Surface Modification of Graphene Nanoplatelets by Organic Acids and Ultrasonic Radiation for Enhance Uremic Toxins Adsorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
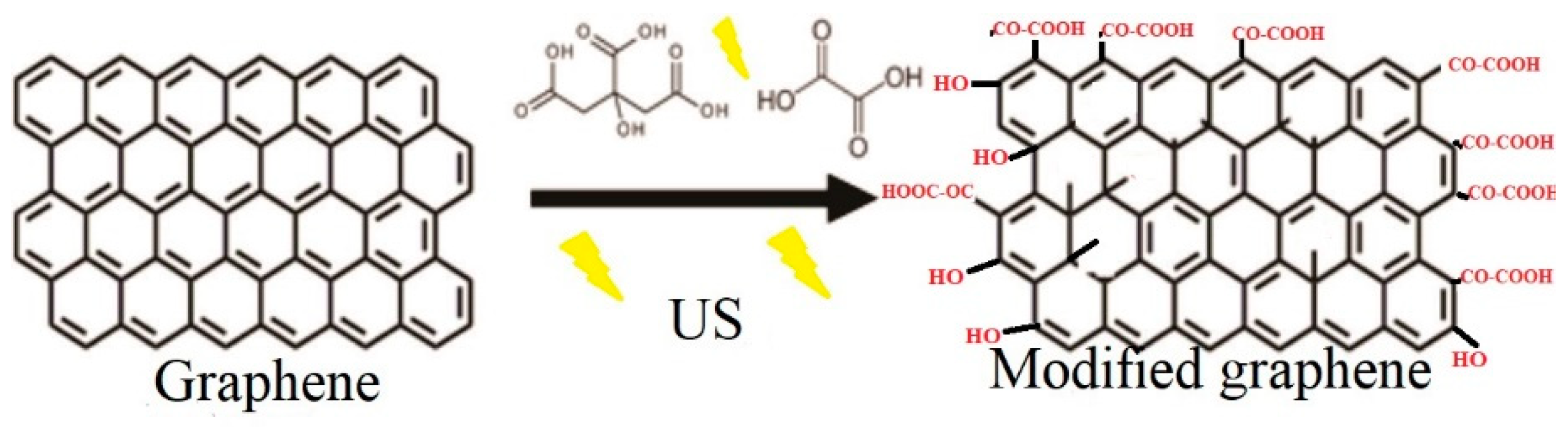
Chemical Modification of Graphene
2.3. Characterization Techniques
2.4. Adsorption of Uremic toxins
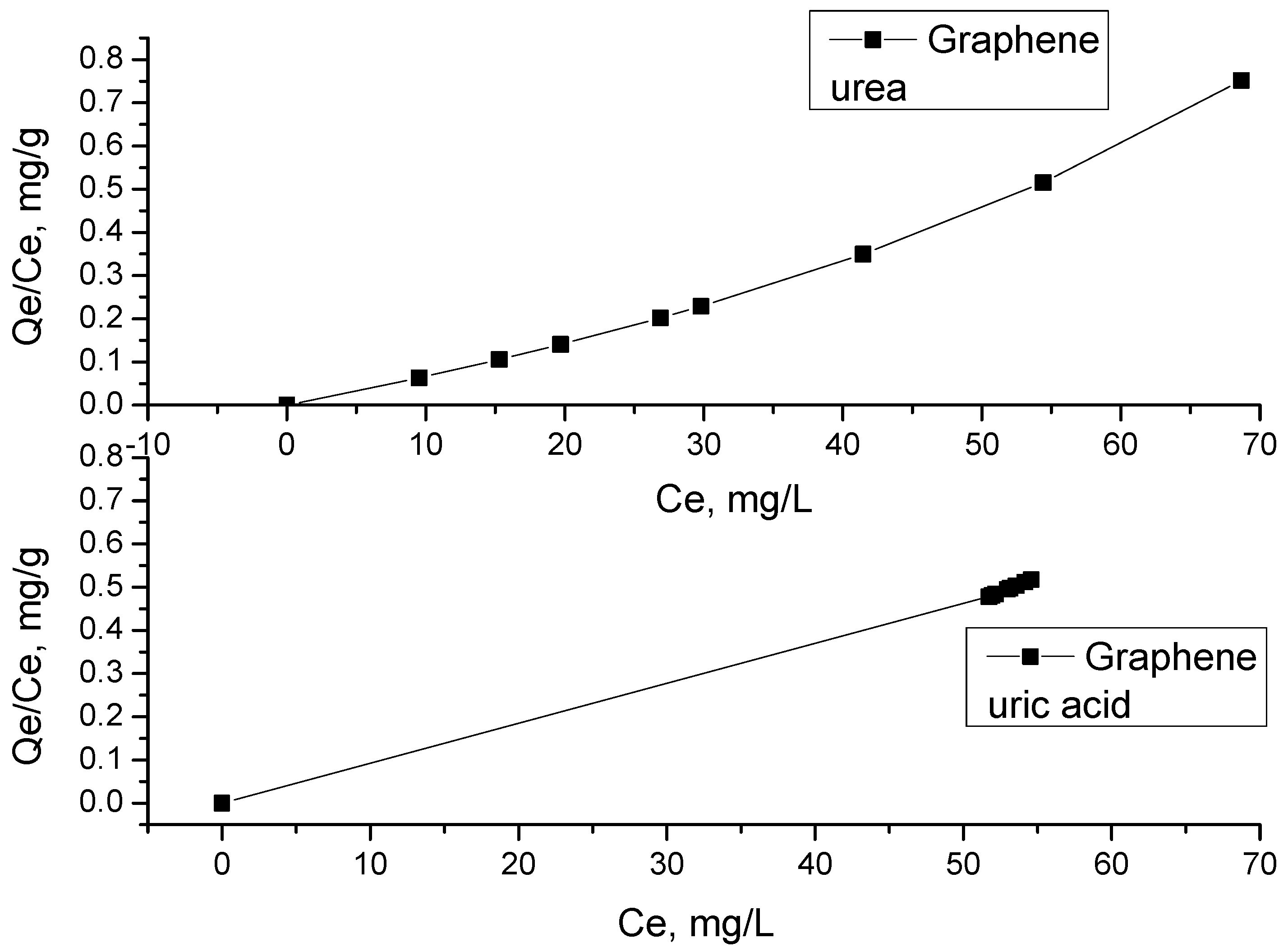
2.5. Adsorption Isotherm
3. Results and Discussion
3.1. Fourier Transform Infrared Spectroscopy (FTIR)
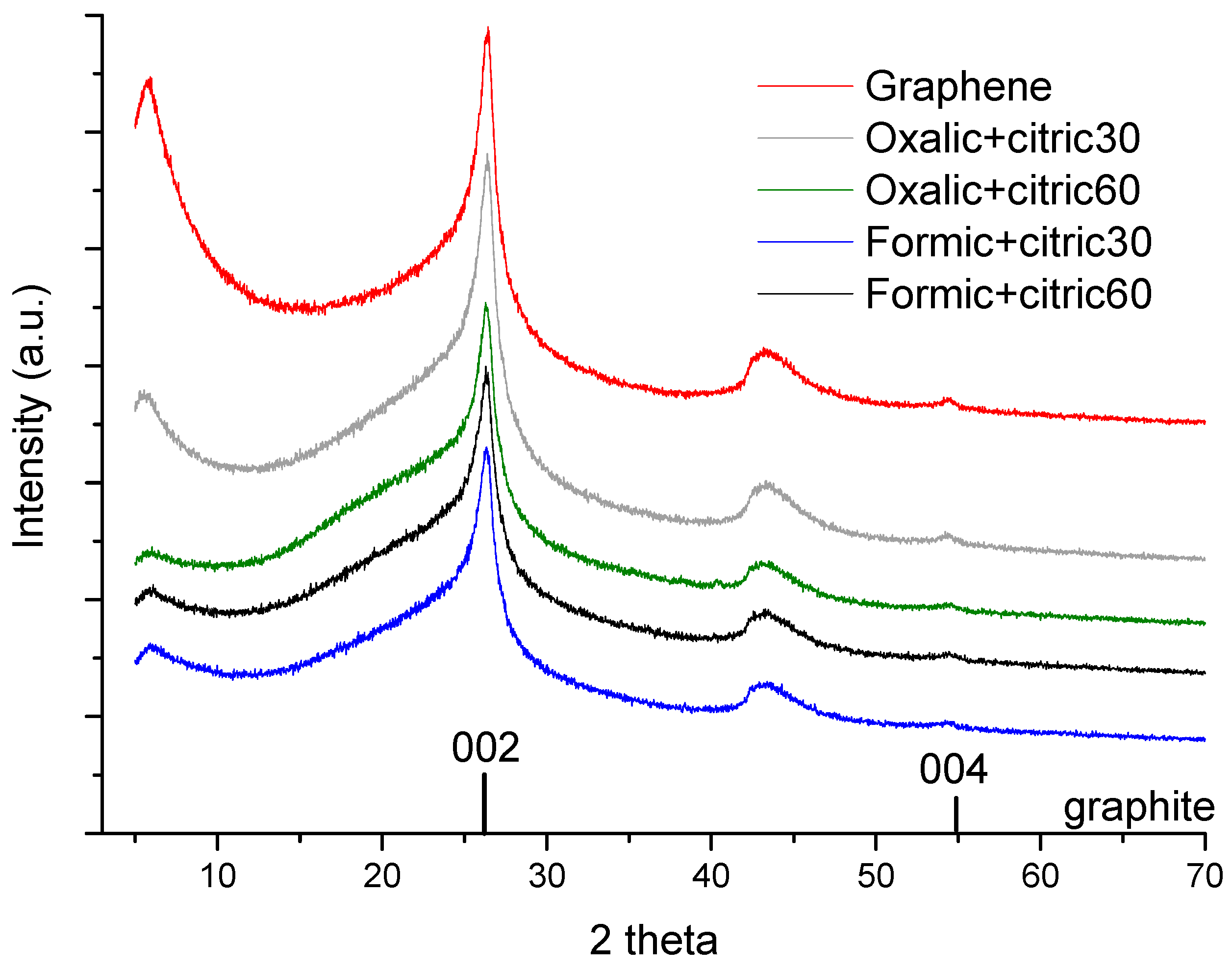
3.2. X-ray Photoelectron Spectroscopy (XPS)
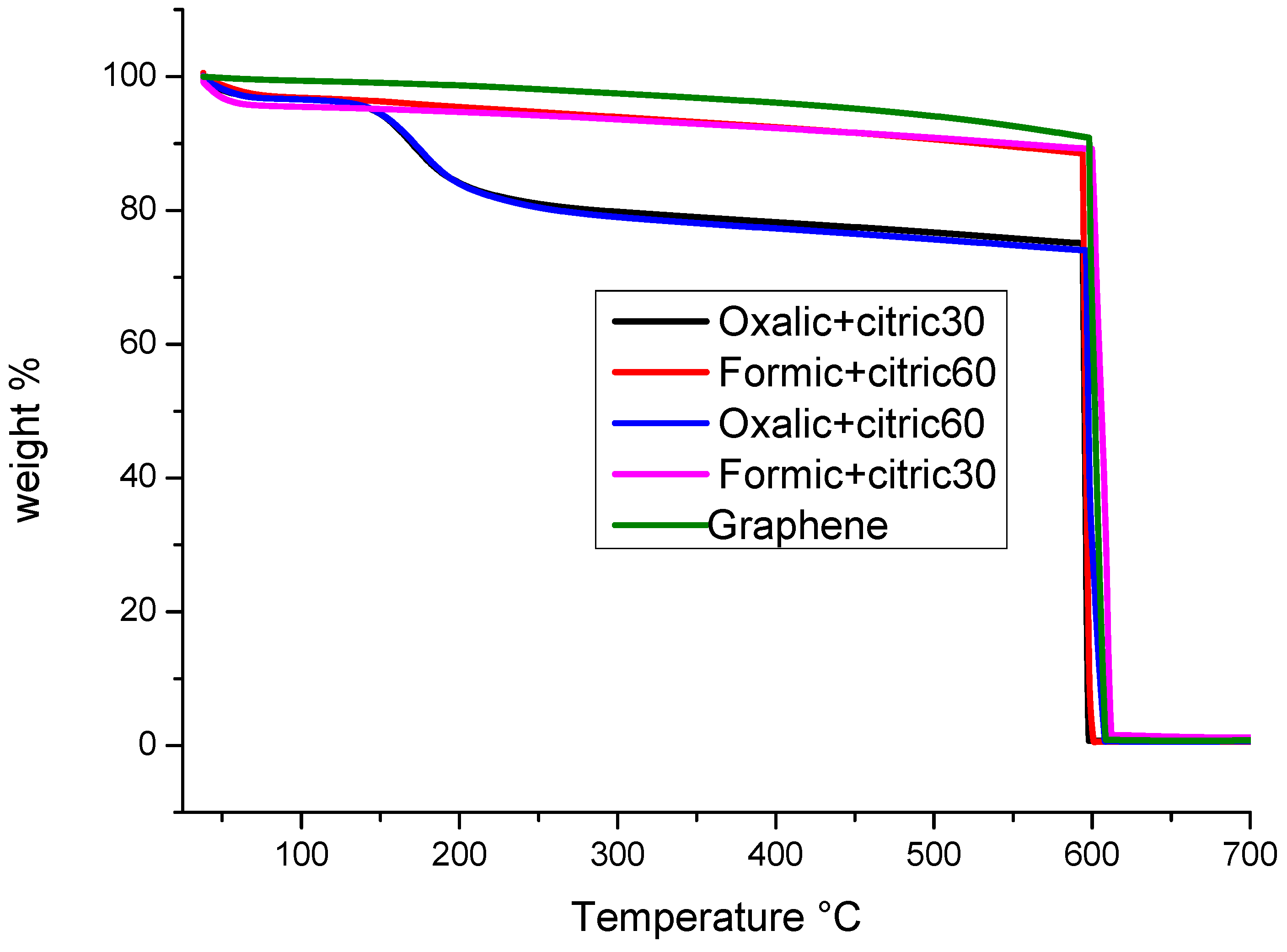
3.3. Thermogravimetric Analysis (TGA)
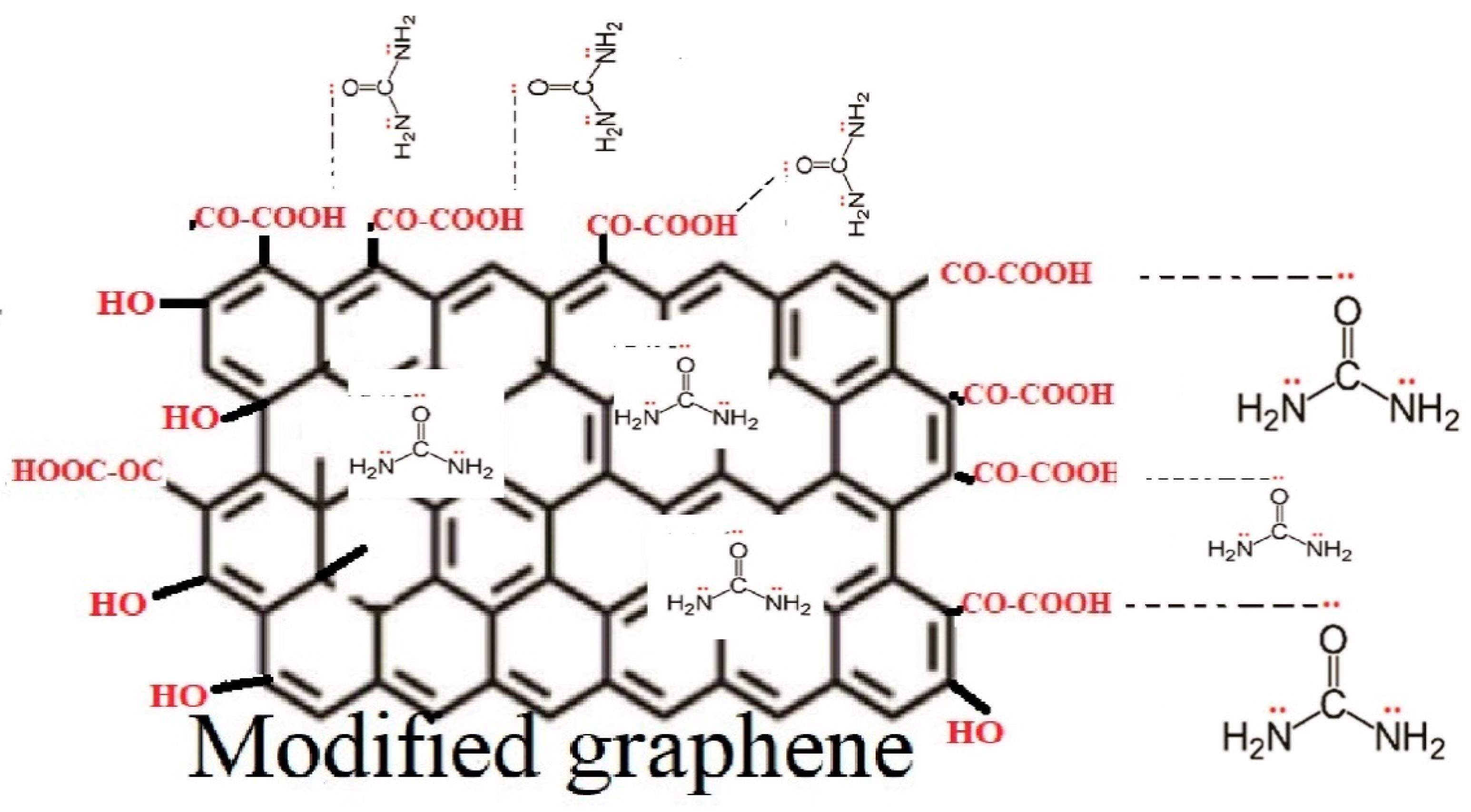
3.4. Scheme of Surface Modification of Graphene Nanoplatelets
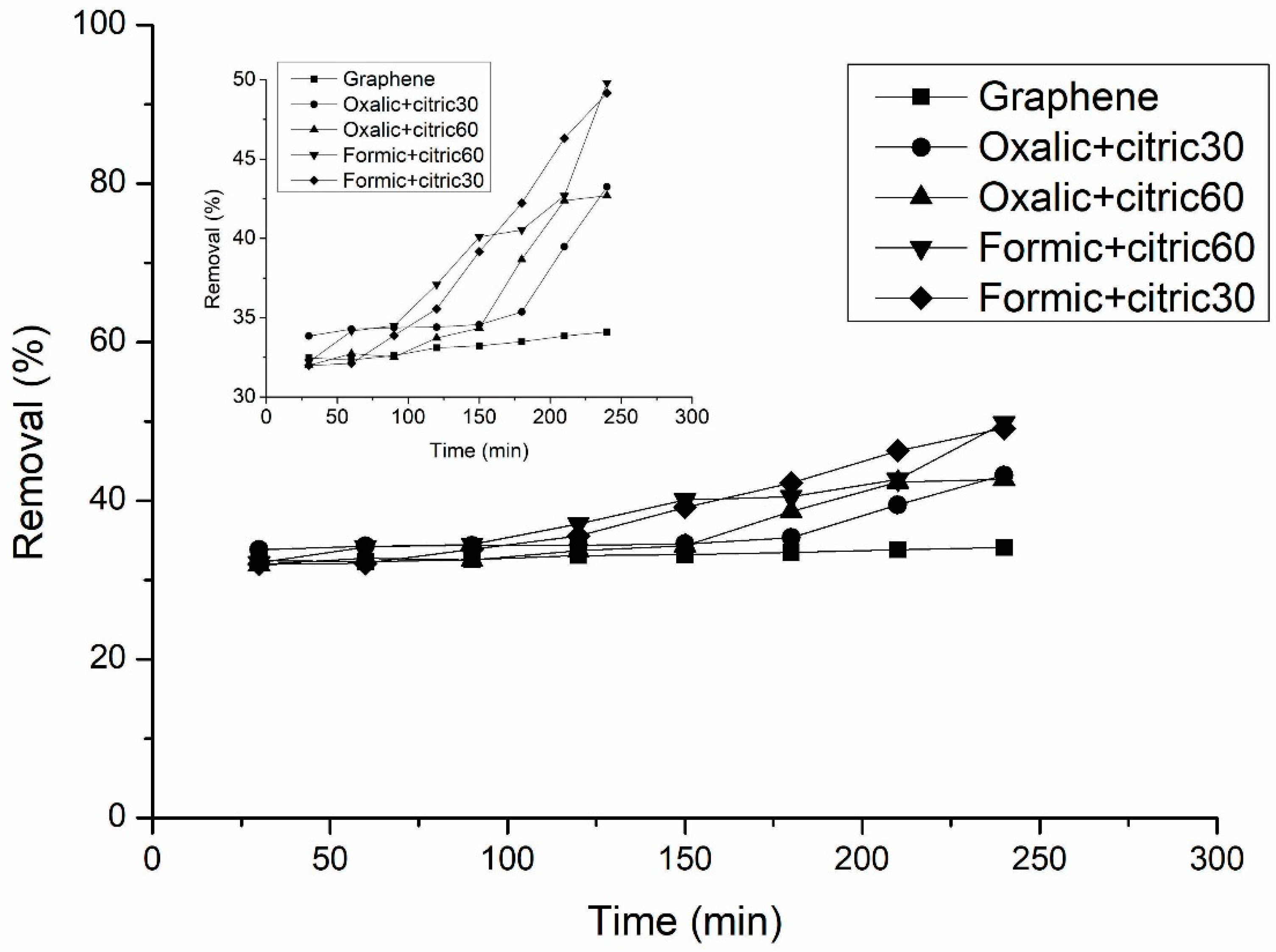
3.5. Adsorption Study of Uremic Toxins
3.6. Scheme of Adsorption of Urea on Graphene Nanoplatelets
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Méndez-Durán, A.; Méndez-Bueno, J.F.; Tapia-Yáñez, T.; Montes, A.M.; Aguilar-Sánchez, L. Epidemiología de la insuficiencia renal crónica en México. Diálisis y Trasplante 2010, 31, 7–11. [Google Scholar] [CrossRef]
- Owen, W.F., Jr.; Lew, N.L.; Liu, Y.; Lowrie, E.G.; Lazarus, J.M. The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N. Engl. J. Med. 1993, 329, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Bergé-Lefranc, D.; Pizzala, H.; Paillaud, J.L.; Schäf, O.; Vagner, C.; Boulet, P.; Denoyel, R. Adsorption of small uremic toxin molecules on MFI type zeolites from aqueous solution. Adsorption 2008, 14, 377–387. [Google Scholar] [CrossRef]
