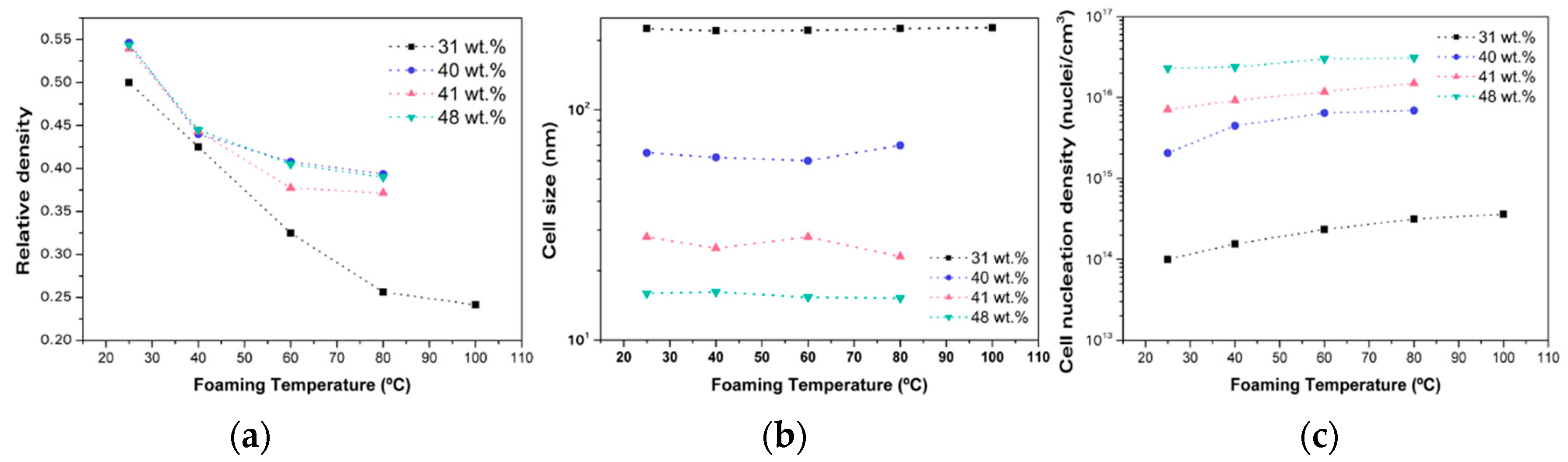
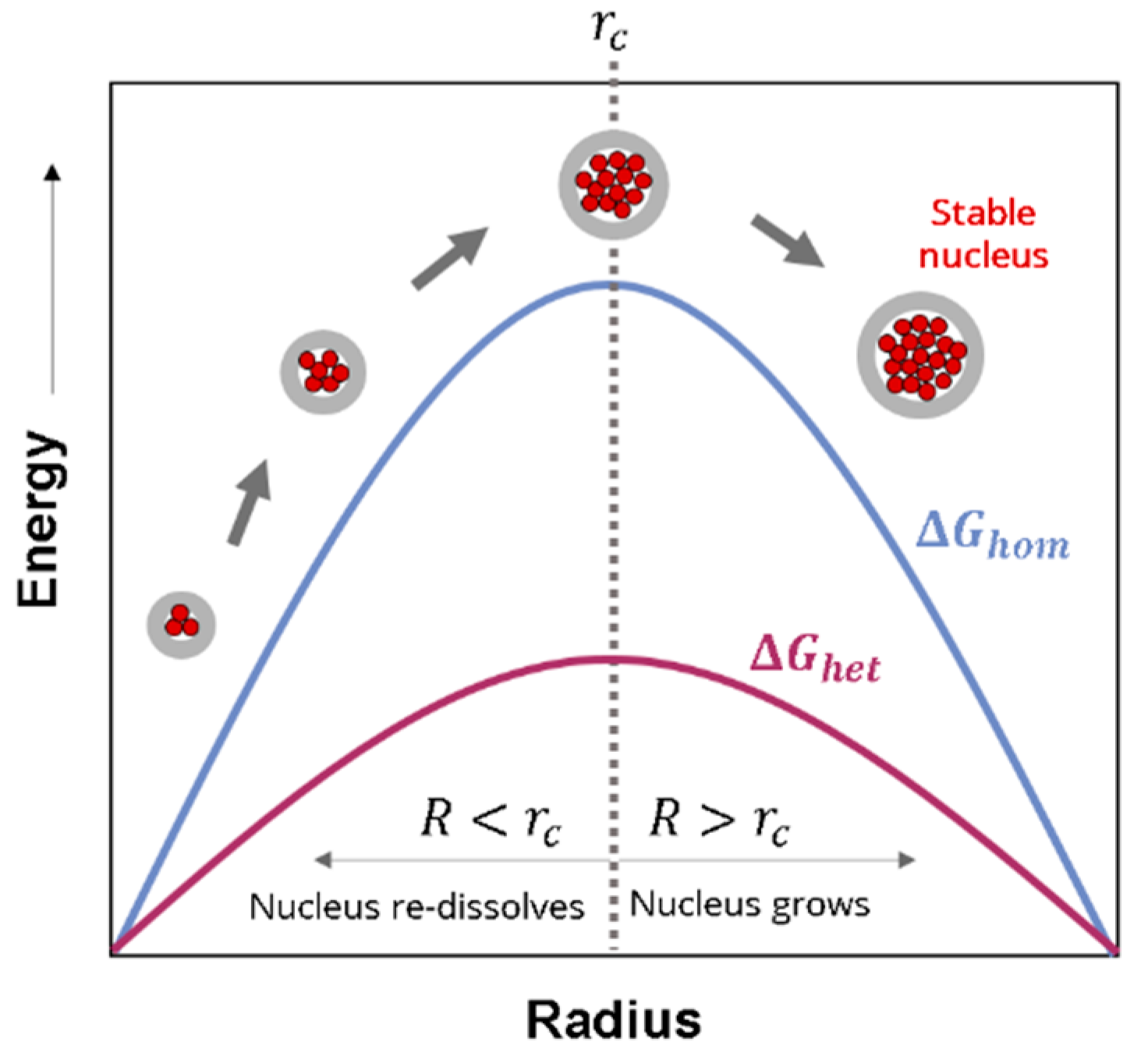
2.1.2. Homogeneous Nucleation
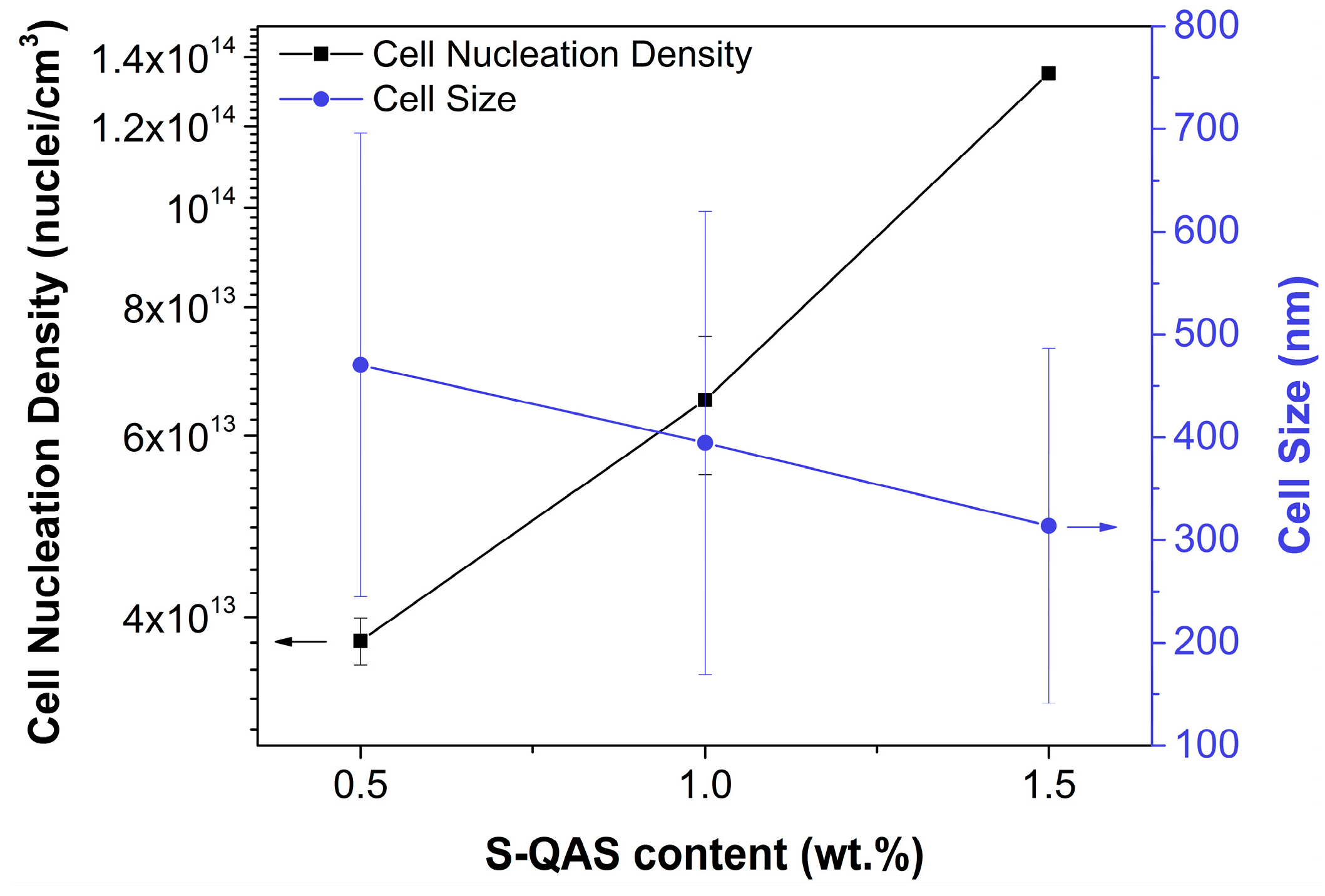
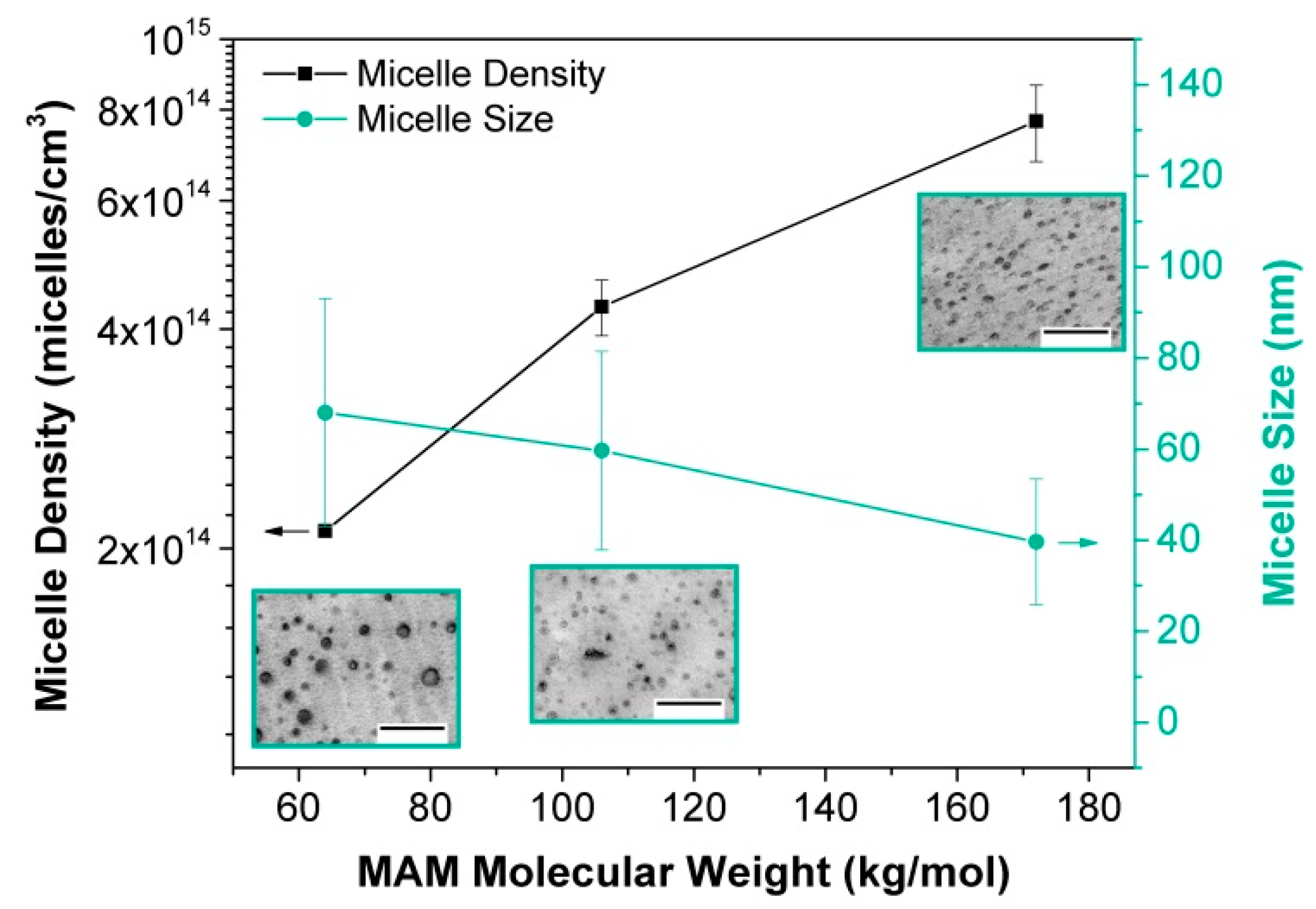
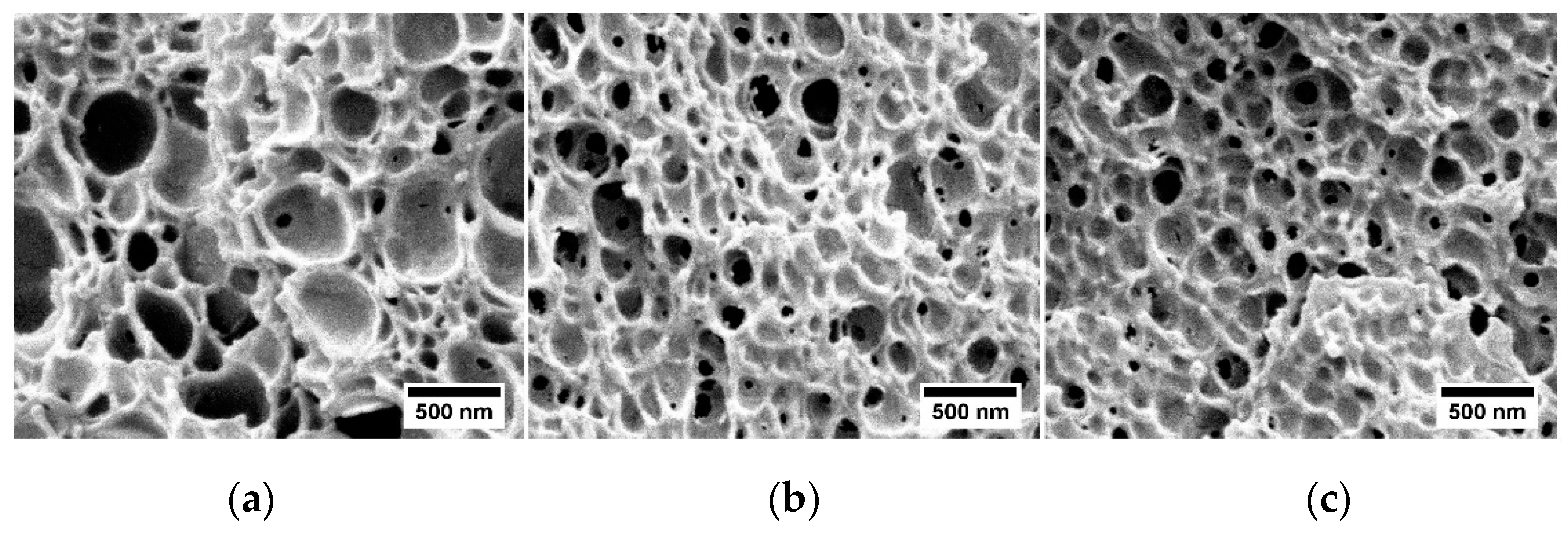
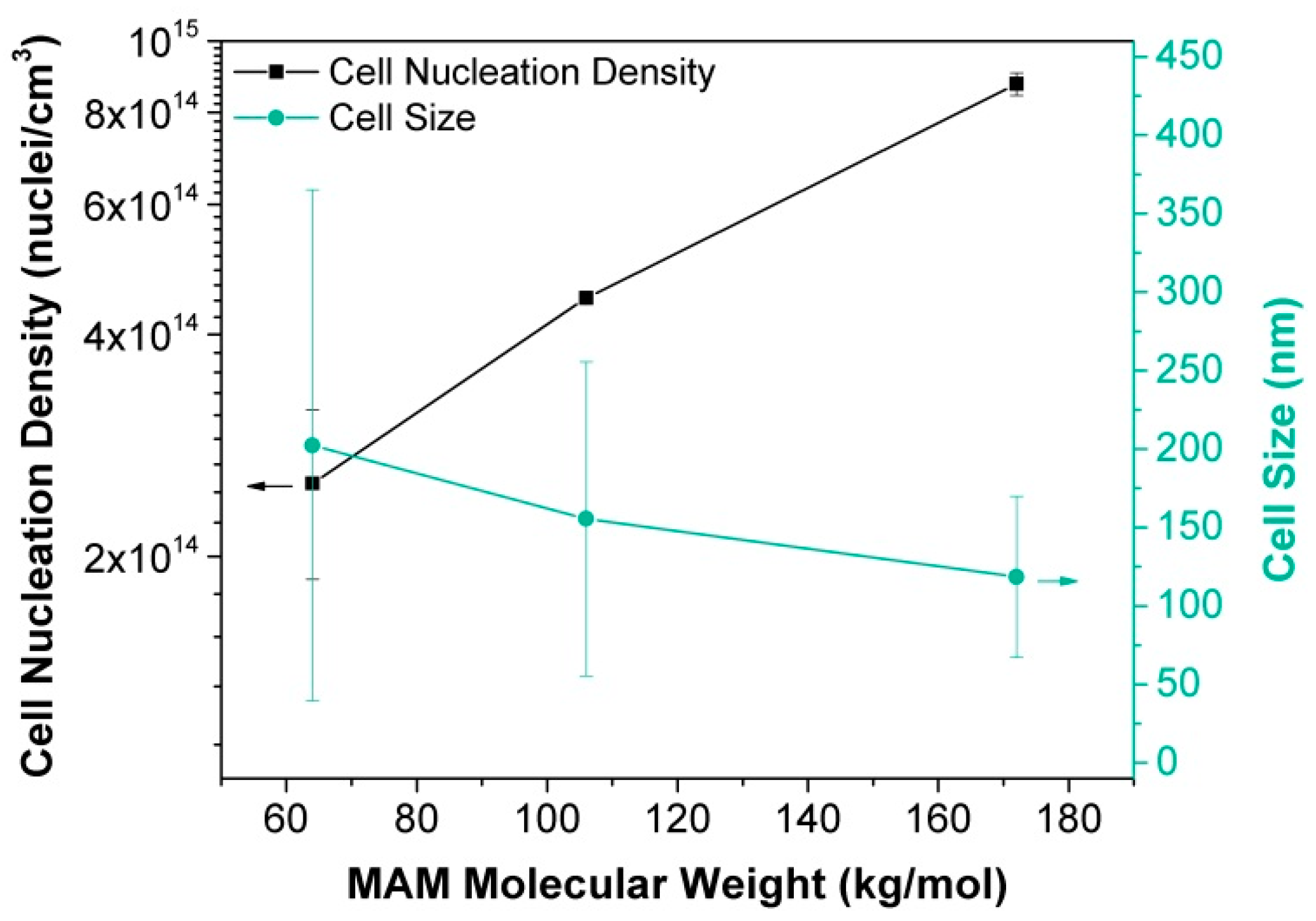
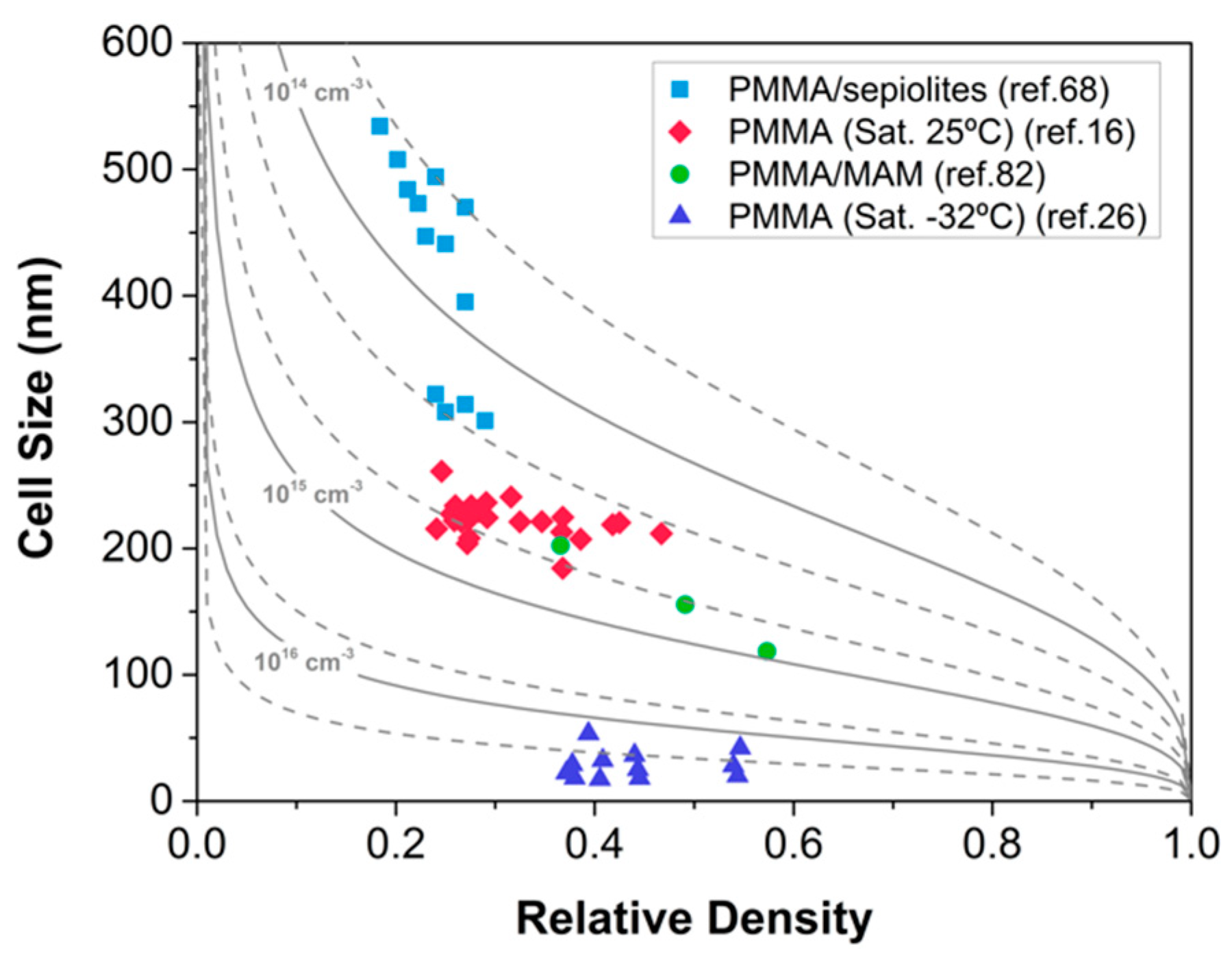
2.1.3. Heterogeneous Nucleation
2.2. Growing and Degeneration Mechanisms
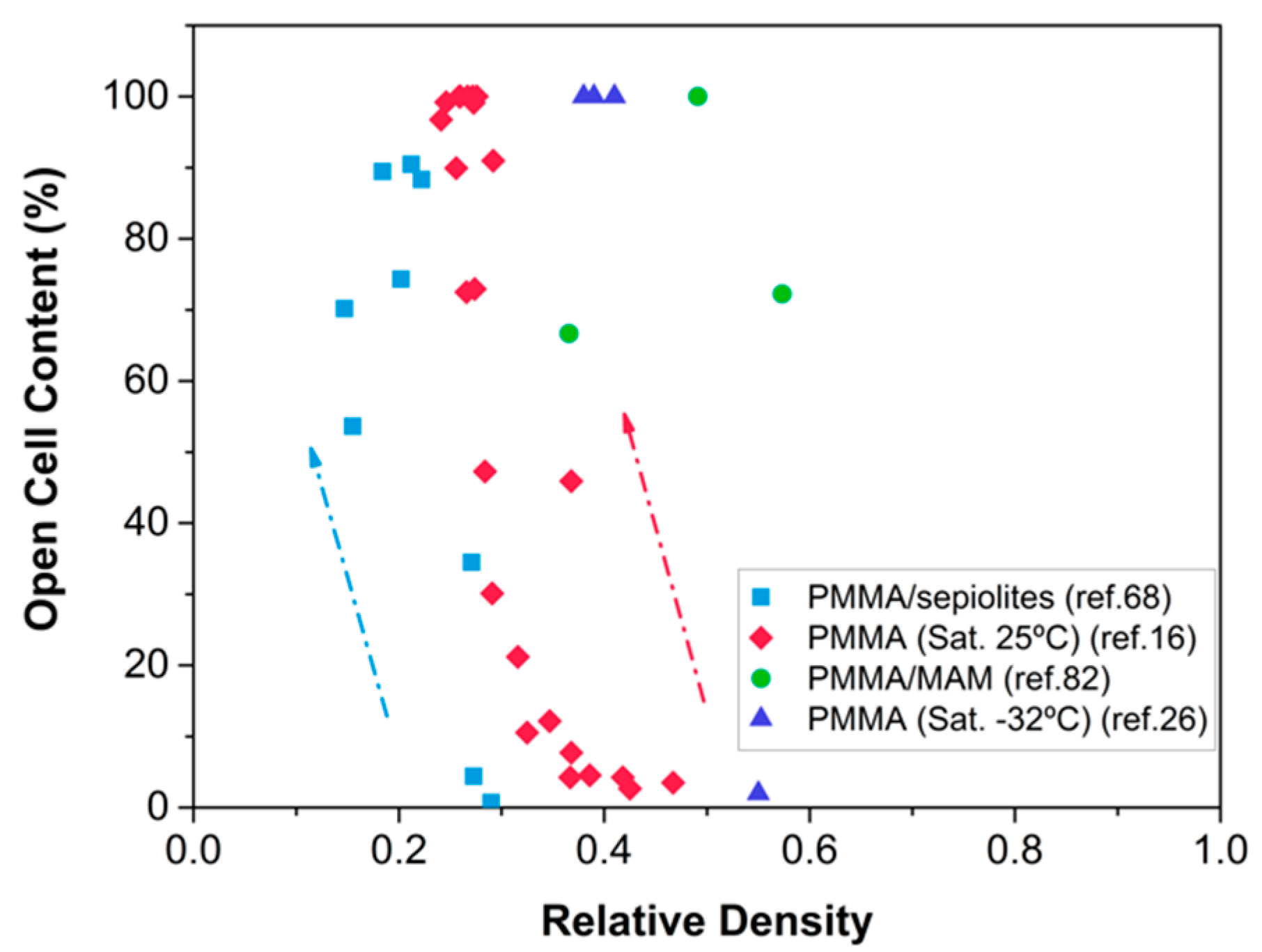
2.3. Skin Formation
3. Limits and Future Trends
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gibson, L.J.; Ashby, M. Cellular Solids; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Rodriguez-Perez, M.A. Crosslinked polyolefin foams: Production, structure, properties, and applications. Adv. Polym. Sci. 2005, 184, 97–126. [Google Scholar] [CrossRef]
- Saiz-Arroyo, C.; Rodriguez-Perez, M.A.; Tirado, J.; López-Gil, A.; de Saja, J.A. Structure-property relationships of medium-density polypropylene foams. Polym. Int. 2013, 62, 1324–1333. [Google Scholar] [CrossRef]
- Eaves, D. Handbook of Polymer Foams; Rapra Technology Limited: Shawbury UK, 2004. [Google Scholar]
- Marketsandmarkets. Available online: http://www.marketsandmarkets.com/Market-Reports/foams-market-1011.html (accessed on 6 March 2019).
- Kumar, V.; Suh, N.P. A process for making microcellular parts. Polym. Eng. Sci. 1990, 30, 1323–1329. [Google Scholar] [CrossRef]
- Park, C.B. Continuous Production of High Density and Low Density Microcellular Plastics in Extrusion in Foam Extrusion; Le, S.T., Ed.; CRC Press: Boca Ratón, FL, USA, 2000. [Google Scholar]
- Shimbo, M.; Higashitani, I.; Miyano, Y. Mechanism of Strength Plastics Having Fine Cell. J. Cell. Plast. 2007, 43, 157–167. [Google Scholar] [CrossRef]
- Kumar, V.; VanderWel, M.; Weller, J.; Seeler, K.A. Experimental Characterization of the Tensile Behavior of Microcellular Polycarbonate Foams. J. Eng. Mater. Technol. 1994, 116, 439. [Google Scholar] [CrossRef]
- Notario, B.; Pinto, J.; Rodriguez-Perez, M.A. Nanoporous polymeric materials: A new class of materials with enhanced properties. Prog. Mater. Sci. 2016, 78–79, 93–139. [Google Scholar] [CrossRef]
- Costeux, S. CO2-blown nanocellular foams. J. Appl. Polym. Sci. 2014, 131, 41293(1)–41293(16). [Google Scholar] [CrossRef]
- Notario, B.; Ballesteros, A.; Pinto, J.; Rodriguez-Perez, M.A. Nanoporous PMMA: A novel system with different acoustic properties. Mater. Lett. 2016, 168, 76–79. [Google Scholar] [CrossRef]
