Ni/Si-Codoped TiO2 Nanostructure Photoanode for Enhanced Photoelectrochemical Water Splitting
Abstract
1. Introduction
2. Materials and Methods
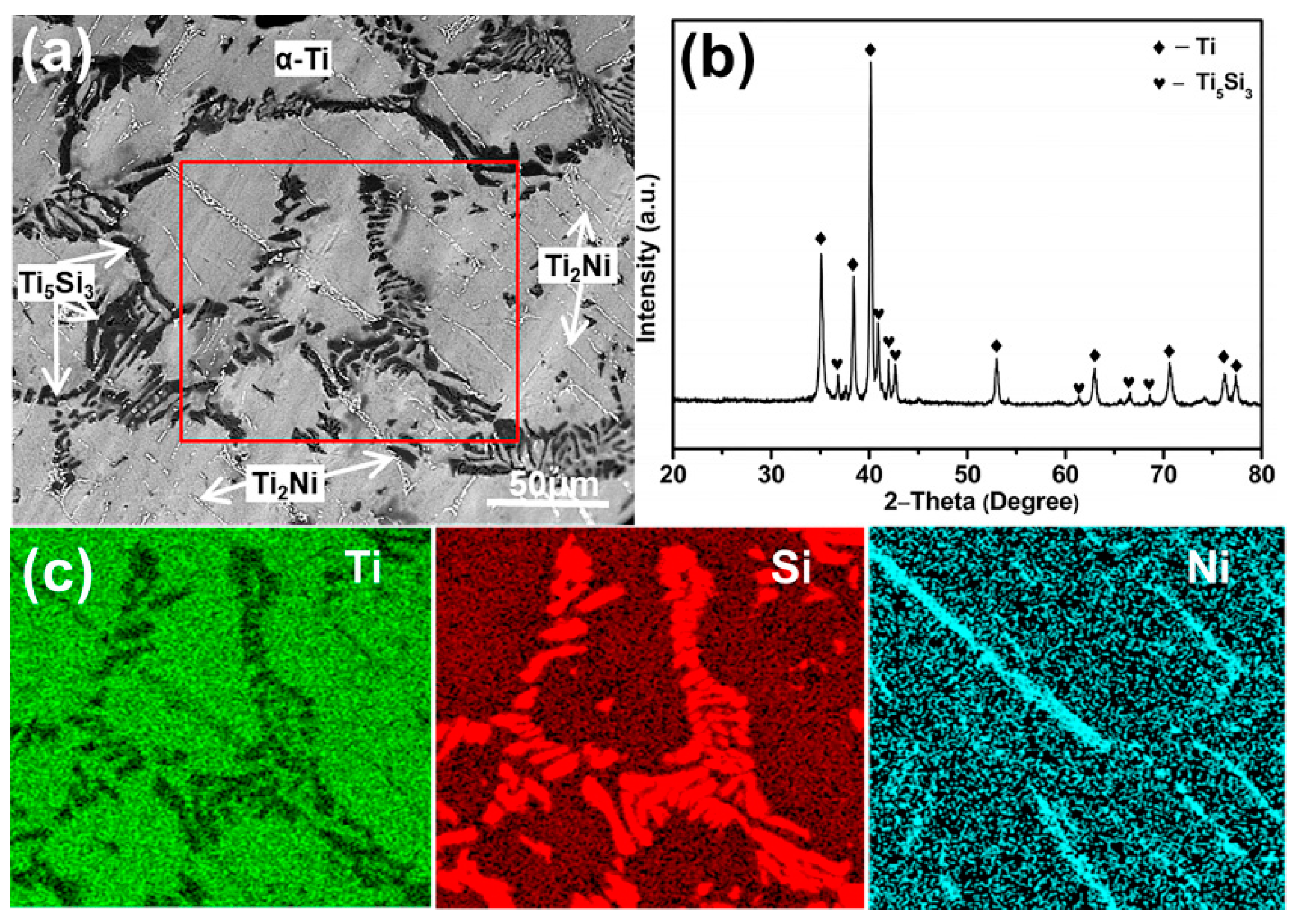
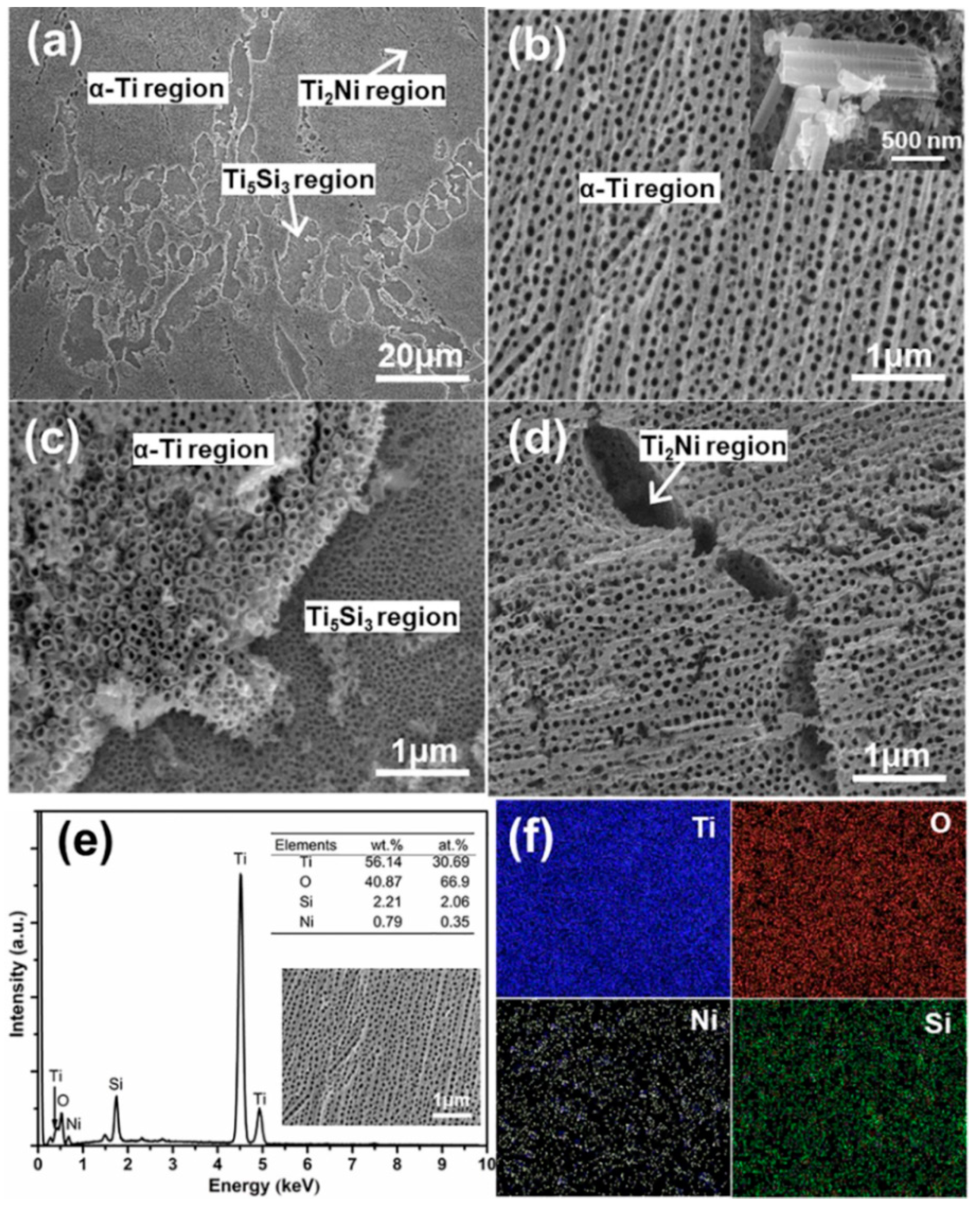
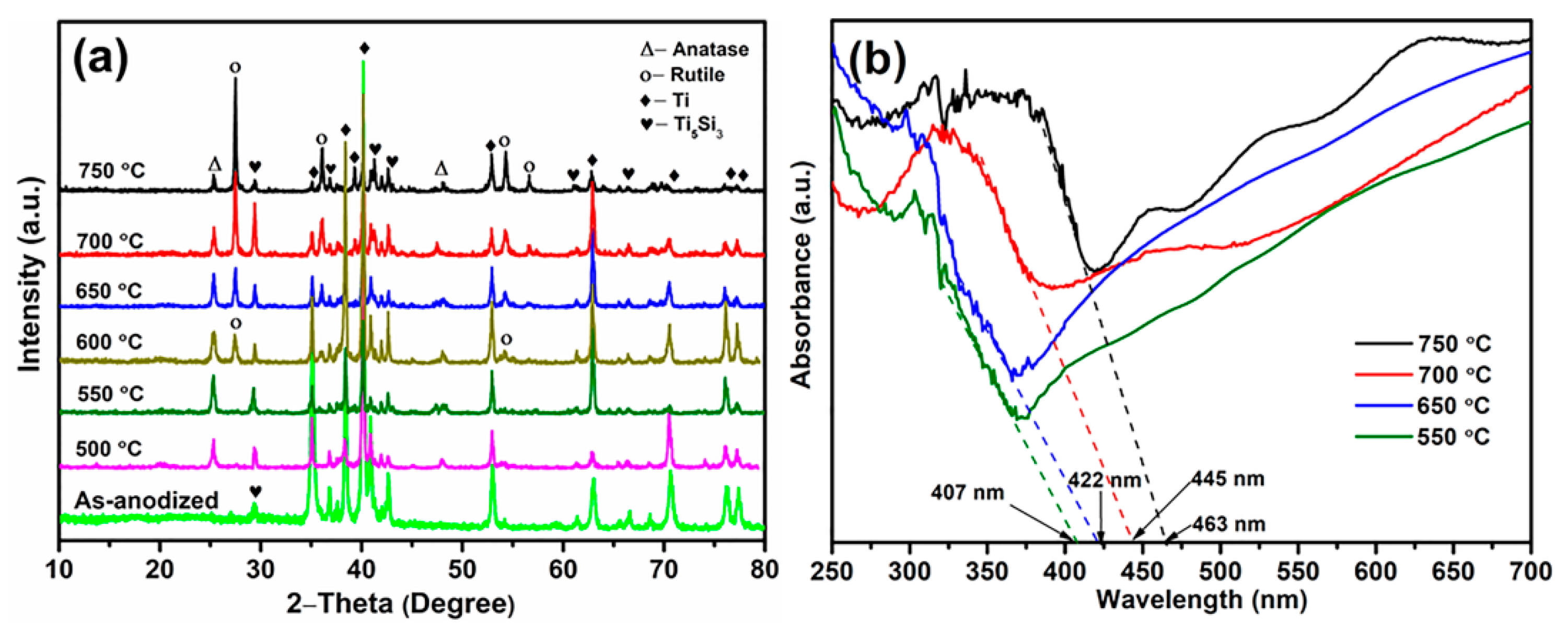
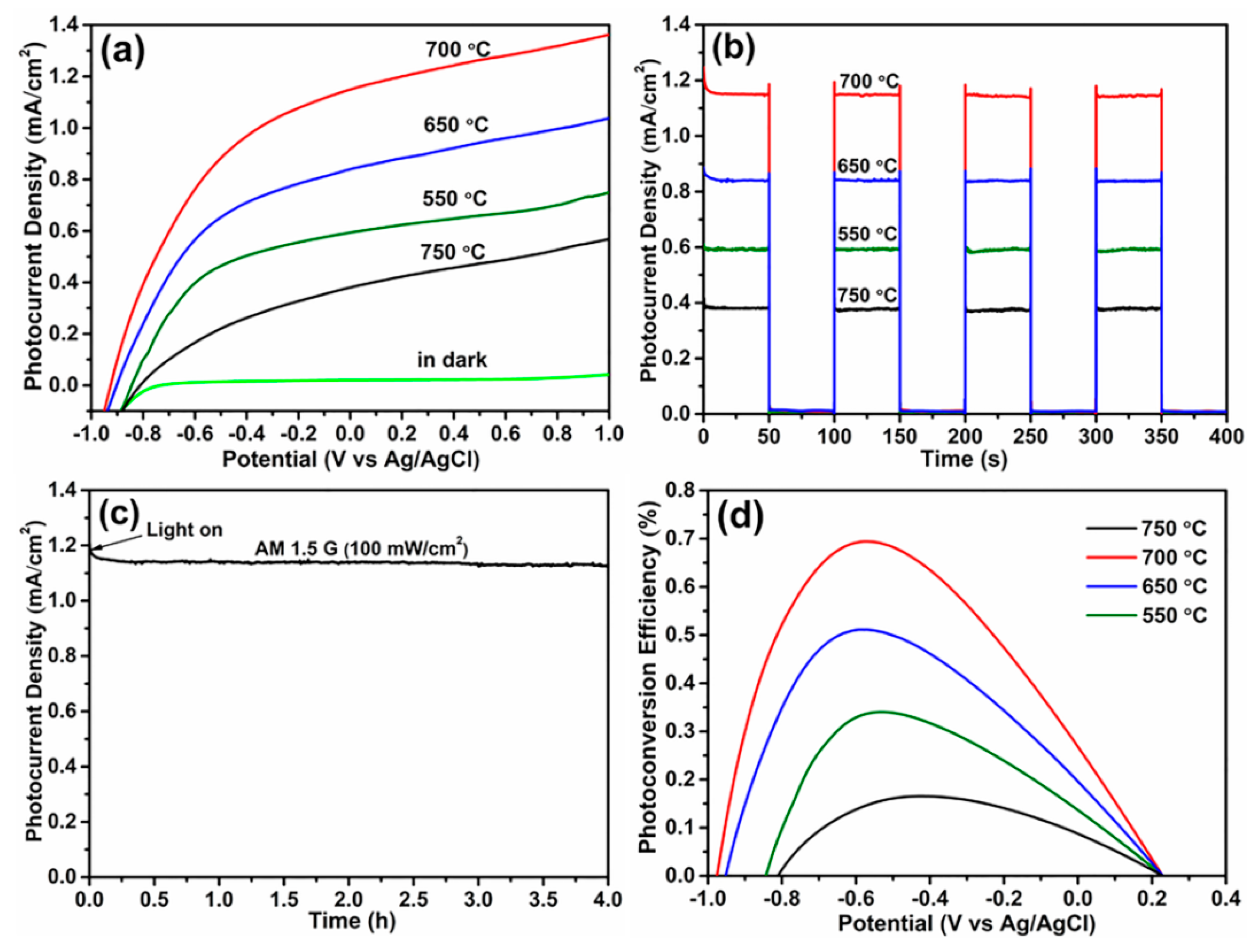
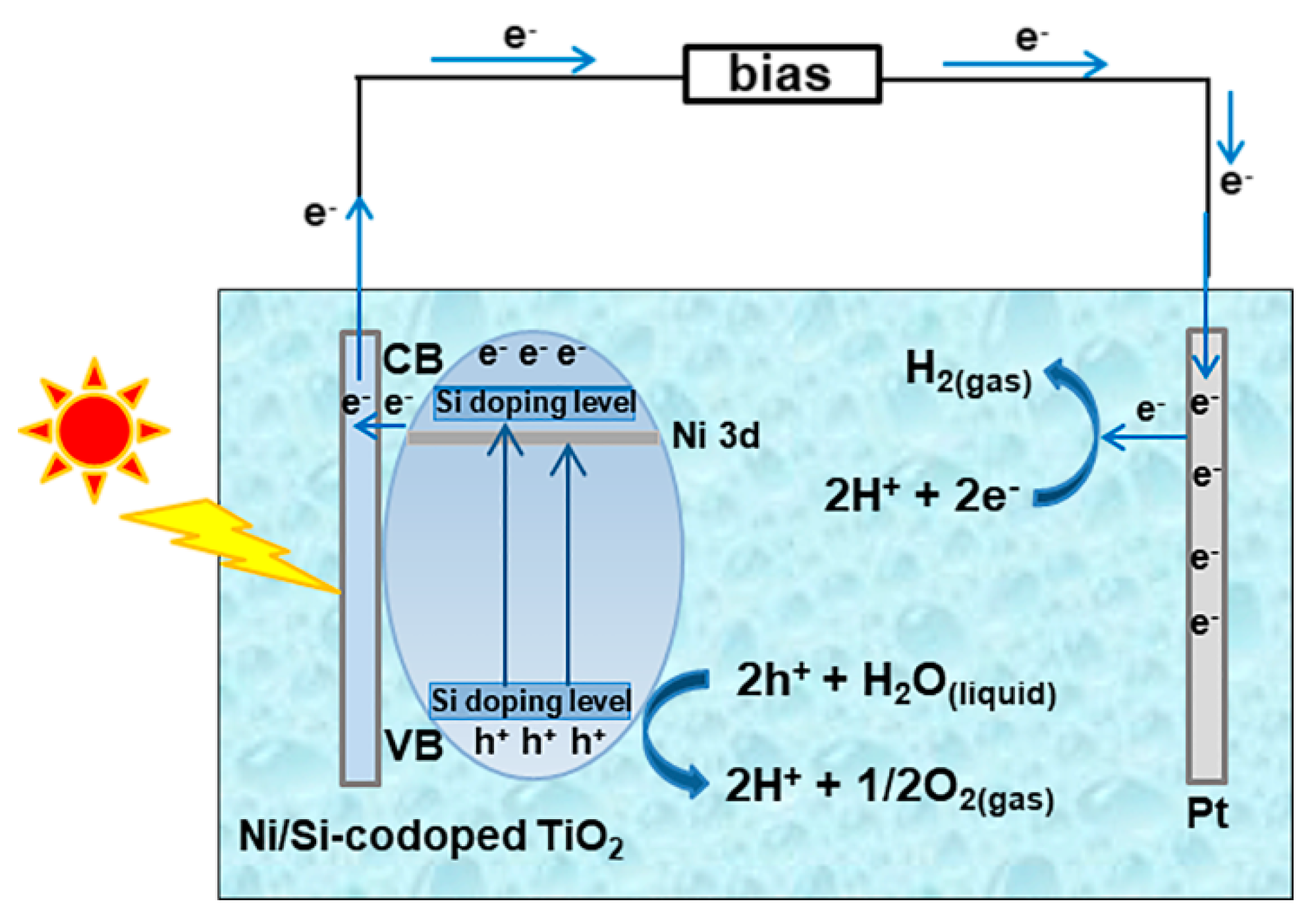
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Momeni, M.M.; Ghayeb, Y.; Davarzadeh, M. Single-step electrochemical anodization for synthesis of hierarchical WO3-TiO2 nanotube arrays on titanium foil as a good photoanode for water splitting with visible light. J. Electroanal. Chem. 2015, 739, 149–155. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37. [Google Scholar] [CrossRef]
- Preethi, L.K.; Mathews, T.; Nand, M.; Jha, S.N.; Gopinath, C.S.; Dash, S. Band alignment and charge transfer pathway in three phase anatase-rutile-brookite TiO2 nanotubes: An efficient photocatalyst for water splitting. Appl. Catal. B 2017, 218, 9–19. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, Y.; Bi, Y. Spatial dual-electric fields for highly enhanced the solar water splitting of TiO2 nanotube arrays. Nano Energy 2019, 57, 542–548. [Google Scholar] [CrossRef]
- Lee, J.S.; You, K.H.; Park, C.B. Highly photoactive, low bandgap TiO2 nanoparticles wrapped by graphene. Adv. Mater. 2012, 2424, 1084–1088. [Google Scholar] [CrossRef]
- Liu, Q.; Ding, D.; Ning, C.; Wang, X. Reduced N/Ni-doped TiO2 nanotubes photoanodes for photoelectrochemical water splitting. RSC Adv. 2015, 55, 95478–95487. [Google Scholar] [CrossRef]
- So, S.G.; Lee, K.; Schmuki, P. Ru-doped TiO2 nanotubes: Improved performance in dye-sensitized solar cells. Phys. Status Solidi RRL 2012, 6, 169–171. [Google Scholar] [CrossRef]
