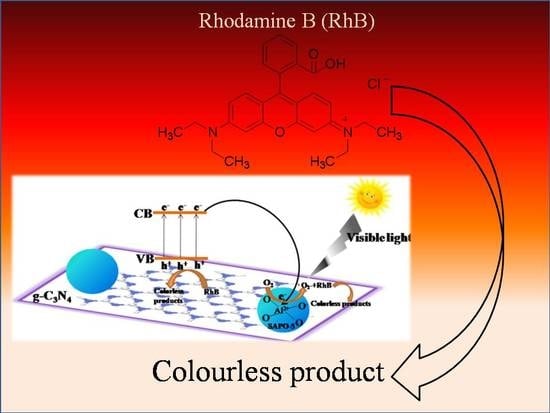
Enhanced Visible-Light Photocatalytic Performance of SAPO-5-Based g-C3N4 Composite for Rhodamine B (RhB) Degradation
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of SAPO-5/g-C3N4 Composite
2.3. Characterization
2.4. Photocatalytic Experiment
3. Results and Discussion
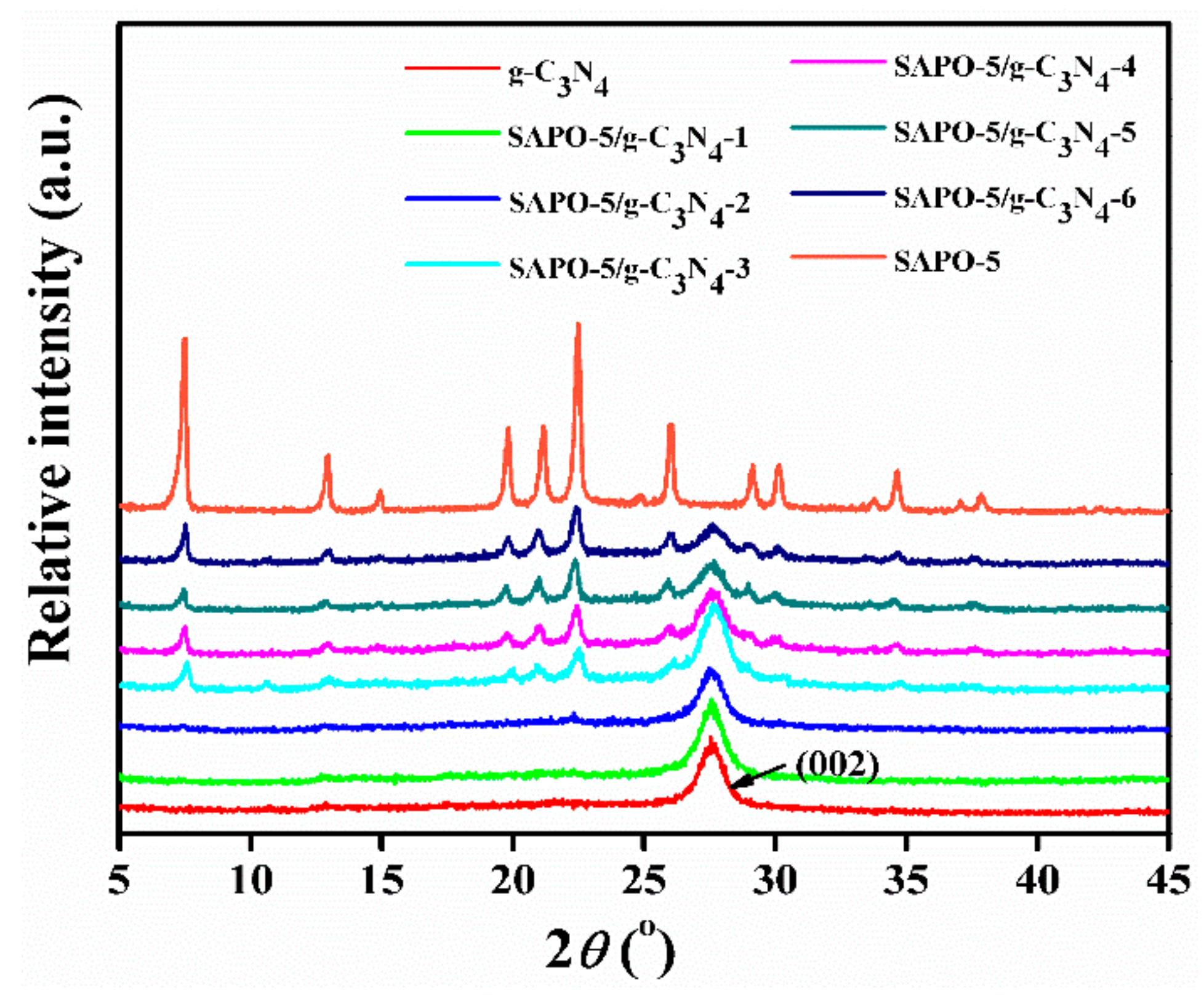
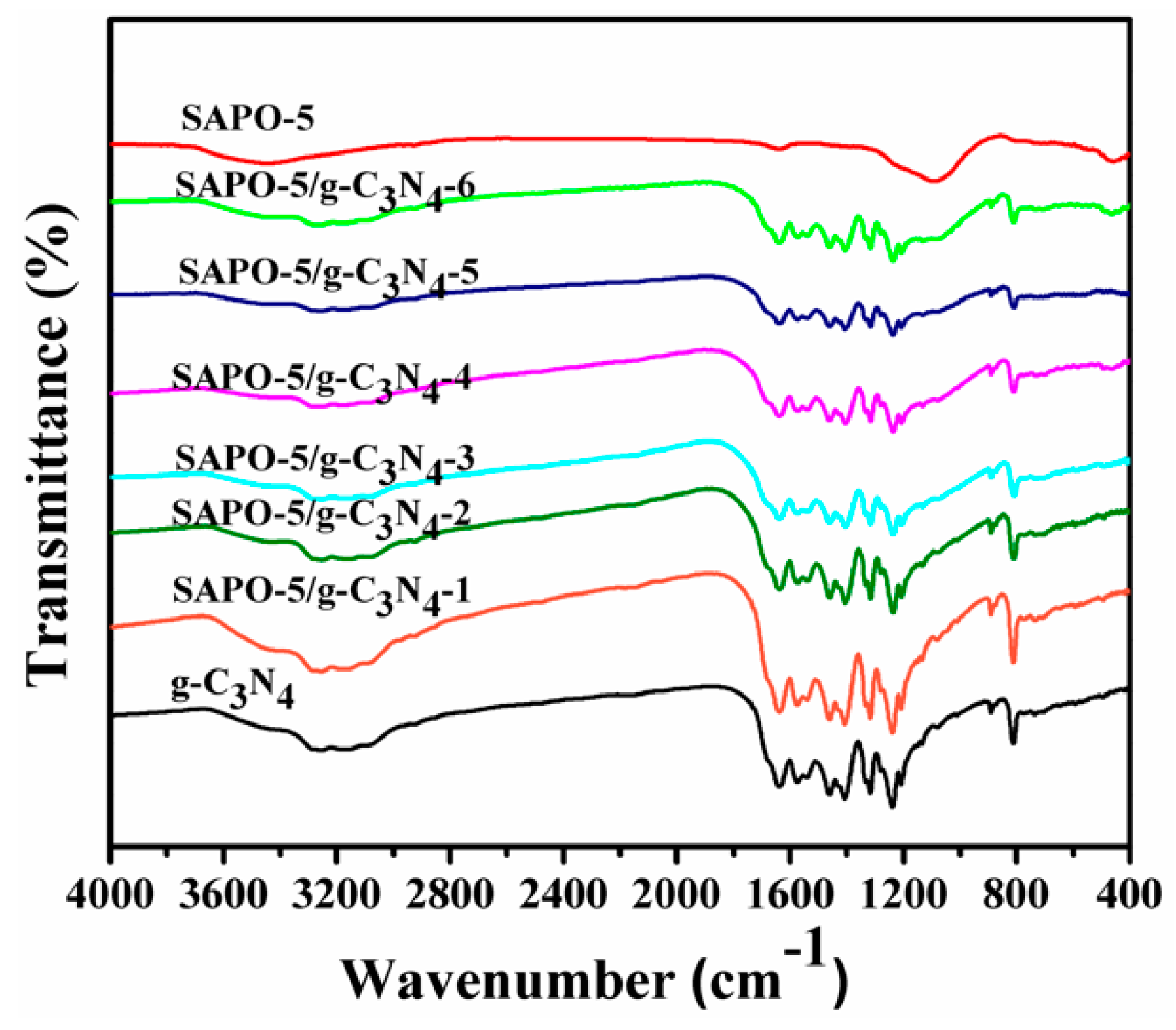
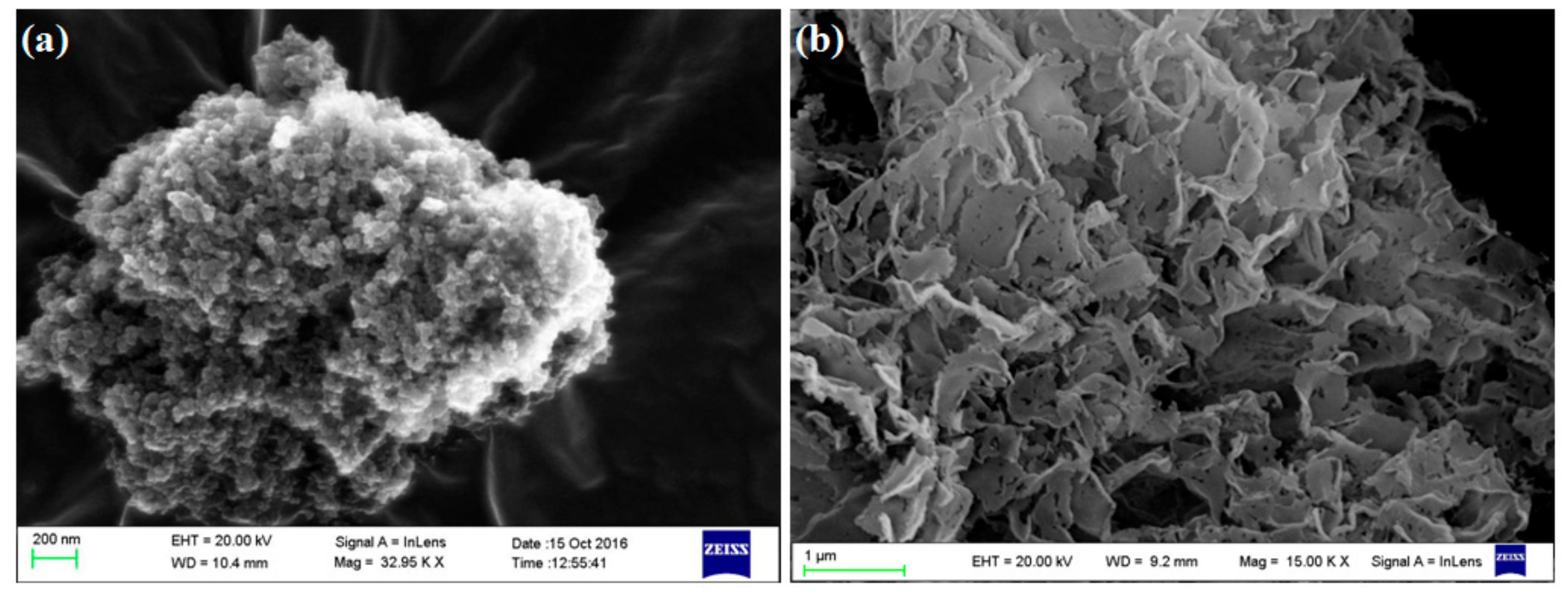
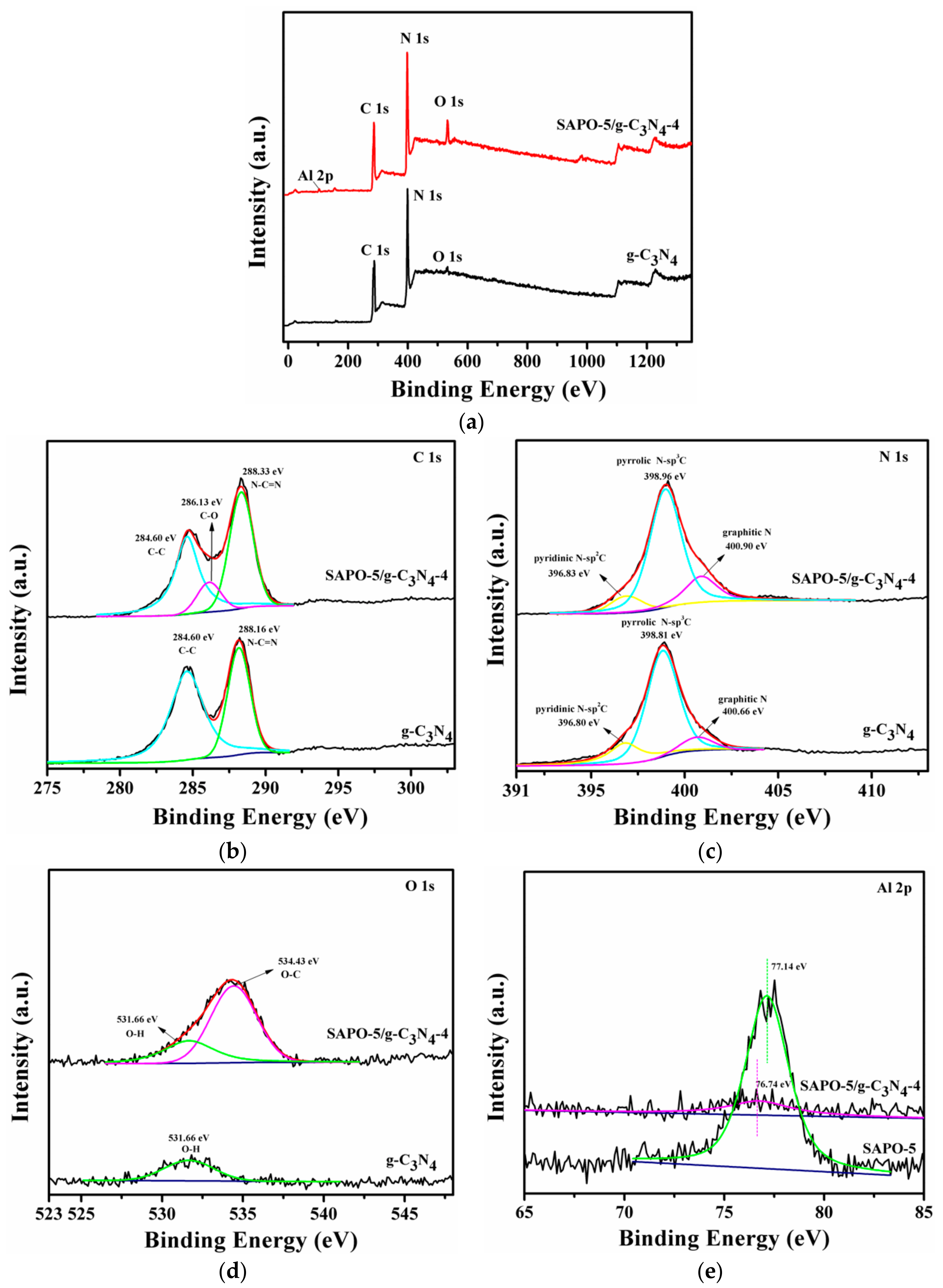
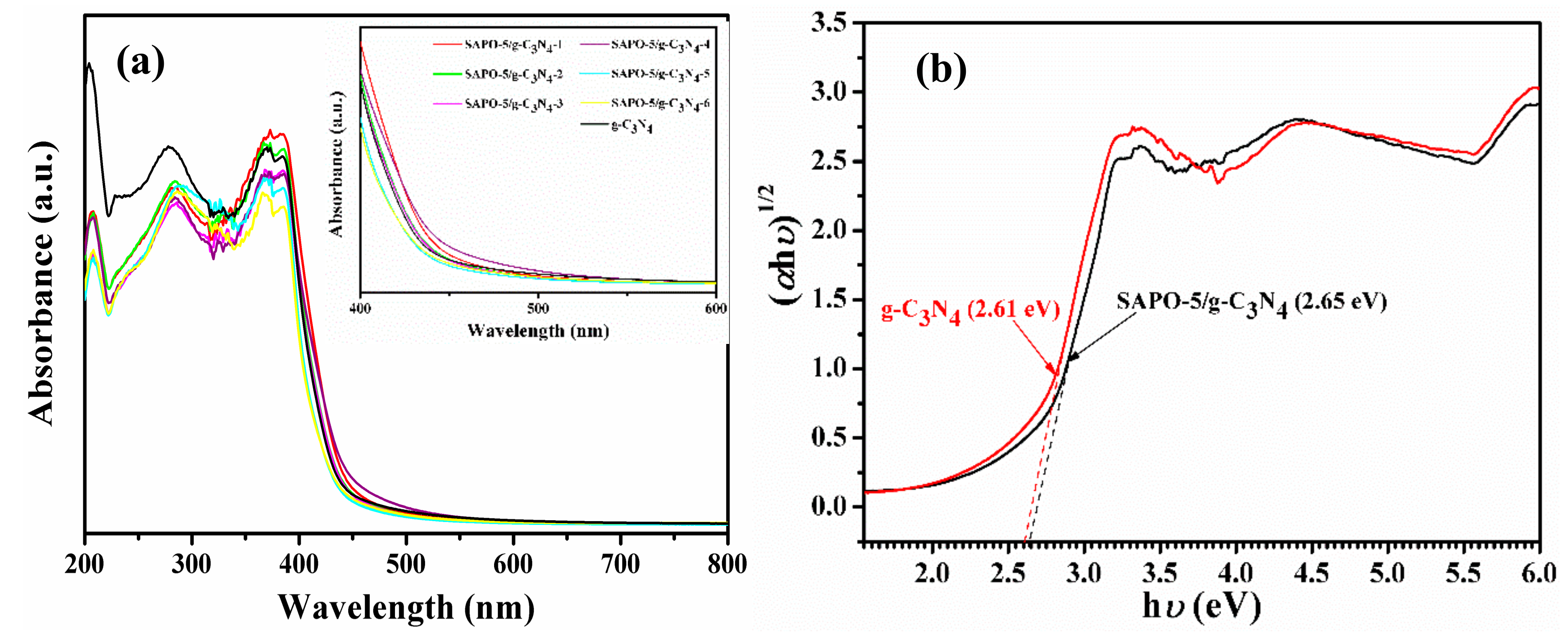
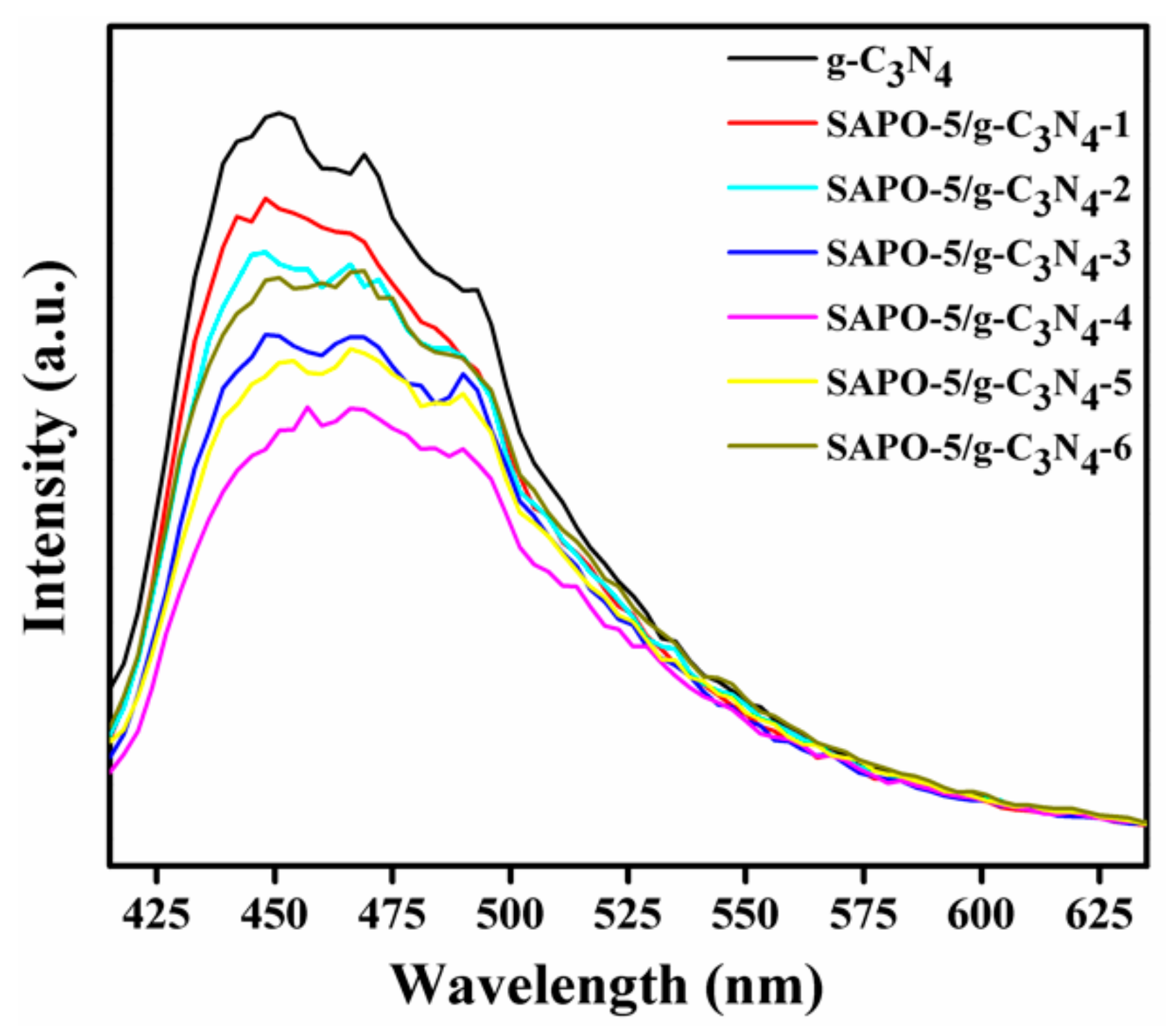
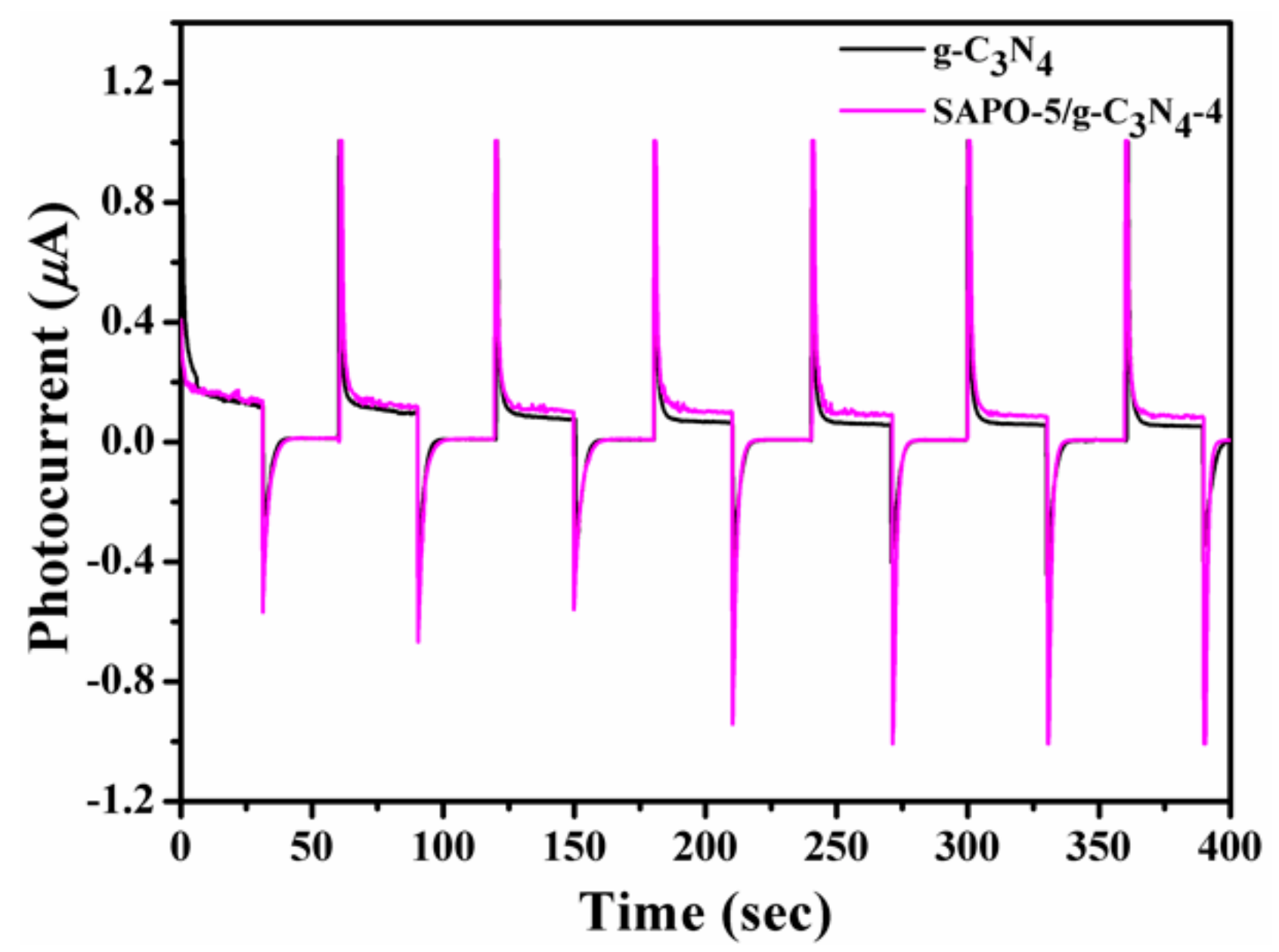
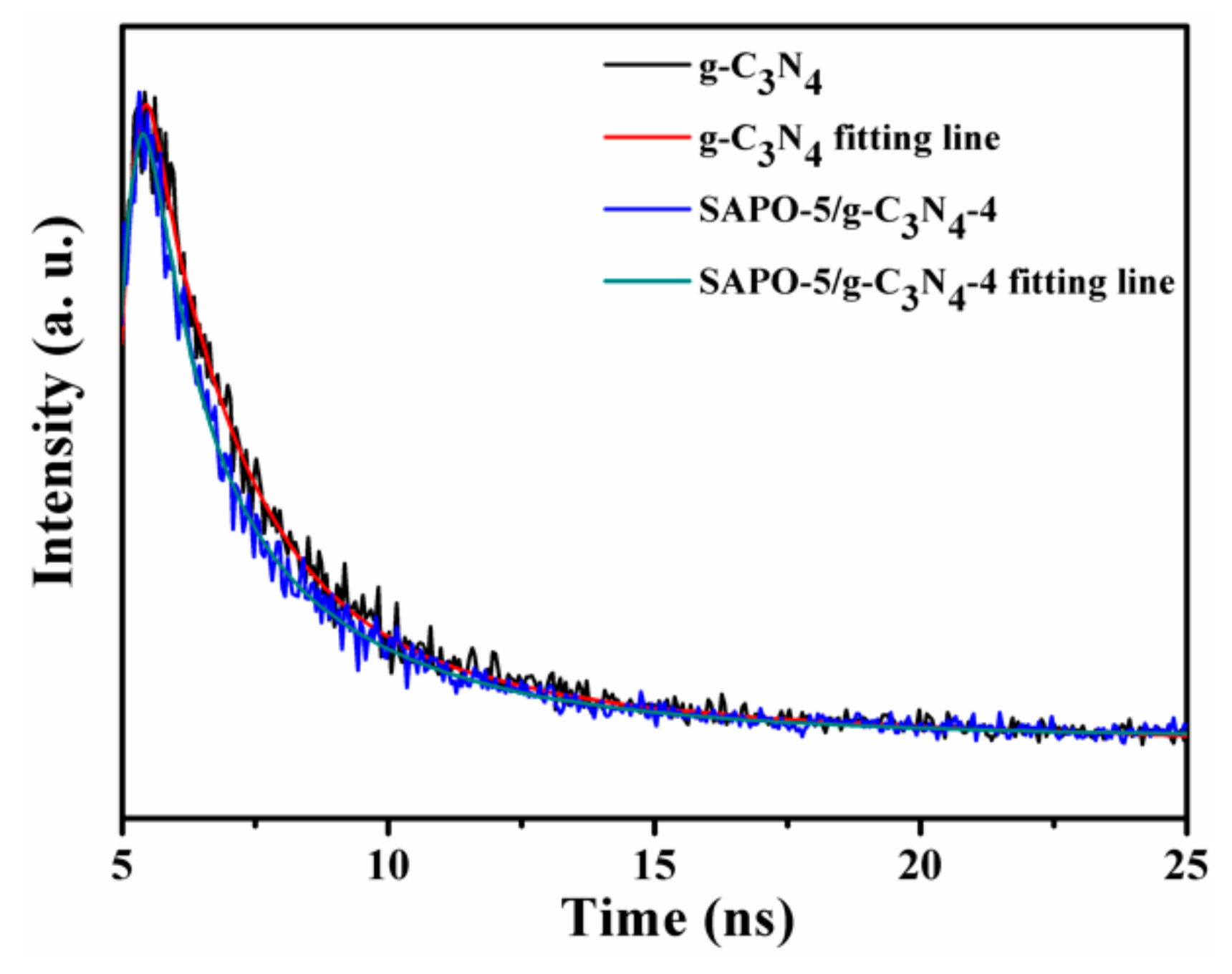
3.1. Characterization of Samples
3.2. Visible-Light Photocatalytic Performance
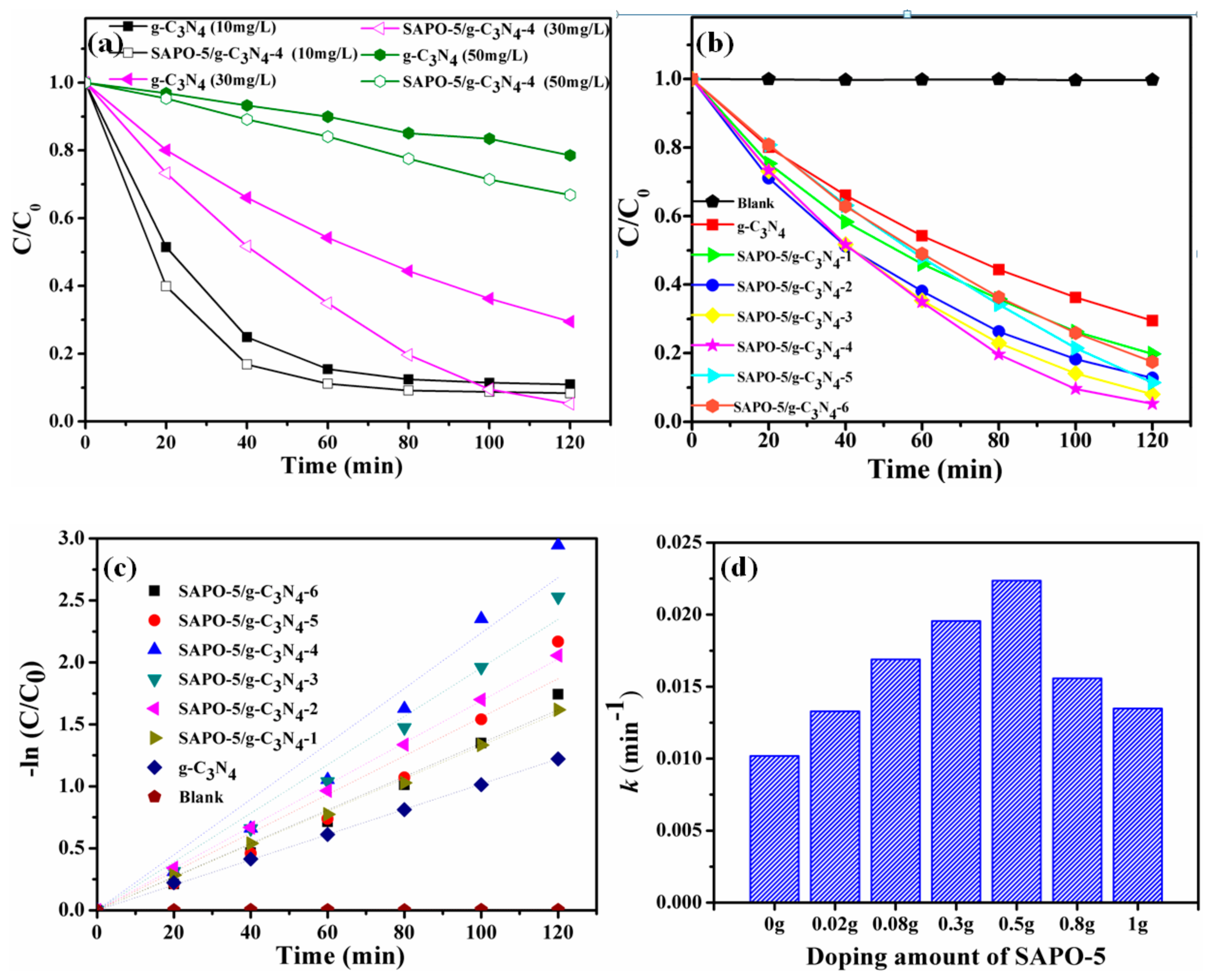
3.2.1. Effect of Initial RhB Concentration
3.2.2. Effect of the SAPO-5 Incorporation Proportion
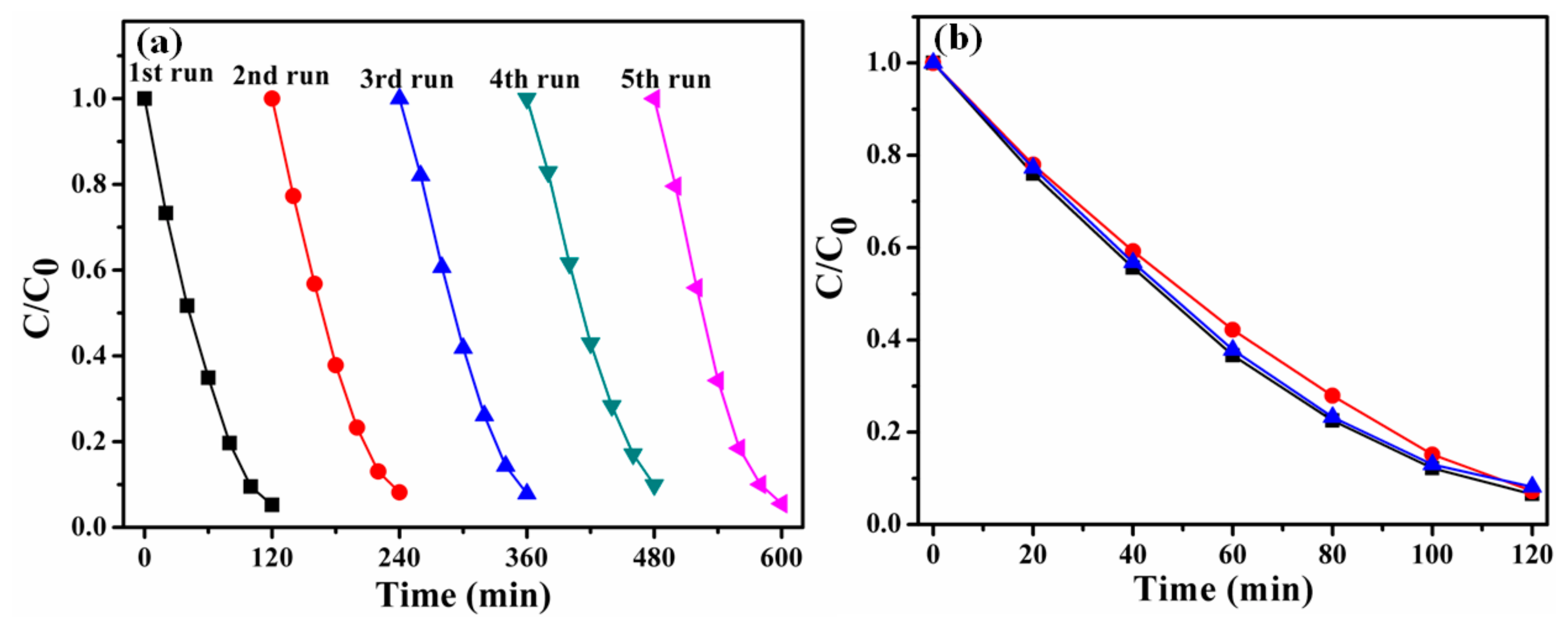
3.3. Reusability
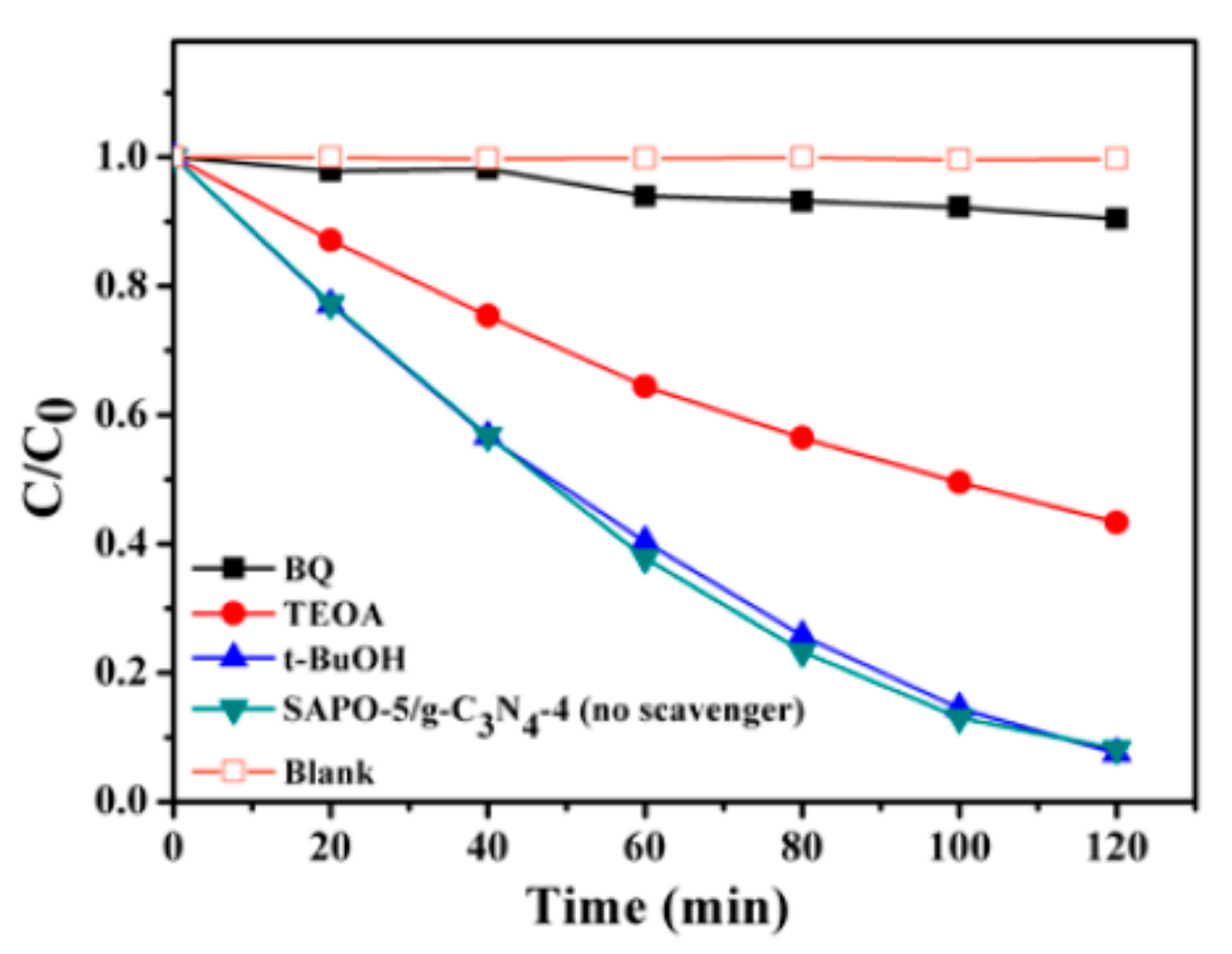
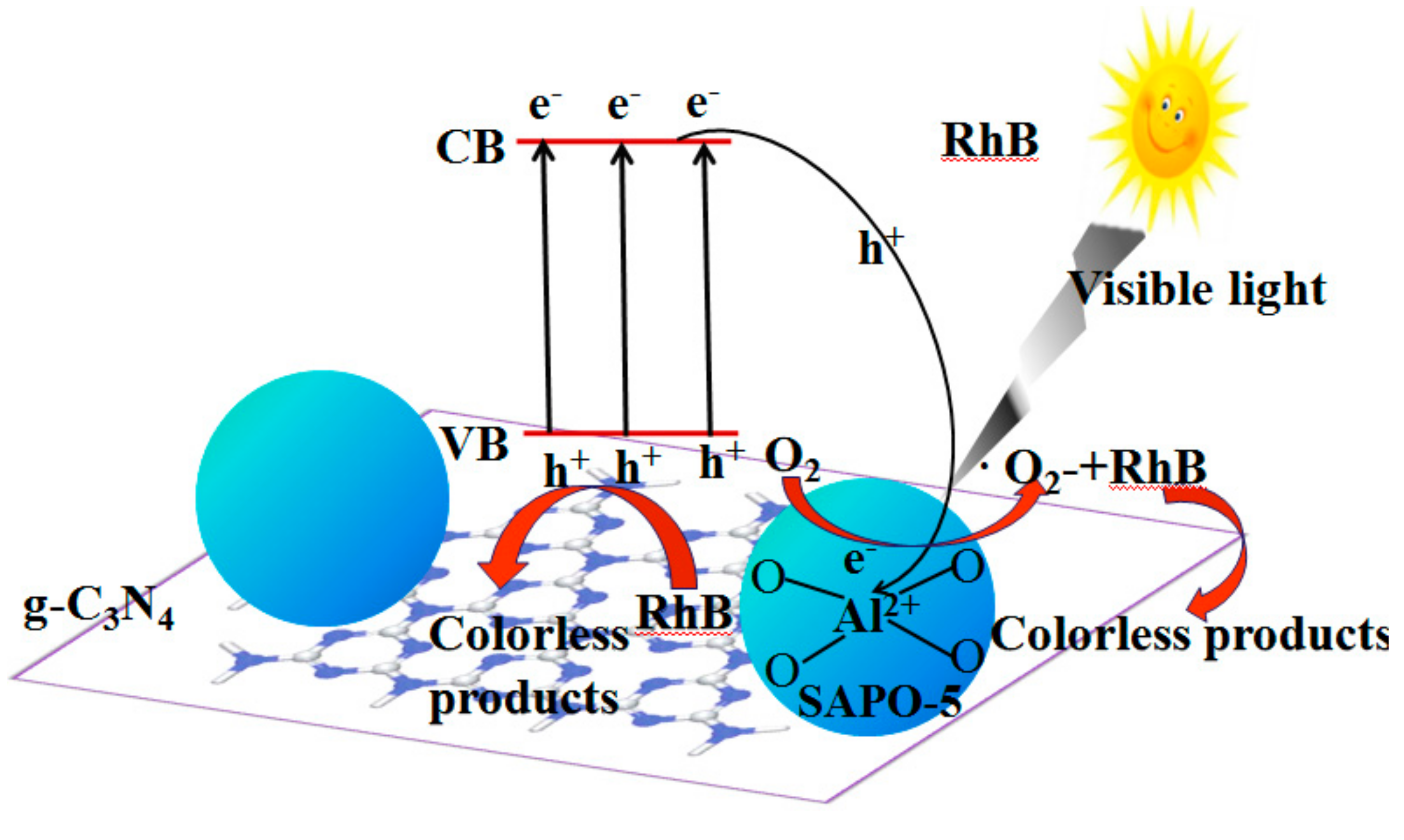
3.4. Synergistic Photocatalytic Degradation Mechanism
