Synthesis and Characterization of TiO2-ZnO-MgO Mixed Oxide and Their Antibacterial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Sample Characterization
2.3. Antibacterial Activity Test
2.4. Statistical Data Analysis
3. Results and Discussion
3.1. Morphological Observation and EDS Analysis
3.2. N2 Physisorption Analysis
3.3. X-Ray Diffraction
3.4. UV-Vis by Diffuse Reflectance
3.5. Infrared FTIR Analysis
3.6. Color and Total Color Difference
3.7. Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jing, F.; Suo, H.; Cui, S.; Tang, X.; Zhang, M.; Shen, X.; Lin, B.; Jiang, G.; Wu, X. Facile synthesis of TiO2/Ag composite aerogel with excellent antibacterial proprieties. J. Sol-Gel Sci. Technol. 2018, 86, 590–598. [Google Scholar] [CrossRef]
- Venkatasubramanian, R.; Srivastava, R.S.; Misra, R.D.K. Comparative study of antimirobial and photocatalytic activity in titania encapsulated composite nanoparticles with different dopants. Mater. Sci. Technol. 2008, 24, 589–595. [Google Scholar] [CrossRef]
- Shi, L.E.; Li, Z.H.; Zheng, W.; Zhao, Y.F.; Jin, Y.F.; Tang, Z.X. Synthesis, antibacterial activity, antibacterial mechanism and food applications of ZnO nanoparticles: A review. Food Addit. Contam. 2014, 31, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Tranquillo, E.; Barrino, F.; Blanco, I.; Dal Poggetto, G.; Naviglio, D. Drug release of hybrid materials containing Fe(II) citrate synthetized by Sol-gel technique. Materials 2018, 11, 2270. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, A.; Siwinska-Ciesielczyk, S.; Jesionowski, T. Titania-based hybrid materials with ZnO, ZrO2 and MoS2: A review. Materials 2018, 11, 2295. [Google Scholar] [CrossRef] [PubMed]