- Lee, C.J.; Hsu, S.T. Preparation of spherical encapsulation of activated carbons and their adsorption capacity of typical uremic toxins. J. Biomed. Mater. Res. 1990, 24, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Zare-Zardini, H.; Amiri, A.; Shanbedi, M.; Taheri-Kafrani, A.; Kazi, S.N.; Chew, B.T.; Razmjou, A. I n vitro and in vivo study of hazardous effects of Ag nanoparticles and Arginine-treated multi walled carbon nanotubes on blood cells: Application in hemodialysis membranes. J. Biomed. Mater. Res. A 2015, 103, 2959–2965. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yang, J.; Park, J.; Gou, X.; Wang, B.; Liu, H.; Yao, J. Facile synthesis and characterization of graphene nanosheets. J. Phys. Chem. C 2008, 112, 8192–8195. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, J.; Li, N.; Shi, M.; Ma, H.; Yan, B.; Ye, M. Amino-functionalization of graphene sheets and the fabrication of their nanocomposites. Polym. Compos. 2010, 31, 1987–1994. [Google Scholar] [CrossRef]
- Zhu, J.; Sadu, R.; Wei, S.; Chen, D.H.; Haldolaarachchige, N.; Luo, Z.; Guo, Z. Magnetic graphene nanoplatelet composites toward arsenic removal. J. Solid State Sci. Technol. 2012, 1, M1–M5. [Google Scholar] [CrossRef]
