Enhanced Activation of Persulfate by Co-Doped Bismuth Ferrite Nanocomposites for Degradation of Levofloxacin Under Visible Light Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of BFO and Co-BFO Catalysts
2.2. Characterization of Catalysts
2.3. Experimental Procedure
2.4. Analytical Methods
3. Results
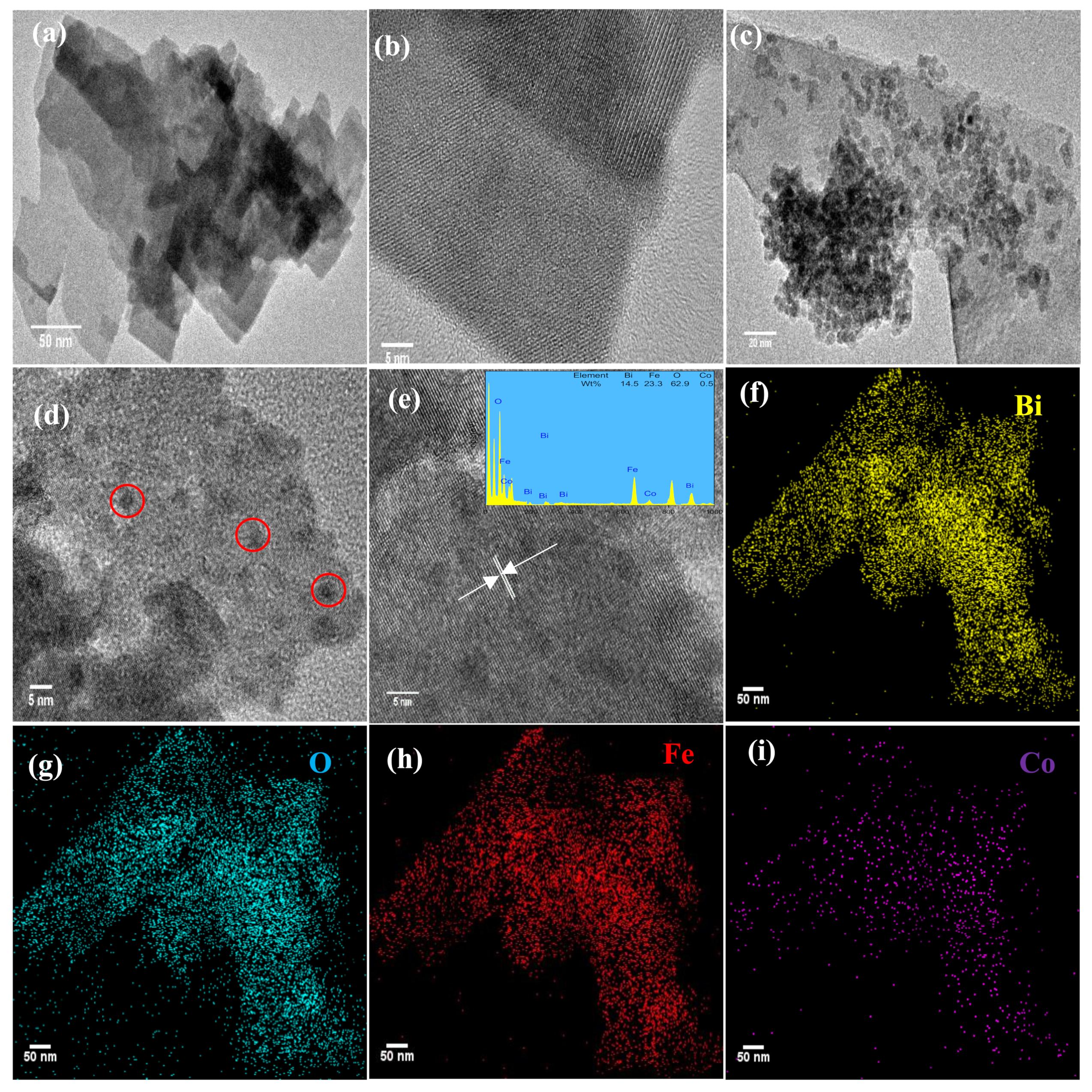
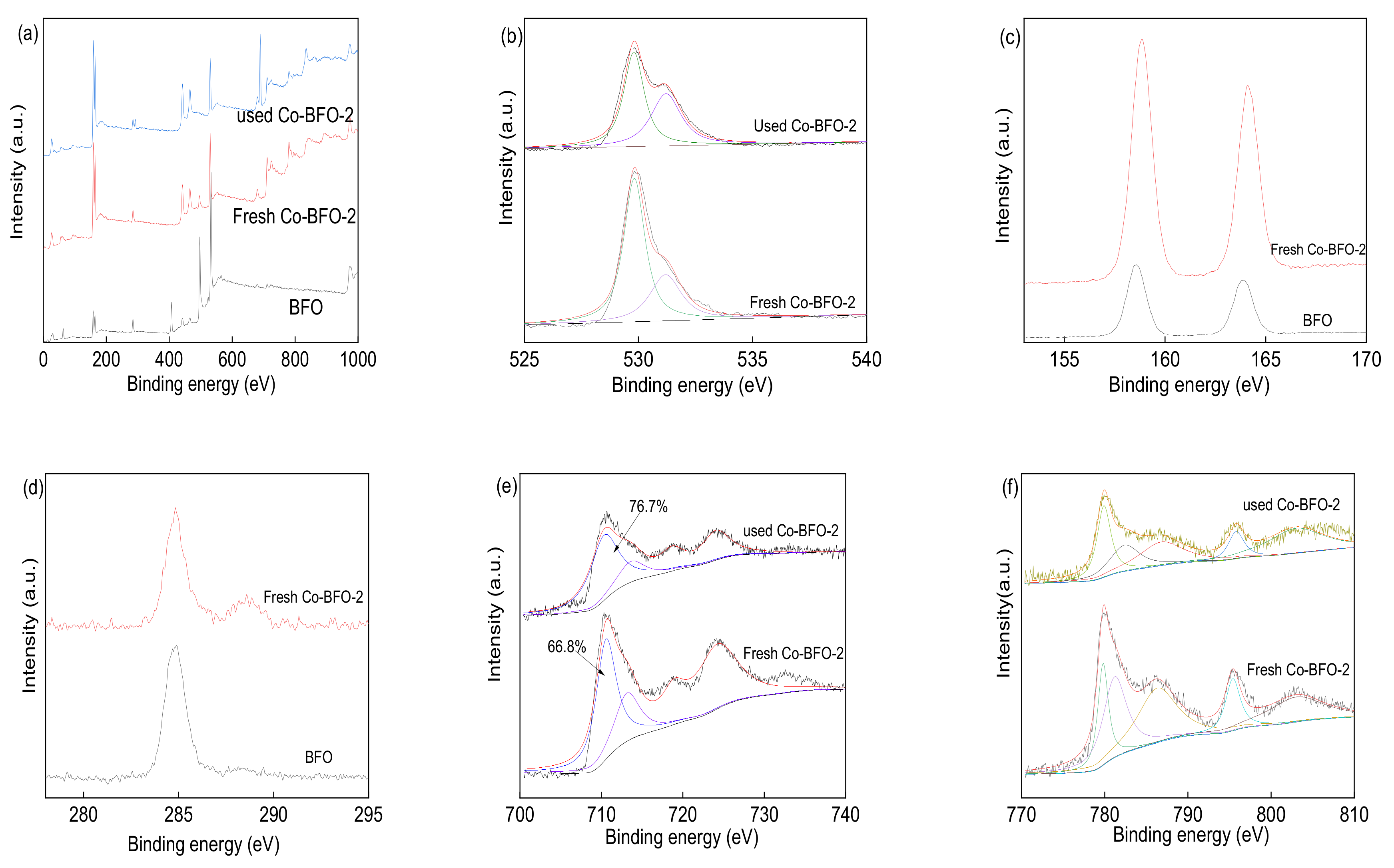
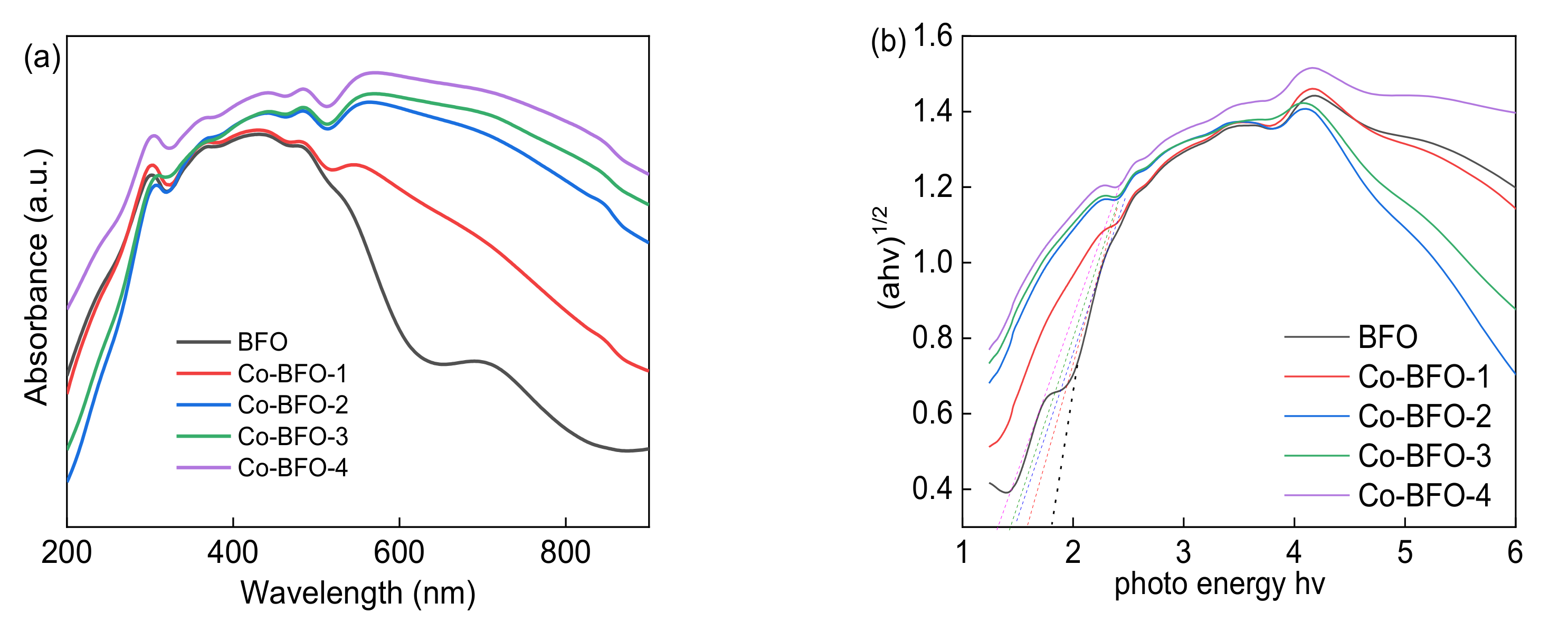
3.1. Catalyst Characterization
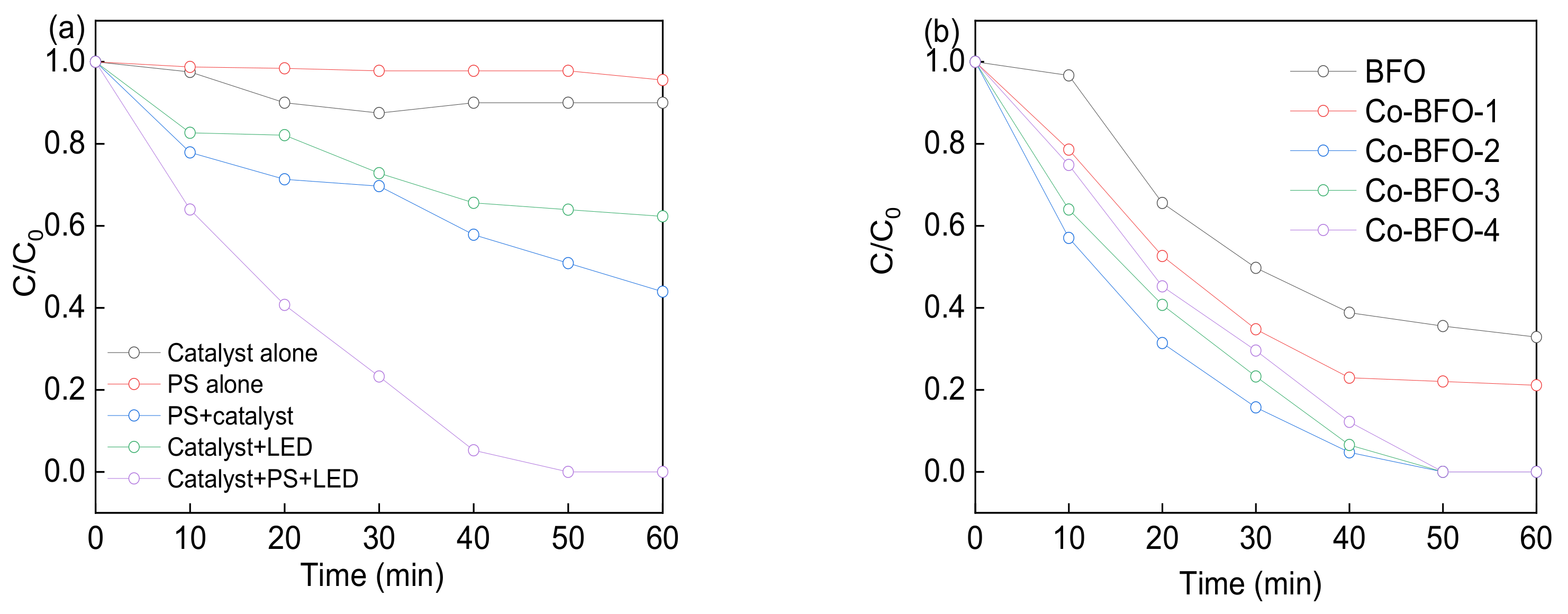
3.2. Photocatalytic Procedures
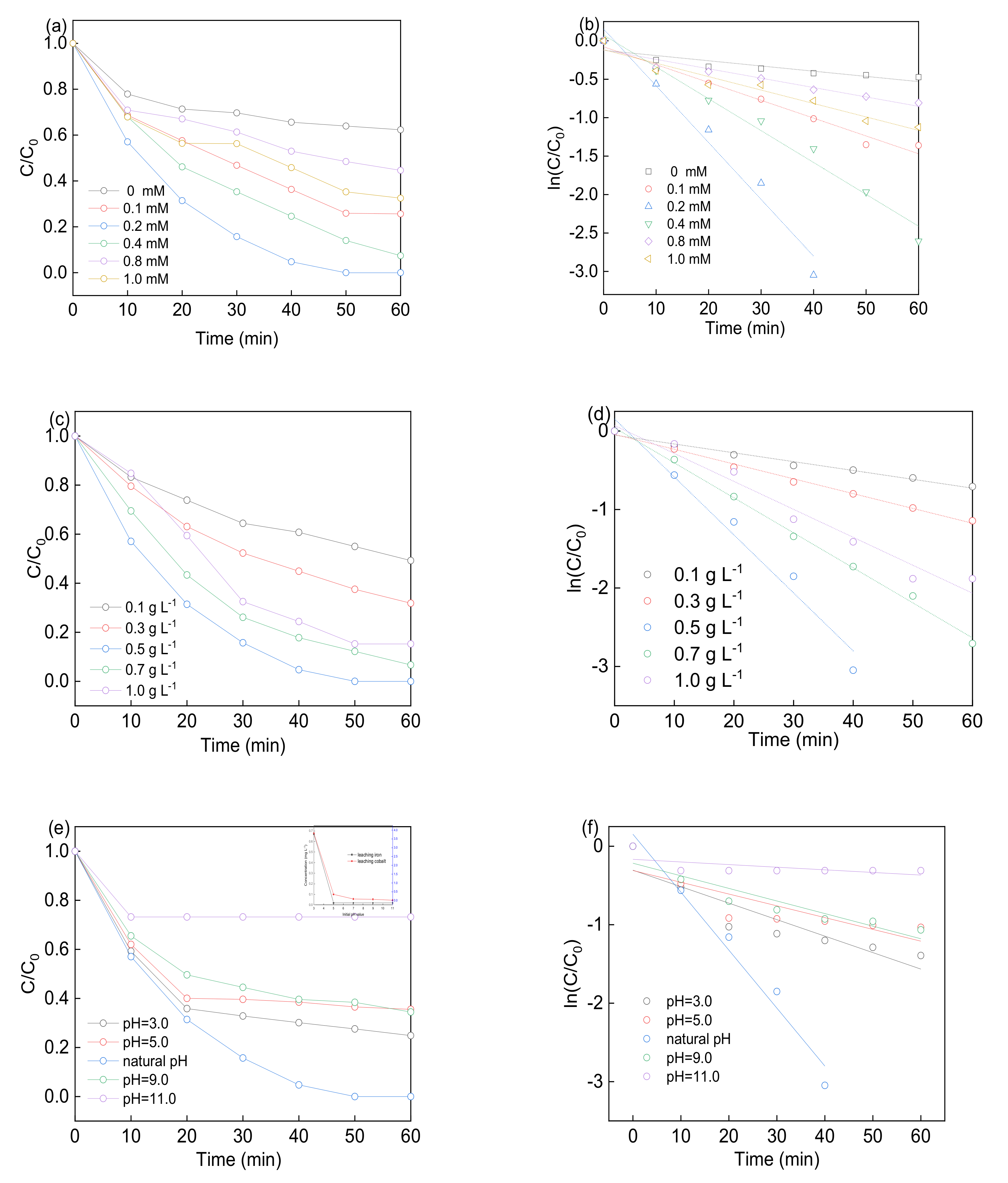
3.3. Effect of Experimental Factors
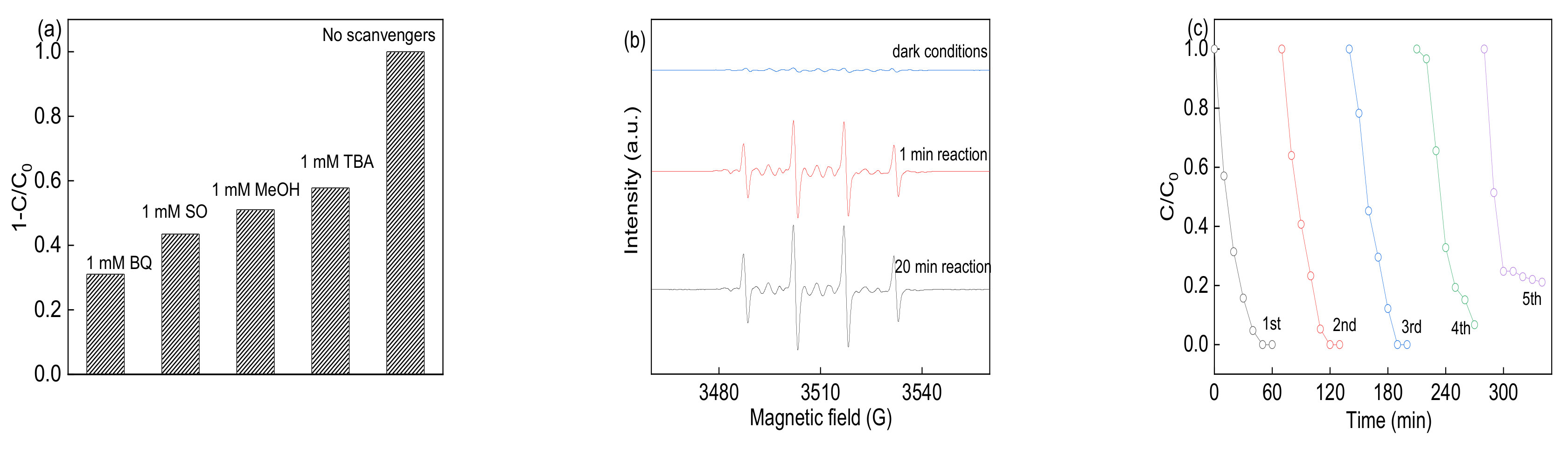
3.4. Identification of Reactive Species and Recycling Tests
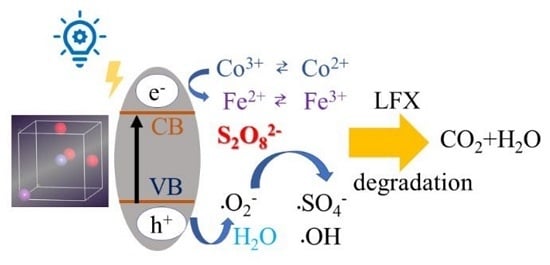
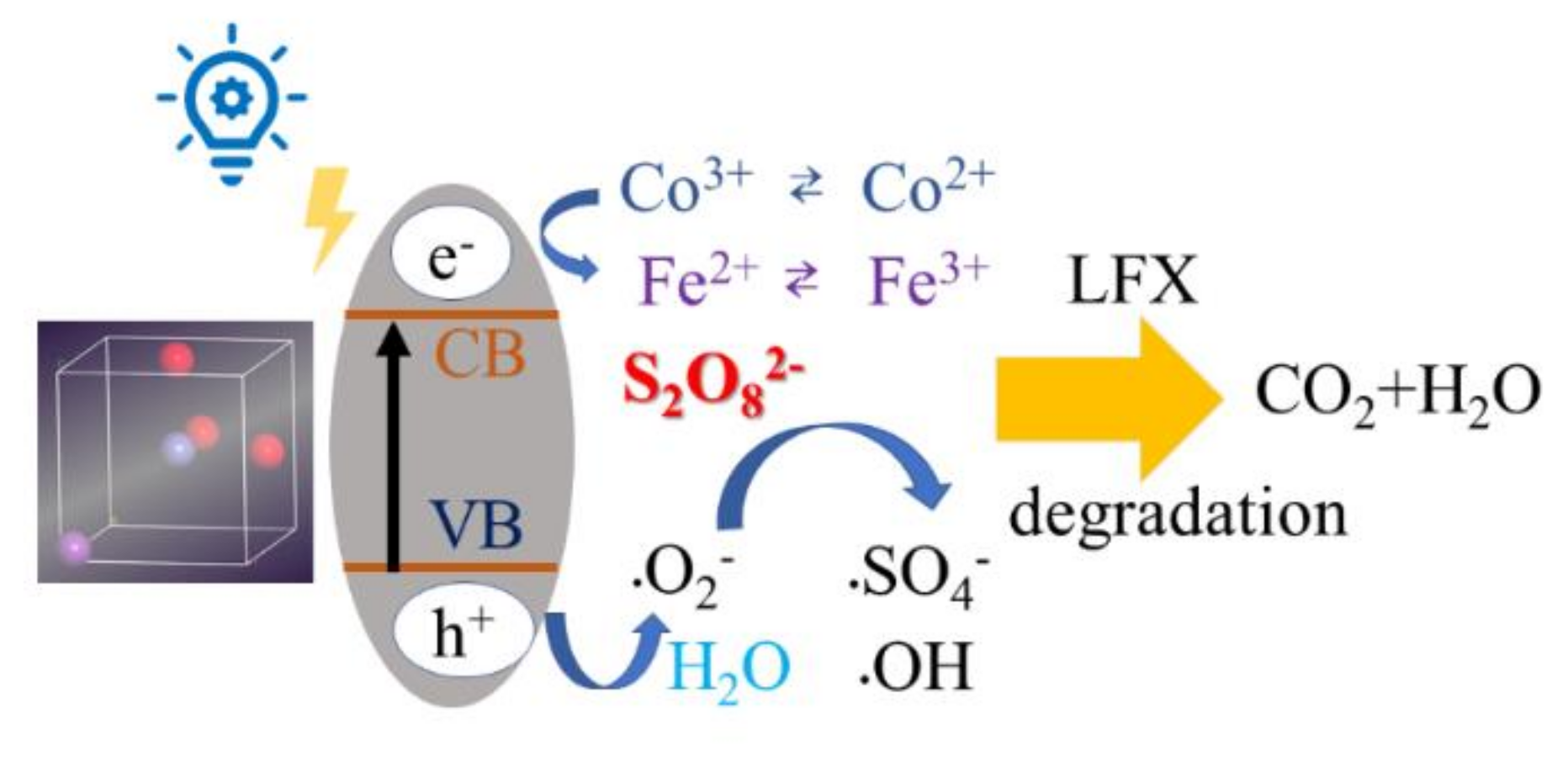
3.5. Potential Degradation Mechanism of LFX by Co-BFO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liao, Z.; Zhu, J.; Jawad, A.; Muzi, J.; Chen, Z.; Chen, Z. Degradation of phenol using peroxymonosulfate activated by a high efficiency and stable CoMgAl-LDH catalyst. Materials 2019, 12, 968. [Google Scholar] [CrossRef] [PubMed]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hubner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Ike, I.A.; Lee, Y.; Hur, J. Impacts of advanced oxidation processes on disinfection byproducts from dissolved organic matter upon post-chlor(am)ination: A critical review. Chem. Eng. J. 2019, 375, 121929. [Google Scholar] [CrossRef]