- Notario, B.; Pinto, J.; Rodriguez-Perez, M.A. Towards a new generation of polymeric foams: PMMA nanocellular foams with enhanced physical properties. Polymer 2015, 63, 116–126. [Google Scholar] [CrossRef]
- Notario, B.; Pinto, J.; Verdejo, R.; Rodriguez-Perez, M.A. Dielectric behavior of porous PMMA: From the micrometer to the nanometer scale. Polymer 2016, 107, 302–305. [Google Scholar] [CrossRef]
- Pinto, J.; Notario, B.; Verdejo, R.; Dumon, M.; Costeux, S.; Rodriguez-Perez, M.A. Molecular confinement of solid and gaseous phases of self-standing bulk nanoporous polymers inducing enhanced and unexpected physical properties. Polymer 2017, 113, 27–33. [Google Scholar] [CrossRef]
- Martín-de León, J.; Bernardo, V.; Rodríguez-Pérez, M. Low Density Nanocellular Polymers Based on PMMA Produced by Gas Dissolution Foaming: Fabrication and Cellular Structure Characterization. Polymers 2016, 8, 265. [Google Scholar] [CrossRef]
- Notario, B.; Pinto, J.; Solorzano, E.; De Saja, J.A.; Dumon, M.; Rodriguez-Perez, M.A. Experimental validation of the Knudsen effect in nanocellular polymeric foams. Polymers 2015, 56, 57–67. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, J.; Mark, L.H.; Wang, G.; Yu, K.; Wang, C.; Park, C.B.; Zhao, G. Ultra-tough and super thermal-insulation nanocellular PMMA/TPU. Chem. Eng. J. 2017, 325, 632–646. [Google Scholar] [CrossRef]
- Grassberger, L.; Koch, K.; Oberhoffer, R.; Müller, A.; Klemmer, H.F.M.; Strey, R. Blowing agent free generation of nanoporous poly (methylmethacrylate) materials. Colloid Polym. Sci. 2017, 295, 379–389. [Google Scholar] [CrossRef]
- Forest, C.; Chaumont, P.; Cassagnau, P.; Swoboda, B.; Sonntag, P. Polymer nano-foams for insulating applications prepared from CO2 foaming. Prog. Polym. Sci. 2015, 41, 122–145. [Google Scholar] [CrossRef]
- Knudsen, M. The Kinetic Theory of Gases; Journal of the Franklin Institute: Methuen, MA, USA; London, UK, 1934. [Google Scholar]
- Li, L.; Schulte, L.; Clausen, L.D.; Hansen, K.M.; Jonsson, G.E.; Ndoni, S. Gyroid nanoporous membranes with tunable permeability. ACS Nano 2011, 5, 7754–7766. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.; Dumon, M.; Rodriguez-Perez, M.A.; Garcia, R.; Dietz, C. Block Copolymers Self-Assembly Allows Obtaining Tunable Micro or Nanoporous Membranes or Depth Filters Based on PMMA; Fabrication Method and Nanostructures. J. Phys. Chem. C 2014, 118, 4656–4663. [Google Scholar] [CrossRef]
