Quantification of the Photocatalytic Self-Cleaning Ability of Non-Transparent Materials
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
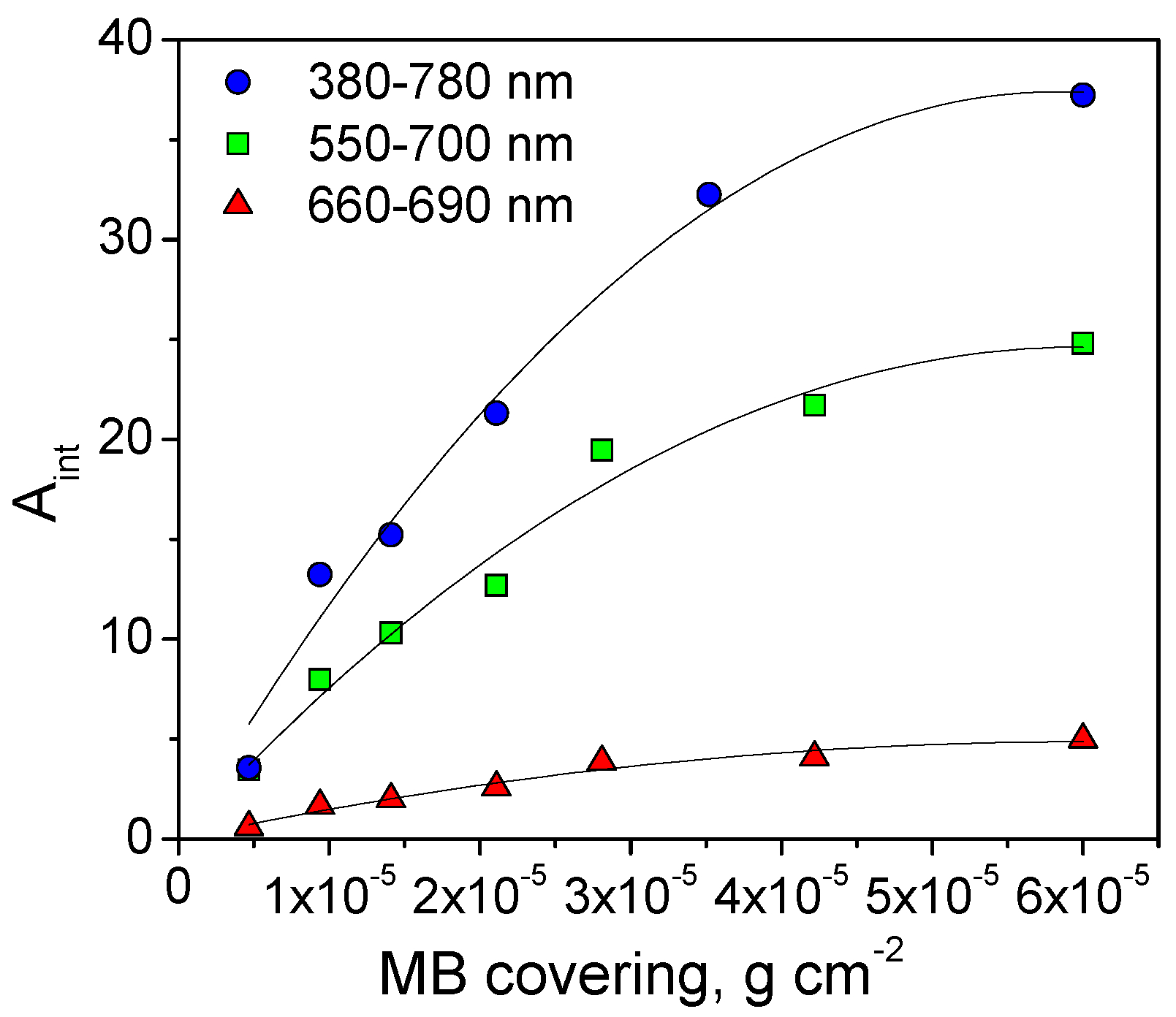
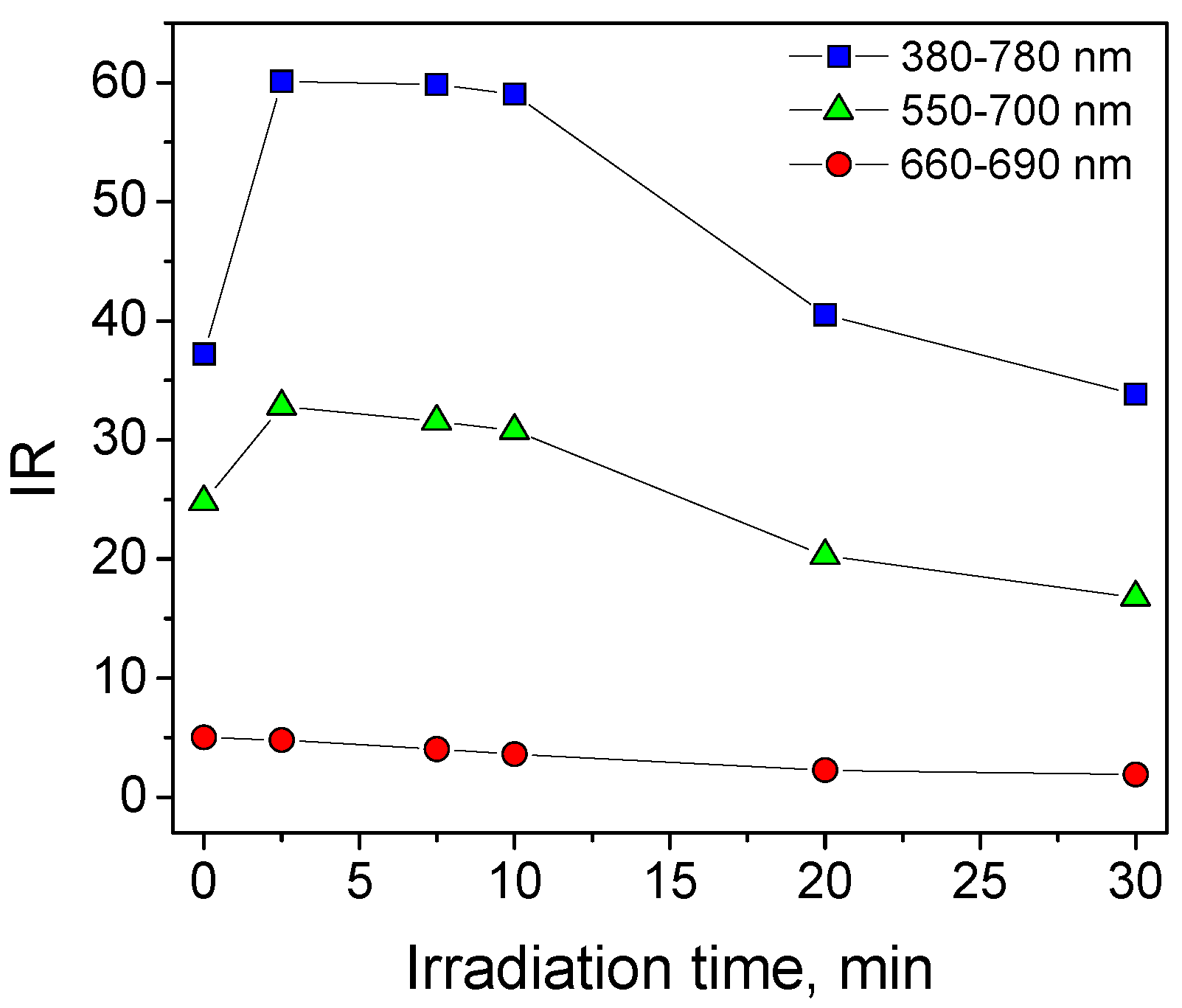
3.1. Methylene Blue Self-Cleaning Test
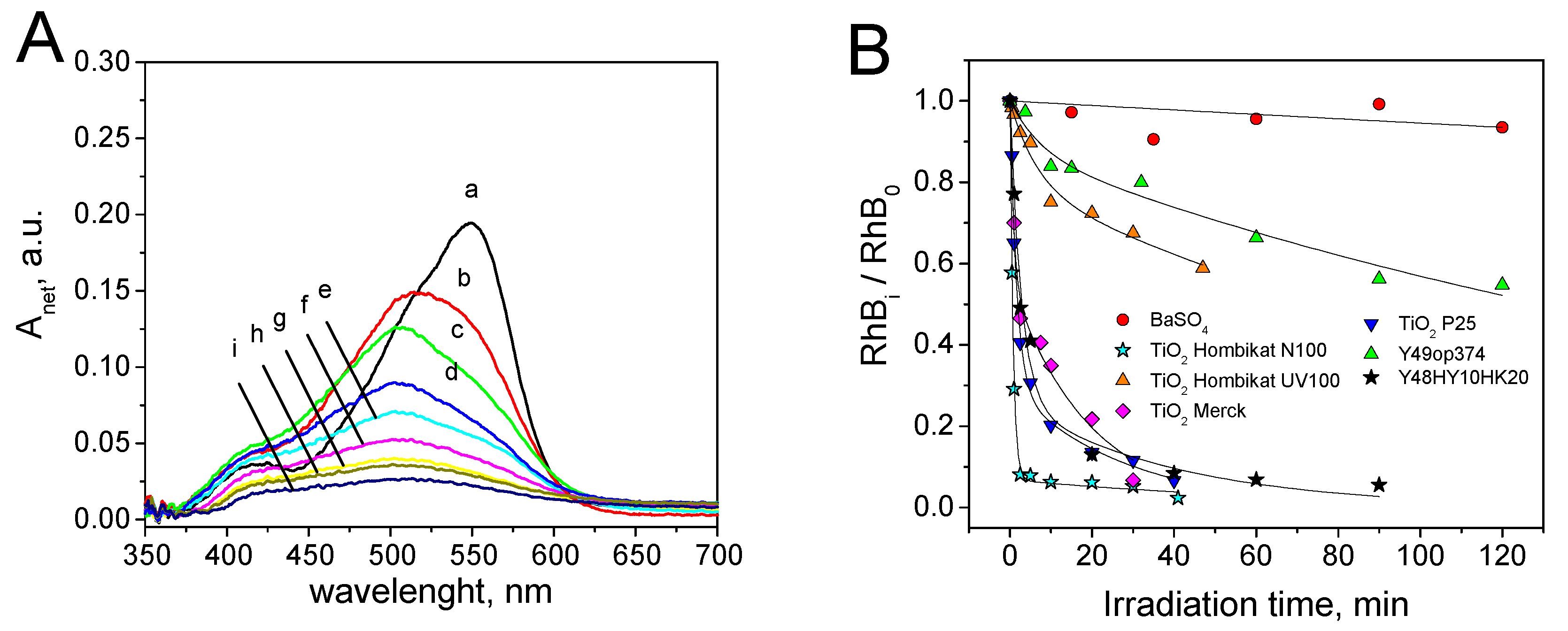
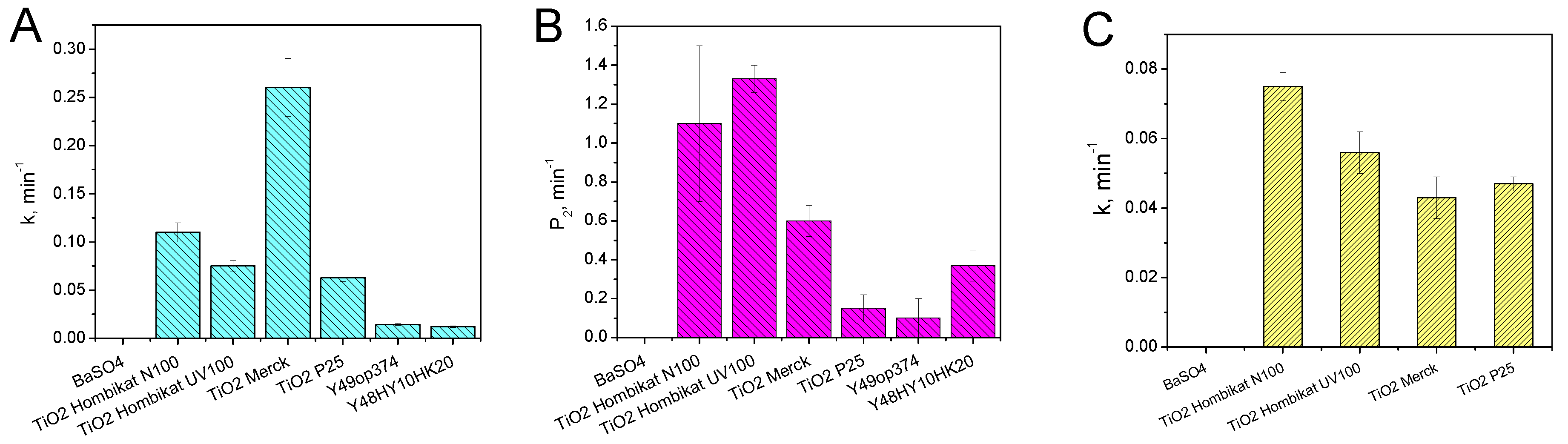
3.2. Rhodamine B Self-Cleaning Test
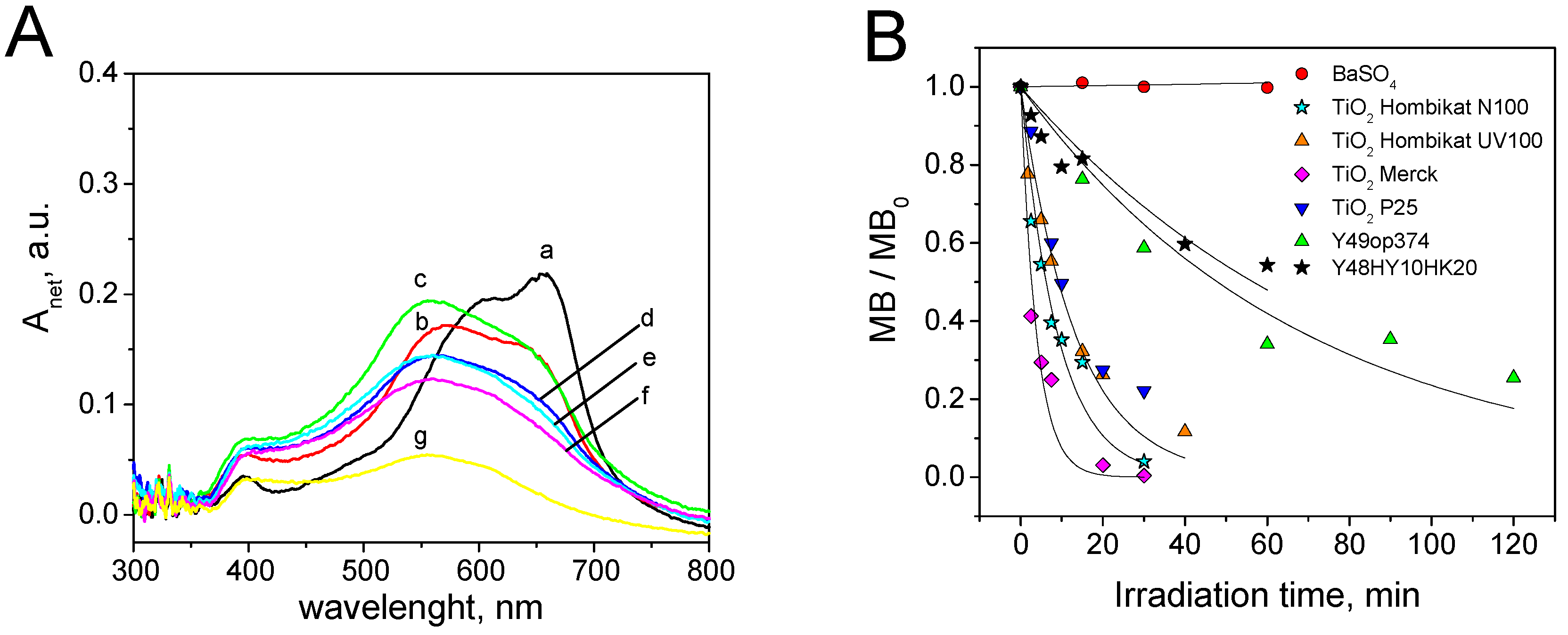
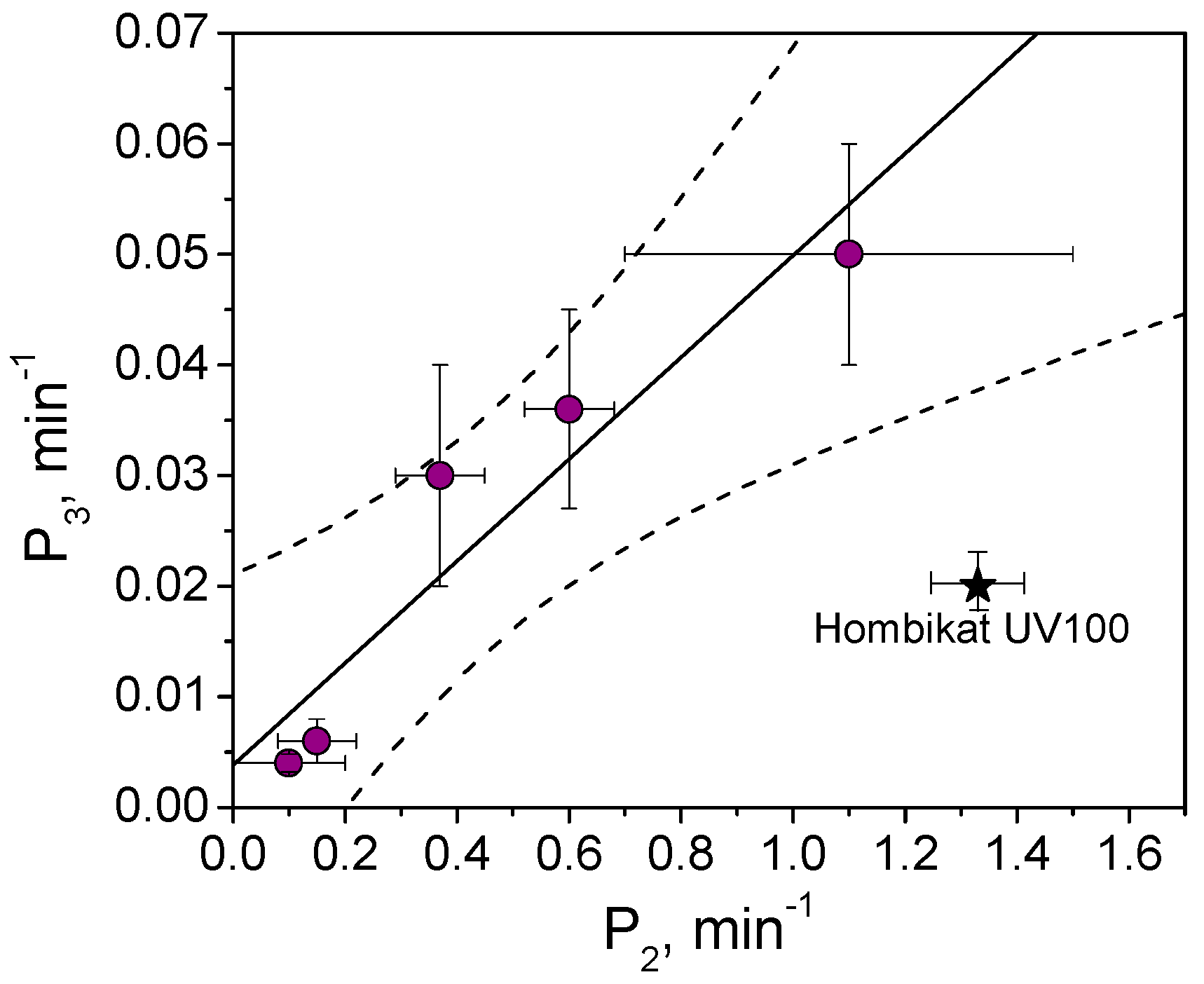
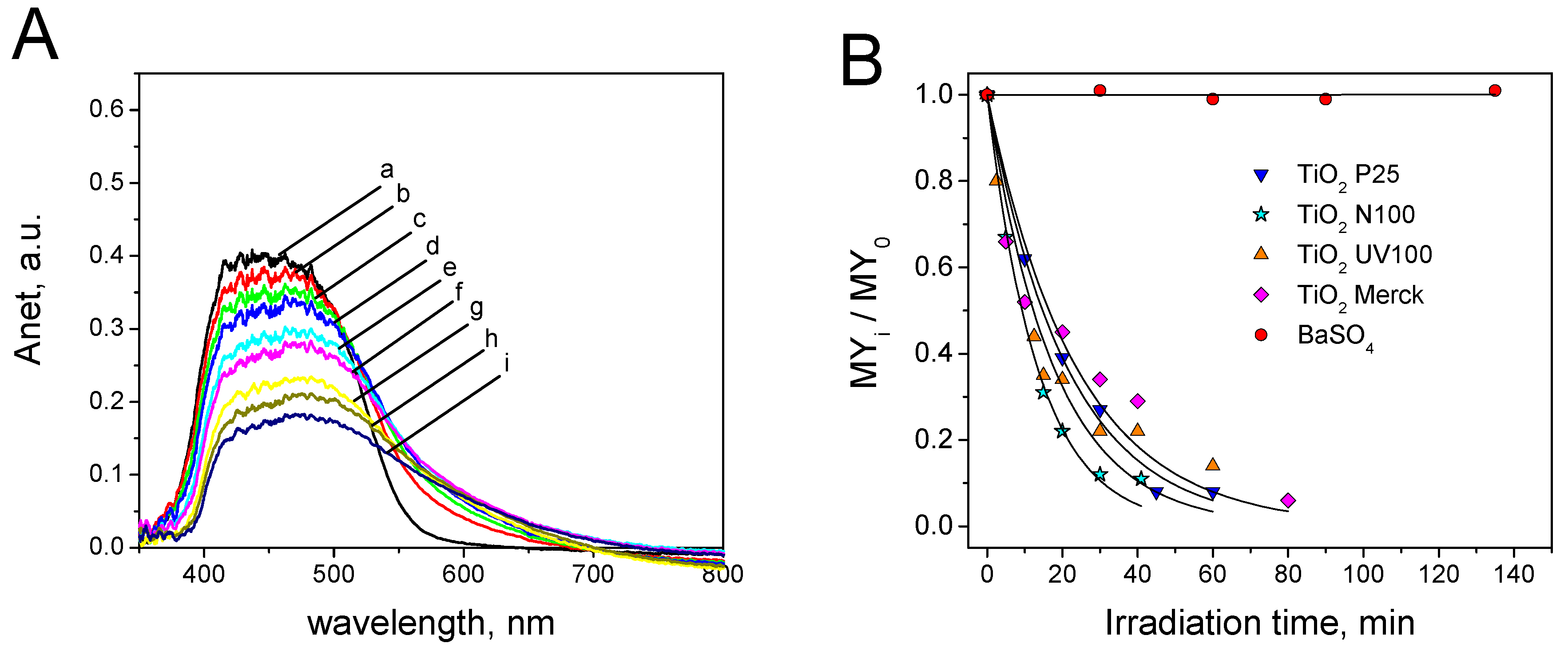
3.3. Metanil Yellow Self-Cleaning Test
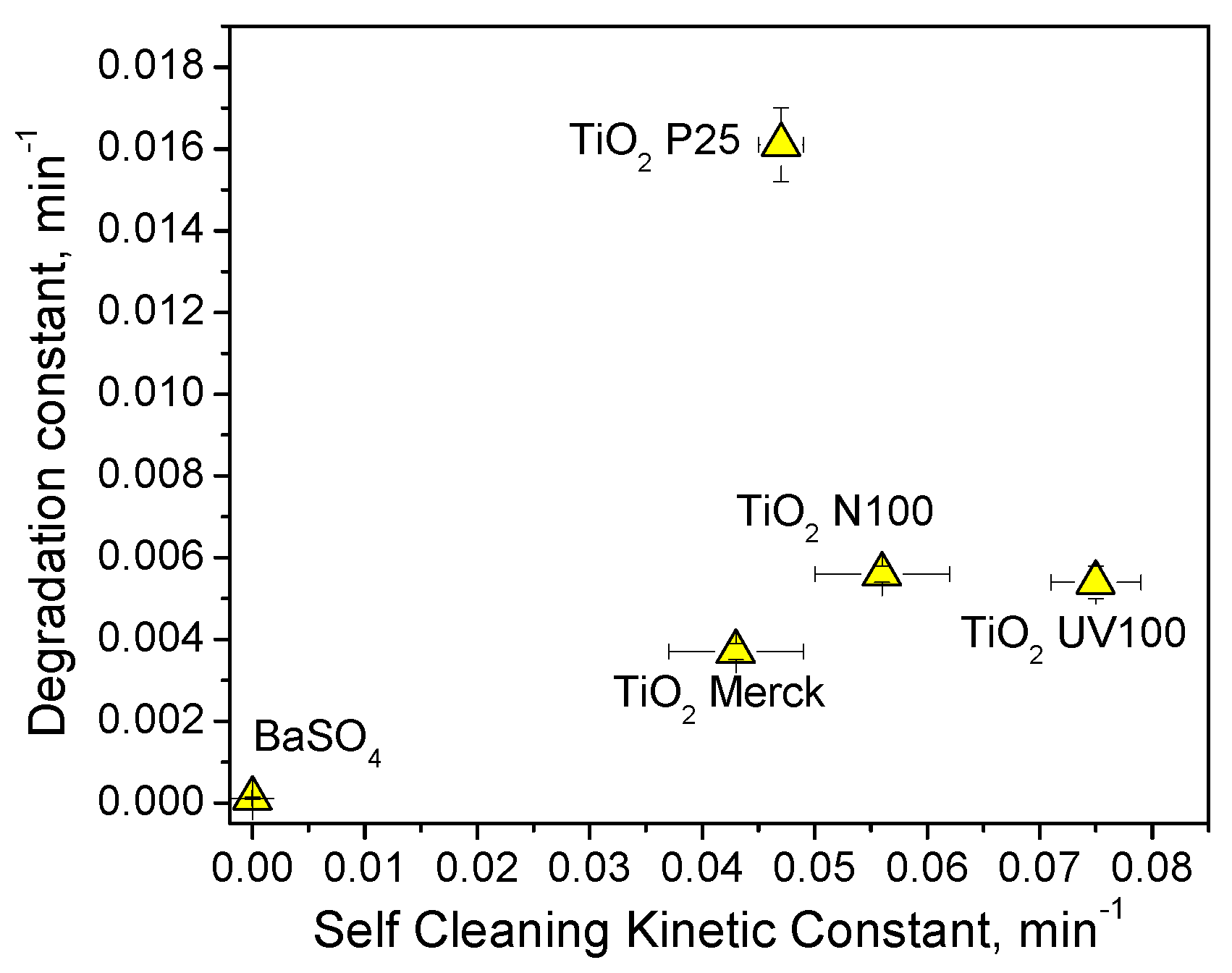
3.4. Comparison among the Results Obtained by Using the Three Dyes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Bisquert, J.; Cahen, D.; Hodes, G.; Ru1hle, S.; Zaba, A. Physical chemical principles of