Undoped and Eu3+ Doped Magnesium-Aluminium Layered Double Hydroxides: Peculiarities of Intercalation of Organic Anions and Investigation of Luminescence Properties
Abstract
1. Introduction
2. Experimental
2.1. Synthesis of LDHs
2.2. Intercalation of Mg3/Al and Mg3/Al0.99Eu0.01 LDHs with Organic Anions
2.3. Characterization
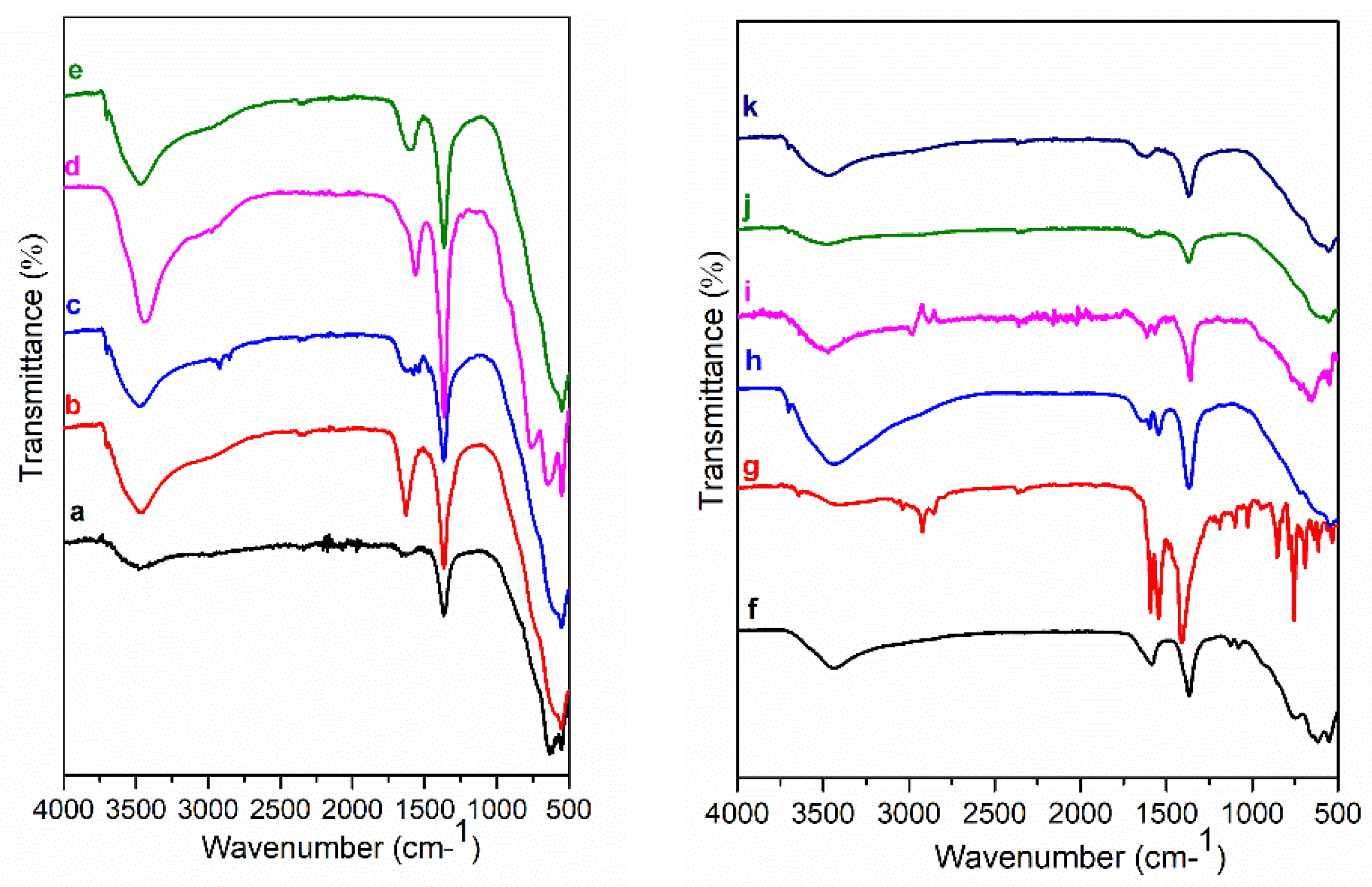
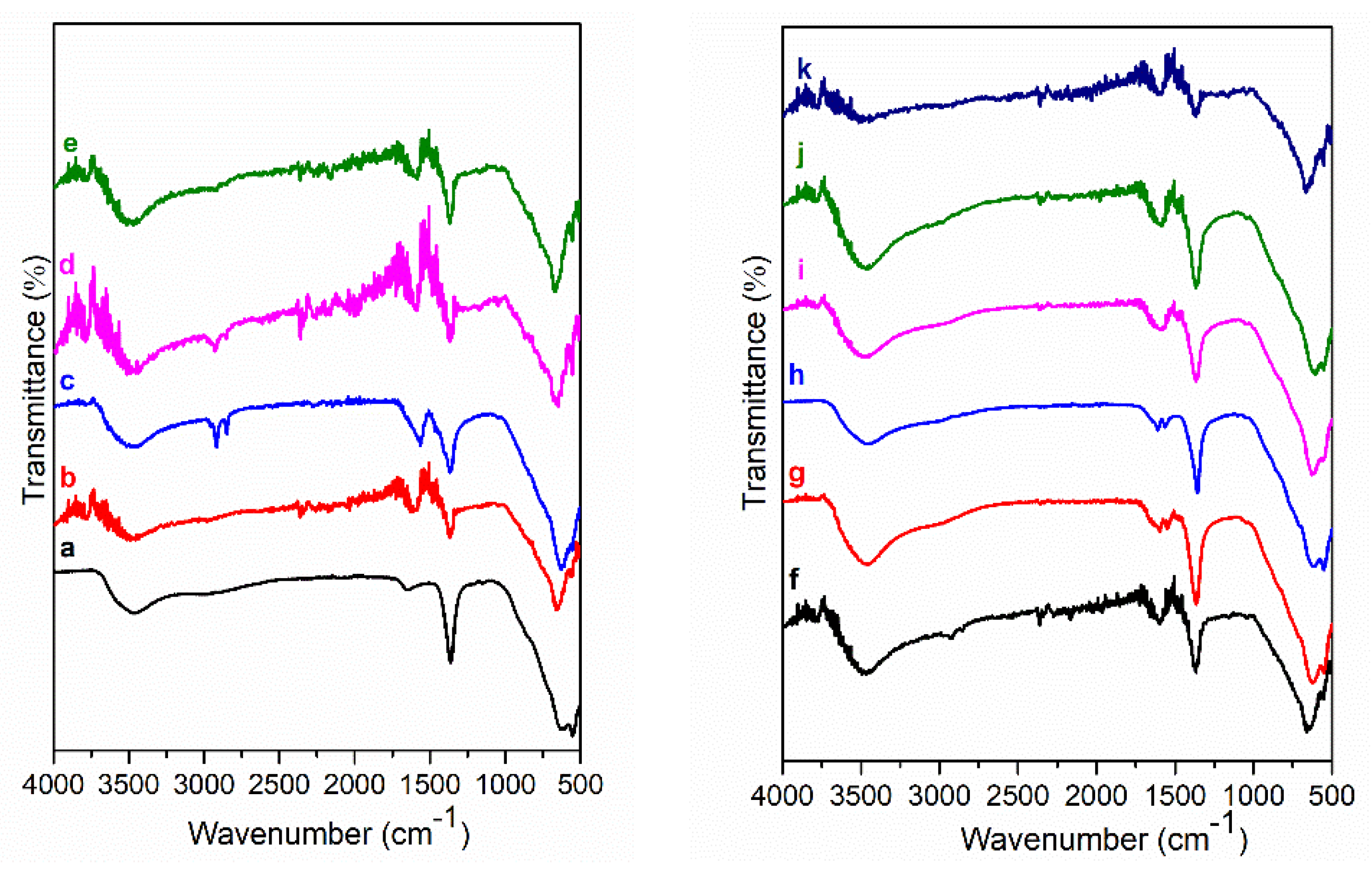
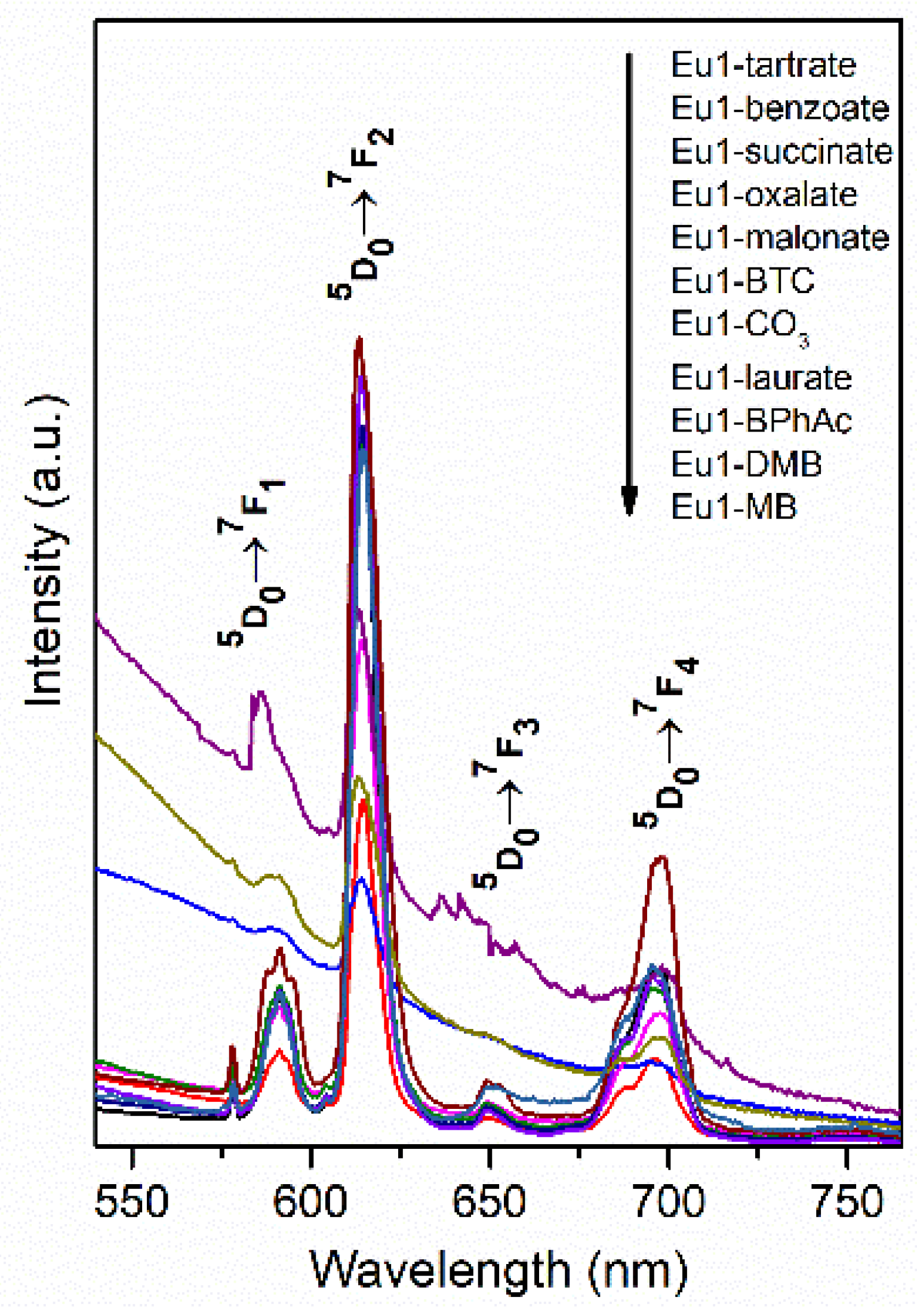
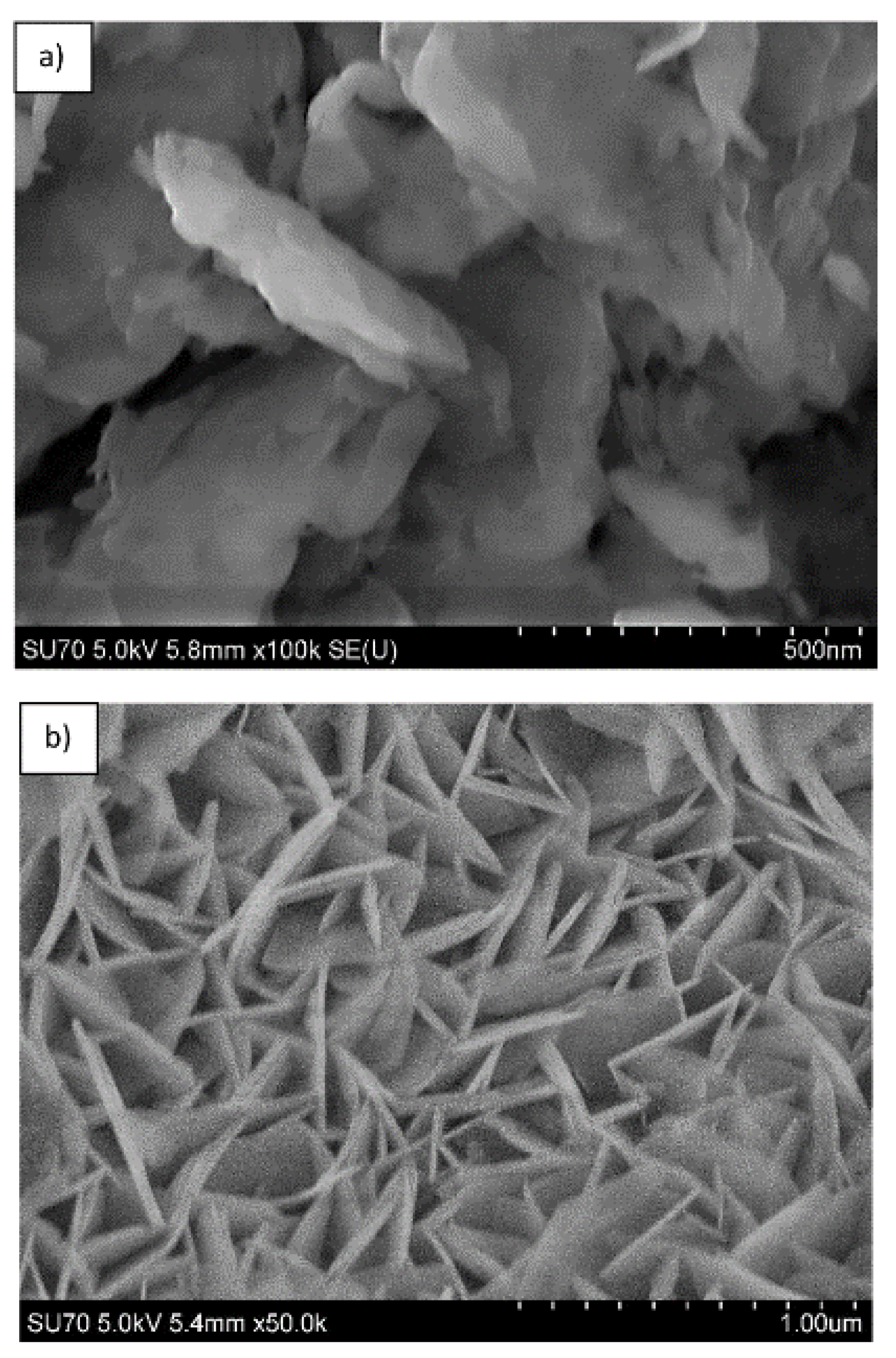
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Evans, D.E.; Slade, R.C.T. Layered Double Hydroxides; Springer-Verlag: Berlin, Germany, 2005; Volume 119, pp. 1–87. [Google Scholar]
- Khan, A.I.; O’Hare, D. Intercalation chemistry of layered double hydroxides: recent developments and applications. J. Mater. Chem. 2002, 12, 3191–3198. [Google Scholar] [CrossRef]
- Miyata, S. Anion-exchange properties of hydrotalcite-like compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Costa, D.G.; Rocha, A.B.; Souza, W.F.; Chiaro, S.S.X.; Leitao, A.A. Structural model proposition and thermodynamic and vibrational analysis of hydrotalcite-like compounds by DFT calculations. J. Phys. Chem. 2010, 114, 14133–14140. [Google Scholar] [CrossRef]