- Lu, G.Q.; Zhao, X.S. Nanoporous Materials—An Overview. In Nanoporous Materials: Science and Engineering; Imperial Collegue Press: London, UK, 2004; ISBN 978-1-86094-210-5. [Google Scholar]
- Kyzioł, A.; Kyzioł, K. Surface Functionalization with Biopolymers via Plasma-Assisted Surface Grafting and Plasma-Induced Graft Polymerization—Materials for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780128104620. [Google Scholar]
- Kumar Thakur, V.; Kumari Thakur, M.; Kessler, M.R. Handbook of Composites from Renewable Materials; Wiley: Hoboken, NJ, USA, 1985; ISBN 9781119223627. [Google Scholar]
- Liu, X.; Chu, P.K.; Ding, C. Surface nano-functionalization of biomaterials. Mater. Sci. Eng. R Rep. 2010, 70, 275–302. [Google Scholar] [CrossRef]
- Perez-Tamarit, S.; Notario, B.; Solorzano, E.; Rodriguez-Perez, M.A. Light transmission in nanocellular polymers: Are semi-transparent cellular polymers possible? Mater. Lett. 2017. [Google Scholar] [CrossRef]
- Martin-de Leon, J.; Bernardo, V.; Rodriguez-Perez, M.A. Key Production Parameters to Obtain Transparent Nanocellular PMMA. Macromol. Mater. Eng. 2017, 1700343(1)–1700343(5). [Google Scholar] [CrossRef]
- Guo, H.; Kumar, V. Solid-state poly(methyl methacrylate) (PMMA) nanofoams. Part I: Low-temperature CO2 sorption, diffusion, and the depression in PMMA glass transition. Polymer 2015, 57, 157–163. [Google Scholar] [CrossRef]
- Guo, H.; Kumar, V. Some thermodynamic and kinetic low-temperature properties of the PC-CO2 system and morphological characteristics of solid-state PC nanofoams produced with liquid CO2. Polymer 2015, 56, 46–56. [Google Scholar] [CrossRef]
- Yeh, S.K.; Liu, Y.C.; Chu, C.C.; Chang, K.C.; Wang, S.F. Mechanical Properties of Microcellular and Nanocellular Thermoplastic Polyurethane Nanocomposite Foams Created Using Supercritical Carbon Dioxide. Ind. Eng. Chem. Res. 2017, 56, 8499–8507. [Google Scholar] [CrossRef]
- Miller, D.; Chatchaisucha, P.; Kumar, V. Microcellular and nanocellular solid-state polyetherimide (PEI) foams using sub-critical carbon dioxide I. Processing and structure. Polymer 2009, 50, 5576–5584. [Google Scholar] [CrossRef]
- Bernardo, V.; Martín-de León, J.; Rodríguez-Pérez, M.A. Production and characterization of nanocellular polyphenylsulfone foams. Mater. Lett. 2016, 178, 155–158. [Google Scholar] [CrossRef]
- Hentze, H.P.; Antonietti, M. Porous polymers and resins for biotechnological and biomedical applications. Rev. Mol. Biotechnol. 2002, 90, 27–53. [Google Scholar] [CrossRef]