- Al-Khateeb, L.A.; Almotiry, S.; Salam, M.A. Adsorption of pharmaceutical pollutants onto graphene nanoplatelets. Chem. Eng. J. 2014, 248, 191–199. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Yao, W.; Wang, J.; Ji, Y.; Ai, Y.; Wang, X. Macroscopic, spectroscopic, and theoretical investigation for the interaction of phenol and naphthol on reduced graphene oxide. Environ. Sci. Technol. 2017, 51, 3278–3286. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.S.K.; Kakan, S.S.; Rajesh, N. A novel amine impregnated graphene oxide adsorbent for the removal of hexavalent chromium. Chem. Eng. J. 2013, 230, 328–337. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Ai, Y.; Tan, X.; Hayat, T.; Hu, W.; Wang, X. Experimental and theoretical studies on competitive adsorption of aromatic compounds on reduced graphene oxides. J. Mater. Chem. A 2016, 4, 5654–5662. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Pang, H.; Yu, S.; Ai, Y.; Ma, X.; Wang, X. Effect of graphene oxide surface modification on the elimination of Co (II) from aqueous solutions. Chem. Eng. J. 2018, 344, 380–390. [Google Scholar] [CrossRef]
- Gong, C.; Hart, D. Ultrasound Induced Cavitation and Sonochemical Yield. J. Acoust. Soc. Am. 1998, 104, 1–16. [Google Scholar] [CrossRef]
- Panwar, V.; Chattree, A.; Pal, K. A new facile route for synthesizing of graphene oxide using mixture of sulfuric–nitric–phosphoric acids as intercalating agent. Physica E 2015, 73, 235–241. [Google Scholar] [CrossRef]
