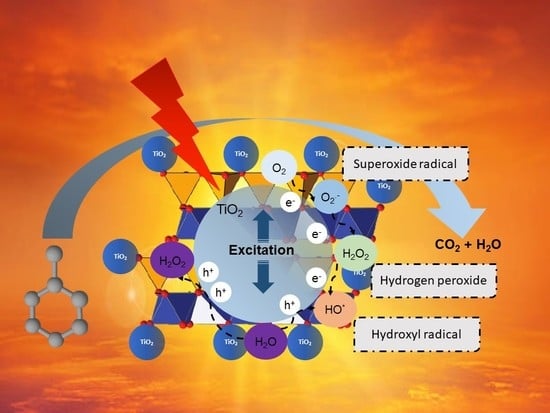
White and Red Brazilian São Simão’s Kaolinite–TiO2 Nanocomposites as Catalysts for Toluene Photodegradation from Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Clay Minerals Purification by Dispersion Decantation
2.2. Intercalation of Kaol and Kaol-R with Dimethylsulfoxide (DMSO)
2.3. Synthesis of Nanocomposite Photocatalysts
2.4. Photodegradation of Toluene from Aqueous solution
2.5. Photolysis of Toluene
2.6. Measurement of Light Intensity
2.7. Characterization Techniques
3. Results and Discussion
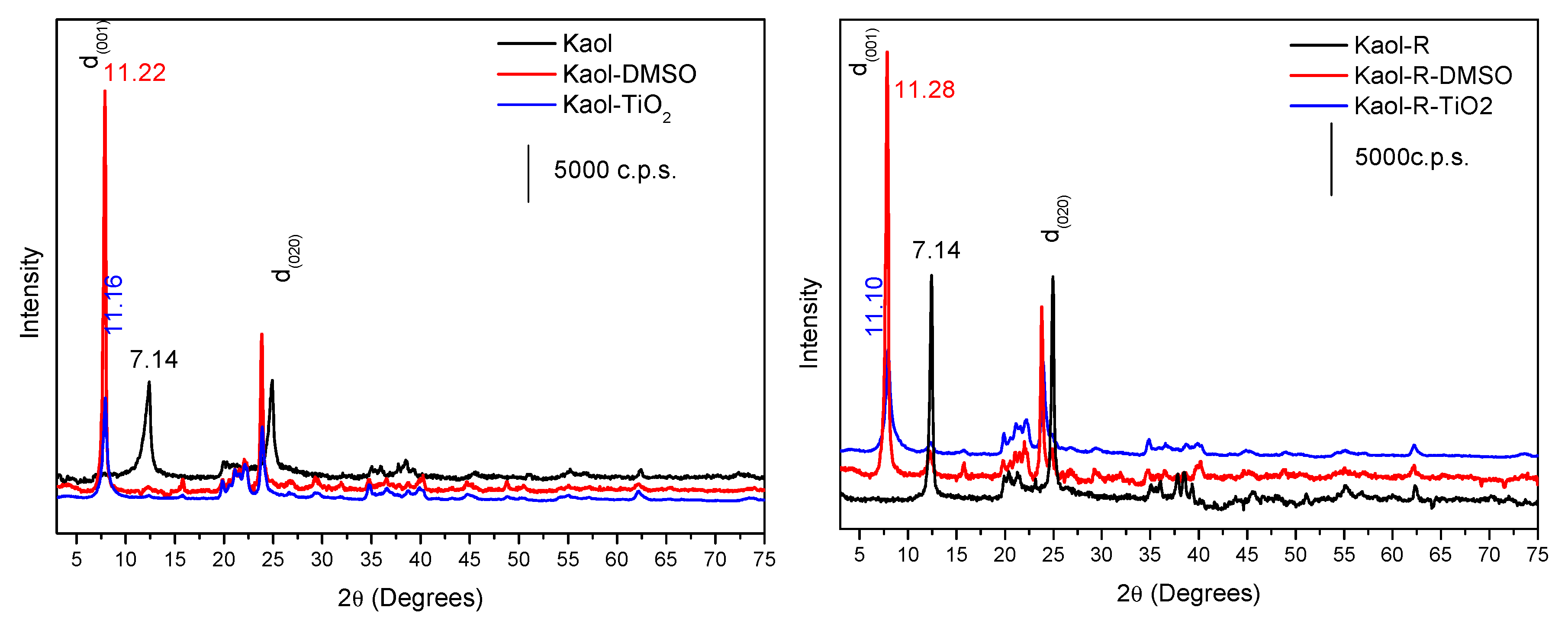
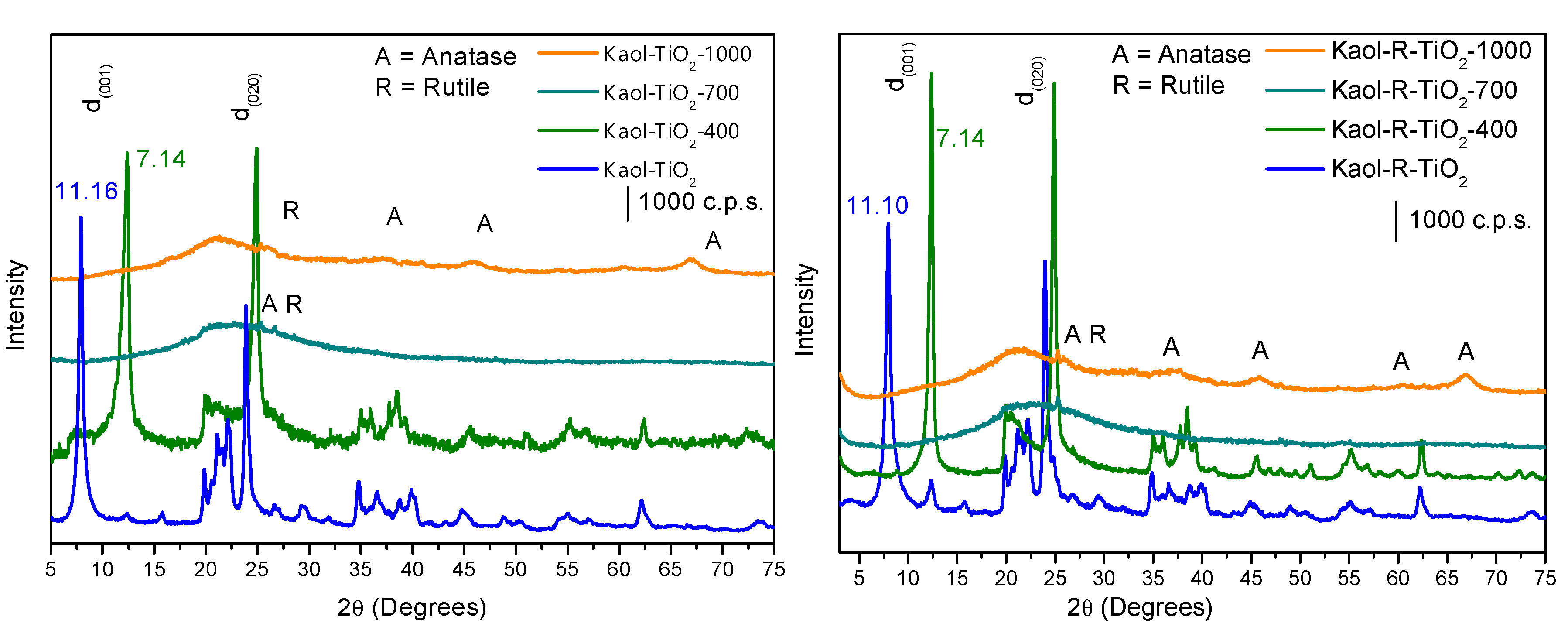
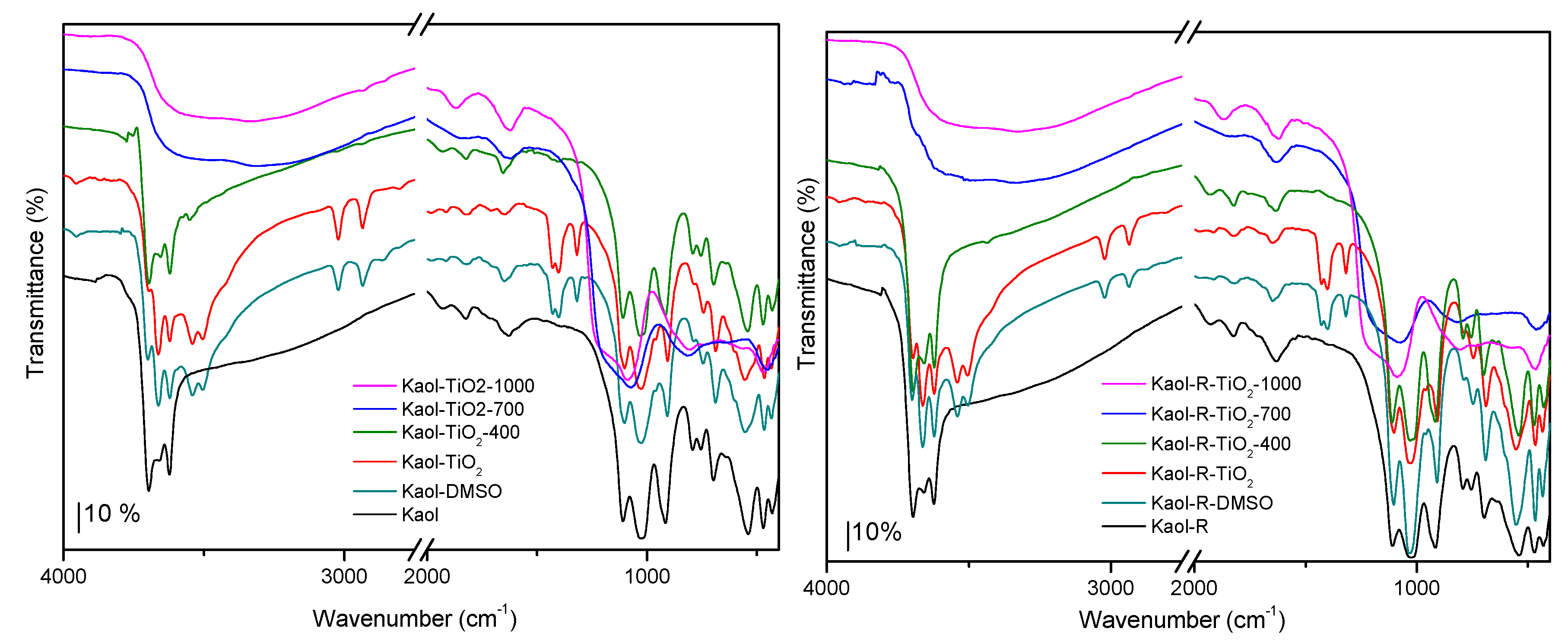
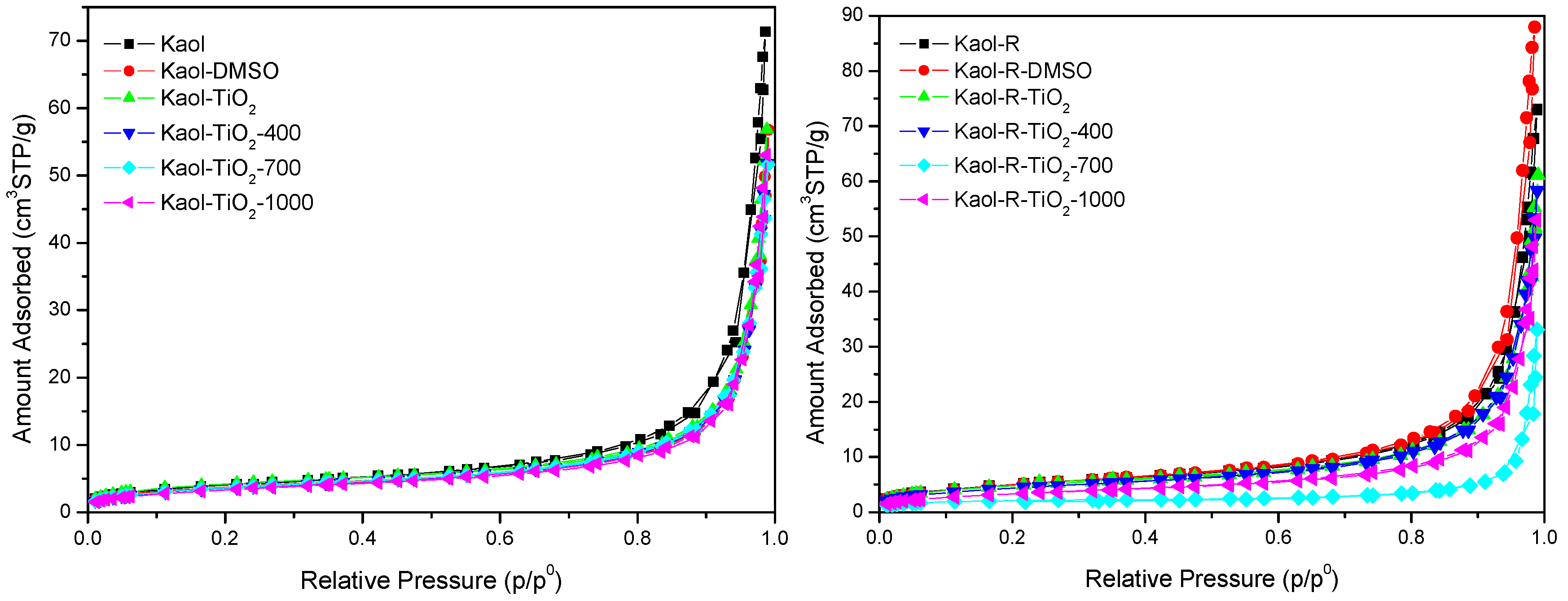
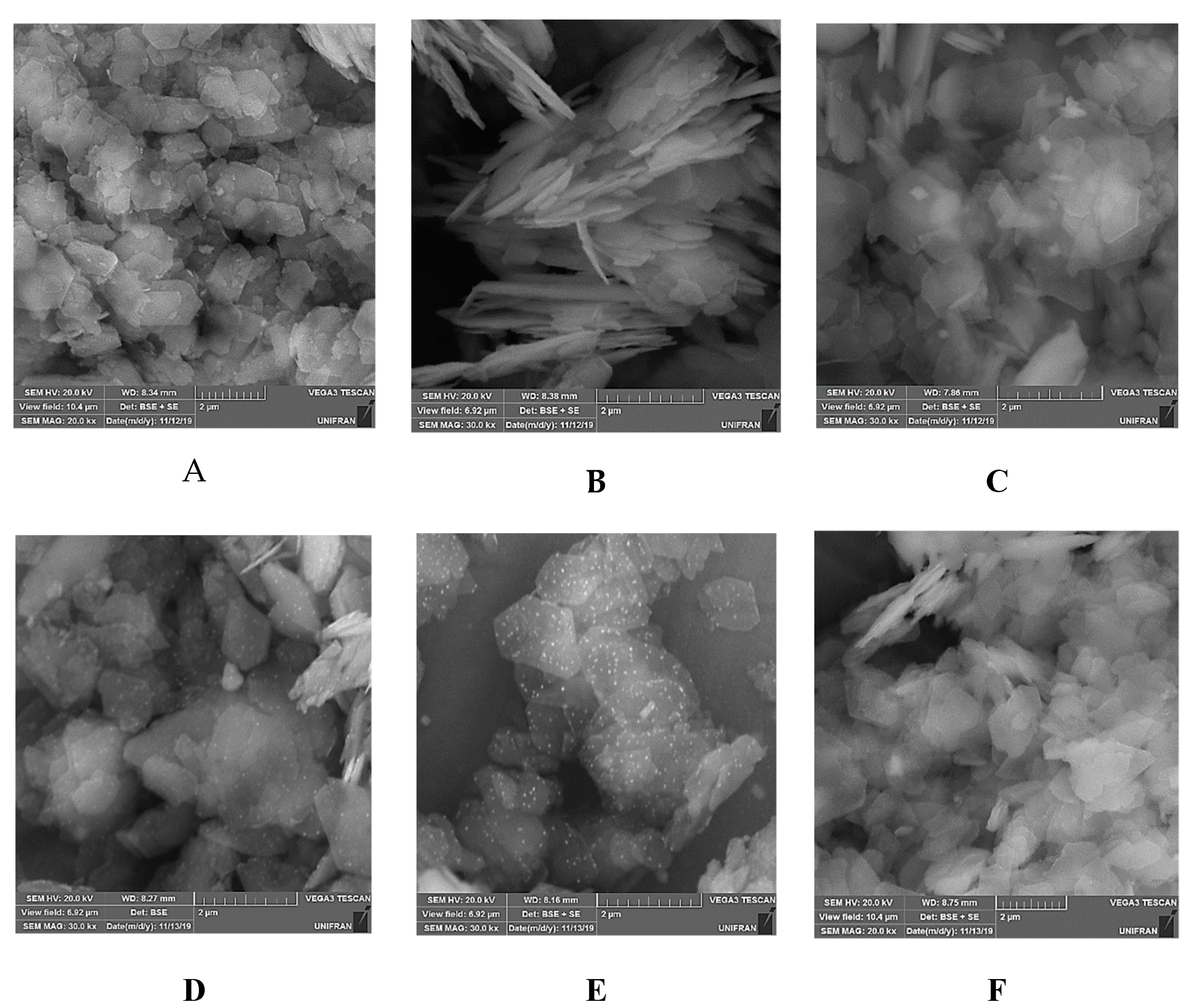
3.1. Characterization of the Photocatalysts