- Kim, N.; Harale, A.; Tsotsis, T.T.; Sahimi, M. Atomistic simulation of nanoporous layered double hydroxide materials and their properties. II. Adsorption and diffusion. J. Chem. Phys. 2007, 127, 224701. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kirkpatrick, G.K.R.J.; Hou, X. Interlayer Structure and Dynamics of Cl−−LiAl2-Layered Double Hydroxide: 35Cl NMR Observations and Molecular Dynamics Modeling. Chem. Mater. 2002, 14, 2078–2085. [Google Scholar]
- Pavlovic, M.; Adok-Sipiczki, M.; Nardin, C.; Pearson, S.; Bourgeat-Lami, E.; Prevot, V.; Szilagyi, I. Effect of MacroRAFT Copolymer Adsorption on the Colloidal Stability of Layered Double Hydroxide Nanoparticles. Langmuir 2015, 31, 12609–12617. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.P.; Jin, Y.; Li, S.; Hao, Z.; Lu, G.Q. Surface charging of layered double hydroxides during dynamic interactions of anions at the interfaces. J. Colloid Interface Sci. 2008, 326, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, M.; Huber, R.; Adok-Sipiczki, M.; Nardina, C.; Szilagyi, I. Ion specific effects on the stability of layered double hydroxide colloids. Soft Matter 2016, 12, 4024–4033. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, M.; Rouster, P.; Oncsik, T.; Szilagyi, I. Tuning Colloidal Stability of Layered Doubl Hydroxides: from Monovalent Ions to Polyelectrolytes. ChemPlusChem 2017, 82, 121–131. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Q.; Gao, C.; Chen, H.; Liu, W.; Tang, Y. Dramatically Enhanced Luminescence of Layered Terbium Hydroxides as Induced by the Synergistic Effect of Gd3+ and Organic Sensitizers. J. Phys. Chem. C 2014, 118, 14511–14520. [Google Scholar] [CrossRef]