- Hillmyer, M.A. Nanoporous Materials from Block Copolymer Precursors. Adv. Polym. Sci. 2005, 190, 137–181. [Google Scholar]
- Olson, D.A.; Chen, L.; Hillmyer, M.A. Templating Nanoporous Polymers with Ordered Block Copolymers. Chem. Mater. 2008, 20, 869–890. [Google Scholar] [CrossRef]
- Forest, C.; Chaumont, P.; Cassagnau, P.; Swoboda, B.; Sonntag, P. CO2 nano-foaming of nanostructured PMMA. Polymer 2015, 58, 76–87. [Google Scholar] [CrossRef]
- Guo, H.; Nicolae, A.; Kumar, V. Solid-state poly(methyl methacrylate) (PMMA) nanofoams. Part II: Low-temperature solid-state process space using CO2 and the resulting morphologies. Polymer 2015, 70, 231–241. [Google Scholar] [CrossRef]
- Durril, P.L.; Griskey, R.G. Diffusion and Solution of gases in s Thermally Softened or Molten Polymers: Part I. Development of Technique and Determination of Data. AIChE. J. 1966, 12, 1147–1151. [Google Scholar] [CrossRef]
- Wilfried, W. Model calculation of the temperature dependence of small molecule diffusion in high polymers. J. Phys. Chem. 1968, 63, 1080–1085. [Google Scholar]
- Weller, J.E.; Kumar, V. Solid State Microcellular PC Foams I. Polym. Eng. Sci. 2007, 47, 21–25. [Google Scholar] [CrossRef]
- Hwang, Y.D.; Cha, S.W. The relationship between gas absorption and the glass transition temperature in a batch microcellular foaming process. Polym. Test. 2002, 21, 269–275. [Google Scholar] [CrossRef]
- Alessi, P.; Cortesi, A.; Kikic, I.; Vecchione, F. Plasticization of Polymers with Supercritical Carbon Dioxide: Experimental Determination of Glass-Transition Temperatures. J. Appl. Polym. Sci. 2003, 88, 2189–2193. [Google Scholar] [CrossRef]
- Zhang, Z.; Handa, Y.P. An in situ study of plasticization of polymers by high-pressure gases. J. Polym. Sci. 1998, 36, 977–982. [Google Scholar] [CrossRef]
- Reglero Ruiz, J.A.; Viot, P.; Dumon, M. Microcellular foaming of polymethylmethacrylate in a batch supercritical CO2 process: Effect of microstructure on compression behavior. J. Appl. Polym. Sci. 2010, 118, 320–331. [Google Scholar] [CrossRef]
- Pinto, J.; Solo, E.; Rodriguez-perez, M.A.; Saja, J.A. De Characterization of the cellular structure based on user-interactive image analysis procedures. J. Cell. Plast. 2013, 49, 555–575. [Google Scholar] [CrossRef]
- Costeux, S.; Khan, I.; Bunker, S.P.; Jeon, H.K. Experimental study and modeling of nanofoams formation from single phase acrylic copolymers. J. Cell. Plast. 2014, 51, 197–221. [Google Scholar] [CrossRef]