3.2. Photocatalysis Experiments
3.2.1. Preliminary Adsorption Tests
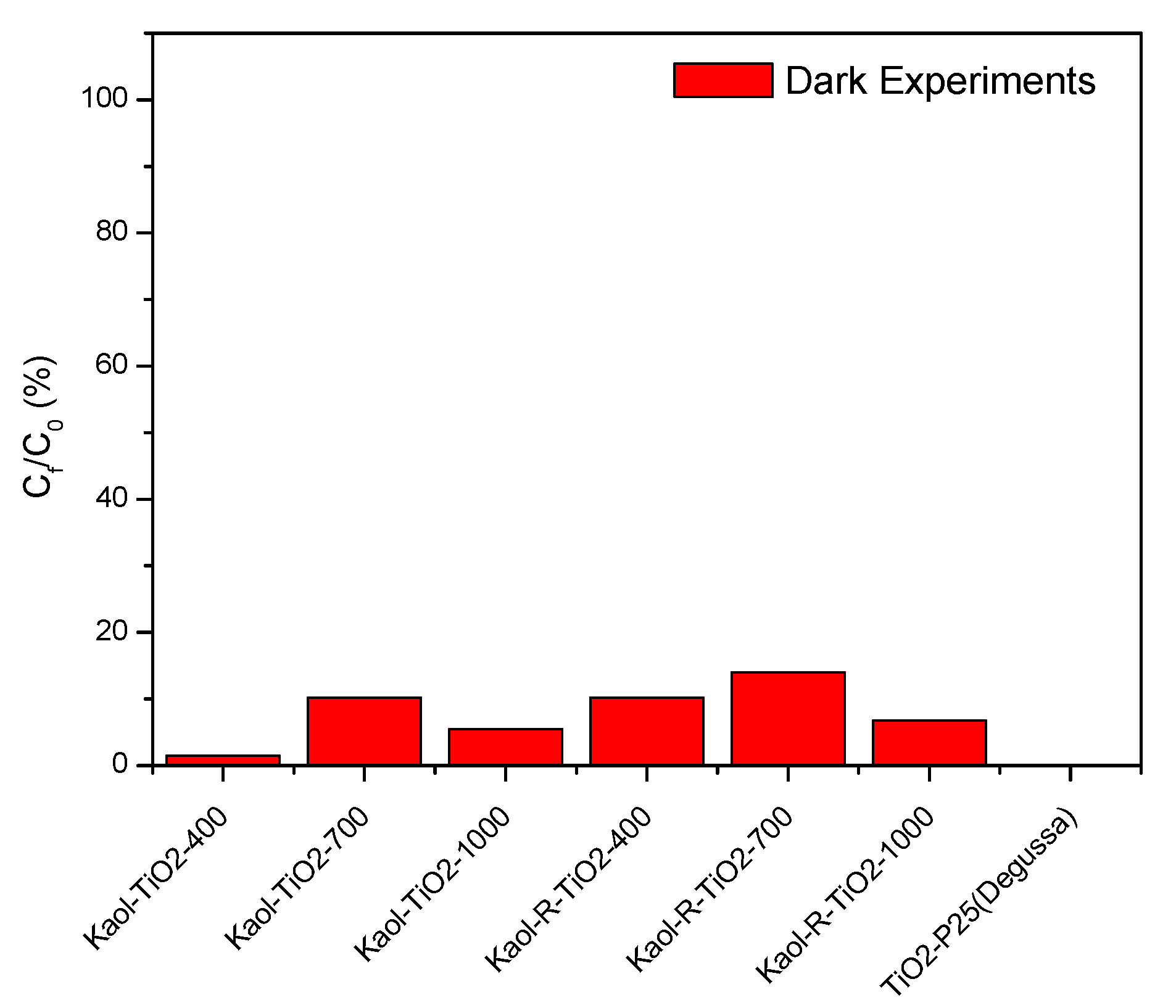
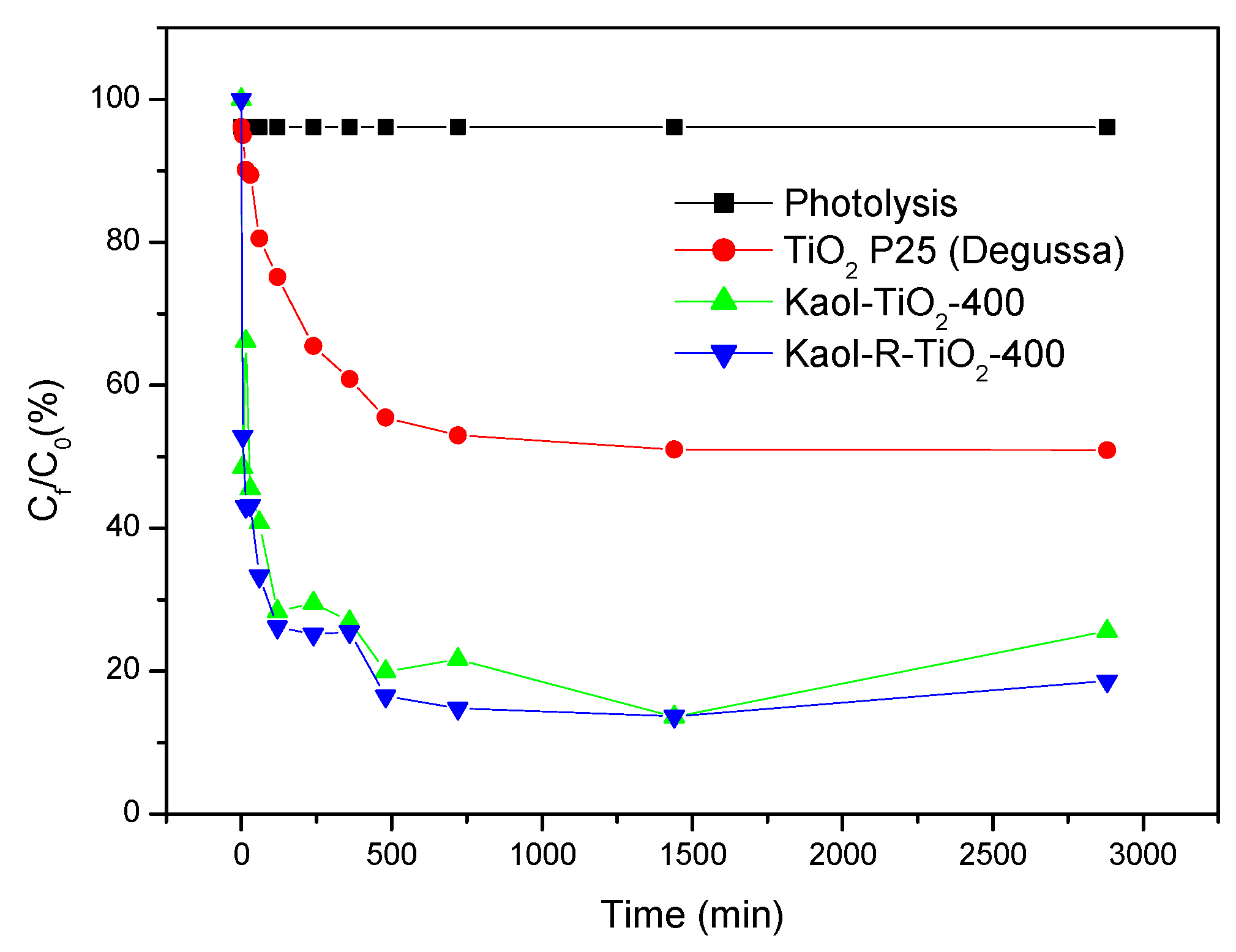
3.2.2. Photolysis and Blank Photocatalytic Tests
3.2.3. Photodegradation of Toluene Using the White and Red Kaolinite–Titanium Dioxide Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Han, L.; Zhou, W.; Li, W.; Qian, Y. Global population exposed to fine particulate pollution by population increase and pollution expansion. Air Qual. Atmos. Health 2017, 10, 1221–1226. [Google Scholar] [CrossRef]
- Nações Unidas Brasil. ONU: 1 em Cada 3 Pessoas No Mundo Não Tem Acesso a Água Potável. Available online: https://nacoesunidas.org/onu-1-em-cada-3-pessoas-no-mundo-nao-tem-acesso-a-agua-potavel/ (accessed on 19 September 2019).