- Cano-Casanova, L.; Amorés-Pérez, A.; Lillo-Ródenas, M.A.; Román-Martínez, M.C. Effect of the preparation method (Sol-gel or hydrothermal) and conditions on the TiO2 properties and activity for propene oxidation. Materials 2018, 11, 2227. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Larios, A.; Hernandez-Gordillo, A.; Morales-Mendoza, G.; Lartundo-Rojas, L.; Mantilla, A.; Gómez, R. Enhancing the H2 evolution from water-methanol solution using Mn2+-Mn+3-Mn4+ redox species of Mn-doped TiO2 sol-gel. Catal. Today 2016, 266, 9–16. [Google Scholar] [CrossRef]
- Catauro, M.; Tranquillo, E.; Dal Poggetto, G.; Pasquali, M.; Dell’Era, A.; Vecchio Ciprioti, S. Influence of the heat treatment on the particle size and on the crystalline phase of TiO2 synthetized by the Sol-gel method. Materials 2018, 11, 2364. [Google Scholar] [CrossRef] [PubMed]
- Siwinska-Stefanska, K.; Kubiak, A.; Piasecki, A.; Goscianska, J.; Nowaczyk, G.; Jurga, S.; Jesionowski, T. TiO2-ZnO binary oxide system: Comprhensive characterization and test of photocatalytic activity. Materials 2018, 11, 841. [Google Scholar] [CrossRef] [PubMed]
- Sharif, H.A.; Rasha, A.A.E.; Ramia, Z.A.L.B. Titanium dioxide content in foodstuff from the Jordanian market: Spectrophonetic evaluation of TiO2 nanoparticles. Int. Food Res. J. 2015, 22, 1024–1029. [Google Scholar]
- Sun, S.; Ding, H.; Hou, X. Preparation of CaCO3-TiO2 composite particles and their pigment properties. Materials 2018, 11, 1131. [Google Scholar] [CrossRef] [PubMed]