- Aubert, J.H.; Clough, R.L. Low-density, microcellular polystyrene foams. Polymer 1985, 26, 2047–2054. [Google Scholar] [CrossRef]
- Clark, J.B.; Hastie, J.W.; Kihlborg, L.H.E.; Metselaar, R.; Thackeray, M.M. Definitions of terms relating to phase transitions of the solid state. Int. Pure Appl. Chem. 1994, 66, 577–594. [Google Scholar] [CrossRef]
- Kalikmanov, V.I. Nucleation Theory; Springer: Berlin, Germany, 2013; ISBN 9789048136421. [Google Scholar]
- Goel, S.K.; Beckman, E.J. Generation of Microcellular Polymeric Foams Using Supercritical Carbon Dioxide. I: Effect of Pressure and Temperature on. Polym. Eng. Sci. 1994, 34, 1137–1147. [Google Scholar] [CrossRef]
- Khan, I.; Adrian, D.; Costeux, S. A model to predict the cell density and cell size distribution in nano-cellular foams. Chem. Eng. Sci. 2015, 138, 634–645. [Google Scholar] [CrossRef]
- Shafi, M.A.; Lee, J.G.; Flumerfelt, R.W. Prediction of cellular structure in free expansion polymer foam processing. Polym. Eng. Sci. 1996, 36, 1950–1959. [Google Scholar] [CrossRef]
- Costeux, S.; Zhu, L. Low density thermoplastic nanofoams nucleated by nanoparticles. Polymer 2013, 54, 2785–2795. [Google Scholar] [CrossRef]
- Pinto, J.; Dumon, M.; Pedros, M.; Reglero, J.; Rodriguez-Perez, M.A. Nanocellular CO2 foaming of PMMA assisted by block copolymer nanostructuration. Chem. Eng. J. 2014, 243, 428–435. [Google Scholar] [CrossRef]
- Spitael, P.; Macosko, C.W.; McClurg, R.B. Block copolymer micelles for nucleation of microcellular thermoplastic foams. Macromolecules 2004, 37, 6874–6882. [Google Scholar] [CrossRef]
- Colton, J.S.; Suh, N.P. Nucleation of Microcellular Foam: Theory and Practice. Polym. Eng. Sci. 1987, 27, 500–503. [Google Scholar] [CrossRef]
- Guo, H.; Nicolae, A.; Kumar, V. Solid-State Microcellular and Nanocellular Polysulfone Foams. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 975–985. [Google Scholar] [CrossRef]
- Ruiz-Reglero, A.J.; Saiz-arroyo, C.; Dumon, M.; Rodriguez-Perez, M.A.; Gonzalez, L. Production, cellular structure and thermal conductivity of microcellular (methyl methacrylate)-(butyl acrylate)-(methyl methacrylate) triblock copolymers. Soc. Chem. Ind. 2010, 60, 146–152. [Google Scholar] [CrossRef]
- Schonhorn, H. Surface Tension-Viscosity Relationship for liquids. J. Chem. Eng. Data 1967, 12, 524–525. [Google Scholar] [CrossRef]
- Zhai, W.; Yu, J.; Wu, L.; Ma, W.; He, J. Heterogeneous nucleation uniformizing cell size distribution in microcellular nanocomposites foams. Polymer 2006, 47, 7580–7589. [Google Scholar] [CrossRef]