- Belver, C.; Bedia, J.; Rodriguez, J.J. Zr-doped TiO2 supported on delaminated clay materials for solar photocatalytic treatment of emerging pollutants. J. Hazard. Mater. 2017, 322, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, B.; Zhu, Y.; Ma, Z.; Liu, B.; Miao, X.; Ma, R.; Wang, C. Enhanced performance of TiO2-based perovskite solar cells with Ru-doped TiO2 electron transport layer. Sol. Energy 2018, 169, 335–342. [Google Scholar] [CrossRef]
- Bhowmick, G.D.; Noori, M.T.; Das, I.; Neethu, B.; Ghangrekar, M.M.; Mitra, A. Bismuth doped TiO2 as an excellent photocathode catalyst to enhance the performance of microbial fuel cell. Int. J. Hydrog. Energy 2018, 43, 7501–7510. [Google Scholar] [CrossRef]
- Bahadur, N.; Pasricha, R.; Govind; Chand, S.; Kotnala, R.K. Effect of Ni doping on the microstructure and high Curie temperature ferromagnetism in sol-gel derived titania powders. Mater. Chem. Phys. 2012, 133, 471–479. [Google Scholar] [CrossRef]
- Dong, Z.; Ding, D.; Li, T.; Ning, C. Facile fabrication of Si-doped TiO2 nanotubes photoanode for enhanced photoelectrochemical hydrogen generation. Appl. Surf. Sci. 2018, 436, 125–133. [Google Scholar] [CrossRef]
- Yu, L.; Li, M.; Huang, C.; Zhang, Y.; He, J.; Zhou, X.; Zhu, H. Photoelectrochemical properties of N doped black TiO2 nanotube arrays. Mater. Lett. 2018, 216, 239–242. [Google Scholar] [CrossRef]
- Wang, K.; Yu, J.; Liu, L.; Hou, L.; Jin, F. Hierarchical P-doped TiO2 nanotubes array@Ti plate: Towards advanced CO2 photocatalytic reduction catalysts. Ceram. Int. 2016, 42, 16405–16411. [Google Scholar] [CrossRef]
- Sharifi, T.; Ghayeb, Y.; Mohammadi, T.; Momeni, M.M. Enhanced photoelectrochemical water splitting of CrTiO2 nanotube photoanodes by the decoration of their surface via the photodeposition of Ag and Au. Dalton Trans. 2018, 47, 11593–11604. [Google Scholar] [CrossRef]
- Ananthakumar, S.; Ramkumar, J.; Moorthy Babu, S. Effect of co-sensitization of CdSe nanoparticles with N3 dye on TiO2 nanotubes. Sol. Energy 2014, 106, 136–142. [Google Scholar] [CrossRef]