- Cabello, C.; Galindo, A.S.; Berumen, C.P.; López, L.L.; Orta, C.Á.; Garza, J.V.; Donías, L.M. Modificación superficial de MWCNTs asistida por ultrasonido con ácido acético y ácido cítrico. Afinidad 2014, 71, 139–145. [Google Scholar]
- Cabello, C.; Rincón, S.; Bartolo, P.; Ruiz-Espinoza, J.; Zepeda, A. Incorporation of organic groups on the surface of multi-walled carbon nanotubes using an ultrasonic tip. Fuller. Nanotub. Carbon Nanostruct. 2018, 26, 502–509. [Google Scholar] [CrossRef]
- Wang, Z.H.; Yue, B.Y.; Teng, J.; Jiao, F.P.; Jiang, X.Y.; Yu, J.G.; Chen, X.Q. Tartaric acid modified graphene oxide as a novel adsorbent for high-efficiently removal of Cu (II) and Pb (II) from aqueous solutions. J. Taiwan Inst. Chem. E 2016, 66, 181–190. [Google Scholar] [CrossRef]
- Avila-Vega, Y.I.; Leyva-Porras, C.C.; Mireles, M.; Quevedo-López, M.; Macossay, J.; Bonilla-Cruz, J. Nitroxide-functionalized graphene oxide from graphite oxide. Carbon 2013, 63, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Monteserín, C.; Blanco, M.; Aranzabe, E.; Aranzabe, A.; Laza, J.M.; Larrañaga-Varga, A.; Vilas, J.L. Effects of Graphene Oxide and Chemically-Reduced Graphene Oxide on the Dynamic Mechanical Properties of Epoxy Amine Composites. Polymers 2017, 9, 449. [Google Scholar] [CrossRef]
- Kim, M.J.; Jeon, I.Y.; Seo, J.M.; Dai, L.; Baek, J.B. Graphene phosphonic acid as an efficient flame retardant. ACS Nano 2014, 8, 2820–2825. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Wang, S.J.; Kim, J.K. Preparation of graphite nanoplatelets and graphene sheets. J. Colloid Interface Sci. 2009, 336, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Tang, J.; Yu, F.; Ye, N. Preparation of graphene nanoplatelets reinforcing copper matrix composites by electrochemical deposition. J. Alloy Compd. 2018, 766, 266–273. [Google Scholar] [CrossRef]
- Akhavan, O. The effect of heat treatment on formation of graphene thin films from graphene oxide nanosheets. Carbon 2010, 48, 509–519. [Google Scholar] [CrossRef]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Ruoff, R.S. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Shang, N.G.; Papakonstantinou, P.; Sharma, S.; Lubarsky, G.; Li, M.; McNeill, D.W.; Blackley, R. Controllable selective exfoliation of high-quality graphene nanosheets and nanodots by ionic liquid assisted grinding. Chem. Commun. 2012, 48, 1877–1879. [Google Scholar] [CrossRef] [PubMed]
- Dave, K.; Park, K.H.; Dhayal, M. Two-step process for programmable removal of oxygen functionalities of graphene oxide: Functional, structural and electrical characteristics. RSC Adv. 2015, 5, 95657–95665. [Google Scholar] [CrossRef]
- Prolongo, S.G.; Jiménez-Suárez, A.; Moriche, R.; Ureña, A. Graphene nanoplatelets thickness and lateral size influence on the morphology and behavior of epoxy composites. Eur. Polym. J. 2014, 53, 292–301. [Google Scholar] [CrossRef]