- Janczarek, M.; Endo, M.; Zhang, D.; Wang, K.; Kowalska, E. Enhanced photocatalytic and antimicrobial performance of cuprous oxide/titania: The effect of titania matrix. Materials 2018, 11, 2069. [Google Scholar] [CrossRef] [PubMed]
- Jesline, A.; Jhon, N.P.; Narayanan, P.M.; Vani, C.; Murugan, S. Antimicrobial activity of zinc and titanium dioxide nanoparticles against biofilm-producing methicillini-resistant Staphyloccus aureus. Appl. Nanosci. 2015, 5, 157–162. [Google Scholar] [CrossRef]
- Venkatasubbu, G.D.; Baskar, R.; Anusuya, T.; Seshan, C.A.; Chelliah, R. Toxicity mechanism of titanium dioxide and zinc oxide nanoparticles against food pathogens. Colloids Surf. B 2016, 148, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Shen, Y.G.; Alajmi, Z.; Yang, S.Y.; Sun, J.M.; Zhang, H.M. Sol-gel preparation and properties of Ag-TiO2 films on surface roughened Ti-6Al-4V alloy. Mater. Sci. Technol. 2015, 31, 501–505. [Google Scholar] [CrossRef]
- Ashok, C.H.; Venkateswara, R.K.; Shilpa-Chakra, C.H. Synthesis and characterization of MgO/TiO2 nanocomposites. Nanomed. Nanotechnol. 2015, 6, 2–5. [Google Scholar]
- Juma, A.O.; Arbab, E.A.A.; Muiva, C.M.; Lepodise, L.M.; Mola, G.T. Synthesis and characterization of CuO-NiO-ZnO mixed metal oxide nanocomposite. J. Alloy. Compd. 2017, 723, 866–872. [Google Scholar] [CrossRef]
- Li, X.; Zhao, R.; Jiang, H.; Zhai, Y.; Ma, P. Preparation and catalytic properties of ZnO-CeO2-TiO2 composite. Synth. React. Inorg. Met-Org. Chem. 2016, 46, 775–782. [Google Scholar] [CrossRef]