- Qu, X.; Alvarez, P.J.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Kim, J.H.; Lee, C.Y.; Jerng, D.W.; Ahn, H.S. Toluene and acetaldehyde removal from air on to graphene-based adsorbents with microsized pores. J. Hazard. Mater. 2018, 344, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, S.J. Description, properties, and degradation of selected volatile organic compounds detected in ground water—A review of selected literature. In Open-File Report 2006–1338; USA Geological Survey: Reston, VA, USA, 2006. [Google Scholar]
- Fujihira, M.; Satoh, Y.; Osa, T. Heterogeneous photocatalytic oxidation of aromatic compounds on TiO2. Nature 1981, 293, 206–208. [Google Scholar] [CrossRef]
- Fujihira, M.; Satoh, Y.; Osa, T. Heterogeneous photocatalytic oxidation of aromatic compounds on semicondutor materials: The Photo-Fenton type reaction. Chem. Lett. 1981, 10, 1053–1056. [Google Scholar] [CrossRef]
- Navio, J.A.; Garcia Gómez, M.; Pradera Adrian, M.A.; Fuentes Mota, J. Partial or complete heterogeneous photocatalytic oxidation of neat toluene and 4-picoline in liquid organic oxygenated dispersions containing pure or iron-doped titania photocatalysts. J. Mol. Catal. A Chem. 1996, 104, 329–339. [Google Scholar] [CrossRef]
- Augugliaro, V.; Loddo, V.; Marci, G.; Palmisano, L.; Sbriziolo, C.; Schiavello, M.; Turco Liveri, M.L. Photocatalytic degradation of toluene in aqueous suspensions of polycrystalline TiO2 in the presence of the surfactant tetradecyldimethylamino-oxide. Stud. Surf. Sci. Catal. 2000, 130, 1973–1978. [Google Scholar]
- Enriquez, R.; Pichat, P. Interactions of humic acid, quinoline, and TiO2 in water in relation to quinoline photocatalytic removal. Langmuir 2001, 17, 6132–6137. [Google Scholar] [CrossRef]
- Tada, H.; Matsui, H.; Shiota, F.; Nomura, M.; Ito, S.; Yoshihara, M.; Esumi, K. Heterosupramolecular photocatalysis: Oxidation of organic compounds in nanospaces between surfactant bilayers formed on TiO2. Chem. Commun. 2002, 2, 1678–1679. [Google Scholar] [CrossRef]
- Marcì, G.; Addamo, M.; Augugliaro, V.; Coluccia, S.; García-López, E.; Loddo, V.; Martra, G.; Palmisano, L.; Schiavello, M. Photocatalytic oxidation of toluene on irradiated TiO2: Comparison of degradation performance in humidified air, in water and in water containing a zwitterionic surfactant. J. Photochem. Photobiol. A Chem. 2003, 160, 105–114. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment e A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Papoulis, D.; Komarneni, S.; Panagiotaras, D.; Stathatos, E.; Christoforidis, K.C.; Fernández-García, M.; Li, H.; Shu, Y. Environmental three-phase nanocomposites of two nanoclays and TiO2: Synthesis, characterization and photacatalytic activities. Appl. Catal. B Environ. 2014, 147, 526–533. [Google Scholar] [CrossRef]
- Barbosa, L.V.; Marçal, L.; Nassar, E.J.; Calefi, P.S.; Vicente, M.A.; Trujillano, R.; Rives, V.; Gil, A.; Korili, S.A.; Ciuffi, K.J.; et al. Kaolinite-titanium oxide nanocomposites prepared via sol-gel as heterogeneous photocatalysts for dyes degradation. Catal. Today 2015, 246, 133–142. [Google Scholar] [CrossRef]