- Okolieocha, C.; Raps, D.; Subramaniam, K.; Altstädt, V. Microcellular to nanocellular polymer foams: Progress (2004–2015) and future directions—A review. Eur. Polym. J. 2015, 73, 500–519. [Google Scholar] [CrossRef]
- Janani, H.; Famili, M.H.N. Investigation of a Strategy for Well Controlled Inducement of Microcellular and Nanocellular Morphologies in Polymers. Polym. Eng. Sci. 2010, 50, 1558–1570. [Google Scholar] [CrossRef]
- Yang, J.; Huang, L.; Zhang, Y.; Chen, F.; Fan, P.; Zhong, M.; Yeh, S. A new promising nucleating agent for polymer foaming: Applications of ordered mesoporous silica particles in polymethyl methacrylate supercritical carbon dioxide microcellular foaming. Ind. Eng. Chem. Res. 2013, 52, 14169–14178. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Ray, S.S.; Okamoto, M.; Ogami, A.; Yamada, K.; Ueda, K. Well-Controlled Biodegradable Nanocomposite Foams: From Microcellular to Nanocellular. Macromol. Rapid Commun. 2003, 24, 457–461. [Google Scholar] [CrossRef]
- Lee, Y.H.; Park, C.B.; Wang, K.H. HDPE-Clay Nanocomposite Foams Blown with Supercritical CO2. J. Cell. Plast. 2005, 41, 487–502. [Google Scholar] [CrossRef]
- Pinto, J.; Morselli, D.; Bernardo, V.; Notario, B.; Fragouli, D.; Rodriguez-Perez, M.A.; Athanassiou, A. Nanoporous PMMA foams with templated pore size obtained by localized in situ synthesis of nanoparticles and CO2 foaming. Polymer 2017, 124, 176–185. [Google Scholar] [CrossRef]
- Siripurapu, S.; DeSimone, J.M.; Khan, S.A.; Spontak, R.J. Controlled Foaming of Polymer Films through Restricted Surface. Macromolecules 2005, 38, 2271–2280. [Google Scholar] [CrossRef]
- Bernardo, V.; Martín-de León, J.; Laguna-Gutiérrez, E.; Rodríguez-Pérez, M.Á. PMMA-sepiolite nanocomposites as new promising materials for the production of nanocellular polymers. Eur. Polym. J. 2017, 96, 10–26. [Google Scholar] [CrossRef]
- Alvarez, A.; Santaren, J.; Esteban-Cubillo, A.; Aparicio, P. Development in Palygorskite-Sepiolite Research; Galan, E., Singer, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9780444536075. [Google Scholar]
- Ruiz-Hitzky, E. Molecular access to intracrystalline tunnels of sepiolite. J. Mater. Chem. 2001, 11, 86–91. [Google Scholar] [CrossRef]
- Liu, M.; Pu, M.; Ma, H. Preparation, structure and thermal properties of polylactide/sepiolite nanocomposites with and without organic modifiers. Compos. Sci. Technol. 2012, 72, 1508–1514. [Google Scholar] [CrossRef]