3.5. Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mamba, G.; Mishra, A.K. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B Environ. 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, X.; Wang, C.; Xu, J.; Du, X.; Li, L. Ti3+ defect mediated g-C3N4/TiO2 Z-scheme system for enhanced photocatalytic redox performance. Appl. Surf. Sci. 2018, 448, 288–296. [Google Scholar] [CrossRef]
- Liang, B.; Han, D.; Sun, C.; Zhang, W.; Qi, Q. Deposition of ZnO flowers on the surface of g-C3N4 by solid phase reaction. Funct. Mater. Lett. 2018, 11, 1850020. [Google Scholar] [CrossRef]
- Yan, H.; Cheng, L.; Guo, L. Combination mechanism and enhanced visible-light photocatalytic activity and stability of CdS/g-C3N4 heterojunctions. J. Mater. Sci. Technol. 2017, 33, 30–38. [Google Scholar]
- Hosein, B.M.; Kazem, M.; Hadi, S. Preparation and application of a nano α-Fe2O3/SAPO-34 photocatalyst for removal of the anti-cancer drug doxorubicin using the taguchi approach. Chem. Open 2016, 14, 267–273. [Google Scholar]
- Bellatreche, S.; Hasnaoui, A.; Boukoussa, B.; García-Aguilar, J.; Berenguer-Murcia, Á.; Cazorla-Amoros, D.; Bengueddach, A. Structural and textural features of TiO2/SAPO-34 nanocomposite prepared by the sol–gel method. Res. Chem. Intermediat. 2016, 42, 1–15. [Google Scholar] [CrossRef]
- Anandan, S.; Yoon, M. Photocatalytic activities of the nano-sized TiO2-supported Y-zeolites. J. Photoch. Photobio. C 2003, 4, 5–18. [Google Scholar] [CrossRef]
- Rajesh, J.T.; Ramchandra, G.K.; Jasra, R.V. Enhanced photocatalytic activity of TiO2-coated NaY and HY zeolites for the degradation of methylene blue in water. Ind. Eng. Chem. Res. 2007, 46, 369–376. [Google Scholar]
- Ökte, A.N.; Yılmaz, Ö. Characteristics of lanthanum loaded TiO2-ZSM-5 photocatalysts: Decolorization and degradation processes of methyl orange. Appl. Catal. A Gen. 2009, 354, 132–142. [Google Scholar] [CrossRef]
- Zhu, S.; Liang, S.; Ying, W.; Zhang, X.; Li, F.; Lin, H.; Zhang, Z.; Wang, X. Ultrathin nanosheets of molecular sieve SAPO-5: A new photocatalyst for efficient photocatalytic reduction of CO2 with H2O to methane. Appl. Catal. B Environ. 2016, 187, 11–18. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, R.; Shi, H.; Qiang, K.; Wang, P.; Wang, J. Preparation of TiO2/SAPO-5 composite zeolite and its photocatalytic properties for the degradation of phenol. J. Adv. Oxid. Technol. 2016, 19, 381–386. [Google Scholar] [CrossRef]
- Qiu, L.; Zhou, Z.W.; Yu, Y.; Zhang, H.; Qian, Y.; Yang, Y.; Duo, S. Fabrication of nanometer-sized high-silica SAPO-5 and its enhanced photocatalytic performance for methyl orange degradation. Res. Chem. Intermediat. 2018, 45, 1457–1473. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, T.; Guo, Y.; Zhang, Z.; Fang, X. Ultrathin g-C3N4 nanosheets coupled with carbon nanodots as 2D/0D composites for efficient photocatalytic H2 evolution. Appl. Catal. B Environ. 2016, 193, 248–258. [Google Scholar] [CrossRef]
- Dong, F.; Wu, L.; Sun, Y.; Fu, M.; Wu, Z.; Lee, S.C. Efficient synthesis of polymeric g-C3N4 layered materials as novel efficient visible light driven photocatalysts. J. Mater. Chem. 2011, 21, 15171–15174. [Google Scholar] [CrossRef]
- Wang, L.; Guo, C.; Yan, S.; Huang, X.; Li, Q. High-silica SAPO-5 with preferred orientation: Synthesis, characterization and catalytic applications. Micropor. Mesopor. Mat. 2003, 64, 63–68. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, T.; Xua, Q.; Li, D.; Meng, S.; Chen, M. Perovskite oxide ultrathin nanosheets/g-C3N4 2D-2D heterojunction photocatalysts with significantly enhanced photocatalytic activity towards the photodegradation of tetracycline. Appl. Catal. B Environ. 2017, 201, 617–628. [Google Scholar] [CrossRef]
- Ma, T.Y.; Tang, Y.; Dai, S.; Qiao, S.Z. Proton-functionalized two-dimensional graphitic carbon nitride nanosheet: An excellent metal-/label-free biosensing platform. Small 2014, 10, 2382–2389. [Google Scholar] [CrossRef]
- Wang, X.; Hong, M.; Zhang, F.; Zhuang, Z.; Yu, Y. Recyclable nanoscale zero valent iron doped g-C3N4/MoS2 for efficient photocatalytic of RhB and Cr(VI) driven by visible light. ACS Sustain. Chem. Eng. 2016, 4, 4055–4063. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Dong, F.; Du, X.; Huang, H. Easily and synchronously ameliorating charge separation and band energy level in porous g-C3N4 for boosting photooxidation and photoreduction ability. J. Phys. Chem. 2016, 120, 10381–10389. [Google Scholar] [CrossRef]
- Bayan, S.; Gogurla, N.; Midya, A.; Ray, S.K. White light emission characteristics of two dimensional graphitic carbon nitride and ZnO nanorod hybrid heterojunctions. Carbon 2016, 108, 335–342. [Google Scholar] [CrossRef]
- Jiang, D.; Yu, H.; Yu, H. Modified g-C3N4/TiO2 nanosheets/ZnO ternary facet coupled heterojunction for photocatalytic degradation of p-toluenesulfonic acid (p-TSA) under visible light. Physica E 2017, 85, 1–6. [Google Scholar] [CrossRef]
- Wei, X.L.; Lu, X.H.; Zhang, T.J.; Chu, X.; Zhou, D.; Nie, R.F.; Xia, Q.H. Synthesis and catalytic application of SAPO-5 by dry-gel conversion for the epoxidation of styrene with air. Microp. Mesop. Mater. 2015, 214, 80–87. [Google Scholar] [CrossRef]
- Singh, A.; Yadav, R.; Sudarsan, V.; Kishore, K.; Upadhyayula, S.; Sakthivel, A. Mesoporous SAPO-5 (MESO-SAPO-5): A potential catalyst for hydroisomerisation of 1-octene. RSC Adv. 2014, 4, 8727–8734. [Google Scholar] [CrossRef]