- Li, J.; Xie, B.; Xia, K.; Li, Y.; Han, J.; Zhao, C. Enhanced antibacterial activity of silver doped titanium dioxide/chitosan composite under visible light. Materials 2018, 11, 1403. [Google Scholar] [CrossRef] [PubMed]
- Arandiyan, H.R.; Parvari, M. Studies on mixed metal oxides solid solutions as heterogeneous catalysts. Braz. J. Chem. Eng. 2009, 26, 63–74. [Google Scholar] [CrossRef]
- Montazer, M.; Pakdel, E. Self-cleaning and color reduction in wool fabric by nano titanium dioxide. J. Text. Inst. 2011, 102, 343–352. [Google Scholar] [CrossRef]
- Kalaiarasi, S.; Jose, M. Streptomycin loaded TiO2 nanoparticles: Preparation, characterization and antibacterial applications. J. Nanostruct. Chem. 2017, 7, 47–53. [Google Scholar] [CrossRef]
- International Union of Pure and Applied Chemistry. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. IUPAC 1985, 57, 603–619. [Google Scholar]
- Deshmane, V.G.; Owen, S.L.; Abrokwah, R.Y.; Kuila, D. Mesoporous nanocrystalline TiO2 supported metal (Cu, Co, Ni, Pd, Zn, and Sn) catalysts: Effect of metal-support interactions on steam reforming of methanol. J. Mol. Catal. A Chem. 2015, 408, 202–213. [Google Scholar] [CrossRef]
- Amorós-Pérez, A.; Cano-Casanova, L.; Castillo-Deltell, A.; Lillo-Ródenas, M.A.; Román-Martínez, M.C. TiO2 Modification with transition metallic species (Cr, Co, Ni, and Cu) for photocatalytic abatement of acetic acid in liquid phase and propene in gas phase. Materials 2019, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Pinzari, F.; Patrono, P.; Costantino, U. Methanol reforming reactions over ZnO/TiO2 catalysts. Catal. Commun. 2006, 7, 696–700. [Google Scholar] [CrossRef]
