Pulsed UV Laser Processing of Carbosilane and Silazane Polymers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Glass Treatment and Coating
2.3. Laser Processing
2.4. Solvent Development
2.5. Pyrolysis
3. Results and Discussion
3.1. Controlling Carbosilane and Silazane Flim Thickness on Silicon and Glass
- Casting with a 127 µm blade gap, then 5 min UV curing.
- Followed by casting with a 254 µm blade gap, then 5 min UV curing.
- Followed by casting with a 381 µm blade gap, then 5 min UV curing.
- Followed by casting with a 508 µm blade gap, then 5 min UV curing.
3.2. Glass Treatment to Facilitate Green Body Release
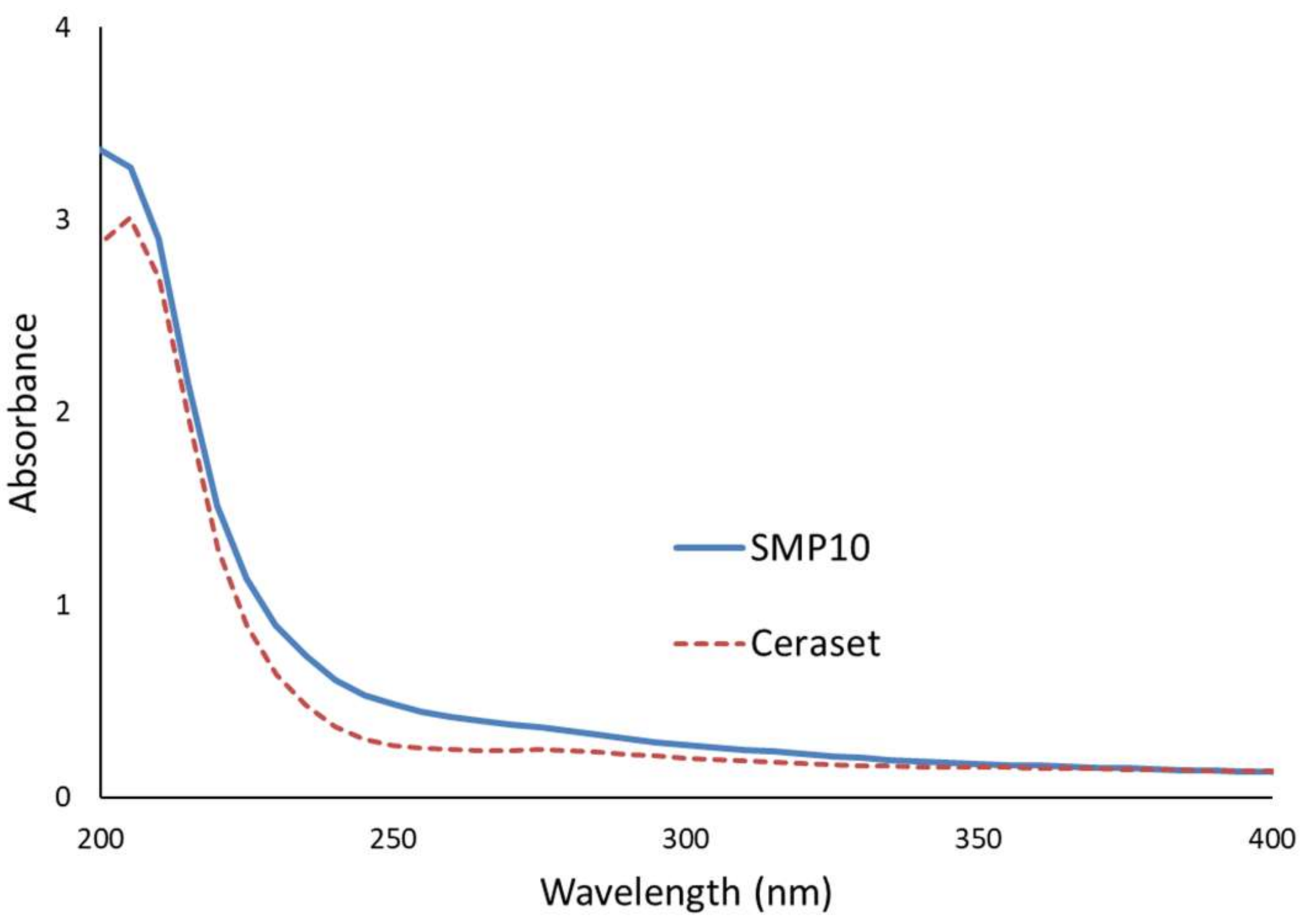
3.3. Spectroscopic Analysis of SMP10 and Ceraset
3.4. Laser Fabrication
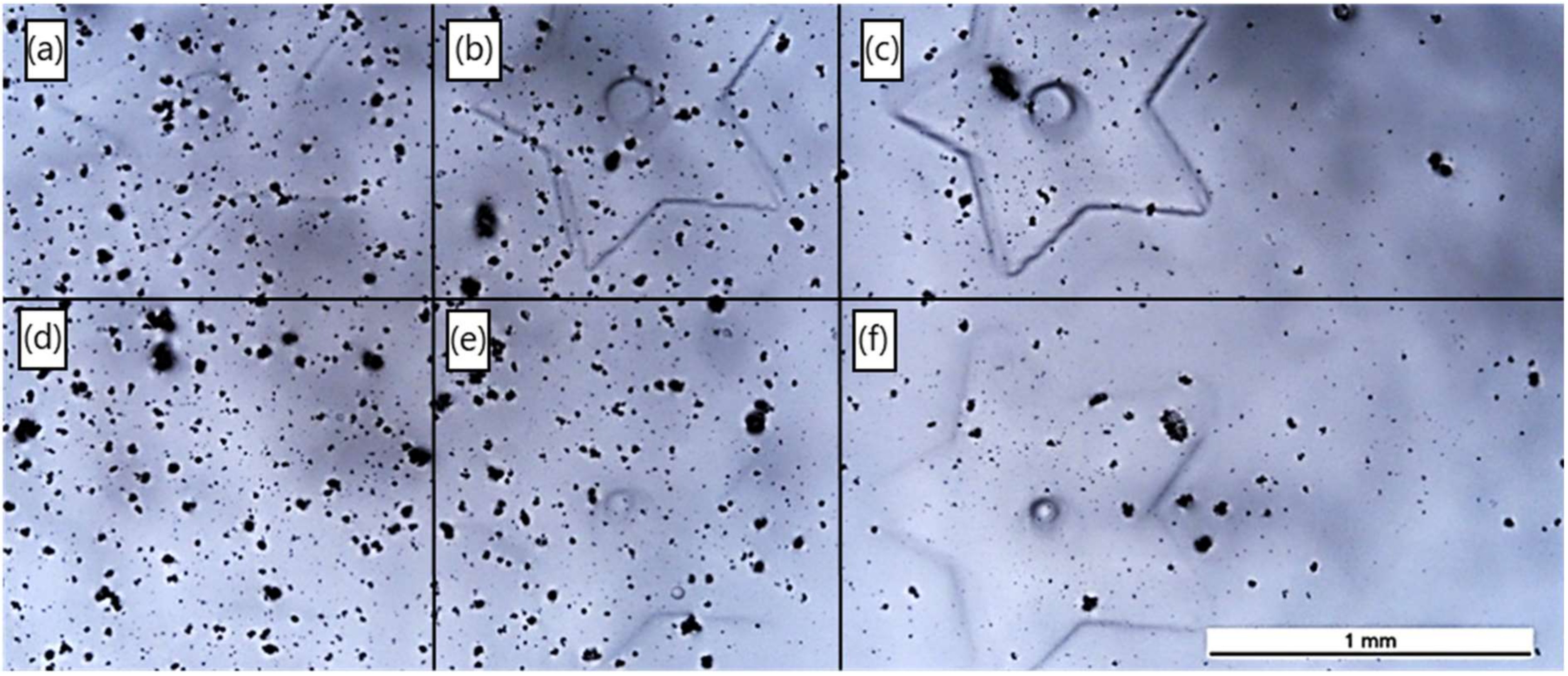
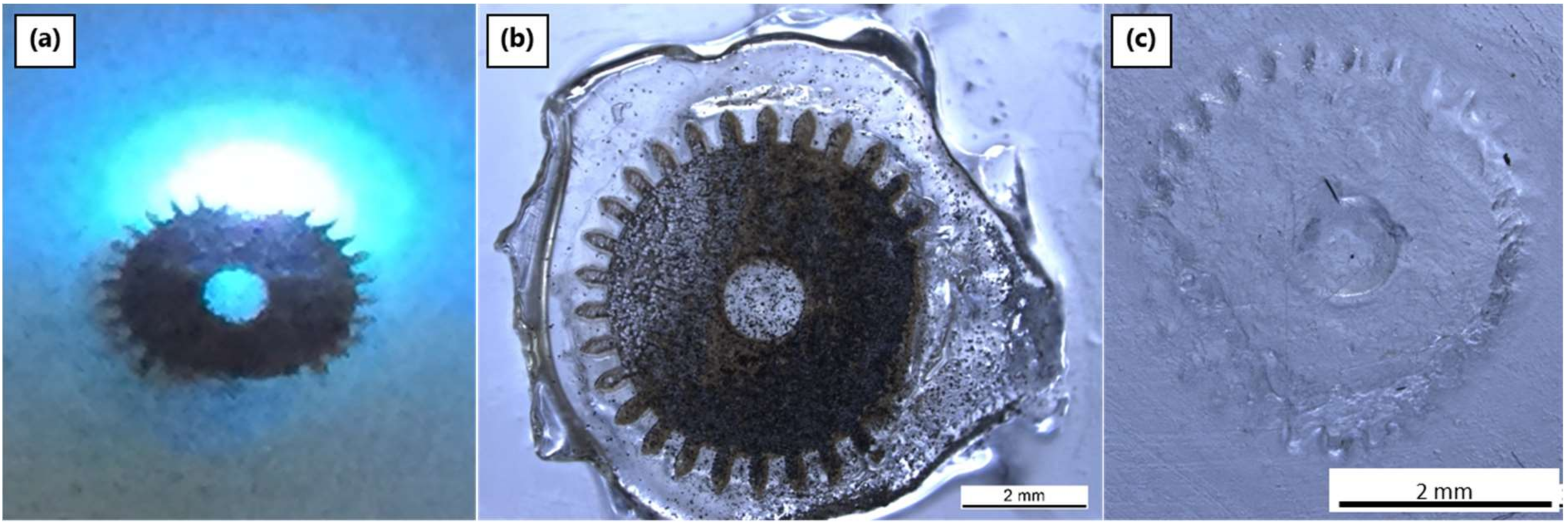
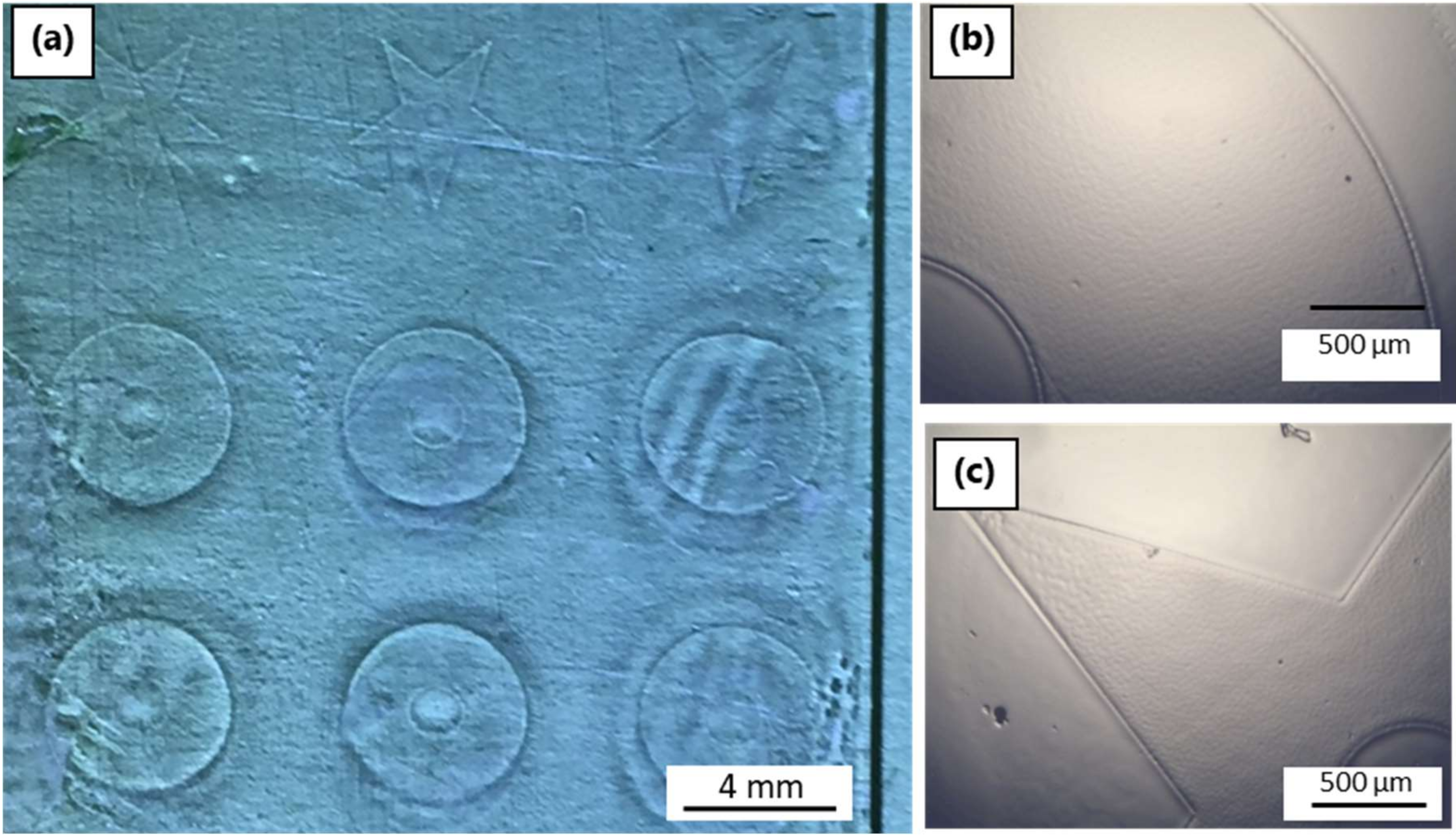
3.4.1. Laser System 1: ns-Pulsed 266 nm
3.4.2. Laser System 2: ns-Pulsed 355 nm
3.4.3. Laser System 3: ps-Pulsed 355 nm
3.5. Thermal Analysis
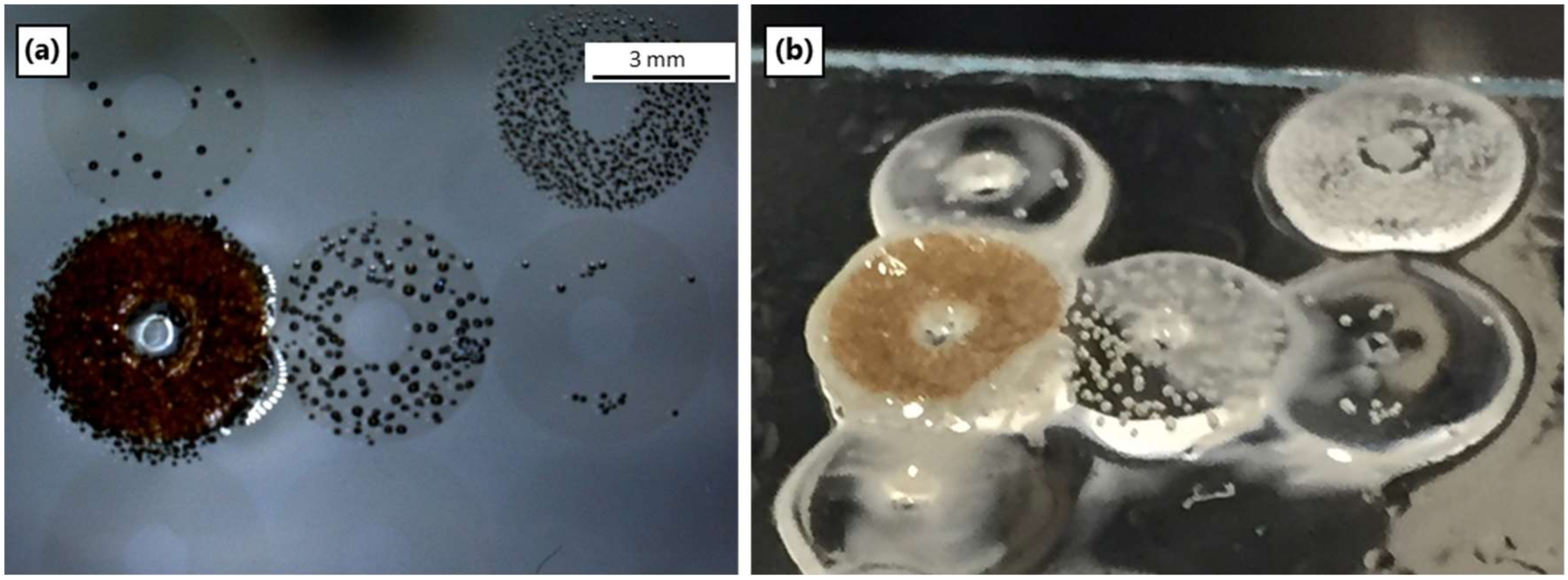
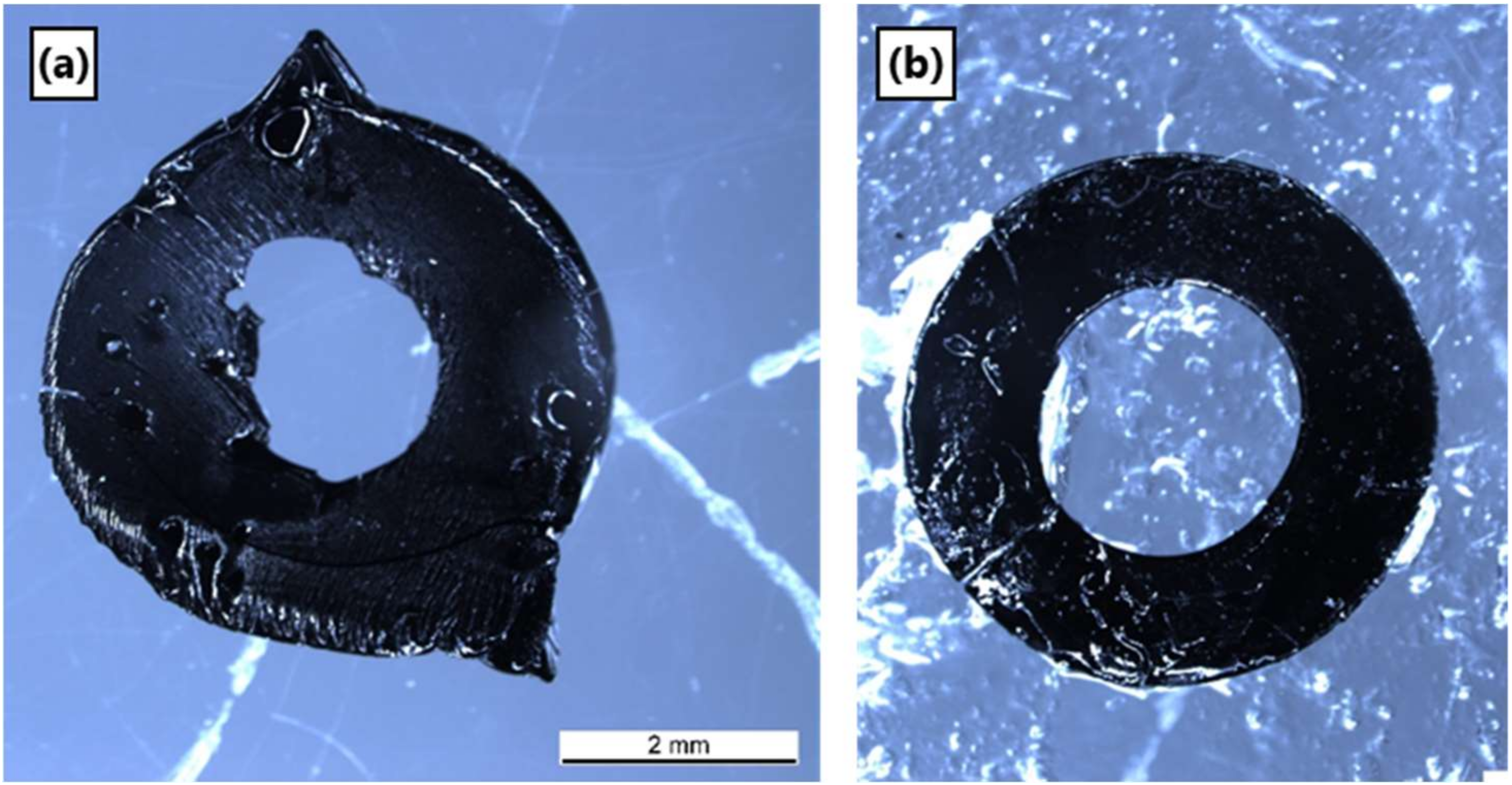
3.6. Pyrolysis of Ceraset Green Bodies
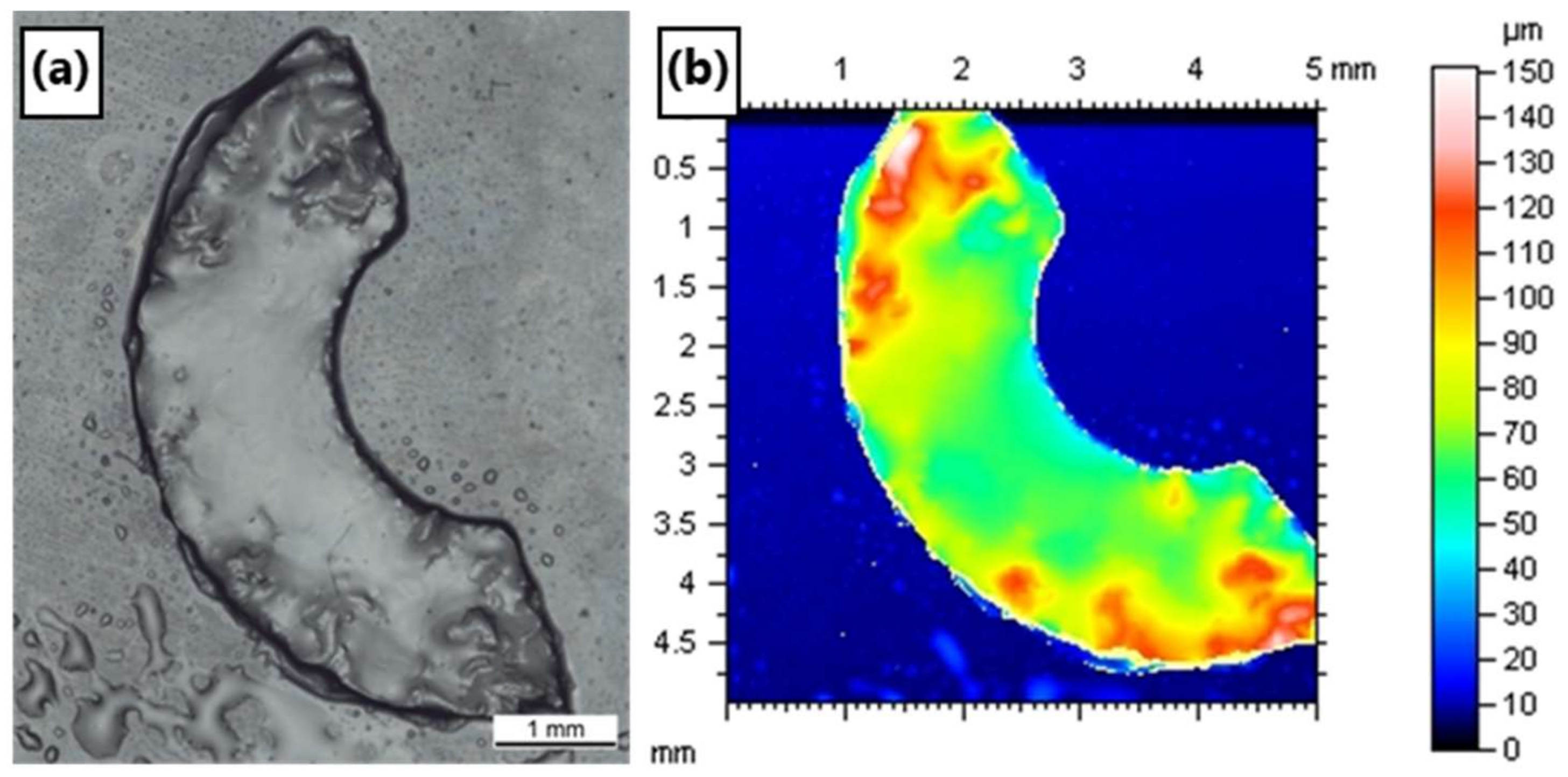
3.7. SEM and Microstructure
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Colombo, P.; Mera, G.; Riedel, R.; Sorarù, G.D. Polymer-Derived Ceramics: 40 Years of Research and Innovation in Advanced Ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Flores, O.; Schmalz, T.; Krenkel, W.; Heymann, L.; Motz, G. Selective cross-linking of oligosilazanes to tailored meltable polysilazanes for the processing of ceramic SiCN fibres. J. Mater. Chem. 2013, 1, 15406–15415. [Google Scholar] [CrossRef]