- Popat, A.; Nidheesh, P.V.; Singh, T.S.A.; Kumar, M.S. Mixed industrial wastewater treatment by combined electrochemical advanced oxidation and biological processes. Chemosphere 2019, 237, 124419. [Google Scholar] [CrossRef]
- Arzate, S.; Pfister, S.; Oberschelp, C.; Sanchez-Perez, J.A. Environmental impacts of an advanced oxidation process as tertiary treatment in a wastewater treatment plant. Sci. Total. Environ. 2019, 694, 133572. [Google Scholar] [CrossRef]
- An, T.; Yang, H.; Li, G.; Song, W.; Cooper, W.J.; Nie, X. Kinetics and mechanism of advanced oxidation processes (AOPs) in degradation of ciprofloxacin in water. Appl. Catal. B Environ. 2010, 94, 288–294. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Xi, Y.; Zhu, J.; Zhu, G.; He, H. Strategies for enhancing the heterogeneous Fenton catalytic reactivity: A review. Appl. Catal. B Environ. 2019, 255, 117739. [Google Scholar] [CrossRef]
- Poblete, R.; Perez, N. Use of sawdust as pretreatment of photo-Fenton process in the depuration of landfill leachate. J. Environ. Manage. 2020, 253, 109697. [Google Scholar] [CrossRef]