- Kovar, P.; Pospisil, M.; Nocchetti, M.; Capkova, P.; Melanova, K. Molecular modeling of layered double hydroxide intercalated with benzoate, modeling and experiment. J. Mol. Model. 2007, 13, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Posati, T.; Costantino, F.; Latterini, L.; Nocchetti, M.; Paolantoni, M.; Tarpani, L. New insights on the incorporation of lanthanide ions into nanosized layered double hydroxides. Inorg. Chem. 2012, 51, 13229. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, J.G.; Fang, F.; Chu, N.; Ma, H.; Yang, X. Structure and luminescence behaviour of as-synthesized, calcined, and restored MgAlEu-LDH with high crystallinity. Dalton Trans. 2012, 41, 12175–12184. [Google Scholar] [CrossRef] [PubMed]
- Salak, A.N.; Tedim, J.; Kuznetsova, A.I.; Ribeiro, J.L.; Vieira, L.G.; Zheludkevich, M.L.; Ferreira, M.G.S. Comparative X-ray diffraction and infrared spectroscopy study of Zn-Al layered double hydroxides: Vanadate vs nitrate. Chem. Phys. 2012, 397, 102–108. [Google Scholar] [CrossRef]
- Constantino, U.; Constantino, F.; Elisei, F.; Latterini, I.; Nocchetti, M. Coupling physical chemical techniques with hydrotalcite-like compounds to exploit their structural features and new multifunctional hybrids with luminescent properties. Phys. Chem. Chem. Phys. 2013, 32, 13254–13269. [Google Scholar] [CrossRef] [PubMed]