- Niu, B.; Wang, S.; Wu, K.; He, H.; Zhang, R. Mesoporous titanium dioxide: Synthesis and applications in photocatalysis, energy and biology. Materials 2018, 11, 1910. [Google Scholar] [CrossRef] [PubMed]
- Tadjarodi, A.; Sedghi, M.; Bijanzad, K. Synthesis and characterization of magnesium oxide mesoporous microstructures using Pluronic F127. J. Nanostruct. 2012, 2, 273–278. [Google Scholar]
- López, R.; Gómez, R.; Llanos, M.E. Photophysical and photocatalytic properties of nanosized copper-doped titania sol-gel catalysts. Catal. Today 2009, 148, 103–108. [Google Scholar] [CrossRef]
- Pérez-Larios, A.; López, R.; Hernández-Gordillo, A.; Tzompantzi, F.; Gómez, R.; Torres-Guerra, L.M. Improved hydrogen production from water splitting using TiO2–ZnO mixed oxides photocatalysts. Fuel 2012, 100, 139–143. [Google Scholar] [CrossRef]
- Das, P.S.; Dey, A.; Mandal, A.K.; Dey, N.; Mukhopadhyay, A.K. Synthesis of Mg(OH)2 micro/nano flowers at room temperature. J. Adv. Ceram. 2013, 2, 173–179. [Google Scholar] [CrossRef]
- López, R.; Gómez, R.; Oros-Cruz, S. Photophysical and photocatalytic properties of TiO2-Cr sol-gel prepared semiconductors. Catal. Today 2012, 166, 159–165. [Google Scholar] [CrossRef]
- Viswanatha, R.; Venkatesh, T.G.; Vidyasagar, C.C.; Nayaka, A. Preparation and characterization of ZnO and Mg-ZnO nanoparticle. Arch. Appl. Sci. Res. 2012, 4, 480–486. [Google Scholar]
- McDevitt, N.T.; Baun, W.L. Infrared absorption study of metal oxides in the low frequency region (700–240 cm−1). Spectrochim. Acta 1964, 20, 799–808. [Google Scholar] [CrossRef]