- Emiru, T.F.; Ayele, D.W. Controlled synthesis, characterization and reduction of graphene oxide: A convenient method for large scale production. Egypt J. Basic Appl. Sci. 2017, 4, 74–79. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron. Spectrosc. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Glassock, R.J. Uremic toxins: What are they? An integrated overview of pathobiology and classification. J. Renal. Nutr. 2008, 18, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Gorin, Y. Nox4 as a potential therapeutic target for treatment of uremic toxicity associated to chronic kidney disease. Kidney Int. 2013, 83, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Idris, A.; Yusof, N.M.; Khairuddin, N.F.M.; Akhmal, H. Surface modification and performance enhancement of nano-hybrid f-MWCNT/PVP90/PES hemodialysis membranes. J. Membr. Sci. 2014, 467, 73–84. [Google Scholar] [CrossRef]
- Gao, B.; Jiang, P.; Lei, H. Studies on adsorption property of novel composite adsorption material PEI/SiO2 for uric acid. Mater. Lett. 2006, 60, 3398–3404. [Google Scholar] [CrossRef]
- Liu, C.; Liang, X.; Liu, X.; Wang, Q.; Zhan, L.; Zhang, R.; Ling, L. Surface modification of pitch-based spherical activated carbon by CVD of NH3 to improve its adsorption to uric acid. Appl. Surf. Sci. 2008, 254, 6701–6705. [Google Scholar] [CrossRef]
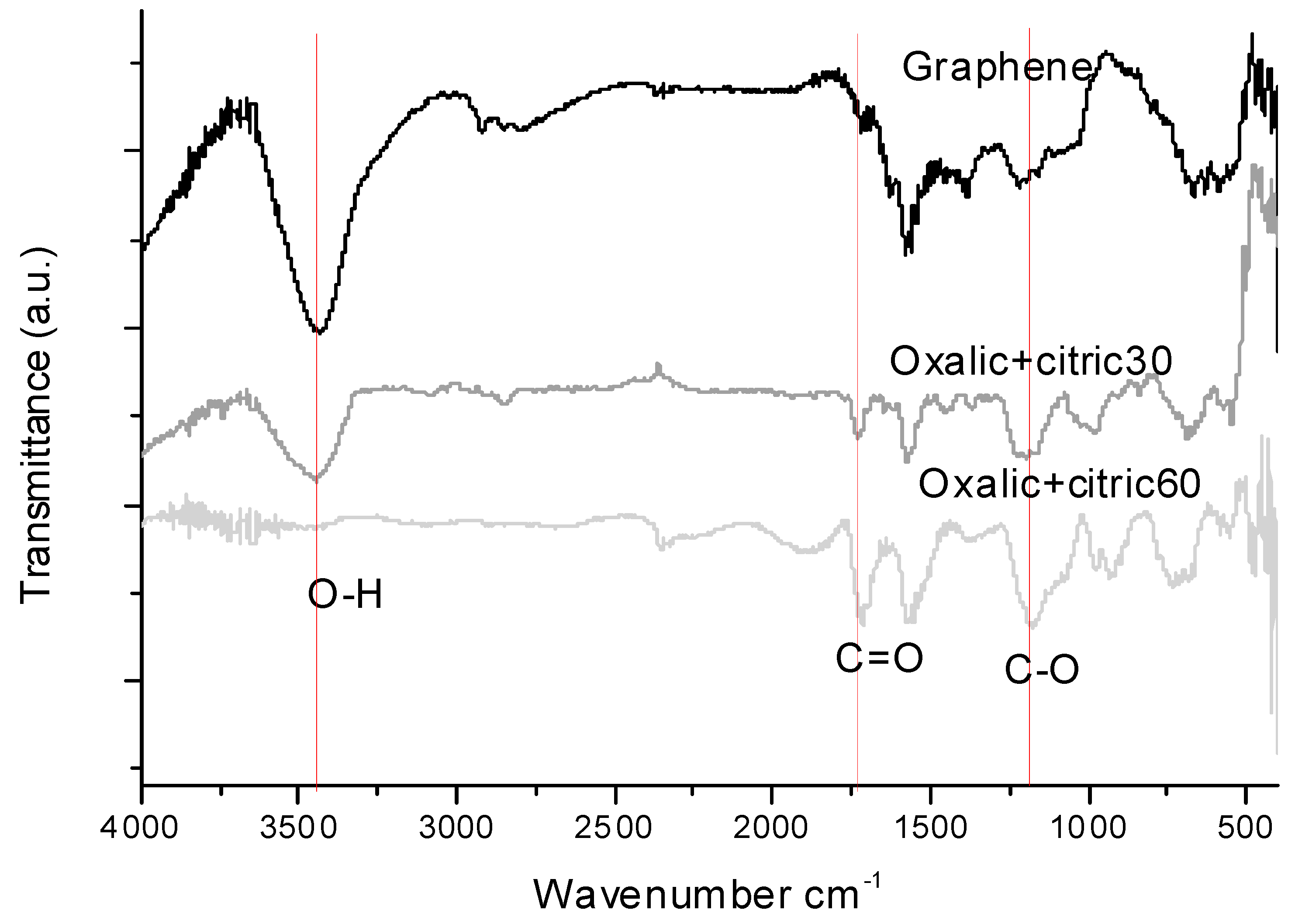
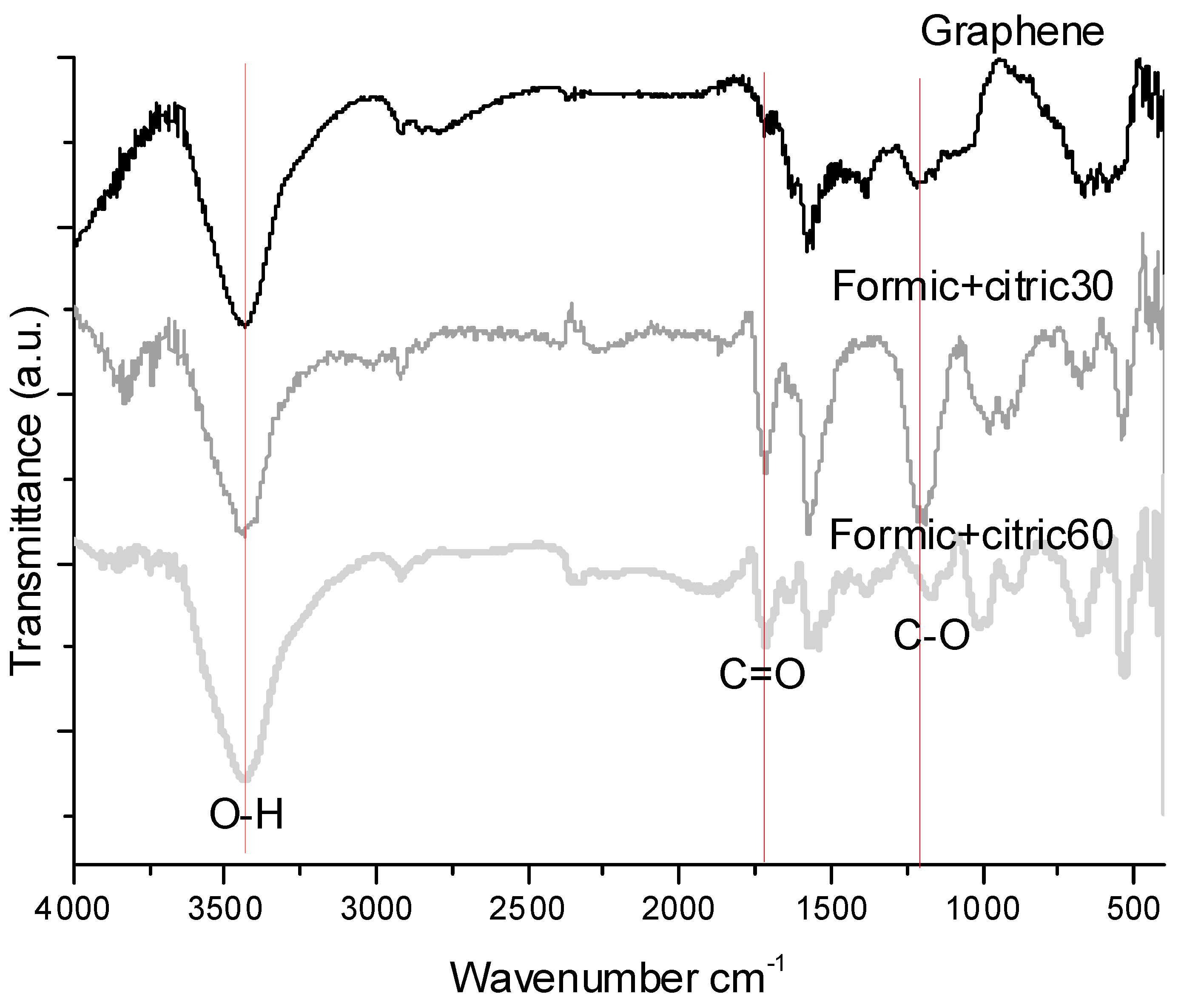

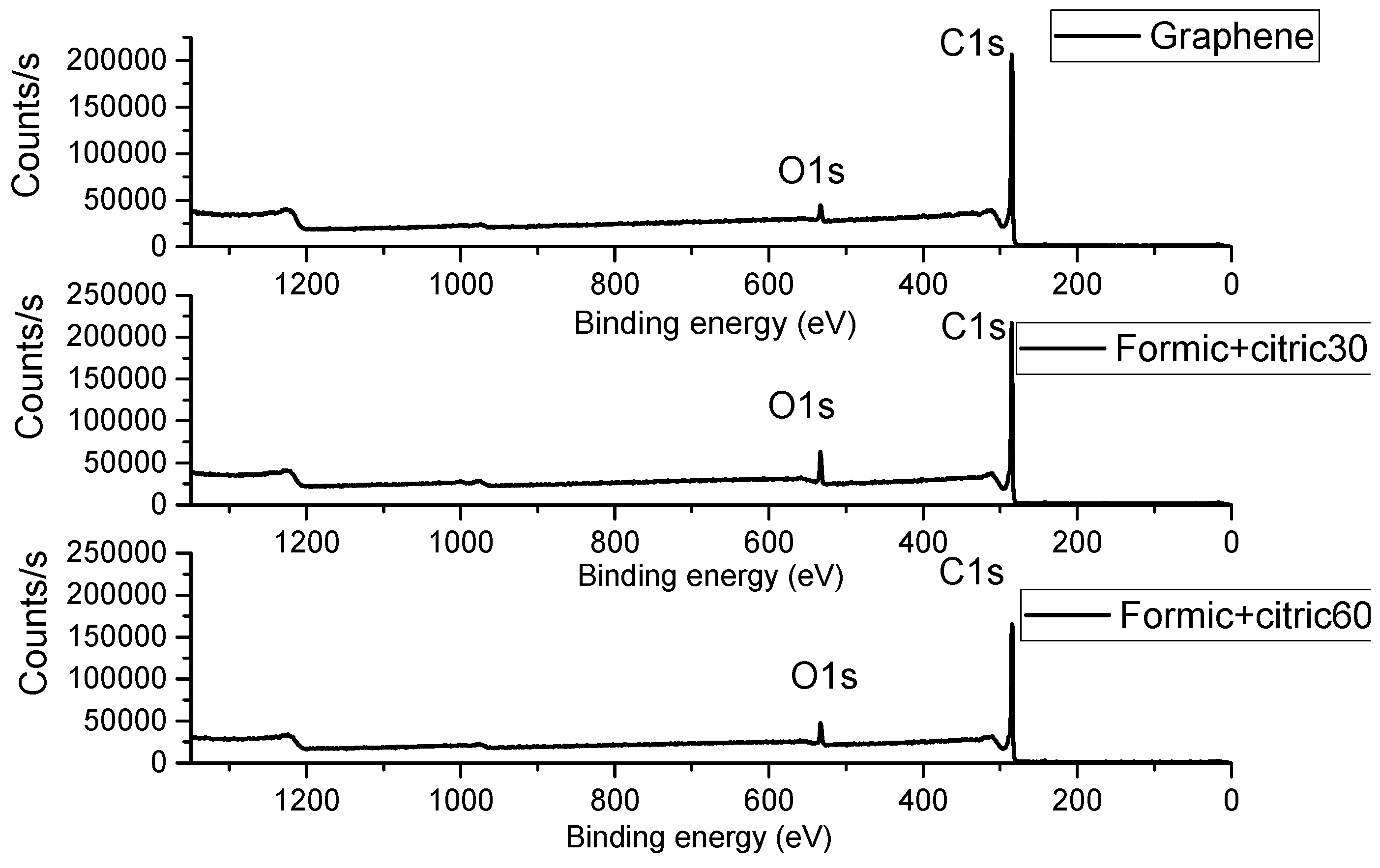
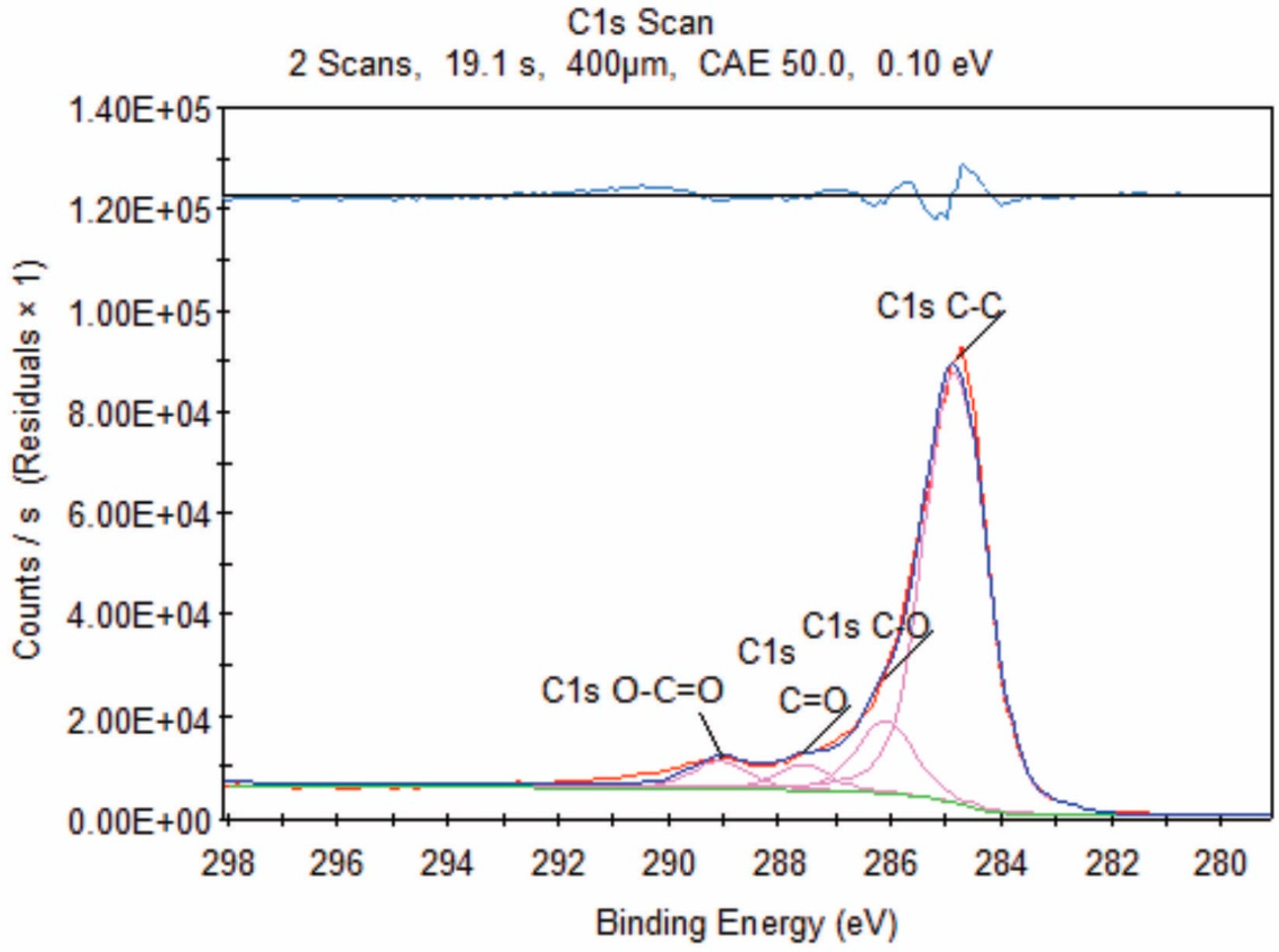


| Sample | Time (min) | Organic Acids Employed | Ratio |
|---|---|---|---|