- García, N.; Guzman, J.; Benito, E.; Esteban-Cubillo, A.; Aguilar, E.; Santaren, J.; Tiemblo, P. Surface Modification of Sepiolite in Aqueous Gels by Using Methoxysilanes and Its Impact on the Nanofiber Dispersion Ability. Langmuir 2011, 27, 3952–3959. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.H.; Chen, Z.J.; Wang, J.Q.; Wei, P. Synergistic effects of sepiolite on intumescent flame retardant polypropylene. Express Polym. Lett. 2010, 4, 743–752. [Google Scholar] [CrossRef]
- Bilotti, E.; Fischer, H.R.; Peijs, T.; Si, À. Polymer Nanocomposites Based on Needle-like Sepiolite Clays: Effect of Functionalized Polymers on the Dispersion of Nanofiller, Crystallinity, and Mechanical Properties. J. Appl. Polym. Sci. 2008, 107, 1116–1123. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, Y. Study on Sepiolite-Reinforced Polymeric Nanocomposites. J. Appl. Polym. Sci. 2006, 99, 2163–2166. [Google Scholar] [CrossRef]
- Lu, P. The Effects of Different Grafted Clays on Thermal Properties of Their PMMA Composites. Polym. Plast. Technol. Eng. 2011, 50, 1541–1545. [Google Scholar] [CrossRef]
- Igualada, J.A.; Feijoo, J.L. Polymer Foams. U.S. Patent 2015/0337103, 26 November 2015. [Google Scholar]
- Santaren, J.; Alvarez, A.; Esteban-Cubillo, A.; Notario, B.; Velasco, D.; Rodrıguez-Perez, M.A. Improving the Cellular Structure and Thermal Conductivity of PS Foams by Using Sepiolites. In Proceedings of the International Conference on Foams and Foams Technology, FOAMS 2012, Barcelona, Spain, 12–13 September 2012; pp. 1–5. [Google Scholar]
- Garcia-Lopez, D.; Fernandez, J.F.; Merino, J.C.; Santaren, J.; Pastor, J.M. Effect of organic modification of sepiolite for PA 6 polymer/organoclay nanocomposites. Compos. Sci. Technol. 2010, 70, 1429–1436. [Google Scholar] [CrossRef]
- Pinto, J.; Dumon, M.; Rodriguez-Perez, M.A. Nanoporous Polymer Foams from Nanostructured Polymer Blends: Preparation, Characterization, and Properties. In Recent Developments in Polymer Macro, Micro and Nano Blends; Visakh, P., Markovic, G., Pasquini, D., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 237–288. [Google Scholar]
- Pinto, J.; Reglero-ruiz, J.A.; Dumon, M.; Rodriguez-Perez, M.A. Temperature influence and CO2 transport in foaming processes of poly (methyl methacrylate)—Block copolymer nanocellular and microcellular foams. J. Supercrit. Fluids 2014, 94, 198–205. [Google Scholar] [CrossRef]