- Rebecca, J.; Priya, G.; Nicola, A.S. Transition metal-doped TiO2 and ZnO-present status of the field. J. Phys. Condens. Matter 2005, 17, 657–689. [Google Scholar]
- Devi, L.G.; Kavitha, R. A review on non metal ion doped titania for the photocatalytic degradation of organic pollutants under UV/solar light: Role of photogenerated charge carrier dynamics in enhancing the activity. Appl. Catal. B 2013, 140, 559–587. [Google Scholar] [CrossRef]
- Sharma, S.D.; Singh, D.; Saini, K.K.; Kant, C.; Sharma, V.; Jain, S.C.; Sharma, C.P. Sol-gel-derived super-hydrophilic nickel doped TiO2 film as active photo-catalyst. Appl. Catal. A 2006, 314, 40–46. [Google Scholar] [CrossRef]
- Barmeh, A.; Nilforoushan, M.R.; Otroj, S. Wetting and photocatalytic properties of Ni-doped TiO2 coating on glazed ceramic tiles under visible light. Thin Solid Films 2018, 666, 137–142. [Google Scholar] [CrossRef]
- Dong, Z.; Ding, D.; Li, T.; Ning, C. Ni-doped TiO2 nanotubes photoanode for enhanced photoelectrochemical water splitting. Appl. Surf. Sci. 2018, 443, 321–328. [Google Scholar] [CrossRef]
- Benjwal, P.; Kar, K.K. One-step synthesis of Zn doped titania nanotubes and investigation of their visible photocatalytic activity. Mater. Chem. Phys. 2015, 160, 279–288. [Google Scholar] [CrossRef]
- Chaves, J.M.; Escada, A.L.A.; Rodrigues, A.D.; Claro, A. Characterization of the structure, thermal stability and wettability of the TiO2 nanotubes growth on the Ti-7.5Mo alloy surface. Appl. Surf. Sci. 2016, 370, 76–82. [Google Scholar] [CrossRef]
- Choi, W.; Termin, A.; Hoffmann, M.R. The role of metal ion dopants in quantum-sized TiO2: Correlation between photoreactivity and charge carrier recombination dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Sood, S.; Umar, A.; Mehta, S.K.; Kansal, S.K. Highly effective Fe-doped TiO2 nanoparticles photocatalysts for visible-light driven photocatalytic degradation of toxic organic compounds. J. Colloid Interface Sci. 2015, 450, 213–223. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.S.R.K. Comparison of modification strategies towards enhanced charge carrier separation and photocatalytic degradation activity of metal oxide semiconductors (TiO2, WO3 and ZnO). Appl. Surf. Sci. 2017, 391, 124–148. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–272. [Google Scholar] [CrossRef]
- Xiao, J.; Pan, Z.; Zhang, B.; Liu, G.; Zhang, H.; Song, X.; Hu, G.; Xiao, C.; Wei, Z.; Zheng, Y. The research of photocatalytic activity on Si doped TiO2 nanotubes. Mater. Lett. 2017, 188, 66–68. [Google Scholar] [CrossRef]
- Chen, C.; Wei, Y.; Yuan, G.; Liu, Q.; Lu, R.; Huang, X.; Cao, Y.; Zhu, P. Synergistic effect of Si doping and heat treatments enhances the photoelectrochemical water oxidation performance of TiO2 nanorod arrays. Adv. Funct. Mater. 2017, 27, 1701575. [Google Scholar] [CrossRef]
- Isimjan, T.T.; Ruby, E.A.; Rohani, S.; Ray, A.K. The fabrication of highly ordered and visible-light-responsive Fe-C-N-Codoped TiO2 nanotubes. Nanotechnology 2010, 21, 055706. [Google Scholar] [CrossRef]
- Varma, R.; Yadav, M.; Tiwari, K.; Makani, N.; Gupta, S.; Kothari, D.C.; Miotello, A.; Patel, N. Roles of vanadium and nitrogen in photocatalytic activity of VN-codoped TiO2 photocatalyst. Photochem. Photobiol. 2018, 94, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Samdarshi, S.K. Correlating oxygen vacancies and phase ratio/interface with efficient photocatalytic activity in mixed phase TiO2. J. Alloys Compd. 2015, 629, 105–112. [Google Scholar] [CrossRef]
- Kang, Q.; Cao, J.; Zhang, Y.; Liu, L.; Xu, H.; Ye, J. Reduced TiO2 nanotube arrays for photoelectrochemical water splitting. J. Mater. Chem. A 2013, 1, 5766–5774. [Google Scholar] [CrossRef]
- Li, T.; Ding, D.; Dong, Z.; Ning, C. Photoelectrochemical water splitting properties of Ti-Ni-Si-O nanostructures on Ti-Ni-Si alloy. Nanomaterials 2017, 7, 359. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ding, D.; Li, N. Anodic fabrication of Ti-Ni-Si-O nanostructures on Ti10Ni5Si alloy. Materials 2019, 12, 1315. [Google Scholar] [CrossRef]