- Gharaee, A.; Khosravi-Nikou, M.R.; Anvaripour, B. Hydrocarbon contaminated soil remediation: A comparison between Fenton, sono-Fenton, photo-Fenton and sono-photo-Fenton processes. J. Ind. Eng. Chem. 2019, 79, 181–193. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Dong, X.X.; Lv, Y.K. Functionalized metal-organic frameworks for photocatalytic degradation of organic pollutants in environment. Chemosphere 2020, 242, 125144. [Google Scholar] [CrossRef]
- Gao, X.; Shang, Y.; Liu, L.; Gao, K. Ag plasmon resonance promoted 2D AgBr-δ-Bi2O3 nanosheets with enhanced photocatalytic ability. J. Alloys. Compounds. 2019, 803, 565–575. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, P.; Chen, H.; Yu, Q. Photocatalytic performance and dispersion stability of nanodispersed TiO2 hydrosol in electrolyte solutions with different cations. J. Environ. Sci. 2020, 88, 59–71. [Google Scholar] [CrossRef]
- Xu, L.; Su, J.; Zheng, G.; Zhang, L. Enhanced photocatalytic performance of porous ZnO thin films by CuO nanoparticles surface modification. Mater. Sci. Eng. B. 2019, 248, 114405. [Google Scholar] [CrossRef]
- Qiu, M.; Wang, R.; Qi, X. Hollow polyhedral α-Fe2O3 prepared by self-assembly and its photocatalytic activities in degradation of RhB. J. Taiwan. Inst. Chem. E 2019, 102, 394–402. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, X.; Li, Y.; Jin, Z. Performance of WO3/g-C3N4 heterojunction composite boosting with NiS for photocatalytic hydrogen evolution. Appl. Surf. Sci. 2020, 499, 143862. [Google Scholar] [CrossRef]