- Hu, L.H.; Raj, R. Semiconductive Behavior of Polymer-Derived SiCN Ceramics for Hydrogen Sensing. J. Am. Ceram. Soc. 2015, 98, 1052–1055. [Google Scholar] [CrossRef]
- Guo, Z.; Parlinska-Wojtan, M.; Blugan, G.; Graule, T.; Reece, M.J.; Kuebler, J. The influence of the grain boundary phase on the mechanical properties of Si3N4–MoSi2 composites. Acta Mater. 2007, 55, 2875–2884. [Google Scholar] [CrossRef]
- Zapata-Solvas, E.; Bonilla, S.; Wilshaw, P.R.; Todd, R.I. Preliminary investigation of flash sintering of SiC. J. Eur. Ceram. Soc. 2013, 33, 2811–2816. [Google Scholar] [CrossRef]
- Li, Q.; Yin, X.; Duan, W.; Hao, B.; Kong, L.; Liu, X. Dielectric and microwave absorption properties of polymer derived SiCN ceramics annealed in N2 atmosphere. J. Eur. Ceram. Soc. 2014, 34, 589–598. [Google Scholar] [CrossRef]
- Bakumov, V.; Blugan, G.; Roos, S.; Graule, T.; Fakhfouri, V.; Grossenbacher, J.; Gullo, M.; Kiefer, T.; Brugger, J.; Parlinska, M.; Kuebler, J. Mechanical and tribological properties of polymer-derived Si/C/N sub-millimetre thick miniaturized components fabricated by direct casting. J. Eur. Ceram. Soc. 2012, 32, 1759–1767. [Google Scholar] [CrossRef]
- Flores, O.; Heymann, L.; Motz, G. Rheological behaviour of tailored polysilazane melts for the processing of SiCN ceramics: viscoelastic properties and thermal stability. Rheol. Acta 2015, 54, 517–528. [Google Scholar] [CrossRef]