photovoltaic conversion with nanoparticulate, mesoporous dye-sensitized solar cells. J. Phys. Chem. B 2004, 108, 8106–8118. [Google Scholar] [CrossRef]
- Khalaji Assadi, M.; Bakhoda, S.; Saidur, R.; Hanaei, H. Recent progress in perovskite solar cells. Renew. Sustain. Energ. Rev. 2018, 81, 2812–2822. [Google Scholar] [CrossRef]
- Tentu, R.D.; Basu, S. Photocatalytic water splitting for hydrogen production. Curr. Opin. Electrochem. 2017, 5, 56–62. [Google Scholar] [CrossRef]
- Serpone, N.; Pelizzetti, E. Photocatalysis: Fundamental and Applications; Wiley: New York, NY, USA, 1989; p. 650. [Google Scholar]
- Pelizzetti, E.; Minero, C. Metal oxides as photocatalysts for environmental detoxification. Comments Inorg. Chem. 1994, 15, 297–337. [Google Scholar] [CrossRef]
- Minero, C.; Maurino, V.; Pelizzetti, E. Mechanism of the photocatalytic transformation of organic compounds. In Semiconductor Photochemistry and Photophysics; Ramamurthy, V., Shanze, K.S., Eds.; Marcel Dekker: New York, NY, USA, 2003; pp. 211–229. [Google Scholar]
- Maurino, V.; Minella, M.; Sordello, F.; Minero, C. A proof of the direct hole transfer in phototcatalysis: The case of melamine. Appl. Catal. A Gen. 2016, 521, 57–67. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Varghese, O.K.; Paulose, M.; LaTempa, T.J.; Grimes, C.A. High-rate solar photocatalytic conversion of CO2 and water vapor to hydrocarbon fuels. Nano Lett. 2009, 9, 731–737. [Google Scholar] [CrossRef]
- Usubharatana, P.; McMartin, D.; Veawab, A.; Tontiwachwuthikul, P. Photocatalytic process for CO2 emission reduction from industrial flue gas streams. Ind. Eng. Chem. Res. 2006, 45, 2558–2568. [Google Scholar] [CrossRef]