| Graphene | 0 | None | 0 |
| Oxalic+Citric30 | 30 | Oxalic and Citric | 1:1 |
| Oxalic+Citric60 | 60 | Oxalic and Citric | 1:1 |
| Formic+Citric30 | 30 | Formic and Citric | 1:1 |
| Formic+Citric60 | 60 | Formic and Citric | 1:1 |
| Sample | C1s Peak (eV) | O1s Peak (eV) | C1s At % | O1s At % |
|---|---|---|---|---|
| Graphene | 285.32 | 532.89 | 95.28 | 4.72 |
| Formic+Citric30 | 284.94 | 532.89 | 93.12 | 6.88 |
| Formic+Citric60 | 284.93 | 532.89 | 95.02 | 4.98 |
| Oxalic+Citric30 | 285.03 | 533 | 91.92 | 8.08 |
| Oxalic+Citric60 | 284.47 | 532.81 | 92.7 | 7.3 |
| Sample | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| k | qmax | R2 | n | Kf | R2 | |
| Graphene | 0.010 | 0.056 | 0.973 | 1.050 | 1.189 | 0.6769 |
| Oxalic + Citric30 | 0.024 | 0.353 | 0.8892 | 0.594 | 6.61 | 0.3972 |
| Oxalic + Citric60 | 0.011 | 0.093 | 0.9382 | 1.128 | 6.68 | 0.9466 |
| Formic + Citric30 | 0.008 | 0.024 | 0.9858 | 1.324 | 6.74 | 0.8687 |
| Formic + Citric60 | 0.010 | 0.056 | 0.9627 | 1.168 | 6.78 | 0.9769 |
| Sample | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| k | qmax | R2 | n | Kf | R2 | |
| Graphene | 0.009 | 0.007 | 0.9993 | 1.17 | 0.001 | 0.9996 |
| Oxalic + Citric30 | 0.010 | 0.022 | 0.9752 | 1.13 | 0.023 | 0.9912 |
| Oxalic + Citric60 | 0.010 | 0.033 | 0.9682 | 1.12 | 0.042 | 0.9842 |
| Formic + Citric30 | 0.011 | 0.061 | 0.9444 | 1.09 | 0.060 | 0.9761 |