- Tian, F.; Li, G.; Zhao, H.; Chen, F.; Li, M.; Liu, Y.; Chen, R. Residual Fe enhances the activity of BiOCl hierarchical nanostructure for hydrogen peroxide activation. J. Catal. 2019, 370, 265–273. [Google Scholar] [CrossRef]
- Ramirez, F.E.N.; Cabrera-Pasca, G.A.; Mestnik-Filho, J.; Carbonari, A.W.; Souza, J.A. Magnetic and transport properties assisted by local distortions in Bi2Mn4O10 and Bi2Fe4O9 multiferroic compounds. J. Alloys. Compounds. 2015, 651, 405–413. [Google Scholar] [CrossRef]
- Wang, K.; Xu, X.; Lu, L.; Li, A.; Han, X.; Wu, Y.; Miao, J.; Jiang, Y. Magnetically recoverable Ag/Bi2Fe4O9 nanoparticles as a visible-light driven photocatalyst. Chem. Phys. Lett. 2019, 715, 129–133. [Google Scholar] [CrossRef]
- Wu, T.; Liu, L.; Pi, M.; Zhang, D.; Chen, S. Enhanced magnetic and photocatalytic properties of Bi2Fe4O9 semiconductor with large exposed (001) surface. Appl. Surf. Sci. 2016, 377, 253–261. [Google Scholar] [CrossRef]
- Oh, W.D.; Dong, Z.; Ronn, G.; Lim, T.T. Surface-active bismuth ferrite as superior peroxymonosulfate activator for aqueous sulfamethoxazole removal: Performance, mechanism and quantification of sulfate radical. J. Hazar. Mater. 2017, 325, 71–81. [Google Scholar] [CrossRef]