- Lu, B.; Wang, Y.; Xu, J. Revisiting the glass-forming ability of Ti-Ni-Si ternary alloys. J. Alloy. Compd. 2009, 475, 157–164. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, Q.; Xu, X. Photodeposition of CdS nanoparticles sensitized TiO2 nanotube arrays for enhanced photoelectrochemical performance. J. Electrochem. Soc. 2014, 161, 656–659. [Google Scholar] [CrossRef]
- Das, S.; Zazpe, R.; Prikryl, J.; Knotek, P.; Krbal, M.; Sopha, H.; Podzemna, V.; Macak, J.M. Influence of annealing temperatures on the properties of low aspect-ratio TiO2 nanotube layers. Electrochim. Acta 2016, 213, 452–459. [Google Scholar] [CrossRef]
- Yadav, A.K.; Khatun, N.; Kumar, S.; Tseng, C.M.; Biring, S.; Sen, S. Size and strain dependent anatase to rutile phase transition in TiO2 due to Si incorporation. J. Mater. Sci.-Mater. Electron. 2017, 28, 19017–19024. [Google Scholar]
- Su, Y.; Chen, S.; Quan, X.; Zhao, H.; Zhang, Y. A silicon-doped TiO2 nanotube arrays electrode with enhanced photoelectrocatalytic activity. Appl. Surf. Sci. 2008, 255, 2167–2172. [Google Scholar] [CrossRef]
- Gole, J.L.; Prokes, S.M.; Glembocki, O.J. Efficient room-temperature conversion of anatase to rutile TiO2 induced by high-spin ion doping. J. Phys. Chem. C 2008, 112, 1782–1788. [Google Scholar] [CrossRef]
- Lakshmi, K.V.D.; Rao, T.S.; Padmaja, J.S.; Raju, I.M.; Kumar, M.R. Structure, photocatalytic and antibacterial activity study of Meso porous Ni and S co-doped TiO2 nano material under visible light irradiation. Chin. J. Chem. Eng. 2019, 27, 1630–1641. [Google Scholar] [CrossRef]
- Tian, J.; Deng, H.; Sun, L.; Kong, H.; Yang, P.; Chu, J. Influence of Ni doping on phase transformation and optical properties of TiO2 films deposited on quartz substrates by sol-gel process. Appl. Surf. Sci. 2012, 258, 4893–4897. [Google Scholar] [CrossRef]