- Maurino, V.; Bedini, A.; Minella, M.; Rubertelli, F.; Minero, C. Glycerol transformation through photocatalysis: A possible route to value added chemicals. J. Adv. Oxid. Technol. 2008, 11, 184–192. [Google Scholar] [CrossRef]
- Minero, C.; Bedini, A.; Maurino, V. Glycerol as a probe molecule to uncover oxidation mechanism in photocatalysis. Appl. Catal. B Environ. 2012, 128, 135–143. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A. Light-induced amphiphilic surfaces. Nature 1997, 88, 431–432. [Google Scholar] [CrossRef]
- Vodišek, N.; Ramanujachary, K.; Brezová, V.; Lavrenčič Štangar, U. Transparent titania-zirconia-silica thin films for self-cleaning and photocatalytic applications. Catal. Today 2017, 142–147. [Google Scholar] [CrossRef]
- Verhovšek, D.; Veronovski, N.; Lavrenčič Štangar, U.; Kete, M.; Žagar, K.; Čeh, M. The synthesis of anatase nanoparticles and the preparation of photocatalytically active coatings based on wet chemical methods for self-cleaning applications. Int. J. Photoenergy 2012, 329796. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Mills, A.; Lee, S.K. A web-based overview of semiconductor photochemistry-based current commercial applications. J. Photochem. Photobiol. A Chem. 2002, 152, 233–247. [Google Scholar] [CrossRef]
- Černigoj, U.; Kete, M.; Lavrenčič Stangar, U. Development of a fluorescence-based method for evaluation of self-cleaning properties of photocatalytic layers. Catal. Today 2010, 151, 46–52. [Google Scholar] [CrossRef]
- Zita, J.; Krýsa, J.; Mills, A. Correlation of oxidative and reductive dye bleaching on TiO2 photocatalyst films. J. Photochem. Photobiol. A Chem. 2009, 203, 119–124. [Google Scholar] [CrossRef]
- Moafi, H.F.; Shojaie, A.F.; Zanjanchi, M.A. The comparative study of photocatalytic self-cleaning properties of synthesized nanoscale titania and zirconia onto polyacrylonitrile fibers. J. Appl. Polym. Sci. 2010, 118, 2062–2070. [Google Scholar] [CrossRef]
- Mills, A.; McFarlane, M. Current and possible future methods of assessing the activities of photocatalyst films. Catal. Today 2007, 129, 22–28. [Google Scholar] [CrossRef]
- ISO. ISO/CD 10678, TC 206 Fine Ceramics. Available online: http://www.iso.org/iso/isocatalogue/catalogue tc/catalogue detail.htm?csnumber=46019 (accessed on 20 December 2018).