- Smalenskaite, A.; Salak, A.N.; Ferreira, M.G.S.; Skaudzius, R.; Kareiva, A. Sol-gel synthesis and characterization of hybrid inorganic-organic Tb(III)-terephthalate containing layered double hydroxides. Opt. Mater. 2018, 80, 186–196. [Google Scholar]
- Tsaryuk, V.I.; Zhuravlev, K.P.; Zolin, V.F.; Kudryashova, V.A.; Legendziewicz, J.; Szostak, R. Luminescence efficiency of aromatic carboxylates of europium and terbium when methylene bridges and nitro groups are present in the ligands. J. Appl. Spectrosc. 2007, 74, 51–59. [Google Scholar] [CrossRef]
- Hu, X.; Gao, X. Multilayer coating of gold nanorods for combined stability and biocompatibility. Phys. Chem. Chem. Phys. 2011, 13, 10028–10035. [Google Scholar]
- Li, C.; Wang, L.; Evans, D.G.; Duan, X. Thermal Evolution and Luminescence Properties of Zn-Al-Layered Double Hydroxides Containing Europium(III) Complexes of Ethylenediaminetetraacetate and Nitrilotriacetate. Ind. Chem. Res. 2009, 48, 2162–2171. [Google Scholar] [CrossRef]
- Sohn, Y. Structural and spectroscopic characteristics of terbium. hydroxide/oxide nanorods and plates. Ceram. Int. 2014, 40, 13803. [Google Scholar] [CrossRef]