- Salami, M.; Mirzaee, O.; Honarbakhsh-Raouf, A.; Lavasani, S.A.N.H.; Moghadam, A.K. Structural, morphological and magnetic parameters investigation of multiferroic (1-x) Bi2Fe4O9-xCoFe2O4 nanocomposite ceramics. Ceram. Int. 2017, 43, 14701–14709. [Google Scholar] [CrossRef]
- Wang, G.; Liu, S.; He, T.; Liu, X.; Deng, Q.; Mao, Y.; Wang, S. Enhanced visible-light-driven photocatalytic activities of Bi2Fe4O9/g-C3N4 composite photocatalysts. Mater. Res. Bull. 2018, 104, 104–111. [Google Scholar] [CrossRef]
- Wang, F.L.; Li, Y.; Wang, N.; Zhu, L.; Jain, A.; Wang, Y.G.; Chen, F.G. Enhanced magnetic, ferroelectric and optical properties of Sr and Co co-doped BiFeO3 powders. J. Alloys. Compounds. 2019, 810, 151941. [Google Scholar] [CrossRef]
- Mohapatra, S.R.; Sahu, B.; Chandrasekhar, M.; Kumar, P.; Kaushik, S.D.; Rath, S.; Singh, A.K. Effect of cobalt substitution on structural, impedance, ferroelectric and magnetic properties of multiferroic Bi2Fe4O9 ceramics. Ceram. Int. 2016, 42(10), 12352–12360. [Google Scholar] [CrossRef]
- Yun, W.C.; Lin, K.Y.A.; Tong, W.C.; Lin, Y.F.; Du, Y. Enhanced degradation of paracetamol in water using sulfate radical-based advanced oxidation processes catalyzed by 3-dimensional Co3O4 nanoflower. Chem. Eng. J. 2019, 373, 1329–1337. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, Y.; Chen, F.; Yao, F.; Sun, J.; Wang, S.; Yi, K.; Hou, L.; Li, X.; Wang, D. Recent advances in photo-activated sulfate radical-advanced oxidation process (SR-AOP) for refractory organic pollutants removal in water. Chem. Eng. J. 2019, 378, 122149. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, X.; Ji, Y.; Wei, J. Sulfate radical-based oxidation of the antibiotics sulfamethoxazole, sulfisoxazole, sulfathiazole, and sulfamethizole: The role of five-membered heterocyclic rings. Sci. Total. Environ. 2019, 692, 201–208. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, S.; Lia, B.; Cheng, X.; Cheng, Q. Fabrication of magnetic Co/BiFeO3 composite and its advanced treatment of pharmaceutical waste water by activation of peroxysulphate. Sep. Purif. Tech. 2018, 202, 242–247. [Google Scholar] [CrossRef]
- Lu, F.C.; Yin, K.; Fu, K.X.; Wang, Y.N.; Ren, J.; Xie, Q. Enhanced magnetic and dielectric properties of Y doped bismuth ferrite nanofiber. Ceram. Int. 2017, 43, 16101–16106. [Google Scholar] [CrossRef]
- Pooladi, M.; Shokrollahi, H.; Lavasani, S.A.N.H.; Yang, H. Investigation of the structural, magnetic and dielectric properties of Mn-doped Bi2Fe4O9 produced by reverse chemical co-precipitation. Mater. Chem. Phys. 2019, 229, 39–48. [Google Scholar] [CrossRef]
- Luo, W.; Hu, F.; Hu, Y.; Dai, H.; Xu, L.; Xu, G.; Jian, Y.; Peng, X. Persulfate enhanced visible light photocatalytic degradation of organic pollutants by construct magnetic hybrid heterostructure. J. Alloys. Compounds. 2019, 806, 1207–1219. [Google Scholar] [CrossRef]