- Sam, E.D.; Urgen, M.; Tepehan, F.Z.; Gunay, V. Self cleaning photoactive TiO2 coatings on SLS glasses by sol-gel dip coating. Key Eng. Mater. 2004, 264–268, 407–410. [Google Scholar] [CrossRef]
- Yamashita, H.; Nakao, H.; Takeuchi, M.; Nakatani, Y.; Anpo, M. Coating of TiO2 photocatalysts on super-hydrophobic porous teflon membrane by an ion assisted deposition method and their self-cleaning performance. Nucl. Instrum. Methods Phys. Res. B 2003, 206, 898–901. [Google Scholar] [CrossRef]
- Folli, A.; Jakobsen, U.H.; Guerrini, G.L.; Macphee, D.E. Rhodamine B discolouration on TiO2 in the cement environment: A look at fundamental aspects of the self-cleaning effect in concretes. J. Adv. Oxid. Technol. 2009, 12, 126–133. [Google Scholar] [CrossRef]
- Lavrenčič Štangar, U.; Tashibi, M.; Fresno, F.; Kete, M.; Gasparotto, A.; Maccato, C.; Barreca, D. Self-cleaning and anti-fogging surfaces based on nanostructured metal oxides. Adv. Sci. Technol. 2014, 91, 39–47. [Google Scholar] [CrossRef]
- Mills, A.; Lepre, A.; Elliott, N.; Bhopal, S.; Parkin, I.P.; O’Neill, S.A. Characterisation of the photocatalyst Pilkington Activ™: A reference film photocatalyst? J. Photochem. Photobiol. A Chem. 2003, 160, 213–224. [Google Scholar] [CrossRef]
- Mills, A.; Wang, J.; Lee, S.-K.; Simonsen, M. An intelligence ink for photocatalytic films. Chem. Commun. 2005, 21, 2721–2723. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.; Wang, J.; McGrady, M. Method of rapid assessment of photocatalytic activities of self-cleaning films. J. Phys. Chem. B 2006, 110, 18324–18331. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.; McGrady, M.; Wang, J.; Hepburn, L. A rapid method of assessing the photocatalytic activity of thin films using an ink based on the redox dye 2,6-dichloroindophenol. Int. J. Photoenergy 2008, 504945. [Google Scholar] [CrossRef]
- CEN European Committee for Standardization. EN 16845-1:2017—Photocatalysis—Anti-Soiling Chemical Activity Using Adsorbed Organics under Solid/Solid Conditions—Part 1: Dyes on Porous Surfaces; CEN: Brussels, Belgium, 2017. [Google Scholar]
- Wyszecki, G.; Stiles, W.S.; Wyszecki, G. Color Science: Concepts and Methods, Quantitative Data and Formulae, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2000; p. 950. [Google Scholar]
- Labidi, N.S. Flotation of barium sulfate contaminants soils. Biodiversity Int. J. 2018, 2, 91–93. [Google Scholar] [CrossRef]
- Wang, P. Aggregation of TiO2 Nanoparticles in Aqueous Media: Effects of pH, Ferric Ion and Humic Acid. Int. J. Environ. Sci. Nat. Res. 2017, 1. [Google Scholar] [CrossRef]
- Kosmulski, M. Surface Charging and Points of Zero Charge; Surfactans Science Series 145; CRC Press: Boca Raton, FL, USA, 2009; p. 1092. [Google Scholar]
- Pelizzetti, E.; Maurino, V.; Minero, C.; Carlin, V.; Tosato, M.L.; Pramauro, E.; Zerbinati, O. Photocatalytic degradation of atrazine and other s-triazine herbicides. Environ. Sci. Technol. 1990, 24, 1559–1565. [Google Scholar] [CrossRef]
- Lakshmi, S.; Renganathan, R.; Fujita, S. Study on TiO2-mediated photocatalytic degradation of methylene blue. J. Photochem. Photobiol. A Chem. 1995, 88, 163–167. [Google Scholar] [CrossRef]
- Mills, A.; Wang, J. Photobleaching of methylene blue sensitised by TiO2: An ambiguous system? J. Photochem. Photobiol. A Chem. 1999, 127, 123–134. [Google Scholar] [CrossRef]
- Gnaser, H.; Savina, M.R.; Calaway, W.F.; Tripa, C.E.; Veryovkin, I.V.; Pell, M.J. Photocatalytic degradation of methylene blue on nanocrystalline TiO2: Surface mass spectrometry of reaction intermediates. Int. J. Mass Spectrom. 2005, 245, 61–67. [Google Scholar] [CrossRef]
- Nozik, A.J.; Memming, R. Physical chemistry of semiconductor-liquid interfaces. J. Phys. Chem. 1996, 100, 13061–13078. [Google Scholar] [CrossRef]