- Jyothy, P.V.; Amrutha, K.A.; Gijo, J.; Unnikrishnan, N.V. Fluorescence enhancement in Tb3+/CdS nanoparticles doped silica xerogels. J. Fluoresc. 2009, 19, 165. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.; Lin, C.; Woo, S.H.; Chao, H.P. Efficient removal of copper and lead by Mg/Al layered double hydroxides intercalated with organic acid anions: adsorption kinetics, isotherms, and thermodynamics. Appl. Clay Sci. 2018, 154, 17–27. [Google Scholar] [CrossRef]
- Li, S.; Qin, H.; Zuo, R.; Bai, Z. Tribological performance of Mg/Al/Ce layered double hydroxides nanoparticles and intercalated products as lubricant additives. Appl. Surf. Sci. 2015, 353, 643–650. [Google Scholar] [CrossRef]
- Costa, D.G.; Rocha, A.B.; Souza, W.F.; Chiaro, S.S.; Leita, A.A. Comparative Structural, thermodynamic and electronic analyses of ZnAlAn− hydrotalcite-like compounds (An −Cl−, F−, Br−, OH−, CO32 − or NO3−): An ab initio study. Appl. Clay. Sci. 2012, 56, 16–22. [Google Scholar] [CrossRef]
- Serdechnova, M.; Salak, A.N.; Barbosa, F.S.; Vieira, D.E.L.; Tedim, J.; Zheludkevich, M.L.; Ferreira, M.G.S. Interlayer intercalation and arrangement of 2-mercaptobenzothiazolate and 1,2,3 benzotriazolate anions in layered double hydroxides: In situ X-ray diffraction study. J. Solid State Chem. 2016, 233, 158–165. [Google Scholar] [CrossRef]
- Smalenskaite, A.; Sen, S.; Salak, A.N.; Ferreira, M.G.S.; Skaudzius, R.; Katelnikovas, A.; Kareiva, A. Sol-gel Synthesis and Characterization of Non-Substituted and Europium-Substituted Layered Double Hydroxides Mg3/Al1-xEux. Curr. Inorg. Chem. 2016, 6, 149–154. [Google Scholar] [CrossRef]