- Blugan, G.; Morawa, K.; Koebel, S.; Graule, T.; Kuebler, J. Development of a tape casting process for making thin layers of Si3N4 and Si3N4 + TiN. J. Eur. Ceram. Soc. 2007, 27, 4789–4795. [Google Scholar] [CrossRef]
- Schmidt, J.; Colombo, P. Digital light processing of ceramic components from polysiloxanes. J. Eur. Ceram. Soc. 2018, 38, 57–66. [Google Scholar] [CrossRef]
- de Hazan, Y.; Penner, D. SiC and SiOC ceramic articles produced by stereolithography of acrylate modified polycarbosilane systems. J. Eur. Ceram. Soc. 2017, 37, 5205–5212. [Google Scholar] [CrossRef]
- Schwarzer, E.; Götz, M.; Markova, D.; Stafford, D.; Scheithauer, U.; Moritz, T. Lithography-based ceramic manufacturing (LCM) – Viscosity and cleaning as two quality influencing steps in the process chain of printing green parts. J. Eur. Ceram. Soc. 2017, 37, 5329–5338. [Google Scholar] [CrossRef]
- Chartier, T.; Dupas, C.; Geffroy, P.M.; Pateloup, V.; Colas, M.; Cornette, J.; Guillemet-Fritsch, S. Influence of irradiation parameters on the polymerization of ceramic reactive suspensions for stereolithography. J. Eur. Ceram. Soc. 2017, 37, 4431–4436. [Google Scholar] [CrossRef]
- Martínez-Crespiera, S.; Ionescu, E.; Schlosser, M.; Flittner, K.; Mistura, G.; Riedel, R.; Schlaak, H.F. Fabrication of silicon oxycarbide-based microcomponents via photolithographic and soft lithography approaches. Sens. Actuators A 2011, 169, 242–249. [Google Scholar]
- Lee, H.J.; Yoon, T.H.; Kim, D.P. Nano-sized patterns derived from a SiCN preceramic polymer: Fabrication and their characterization. J. Phys. Chem. Solids 2008, 69, 2131–2136. [Google Scholar] [CrossRef]