- Lun, K.; Kin, W.; Yao, N.; Cao, S. Reactivity and antimicrobial properties of nanostructured titanium dioxide. Catal. Today 2009, 143, 218–224. [Google Scholar]
- Simonsen, M.E.; Sogaard, E.G. Sol-gel reactions of titanium alkoxides and wáter: Influence of pH and alkoxy group on cluster formation and properties of the resulting products. J. Sol-Gel Sci. Technol. 2010, 53, 485–497. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habid, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef] [PubMed]
- Salih, F. Enhancement of solar inactivation of Escherichia coli by titanium dioxide photocatalytic oxidation. J. Appl. Microbiol. 2002, 92, 920–926. [Google Scholar] [CrossRef] [PubMed]
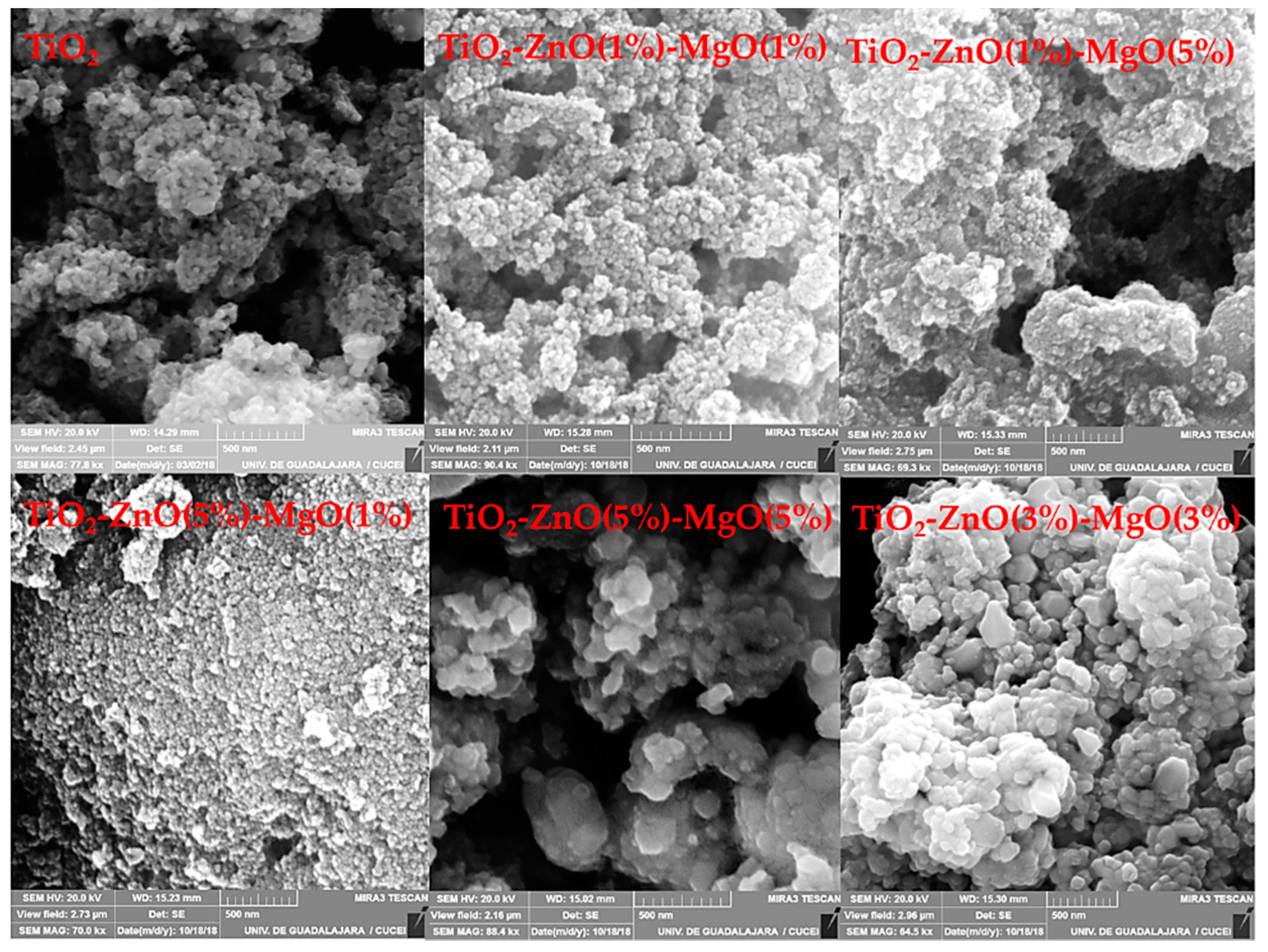
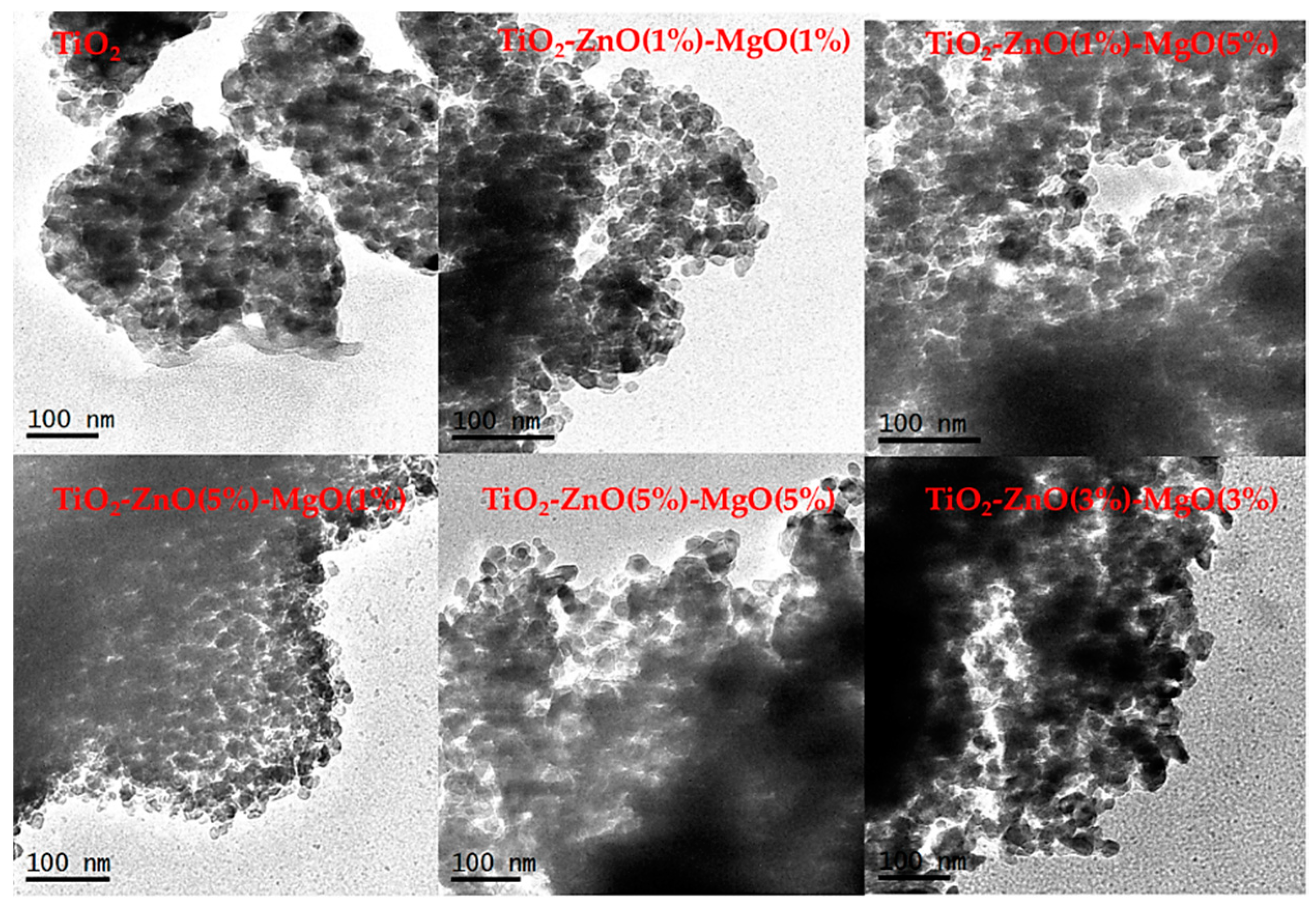
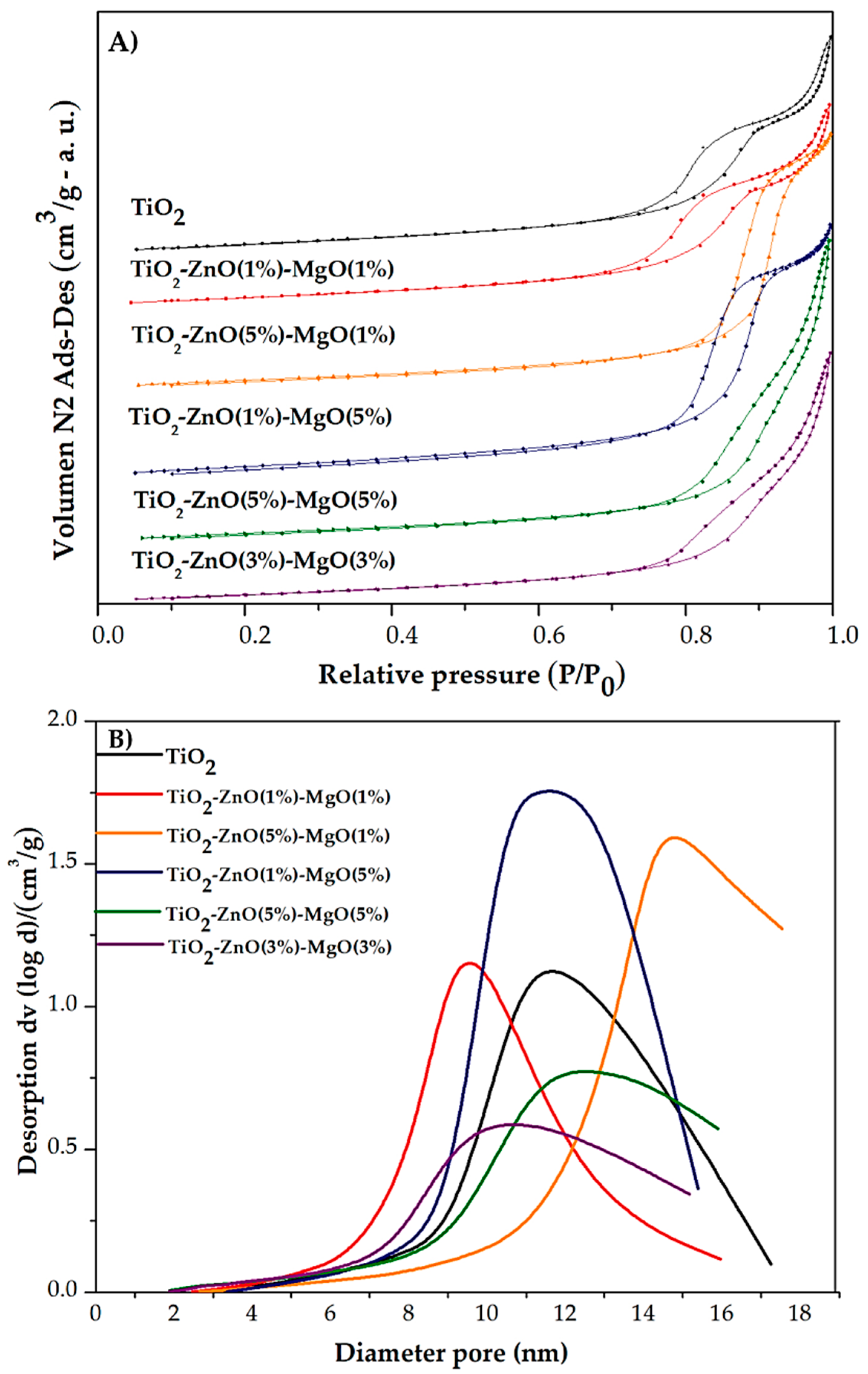
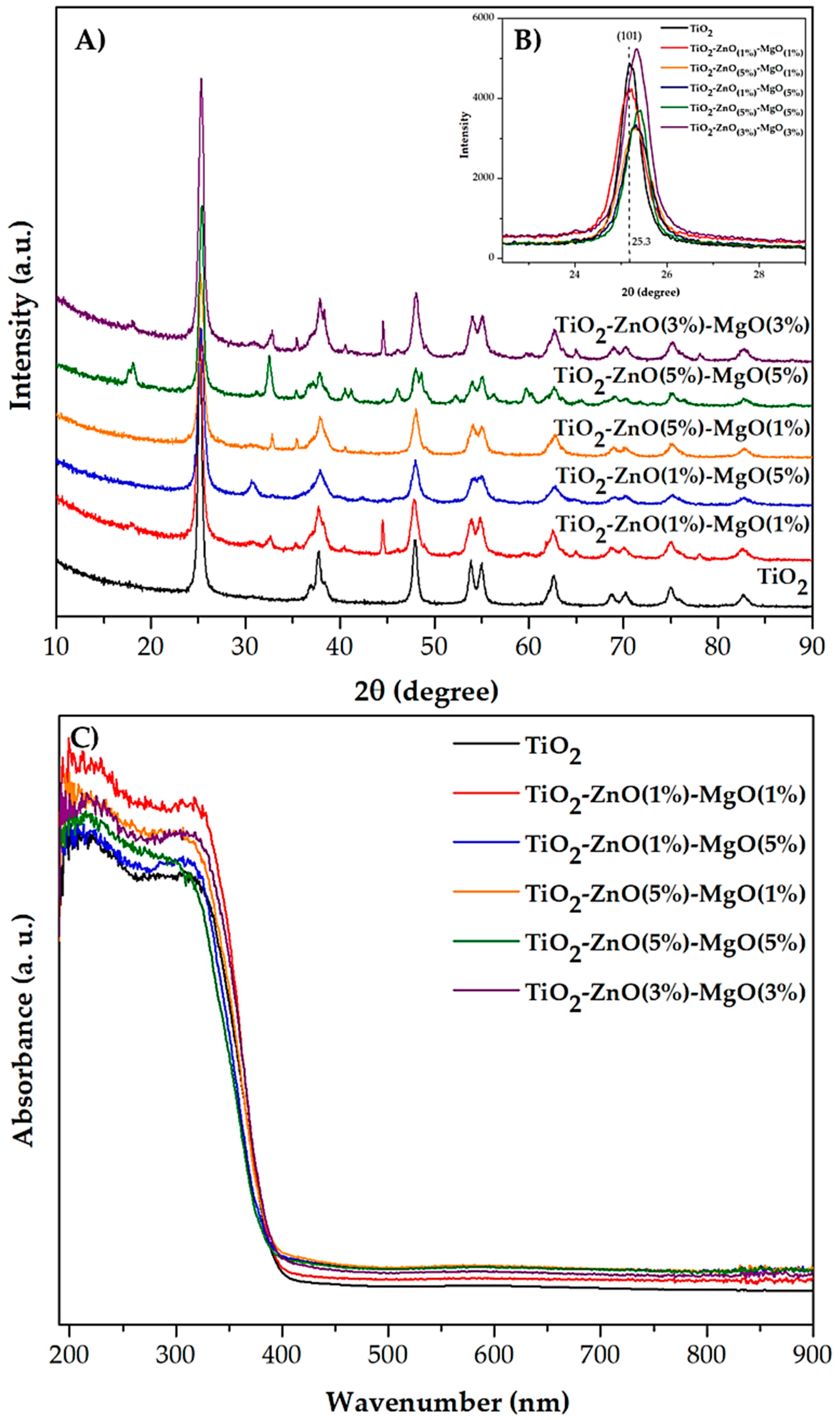

| Sample | Code | Element | |||
|---|---|---|---|---|---|
| Ti | Zn | Mg | O | ||
| Undoped TiO2 | - | 44.90 | 55.10 | ||
| TiO2-ZnO(1 wt.%)-MgO(1 wt.%) | T1 | 55.91 | 0.95 | 0.75 | 42.39 |
| TiO2-ZnO(5 wt.%)-MgO(1 wt.%) | T2 | 59.38 | 5.02 | 0.90 | 34.70 |
| TiO2-ZnO(1 wt.%)-MgO(5 wt.%) | T3 | 51.31 | 0.85 | 4.75 | 43.09 |
| TiO2-ZnO(5 wt.%)-MgO(5 wt.%) | T4 | 68.72 | 5.70 | 5.29 | 20.28 |
| TiO2-ZnO(3 wt.%)-MgO(3 wt.%) | T5 | 45.21 | 3.25 | 3.10 | 48.44 |
| Treatment | Code | Eg (eV) | SSA (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|---|---|
| Undoped TiO2 | - | 3.13 | 61.53 | 0.20 | 9.97 |
| TiO2-ZnO(1 wt.%)-MgO(1 wt.%) | T1 | 3.12 | 58.90 | 0.20 | 9.72 |
| TiO2-ZnO(5 wt.%)-MgO(1 wt.%) | T2 | 3.14 | 57.52 | 0.27 | 14.78 |