- Li, Z.; Ding, D.; Liu, Q.; Ning, C.; Wang, X. Ni-doped TiO2 nanotubes for wide-range hydrogen sensing. Nanoscale Res. Lett. 2014, 9, 118. [Google Scholar] [CrossRef]
- Iwamoto, S.; Iwamoto, S.; Inoue, M.; Yoshida, H.; Tanaka, T.; Kagawa, K. XANES and XPS study of silica-modified titanias prepared by the glycothermal method. Chem. Mater. 2005, 17, 650–655. [Google Scholar] [CrossRef]
- Ding, Z.; Zhu, H.Y.; Greenfield, P.F.; Lu, G.Q. Characterization of pore structure and coordination of titanium in TiO2 and SiO2-TiO2 sol-pillared clays. J. Colloid Interface Sci. 2001, 238, 267–272. [Google Scholar] [CrossRef]
- Chen, Q.; Shi, H.; Shi, W.; Xu, Y.; Wu, D. Enhanced visible photocatalytic activity of titania-silica photocatalysts: Effect of carbon and silver doping. Catal. Sci. Technol. 2012, 2, 1213–1220. [Google Scholar] [CrossRef]
- Lin, Y.M.; Jiang, Z.Y.; Zhu, C.Y.; Hu, X.Y.; Zhang, X.D.; Fan, J. Visible-light photocatalytic activity of Ni-doped TiO2 from ab initio calculations. Mater. Chem. Phys. 2012, 133, 746–750. [Google Scholar] [CrossRef]
- Cui, H.; Zhao, W.; Yang, C.; Yin, H.; Lin, T.; Shan, Y.; Xie, Y.; Gu, H.; Huang, F. Black TiO2 nanotube arrays for high-efficiency photoelectrochemical water-splitting. J. Mater. Chem. A 2014, 2, 8612–8616. [Google Scholar] [CrossRef]
- Dong, Z.; Ding, D.; Li, T.; Ning, C. Facile preparation of Ti3+/Ni co-doped TiO2 nanotubes photoanode for efficient photoelectrochemical water splitting. Appl. Surf. Sci. 2019, 480, 219–228. [Google Scholar] [CrossRef]
- Edelmannova, M.; Dubnova, L.; Reli, M.; Meinhardova, V.; Huo, P.; Lavrencic Stangar, U.; Capek, L.; Koci, K. The role of fluorine in F-La/TiO2 photocatalysts on photocatalytic decomposition of methanol-water solution. Materials 2019, 12, 2867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Chen, D.; Ma, N.; Hua, X.; Wang, H. Si doping effects on the photocatalytic activity of TiO2 nanotubes film prepared by an anodization process. Scr. Mater. 2009, 60, 543–546. [Google Scholar] [CrossRef]