- Gago, S.; Pillinger, M.; Ferreira, R.A.S.; Carlos, L.D.; Santos, T.M.; Goncalves, I.S. Immobilization of lanthanide ions in a pillared layered double hydroxide. Chem. Mater. 2005, 17, 5803–5809. [Google Scholar] [CrossRef]
- Yan, B.; Wang, W.-J.; Song, Y.-S. Photophysical properties of praseodymium complexes with aromatic carboxylic acids: double light conversion both in ultraviolet and visible region. Spectrochim. Acta Part A 2007, 66, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Bryce-Smith, D.; Gilbert, A.; Orger, B.; Twitchett, P. Photoaddition of ethylenes and acetylenes to hexafluorobenzene. Chem. Soc. 1978, 1, 241–245. [Google Scholar] [CrossRef]
- Hilder, M.; Junk, P.C.; Kynast, U.H.; Lezhnina, M.M. Spectroscopic properties of lanthanoid benzene carboxylates in the solid state: Part 1. J. Photochem. Photobiol. A 2009, 202, 10–20. [Google Scholar] [CrossRef]
- Xu, Z.P.; Braterman, P.S. Synthesis, structure and morphology of organic layered double hydroxide (LDH) hybrids: Comparison between aliphatic anions and their oxygenated analogs. Appl. Clay Sci. 2010, 48, 235–242. [Google Scholar] [CrossRef]
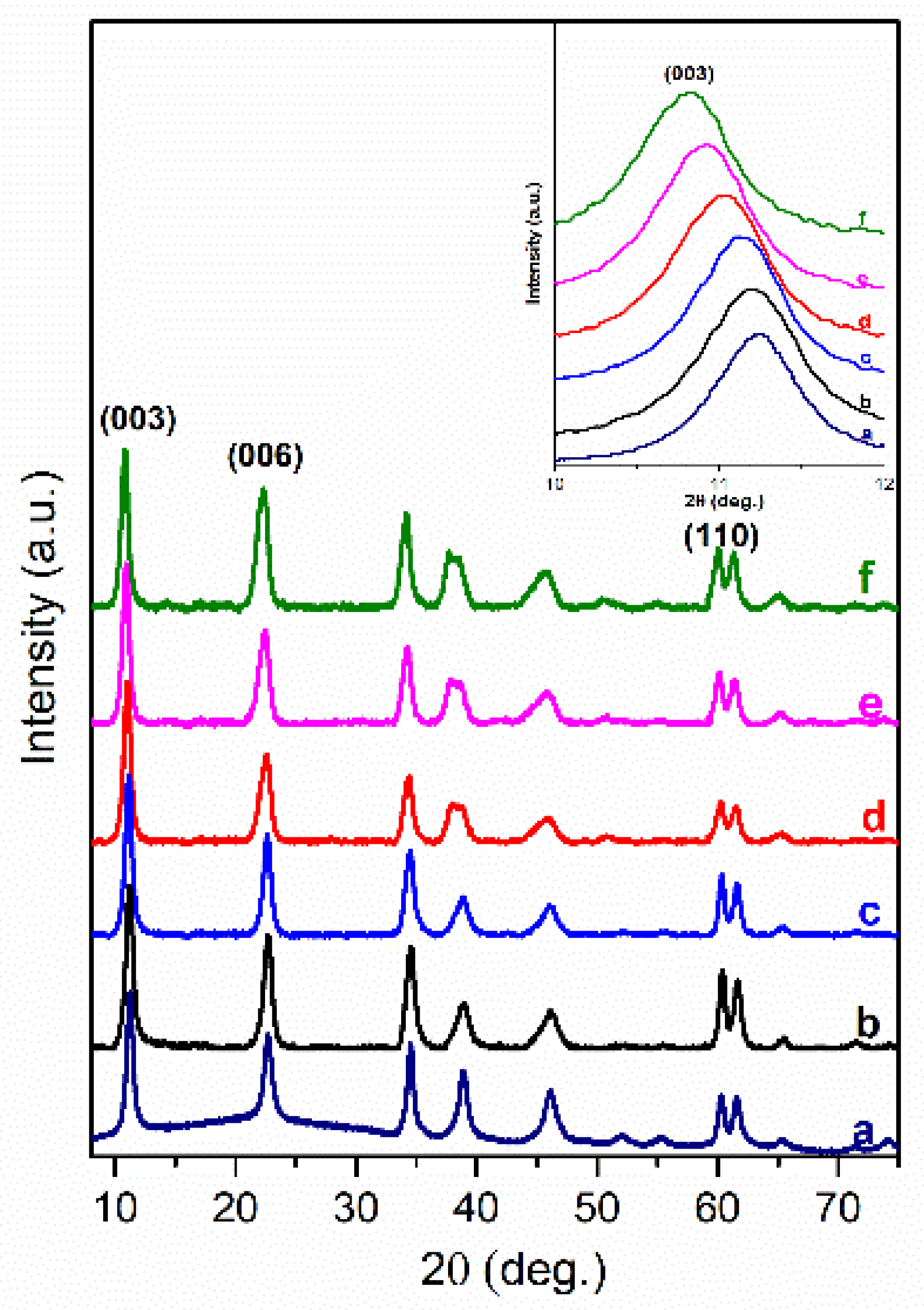
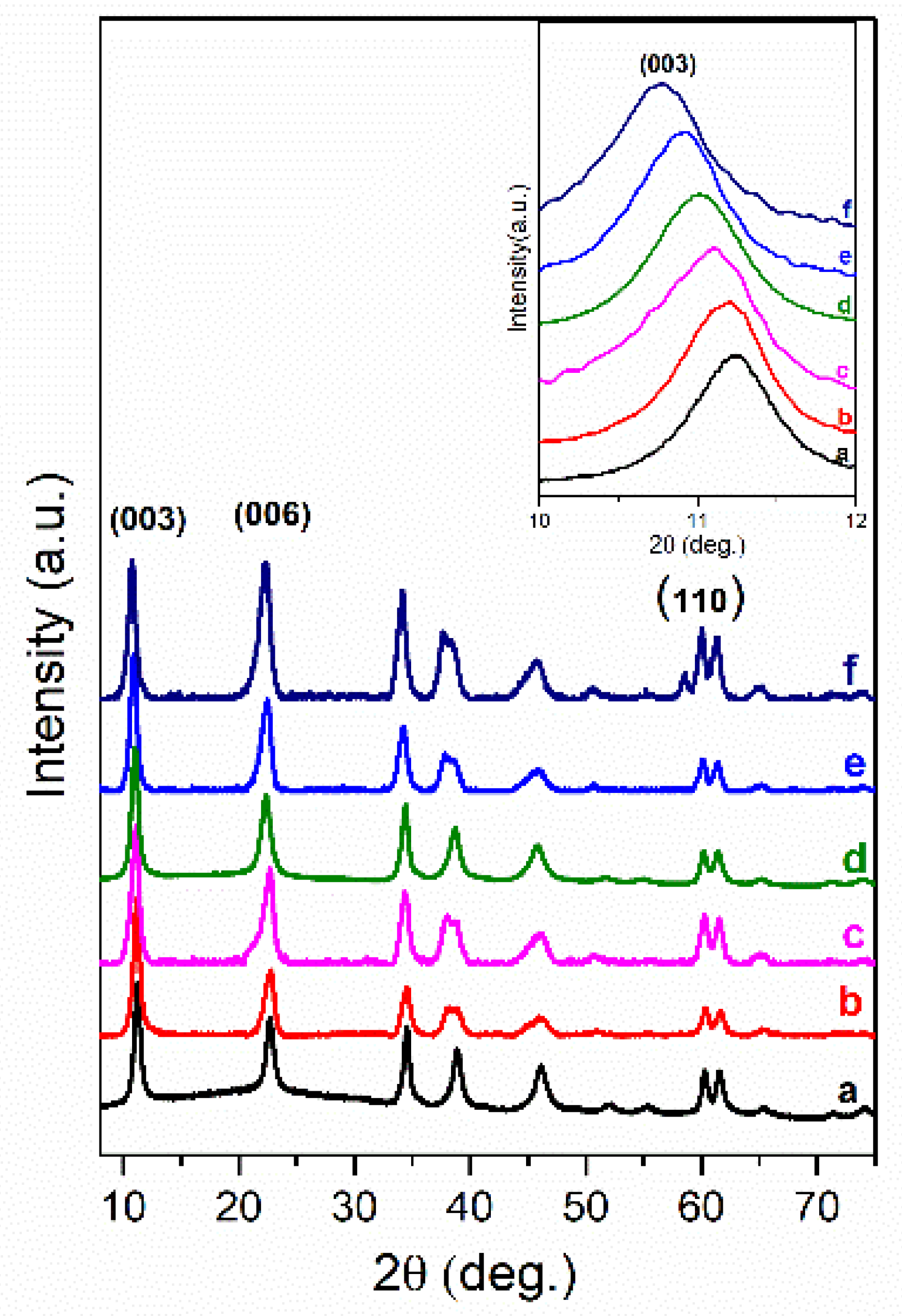
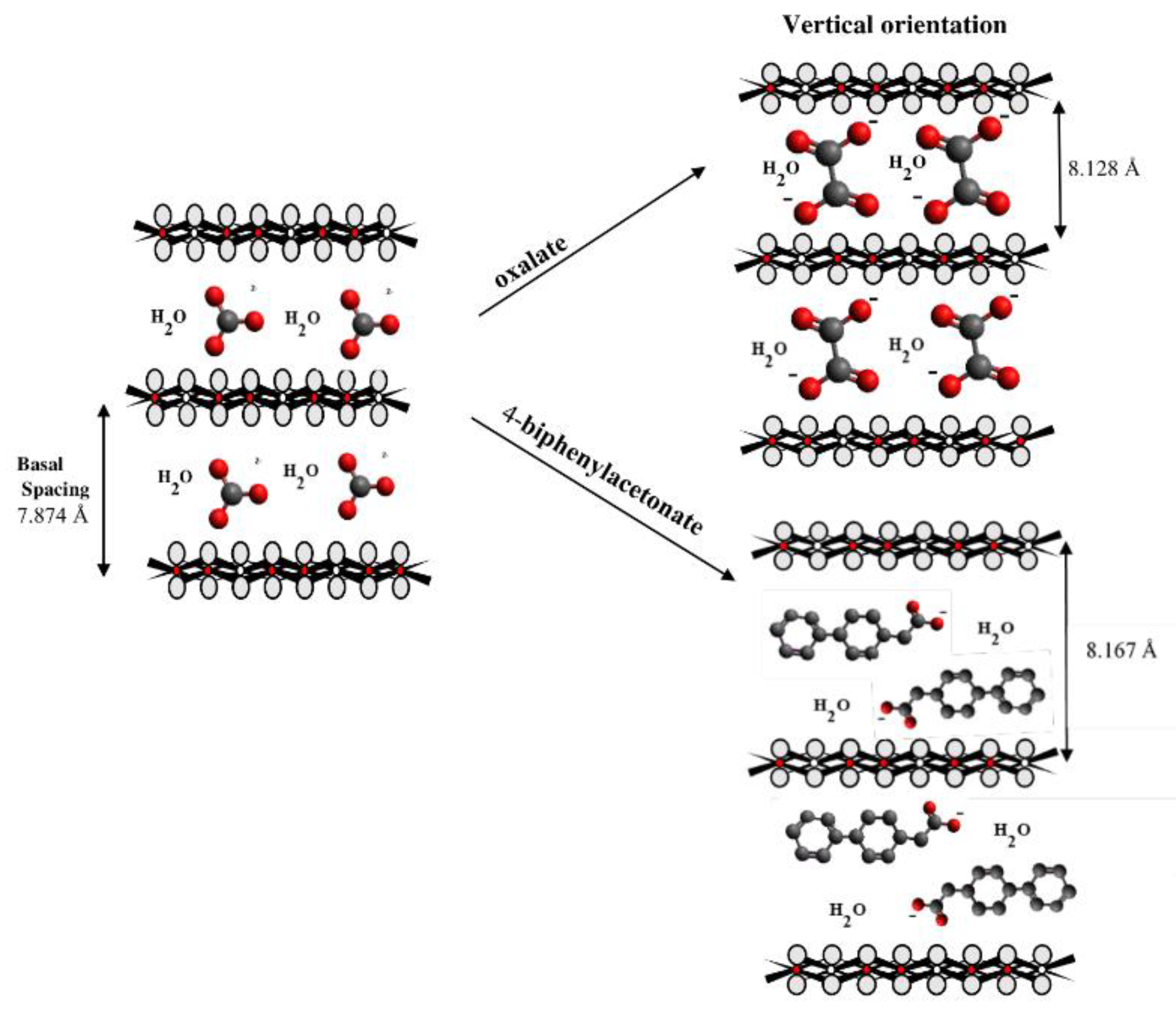
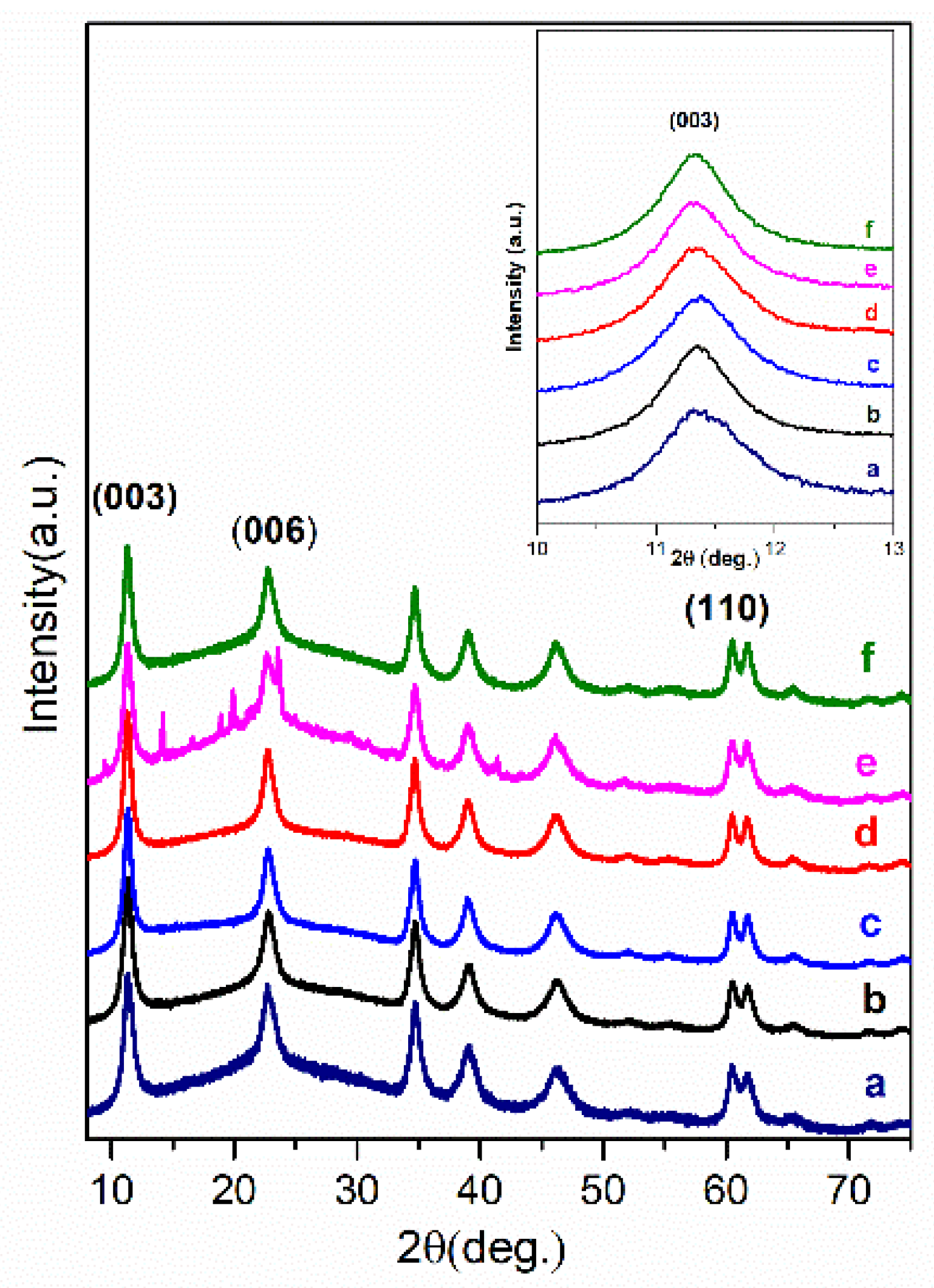

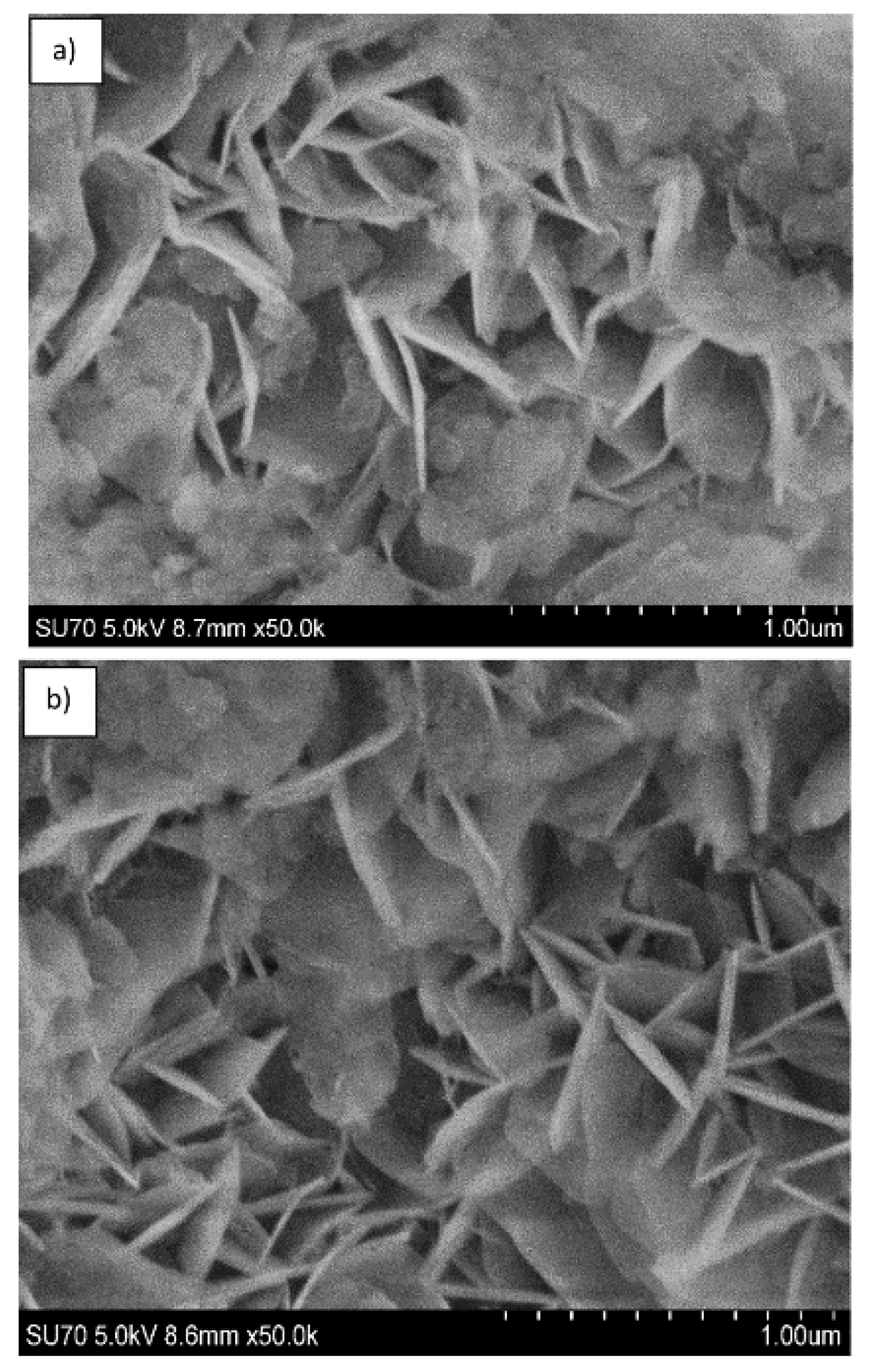
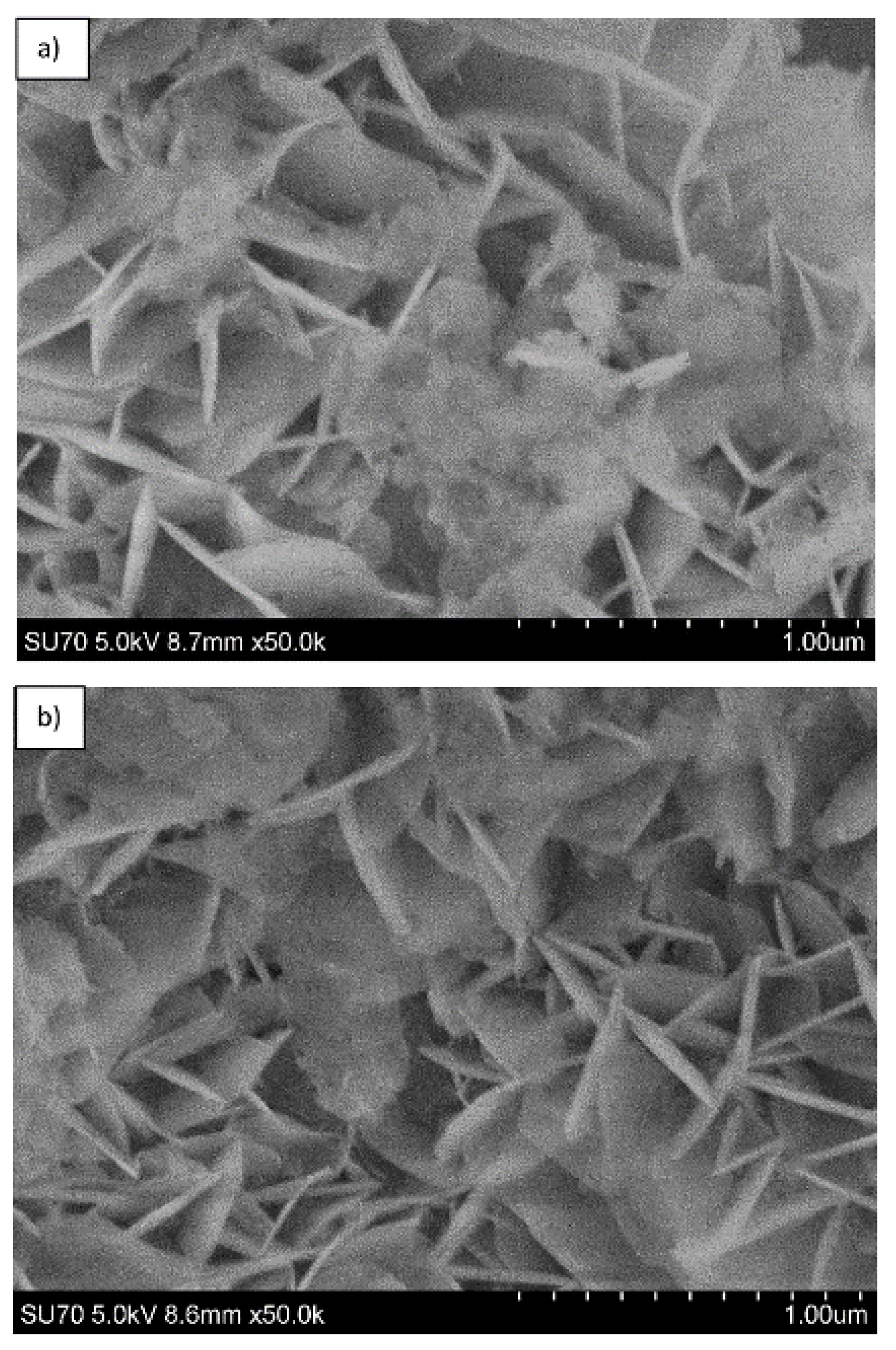