- Greenberg, S.; Clendenning, S.B.; Liu, K.; Manners, I.; Aouba, S.; Ruda, H.E. Synthesis and Lithographic Patterning of Polycarbosilanes with Pendant Cobalt Carbonyl Clusters. Macromolecules 2005, 38, 2023–2026. [Google Scholar] [CrossRef]
- Liew, L.A.; Liu, Y.; Luo, R.; Cross, T.; An, L.; Bright, V.M.; Dunn, M.L.; Daily, J.W.; Raj, R. Fabrication of SiCN MEMS by photopolymerization of pre-ceramic polymer. Sens. Actuators A 2002, 95, 120–134. [Google Scholar] [CrossRef]
- Chartier, T.; Badev, A.; Abouliatim, Y.; Lebaudy, P.; Lecamp, L. Stereolithography process: Influence of the rheology of silica suspensions and of the medium on polymerization kinetics—Cured depth and width. J. Eur. Ceram. Soc. 2012, 32, 1625–1634. [Google Scholar] [CrossRef]
- D’Elia, R.; Dusserre, G.; Del Confetto, S.; Eberling-Fux, N.; Descamps, C.; Cutard, T. Cure kinetics of a polysilazane system: Experimental characterization and numerical modelling. Eur. Polym. J. 2016, 76, 40–52. [Google Scholar] [CrossRef]
- Lukacs, A. Polysilazane precursors to advanced ceramics. Am. Ceram. Soc. Bull. 2007, 86, 9301–9306. [Google Scholar]
- Dalcanale, F.; Grossenbacher, J.; Blugan, G.; Gullo, M.R.; Brugger, J.; Tevaearai, H.; Graule, T.; Kuebler, J. Rapid carbon nanotubes suspension in organic solvents using organosilicon polymers. J. Colloid Interface Sci. 2016, 470, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Zemanová, M.; Lecomte, E.; Šajgalı́k, P.; Riedel, R. Polysilazane derived micro/nano Si3N4/SiC composites. J. Eur. Ceram. Soc. 2002, 22, 2963–2968. [Google Scholar] [CrossRef]