- Bernardo, V.; Martin-de Leon, J.; Laguna-Gutierrez, E.; Catelani, T.; Pinto, J.; Athanassiou, A.; Rodriguez-Perez, M.A. Understanding the role of MAM molecular weight in the production of PMMA/MAM nanocellular polymers. Polymer 2018, 153, 262–270. [Google Scholar] [CrossRef]
- Kumar, V.; Weller, J.E. Model for the unfoamed skin on microcellular foams. Polym. Eng. Sci. 1994, 34, 169–173. [Google Scholar] [CrossRef]
- Pinto, J.; Pardo, S.; Solórzano, E.; Rodríguez-Pérez, M.A.; Dumon, M.; de Saja, J.A. Solid Skin Characterization of PMMA/MAM Foams Fabricated by Gas Dissolution Foaming over a Range of Pressures. Defect Diffus. Forum 2012, 326–328, 434–439. [Google Scholar] [CrossRef]
- Nadella, K.; Kumar, V.; Li, W. Constrained solid-state foaming of microcellular panels. Cell. Polym. 2005, 24, 71–90. [Google Scholar] [CrossRef]
- Siripurapu, S.; Coughlan, J.A.; Spontak, R.J.; Khan, S.A. Surface-Constrained Foaming of Polymer Thin Films with Supercritical Carbon Dioxide. Macromolecules 2004, 37, 9872–9879. [Google Scholar] [CrossRef]
- Miller, D.; Kumar, V. Microcellular and nanocellular solid-state polyetherimide (PEI) foams using sub-critical carbon dioxide II. Tensile and impact properties. Polymer 2011, 52, 2910–2919. [Google Scholar] [CrossRef]
- Forest, C.; Chaumont, P.; Cassagnau, P.; Swoboda, B.; Sonntag, P. Nanofoaming of PMMA using a batch CO2 process: Influence of the PMMA viscoelastic behaviour. Polymer 2015, 77, 1–9. [Google Scholar] [CrossRef]
- Liao, Z.; Yeh, S.; Chu, C.; Tseng, T. Critical Parameters of Generating PMMA Nanocellular Foam. In Proceedings of the Annual technical conference/Society of Plastics, Indianapolis, IN, USA, 23–25 May 2016; pp. 1816–1821. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-de León, J.; Bernardo, V.; Rodríguez-Pérez, M.Á. Nanocellular Polymers: The Challenge of Creating Cells in the Nanoscale. Materials 2019, 12, 797. https://doi.org/10.3390/ma12050797
Martín-de León J, Bernardo V, Rodríguez-Pérez MÁ. Nanocellular Polymers: The Challenge of Creating Cells in the Nanoscale. Materials. 2019; 12(5):797. https://doi.org/10.3390/ma12050797
Chicago/Turabian StyleMartín-de León, Judith, Victoria Bernardo, and Miguel Ángel Rodríguez-Pérez. 2019. "Nanocellular Polymers: The Challenge of Creating Cells in the Nanoscale" Materials 12, no. 5: 797. https://doi.org/10.3390/ma12050797
APA StyleMartín-de León, J., Bernardo, V., & Rodríguez-Pérez, M. Á. (2019). Nanocellular Polymers: The Challenge of Creating Cells in the Nanoscale. Materials, 12(5), 797. https://doi.org/10.3390/ma12050797