| TiO2-ZnO(1 wt.%)-MgO(5 wt.%) | T3 | 3.13 | 71.36 | 0.31 | 12.14 |
| TiO2-ZnO(5 wt.%)-MgO(5 wt.%) | T4 | 3.14 | 54.85 | 0.21 | 11.82 |
| TiO2-ZnO(3 wt.%)-MgO(3 wt.%) | T5 | 3.09 | 59.14 | 0.18 | 10.78 |
| Treatment | Code | Luminosity | a | b | Chrome | hue | TCD |
|---|---|---|---|---|---|---|---|
| Undoped TiO2 | - | 95.10 ± 0.04 b | −0.41 ± 0.01 b,c | 1.24 ± 0.03 b | 1.30 ± 0.02 a,b | 108.53 ± 0.40 c,d | - |
| TiO2-ZnO(1 wt.%)-MgO(1 wt.%) | T1 | 94.63 ± 0.05 b | −0.31 ± 0.02 a,b | 1.12 ± 0.06 c | 1.15 ± 0.05 b | 105.30 ± 1.57 d | 0.49 |
| TiO2-ZnO(5 wt.%)-MgO(1 wt.%) | T2 | 96.73 ± 0.14 a | −0.77 ± 0.04 c | 1.60 ± 0.03 a | 1.56 ± 0.33 a | 114.93 ± 0.99 c | 1.70 |
| TiO2-ZnO(1 wt.%)-MgO(5 wt.%) | T3 | 86.21 ± 1.18 c | −0.11 ± 0.02 a | 0.17 ± 0.06 e | 0.17 ± 0.02 d | 125.86 ± 1.56 b | 8.95 |
| TiO2-ZnO(5 wt.%)-MgO(5 wt.%) | T4 | 85.84 ± 0.68 c | −-0.40 ± 0.03 b | 0.48 ± 0.01 d | 0.59 ± 0.06 c | 134.00 ± 1.52 a | 9.29 |
| TiO2-ZnO(3 wt.%)-MgO(3 wt.%) | T5 | 97.34 ± 0.09 a | −0.62 ± 0.01 c | 1.37 ± 0.02 b | 1.52 ± 0.01 a,b | 115.90 ± 1.43 c | 2.25 |
| Treatment | Code | E. coli (mm) | S. paratyphi (mm) | S. aureus (mm) | L. monocytogenes (mm) |
|---|---|---|---|---|---|
| Ampicillin (C+) | - | 22.33 ± 0.51 a | 25.66 ± 1.52 a | 18.33 ± 0.57 a | 17.33 ± 1.15 a |
| Distilled water (C-) | - | 0 | 0 | 0 | 0 |
| Undoped TiO2 | - | 9.33 ± 0.57 c | 9.33 ± 0.57 c | 8.00 ± 1.00 e | 5.66 ± 0.57 d |
| TiO2-ZnO(1 wt.%)-MgO(1 wt.%) | T1 | 14.05 ± 0.42 b | 16.00 ± 1.24 b | 12.33 ± 0.87 d | 8.83 ± 0.75 b,c |
| TiO2-ZnO(5 wt.%)-MgO(1 wt.%) | T2 | 14.00 ± 0.89 b | 17.00 ± 1.26 b | 12.00 ± 0.94 d | 9.58 ± 0.66 b,c |