- Li, Y.H.; Li, X.D.; Kim, D.P. Chemical development of preceramic polyvinylsilazane photoresist for ceramic patterning. J. Electroceram. 2007, 23, 133. [Google Scholar] [CrossRef]
- Ligon, S.C.; Blugan, G.; Dalcanale, F.; Kuebler, J. Production of improved SiC and SiCN ceramics from polycarbosilane and polysilazane composites. AIP Conf. Proc. 2018, 1981, 020037. [Google Scholar]
- Ligon, S.C.; Blugan, G.; Kuebler, J. Maskless lithography of silazanes for fabrication of ceramic micro-components. Ceram. Int. 2019, 45, 2345–2350. [Google Scholar] [CrossRef]
- Knowles, M.R.H.; Rutterford, G.; Karnakis, D.; Ferguson, A. Micro-machining of metals, ceramics and polymers using nanosecond lasers. Int. J. Adv. Manuf. Technol. 2007, 33, 95–102. [Google Scholar] [CrossRef]
- Rihakova, L.; Chmelickova, H. Laser Micromachining of Glass, Silicon, and Ceramics. Adv. Mater. Sci. Eng. 2015, 584952. [Google Scholar] [CrossRef]
- Tshabalala, L.C.; Pityana, S. Surface texturing of Si3N4–SiC ceramic tool components by pulsed laser machining. Surf. Coat. Technol. 2016, 289, 52–60. [Google Scholar] [CrossRef]
- Rudolph, P.; Kautek, W. Composition influence of non-oxidic ceramics on self-assembled nanostructures due to fs-laser irradiation. Thin Solid Films 2004, 453, 537–541. [Google Scholar] [CrossRef]
- Park, S.; Lee, D.H.; Ryoo, H.I.; Lim, T.W.; Yang, D.Y.; Kim, D.P. Fabrication of three-dimensional SiC ceramic microstructures with near-zero shrinkage via dual crosslinking induced stereolithography. Chem. Commun. 2009, 32, 4880–4882. [Google Scholar] [CrossRef]