- Tang, X.; Feng, Q.; Liu, K.; Luo, X.; Huang, J.; Li, Z. A simple and innovative route to remarkably enhance the photocatalytic performance of TiO2: Using micro-meso porous silica nanofibers as carrier to support highly-dispersed TiO2 nanoparticles. Microporous Mesoporous Mater. 2018, 258, 251–261. [Google Scholar] [CrossRef]
- Alhosseini, A.F. Passivity of AISI 321 stainless steel in 0.5 M H2SO4 solution studied by Mott-Schottky analysis in conjunction with the point defect model. Arab. J. Chem. 2016, 9, S1342–S1348. [Google Scholar] [CrossRef]
- Fernández-Domene, R.M.; Blasco-Tamarit, E.; García-García, D.M.; García Antón, J. Passivity breakdown of titanium in LiBr solutions. J. Electrochem. Soc. 2013, 161, C25–C35. [Google Scholar] [CrossRef]
- Fernández-Domene, R.M.; Sánchez-Tovar, R.; Sánchez-González, S.; García-Antón, J. Photoelectrochemical characterization of anatase-rutile mixed TiO2 nanosponges. Int. J. Hydrog. Energy 2016, 41, 18380–18388. [Google Scholar] [CrossRef]
- Sánchez-Tovar, R.; Fernández-Domene, R.M.; García-García, D.M.; García-Antón, J. Enhancement of photoelectrochemical activity for water splitting by controlling hydrodynamic conditions on titanium anodization. J. Power Sources 2015, 286, 224–231. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Sun, P.; Lu, S.; Wang, L.; Wang, C.; Liu, Y. Photoelectrochemical water splitting with rutile TiO2 nanowires array: Synergistic effect of hydrogen treatment and surface modification with anatase nanoparticles. Electrochim. Acta 2014, 130, 290–295. [Google Scholar] [CrossRef]
- Parmar, K.P.S.; Kang, H.J.; Bist, A.; Dua, P.; Jang, J.S.; Lee, J.S. Photocatalytic and photoelectrochemical water oxidation over metal-doped monoclinic BiVO4 photoanodes. ChemSusChem 2012, 5, 1926–1934. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, L.; Jin, B.W.; Huang, L.; Gan, Y. Enhanced photoelectrochemical properties and water splitting activity of self-ordered MoO3-TiO2 nanotubes. Appl. Surf. Sci. 2016, 364, 410–415. [Google Scholar] [CrossRef]