| TiO2-ZnO(1 wt.%)-MgO(5 wt.%) | T3 | 14.91 ± 0.66 b | 18.33 ± 0.63 b | 15.16 ± 0.30 b,c | 10.00 ± 0.89 b,c |
| TiO2-ZnO(5 wt.%)-MgO(5 wt.%) | T4 | 14.66 ± 0.51 b | 17.50 ± 1.04 b | 14.66 ± 0.81 b | 8.66 ± 0.81 b |
| TiO2-ZnO(3 wt.%)-MgO(3 wt.%) | T5 | 14.76 ± 0.35 b | 17.76 ± 0.30 b | 13.83 ± 0.75 c | 8.33 ± 0.51 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anaya-Esparza, L.M.; Montalvo-González, E.; González-Silva, N.; Méndez-Robles, M.D.; Romero-Toledo, R.; Yahia, E.M.; Pérez-Larios, A. Synthesis and Characterization of TiO2-ZnO-MgO Mixed Oxide and Their Antibacterial Activity. Materials 2019, 12, 698. https://doi.org/10.3390/ma12050698
Anaya-Esparza LM, Montalvo-González E, González-Silva N, Méndez-Robles MD, Romero-Toledo R, Yahia EM, Pérez-Larios A. Synthesis and Characterization of TiO2-ZnO-MgO Mixed Oxide and Their Antibacterial Activity. Materials. 2019; 12(5):698. https://doi.org/10.3390/ma12050698
Chicago/Turabian StyleAnaya-Esparza, Luis M., Efigenia Montalvo-González, Napoleón González-Silva, María D. Méndez-Robles, Rafael Romero-Toledo, Elhadi M. Yahia, and Alejandro Pérez-Larios. 2019. "Synthesis and Characterization of TiO2-ZnO-MgO Mixed Oxide and Their Antibacterial Activity" Materials 12, no. 5: 698. https://doi.org/10.3390/ma12050698
APA StyleAnaya-Esparza, L. M., Montalvo-González, E., González-Silva, N., Méndez-Robles, M. D., Romero-Toledo, R., Yahia, E. M., & Pérez-Larios, A. (2019). Synthesis and Characterization of TiO2-ZnO-MgO Mixed Oxide and Their Antibacterial Activity. Materials, 12(5), 698. https://doi.org/10.3390/ma12050698