- Lim, T.W.; Son, Y.; Yang, D.Y.; Pham, T.A.; Kim, D.P.; Yang, B.-Il; Lee, K.-S.; Park, S.H. Net Shape Manufacturing of Three-Dimensional SiCN Ceramic Microstructures Using an Isotropic Shrinkage Method by Introducing Shrinkage Guiders. Int. J. Appl. Ceram. Technol. 2008, 5, 258–264. [Google Scholar] [CrossRef]
- Pham, T.A.; Kim, D.-P.; Lim, T.-W.; Park, S.-H.; Yang, D.-Y.; Lee, K.-S. Three-Dimensional SiCN Ceramic Microstructures via Nano-Stereolithography of Inorganic Polymer Photoresists. Adv. Funct. Mater. 2006, 16, 1235–1241. [Google Scholar] [CrossRef]
- Qian, B.; Shen, Z. Laser sintering of ceramics. J. Asian Ceram. Soc. 2013, 1, 315–321. [Google Scholar] [CrossRef]
- Rekštytė, S.; Jonavičius, T.; Malinauskas, M. Direct laser writing of microstructures on optically opaque and reflective surfaces. Opt. Laser Eng. 2014, 53, 90–97. [Google Scholar] [CrossRef]
- Pola, J.; Galíková, A.; Bastl, Z.; Šubrt, J.; Vacek, K.; Brus, J.; Ouchi, A. UV laser deposition of nanostructured Si/C/O/N/H from disilazane precursors and evolution to silicon oxycarbonitride. Appl. Organomet. Chem. 2006, 20, 648–655. [Google Scholar] [CrossRef]
- Exner, H.; Streek, A. High Resolution Laser Micro Sintering/Melting Using Q-Switched and High Brilliant Laser Radiation; SPIE LASE, SPIE: San Francisco, CA, USA, 12 February 2015; Volume 9353, p. 9353–24. [Google Scholar]
- Chavez, R.; Ionescu, E.; Balan, C.; Fasel, C.; Riedel, R. Effect of ambient atmosphere on crosslinking of polysilazanes. J. Appl. Polym. Sci. 2011, 119, 794–802. [Google Scholar] [CrossRef]