- Miao, X.L.; Shen, X.P.; Wu, J.J.; Ji, Z.Y.; Wang, J.H.; Kong, L.R.; Liu, M.M.; Song, C.S. Fabrication of an all solid Z-scheme photocatalyst g-C3N4/GO/AgBr with enhanced visible light photocatalytic activity. Appl. Catal. A Gen. 2017, 539, 104–113. [Google Scholar] [CrossRef]
- Bharat, L.K.; Nagaraju, G.; Krishna, K.G.; Yu, J.S. Controlled synthesis of yttrium gallium garnet spherical nanostructures modified by silver oxide nanoparticles for enhanced photocatalytic properties. Crystengcomm. 2016, 18, 8915–8925. [Google Scholar] [CrossRef]
- Qi, C.; He, Q.; Lv, M.; Liu, X.; Jin, W.; Lv, J. The vital role of PANI for the enhanced photocatalytic activity of magnetically recyclable N-K2Ti4O9/MnFe2O4/PANI composites. Appl. Surf. Sci. 2014, 311, 230–238. [Google Scholar]
- Dubey, N.; Rayalu, S.S.; Labhsetwar, N.K.; Naidu, R.R.; Chatti, R.V.; Devotta, S. Photocatalytic properties of zeolite-based materials for the photoreduction of methyl orange. Appl. Catal. A Gen. 2006, 303, 152–157. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, L.; Zhou, Z.; Ma, M.; Li, P.; Lu, J.; Hou, Y.; Chen, X.; Duo, S. Enhanced Visible-Light Photocatalytic Performance of SAPO-5-Based g-C3N4 Composite for Rhodamine B (RhB) Degradation. Materials 2019, 12, 3948. https://doi.org/10.3390/ma12233948
Qiu L, Zhou Z, Ma M, Li P, Lu J, Hou Y, Chen X, Duo S. Enhanced Visible-Light Photocatalytic Performance of SAPO-5-Based g-C3N4 Composite for Rhodamine B (RhB) Degradation. Materials. 2019; 12(23):3948. https://doi.org/10.3390/ma12233948
Chicago/Turabian StyleQiu, Lingfang, Zhiwei Zhou, Mengfan Ma, Ping Li, Jinyong Lu, Yingying Hou, Xiangshu Chen, and Shuwang Duo. 2019. "Enhanced Visible-Light Photocatalytic Performance of SAPO-5-Based g-C3N4 Composite for Rhodamine B (RhB) Degradation" Materials 12, no. 23: 3948. https://doi.org/10.3390/ma12233948
APA StyleQiu, L., Zhou, Z., Ma, M., Li, P., Lu, J., Hou, Y., Chen, X., & Duo, S. (2019). Enhanced Visible-Light Photocatalytic Performance of SAPO-5-Based g-C3N4 Composite for Rhodamine B (RhB) Degradation. Materials, 12(23), 3948. https://doi.org/10.3390/ma12233948