| Sample | Basal Spacing/Å | Cell Parameter/Å | ||
|---|---|---|---|---|
| d(003) | d(110) | a | c | |
| Mg3/Al-CO3 | 7.8744 | 1.5350 | 3.068 | 23.613 |
| Mg3/Al-oxalate | 8.1286 | 1.5385 | 3.076 | 24.375 |
| Mg3/Al-laurate | 8.0905 | 1.5380 | 3.075 | 24.261 |
| Mg3/Al-tartarate | 7.9970 | 1.5359 | 3.070 | 23.981 |
| Mg3/Al-malonate | 7.9568 | 1.5343 | 3.067 | 23.860 |
| Mg3/Al-succinate | 7.9454 | 1.5333 | 3.065 | 23.826 |
| Mg3/Al-4-biphenylacetonate | 8.1675 | 1.5396 | 3.078 | 24.492 |
| Mg3/Al-benzoate | 8.0875 | 1.5384 | 3.075 | 24.252 |
| Mg3/Al-4-methylbenzoate | 8.0564 | 1.5383 | 3.075 | 24.159 |
| Mg3/Al-1,3,5-benzentricarboxylate | 8.0328 | 1.5373 | 3.073 | 24.088 |
| Mg3/Al-4-dimethylaminobenzoate | 7.8907 | 1.5324 | 3.063 | 23.662 |
| Anion | Chemical Formula | Structural Formula and Dimensions |
|---|---|---|
| Oxalate | (C2H4)2− |  |
| Laurate | (C12H23O2)2− | 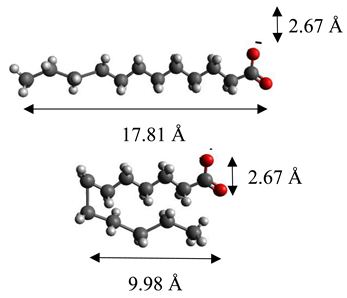 |
| Malonate | (C3H2O4)2− | 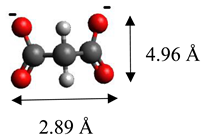 |
| Succinate | (C3H4O4)2− | 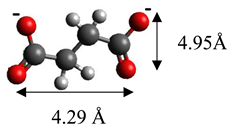 |
| Tartrate | (C4H4O6)2− | 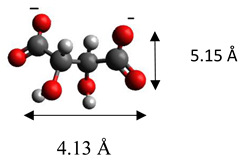 |
| Benzoate | (C7H5O2)− | 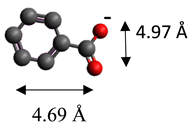 |
| 1,3,5-benzentricarboxylate | (C9H5O6)3− | 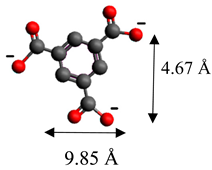 |
| 4-methylbenzoate | (C8H7O2)− | 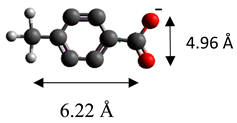 |