- Pleskov, Y.V.; Gurevich, Y.Y. Semiconductor Photoelectrochemistry; Consultants Bureau: New York, NY, USA, 1986; p. 430. [Google Scholar]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Zhang, T.; Oyama, T.; Aoshima, A.; Hidaka, H.; Zhao, J.; Serpone, N. Photooxidative N-demethylation of methylene blue in aqueous TiO2 dispersions under UV irradiation. J. Photochem. Photobiol. A 2001, 140, 163–172. [Google Scholar] [CrossRef]
- Zhang, T.; Oyama, T.; Horikoshi, S.; Hidaka, H.; Zhao, J.; Serpone, N. Photocatalyzed N-demethylation and degradation of methylene blue in titania dispersions exposed to concentrated sunlight. Sol. Energy Mater. Sol. Cells 2002, 73, 287–303. [Google Scholar] [CrossRef]
- Lee, J.; Choi, W. Effect of platinum deposits on TiO2 on the anoxic photocatalytic degradation pathways of alkylamines in water: dealkylation and n-alkylation. Environ. Sci. Technol. 2004, 38, 4026–4033. [Google Scholar] [CrossRef]
- Lu, C.-S.; Chen, C.-C.; Mai, F.-D.; Li, H.-K. Identification of the degradation pathways of alkanolamines with TiO2 photocatalysis. J. Hazard. Mater. 2009, 165, 306–316. [Google Scholar] [CrossRef]
- Minella, M.; Sordello, F.; Minero, C. Photocatalytic process in TiO2/graphene hybrid materials. Evidence of charge separation by electron transfer from reduced graphene oxide to TiO2. Catal. Today 2017, 281, 29–37. [Google Scholar] [CrossRef]
- UNI. UNI 11259:2008—Determination of the Photocatalytic Activity of Hydraulic Binders—Rodammina Test Method. Available online: http://webstore.uni.com (accessed on 20 December 2018).
- Minero, C.; Mariella, G.; Maurino, V.; Pelizzetti, E. Photocatalytic transformation of organic compounds in the presence of inorganic anions. 1. Hydroxyl-mediated and direct electron-transfer reactions of phenol on a titanium dioxide−fluoride system. Langmuir 2000, 16, 2632–2641. [Google Scholar] [CrossRef]
- Ryu, J.; Choi, W. Substrate-specific photocatalytic activities of TiO2 and multiactivity test for water treatment application. Environ. Sci. Technol. 2008, 42, 294–300. [Google Scholar] [CrossRef] [PubMed]
| Sample Name/Producer | Material | Average Particle Size, nm |
|---|---|---|
| BaSO4 (Rockwood Italia S.p.A.) | Barite | 700–1000 |
| TiO2 Hombikat N100 (Sachtleben Chemie GmbH) | Titania (Anatase) | 20 |
| TiO2 Hombikat UV100 (Sachtleben Chemie GmbH) | Titania (Anatase) | 10 |
| TiO2 Merck | Titania (Anatase) | 40–300 |
| TiO2 P25 (Evonik) | Titania (Anatase 80%, Rutile 20%) | 40 |
| Y49 (Rockwood Italia S.p.a.) | Goethite | Acicular shape l = 1000–1300 w = 100 |
| Y48HY10HK20 (Rockwood Italia S.p.a.) | Goethite | Acicular shape l = 1500–2000 w = 100–200 |
| Dye | Standard Covering (SC) | Molar Extinction Coefficient at λmax (M−1·cm−1) | Maximum Absorbance (λmax, nm) | Interval of Absorbance Integration (nm) | |
|---|---|---|---|---|---|
| (g·cm−2) | (molecule·cm−2) | ||||
| Methylene Blue (MB) | 4 × 10−5 | 6.4 × 1016 | 23,430 | 657 | 660–690 |
| Rhodamine B (RhB) | 2 × 10−5 | 2.5 × 1016 | 60,750 | 556 | 510–550 |
| Metanil Yellow (MY) | 4 × 10−5 | 3.2 × 1016 | 21,750 | 411 | 410–440 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minella, M.; Minero, C. Quantification of the Photocatalytic Self-Cleaning Ability of Non-Transparent Materials. Materials 2019, 12, 508. https://doi.org/10.3390/ma12030508
Minella M, Minero C. Quantification of the Photocatalytic Self-Cleaning Ability of Non-Transparent Materials. Materials. 2019; 12(3):508. https://doi.org/10.3390/ma12030508
Chicago/Turabian StyleMinella, Marco, and Claudio Minero. 2019. "Quantification of the Photocatalytic Self-Cleaning Ability of Non-Transparent Materials" Materials 12, no. 3: 508. https://doi.org/10.3390/ma12030508
APA StyleMinella, M., & Minero, C. (2019). Quantification of the Photocatalytic Self-Cleaning Ability of Non-Transparent Materials. Materials, 12(3), 508. https://doi.org/10.3390/ma12030508