- Janakiraman, N.; Aldinger, F. Fabrication and characterization of fully dense Si–C–N ceramics from a poly(ureamethylvinyl)silazane precursor. J. Eur. Ceram. Soc. 2009, 29, 163–173. [Google Scholar] [CrossRef]
- Vakifahmetoglu, C.; Zeydanli, D.; Colombo, P. Porous polymer derived ceramics. Mater. Sci. Eng. R Rep. 2016, 106, 1–30. [Google Scholar] [CrossRef]
- Konegger, T.; Williams, L.F.; Bordia, R.K. Planar, Polysilazane-Derived Porous Ceramic Supports for Membrane and Catalysis Applications. J. Am. Ceram. Soc. 2015, 98, 3047–3053. [Google Scholar] [CrossRef]
- Bernard, S.; Miele, P. Ordered mesoporous polymer-derived ceramics and their processing into hierarchically porous boron nitride and silicoboron carbonitride monoliths. New J. Chem. 2014, 38, 1923–1931. [Google Scholar] [CrossRef]
- Vakifahmetoglu, C.; Menapace, I.; Hirsch, A.; Biasetto, L.; Hauser, R.; Riedel, R.; Colombo, P. Highly porous macro- and micro-cellular ceramics from a polysilazane precursor. Ceram. Int. 2009, 35, 3281–3290. [Google Scholar] [CrossRef]




| Formulation | Substrate | Speed (rpm) | Thickness (µm) |
|---|---|---|---|
| 50% in xylene | silicon | 1000 | 3.80 ± 0.03 |
| 50% in xylene | silicon | 500 | 5.80 ± 0.02 |
| 50% in xylene | glass | 500 | 5.90 ± 0.16 |
| Blade Height | Ceraset & 5 wt % PI | SMP10 & 5 wt % PI |
|---|---|---|
| (µm) | Thickness (µm) | Thickness (µm) |
| 127 | 70.0 ± 0.5 | 65 ± 24 |
| 254 | 140 ± 4 | 120 ± 42 |
| 381 | 210 ± 2 | 198 ± 40 |
| 508 | 280 ± 5 | 247 ± 44 |
| Test | Power (mW) | Hatching (µm) | Repetitions | Thickness (µm) | Comments |