- Liao, W.; Yang, J.; Zhou, H.; Murugananthan, M.; Zhang, Y. Electrochemically self-doped TiO2 nanotube arrays for efficient visible light photoelectrocatalytic degradation of contaminants. Electrochim. Acta 2014, 136, 310–317. [Google Scholar] [CrossRef]
- Woo, S.R.; Sung, Y.M. Enhanced photoelectrochemical water splitting of micro-arc oxidized TiO2 via anatase/rutile phase control and nitrogen doping. J. Electrochem. Soc. 2016, 163, H278–H285. [Google Scholar] [CrossRef]
- Choi, J.Y.; Sung, Y.H.; Choi, H.J.; Kim, Y.D.; Huh, D.; Lee, H. Fabrication of Au nanoparticle-decorated TiO2 nanotube arrays for stable photoelectrochemical water splitting by two-step anodization. Ceram. Int. 2017, 43, 14063–14067. [Google Scholar] [CrossRef]
- Liang, Z.; Hou, H.; Fang, Z.; Gao, F.; Wang, L.; Chen, D.; Yang, W. Hydrogenated TiO2 nanorod arrays decorated with carbon quantum dots toward efficient photoelectrochemical water splitting. ACS Appl. Mater. Interfaces 2019, 11, 19167–19175. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Ding, D. Ni/Si-Codoped TiO2 Nanostructure Photoanode for Enhanced Photoelectrochemical Water Splitting. Materials 2019, 12, 4102. https://doi.org/10.3390/ma12244102
Li T, Ding D. Ni/Si-Codoped TiO2 Nanostructure Photoanode for Enhanced Photoelectrochemical Water Splitting. Materials. 2019; 12(24):4102. https://doi.org/10.3390/ma12244102
Chicago/Turabian StyleLi, Ting, and Dongyan Ding. 2019. "Ni/Si-Codoped TiO2 Nanostructure Photoanode for Enhanced Photoelectrochemical Water Splitting" Materials 12, no. 24: 4102. https://doi.org/10.3390/ma12244102
APA StyleLi, T., & Ding, D. (2019). Ni/Si-Codoped TiO2 Nanostructure Photoanode for Enhanced Photoelectrochemical Water Splitting. Materials, 12(24), 4102. https://doi.org/10.3390/ma12244102