| Formic + Citric60 | 0.011 | 0.073 | 0.9445 | 1.09 | 0.081 | 0.968 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade-Guel, M.; Cabello-Alvarado, C.; Cruz-Delgado, V.J.; Bartolo-Perez, P.; De León-Martínez, P.A.; Sáenz-Galindo, A.; Cadenas-Pliego, G.; Ávila-Orta, C.A. Surface Modification of Graphene Nanoplatelets by Organic Acids and Ultrasonic Radiation for Enhance Uremic Toxins Adsorption. Materials 2019, 12, 715. https://doi.org/10.3390/ma12050715
Andrade-Guel M, Cabello-Alvarado C, Cruz-Delgado VJ, Bartolo-Perez P, De León-Martínez PA, Sáenz-Galindo A, Cadenas-Pliego G, Ávila-Orta CA. Surface Modification of Graphene Nanoplatelets by Organic Acids and Ultrasonic Radiation for Enhance Uremic Toxins Adsorption. Materials. 2019; 12(5):715. https://doi.org/10.3390/ma12050715
Chicago/Turabian StyleAndrade-Guel, M., C. Cabello-Alvarado, V. J. Cruz-Delgado, P. Bartolo-Perez, P. A. De León-Martínez, A. Sáenz-Galindo, G. Cadenas-Pliego, and C. A. Ávila-Orta. 2019. "Surface Modification of Graphene Nanoplatelets by Organic Acids and Ultrasonic Radiation for Enhance Uremic Toxins Adsorption" Materials 12, no. 5: 715. https://doi.org/10.3390/ma12050715
APA StyleAndrade-Guel, M., Cabello-Alvarado, C., Cruz-Delgado, V. J., Bartolo-Perez, P., De León-Martínez, P. A., Sáenz-Galindo, A., Cadenas-Pliego, G., & Ávila-Orta, C. A. (2019). Surface Modification of Graphene Nanoplatelets by Organic Acids and Ultrasonic Radiation for Enhance Uremic Toxins Adsorption. Materials, 12(5), 715. https://doi.org/10.3390/ma12050715