| 4-dimethylaminobenzoate | (C9H10O2)− | 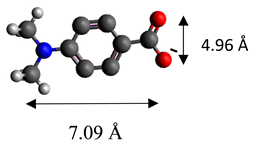 |
| 4-biphenylacetonate | (C14H11O2)− | 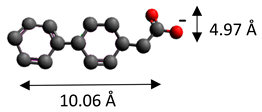 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smalenskaite, A.; Pavasaryte, L.; Yang, T.C.K.; Kareiva, A. Undoped and Eu3+ Doped Magnesium-Aluminium Layered Double Hydroxides: Peculiarities of Intercalation of Organic Anions and Investigation of Luminescence Properties. Materials 2019, 12, 736. https://doi.org/10.3390/ma12050736
Smalenskaite A, Pavasaryte L, Yang TCK, Kareiva A. Undoped and Eu3+ Doped Magnesium-Aluminium Layered Double Hydroxides: Peculiarities of Intercalation of Organic Anions and Investigation of Luminescence Properties. Materials. 2019; 12(5):736. https://doi.org/10.3390/ma12050736
Chicago/Turabian StyleSmalenskaite, Aurelija, Lina Pavasaryte, Thomas C. K. Yang, and Aivaras Kareiva. 2019. "Undoped and Eu3+ Doped Magnesium-Aluminium Layered Double Hydroxides: Peculiarities of Intercalation of Organic Anions and Investigation of Luminescence Properties" Materials 12, no. 5: 736. https://doi.org/10.3390/ma12050736
APA StyleSmalenskaite, A., Pavasaryte, L., Yang, T. C. K., & Kareiva, A. (2019). Undoped and Eu3+ Doped Magnesium-Aluminium Layered Double Hydroxides: Peculiarities of Intercalation of Organic Anions and Investigation of Luminescence Properties. Materials, 12(5), 736. https://doi.org/10.3390/ma12050736