|---|---|---|---|---|---|
| 1 | 300 | 10 | 1 | 50 | partial ablation |
| 2 | 40 | 10 | 10 | 70 | stable green body |
| 3 | 150 | 5 | 5 | 200 | poor crosslinking |
| 4 | 150 | 2 | 5 | 1000 | crosslinking; bubbles |
| 5 | 90 | 2 | 5 | 1000 | stable green body |
| Test | Power (mW) | Pulse Length (ns) | Scan Speed (mm s−1) | Repetitions | Comments |
|---|---|---|---|---|---|
| 1 | 150 | 30 | 20 | 1 | ablation |
| 2 | 150 | 40 | 20 | 1 | crosslinking; poor resolution |
| 3 | 150 | 40 | 100 | 5 | crosslinking; decent resolution |
| 4 | 150 | 40 | 500 | 10 | no crosslinking |
| 5 | 80 | 40 | 50 | 10 | crosslinking; poor resolution |
| 6 | 50 | 40 | 50 | 10 | crosslinking; better resolution |
| 7 | 35 | 40 | 50 | 10 | insufficient crosslinking |
| 8 | 15 | 40 | 50 | 10 | no crosslinking |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ligon, S.C.; Blugan, G.; Kuebler, J. Pulsed UV Laser Processing of Carbosilane and Silazane Polymers. Materials 2019, 12, 372. https://doi.org/10.3390/ma12030372
Ligon SC, Blugan G, Kuebler J. Pulsed UV Laser Processing of Carbosilane and Silazane Polymers. Materials. 2019; 12(3):372. https://doi.org/10.3390/ma12030372
Chicago/Turabian StyleLigon, Samuel Clark, Gurdial Blugan, and Jakob Kuebler. 2019. "Pulsed UV Laser Processing of Carbosilane and Silazane Polymers" Materials 12, no. 3: 372. https://doi.org/10.3390/ma12030372
APA StyleLigon, S. C., Blugan, G., & Kuebler, J. (2019). Pulsed UV Laser Processing of Carbosilane and Silazane Polymers. Materials, 12(3), 372. https://doi.org/10.3390/ma12030372