- Yang, L.; Bai, X.; Shi, J.; Du, X.; Xu, L.; Jin, P. Quasi-full-visible-light absorption by D35-TiO2/g-C3N4 for synergistic persulfate activation towards efficient photodegradation of micropollutants. Appl. Catal. B Environ. 2019, 256, 117759. [Google Scholar] [CrossRef]
- Miao, S.; Zha, Z.; Li, Y.; Geng, X.; Yang, J.; Cui, S.; Yang, J. Visible-light-driven MIL-53(Fe)/BiOCl composite assisted by persulfate: Photocatalytic performance and mechanism. J. Photoch. Photobiol. A. 2019, 380, 111862. [Google Scholar] [CrossRef]
- Kilic, M.Y.; Abdelraheem, W.H.; He, X.; Kestioglu, K.; Dionysiou, D.D. Photochemical treatment of tyrosol, a model phenolic compound present in olive mill wastewater, by hydroxyl and sulfate radical-based advanced oxidation processes (AOPs). J. Hazar. Mater. 2019, 367, 734–742. [Google Scholar] [CrossRef]
- Cashman, M.A.; Kirschenbaum, L.; Holowachuk, J.; Boving, T.B. Identification of hydroxyl and sulfate free radicals involved in the reaction of 1,4-dioxane with peroxone activated persulfate oxidant. J. Hazar. Mater. 2019, 380, 120875. [Google Scholar] [CrossRef]

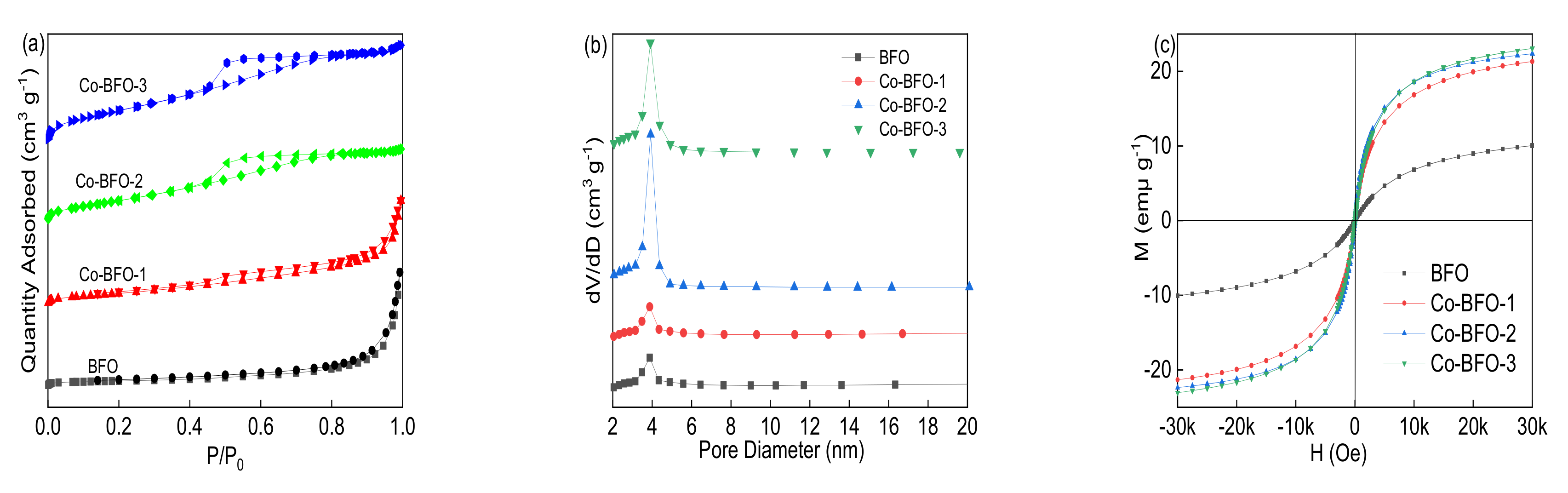
| Samples | BET Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) | Ms (emμ g−1) |
|---|---|---|---|---|
| BFO | 11.8004 | 0.034523 | 3.80215 | 9.76 |
| Co-BFO-1 | 29.9661 | 0.047210 | 3.90179 | 21.26 |
| Co-BFO-2 | 58.1761 | 0.061675 | 4.24059 | 22.17 |
| Co-BFO-3 | 78.8946 | 0.078730 | 3.99163 | 23.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, X.; Zou, Z.-S.; Wang, H.-L.; Huang, W.; Zhou, B.-X. Enhanced Activation of Persulfate by Co-Doped Bismuth Ferrite Nanocomposites for Degradation of Levofloxacin Under Visible Light Irradiation. Materials 2019, 12, 3952. https://doi.org/10.3390/ma12233952
Zhong X, Zou Z-S, Wang H-L, Huang W, Zhou B-X. Enhanced Activation of Persulfate by Co-Doped Bismuth Ferrite Nanocomposites for Degradation of Levofloxacin Under Visible Light Irradiation. Materials. 2019; 12(23):3952. https://doi.org/10.3390/ma12233952
Chicago/Turabian StyleZhong, Xin, Zheng-Shuo Zou, Hu-Lin Wang, Wei Huang, and Bin-Xue Zhou. 2019. "Enhanced Activation of Persulfate by Co-Doped Bismuth Ferrite Nanocomposites for Degradation of Levofloxacin Under Visible Light Irradiation" Materials 12, no. 23: 3952. https://doi.org/10.3390/ma12233952
APA StyleZhong, X., Zou, Z.-S., Wang, H.-L., Huang, W., & Zhou, B.-X. (2019). Enhanced Activation of Persulfate by Co-Doped Bismuth Ferrite Nanocomposites for Degradation of Levofloxacin Under Visible Light Irradiation. Materials, 12(23), 3952. https://doi.org/10.3390/ma12233952