- Kibanova, D.; Trejo, M.; Destaillats, H.; Cervini-Silva, J. Synthesis of hectorite-TiO2 and kaolinite-TiO2 nanocomposites with photocatalytic activity for the degradation of model air pollutants. Appl. Clay Sci. 2009, 42, 563–568. [Google Scholar] [CrossRef]
- Chen, J.; Li, G.; He, Z.; An, T. Adsorption and degradation of model volatile organic compounds by a combined titania-montmorillonite-silica photocatalyst. J. Hazard. Mater. 2011, 190, 416–423. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Sharma, M.; Basu, S. Enhanced heterogeneous photodegradation of VOC and dye using microwave synthesized TiO2/clay nanocomposites: A comparison study of different type of clays. J. Alloys Compd. 2017, 694, 574–580. [Google Scholar] [CrossRef]
- Fan, W.; Lai, Q.; Zhang, Q.; Wang, Y. Nanocomposites of TiO2 and reduced graphene oxide as efficient photocatalysts for hydrogen evolution. J. Phys. Chem. C 2011, 115, 10694–10701. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Z.-R.; Fu, X.; Xu, Y.-J. Engineering the unique 2D mat of graphene to achieve graphene-TiO2 nanocomposite for photocatalytic selective transformation: What advantage does graphene have over its forebear carbon nanotube? ACS Nano 2011, 5, 7426–7435. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, M.Q.; Liu, S.; Sun, Y.; Xu, Y.J. Waltzing with the versatile platform of graphene to synthesize composite photocatalysts. Chem. Rev. 2015, 115, 10307–10377. [Google Scholar] [CrossRef]
- De Faria, E.H.; Ciuffi, K.J.; Nassar, E.J.; Vicente, M.A.; Trujillano, R.; Calefi, P.S. Novel reactive amino-compound: Tris(hydroxymethyl)aminomethane covalently grafted on kaolinite. Appl. Clay Sci. 2010, 48, 516–521. [Google Scholar] [CrossRef]
- Da Silva, T.H.; De Souza, T.F.M.; Ribeiro, A.O.; Ciuffi, K.J.; Nassar, E.J.; Silva, M.L.A.; De Faria, E.H.; Calefi, P.S. Immobilization of metallophthalocyanines on hybrid materials and in-situ synthesis of pseudo-tubular structures from an aminofunctionalized kaolinite. Dyes Pigment. 2014, 100, 17–23. [Google Scholar] [CrossRef]
- Letaief, S.; Detellier, C. Functionalized nanohybrid materials obtained from the interlayer grafting of aminoalcohols on kaolinite. Chem. Commun. 2007, 1, 2613–2615. [Google Scholar] [CrossRef] [PubMed]
- Hang, P.T.; Brindley, G.W. Methylene blue absorption by clay minerals. Determination of surface areas and cation exchange capacities (clay-organic studies XVIII). Clays Clay Miner. 1970, 18, 203–212. [Google Scholar] [CrossRef]
- Maček, M.; Mauko, A.; Mladenovič, A.; Majes, B.; Petkovšek, A. A comparison of methods used to characterize the soil specific surface area of clays. Appl. Clay Sci. 2013, 83–84, 144–152. [Google Scholar] [CrossRef]
- Avila, L.R.; de Faria, E.H.; Ciuffi, K.J.; Nassar, E.J.; Calefi, P.S.; Vicente, M.A.; Trujillano, R. New synthesis strategies for effective functionalization of kaolinite and saponite with silylating agents. J. Colloid Interface Sci. 2010, 341, 186–193. [Google Scholar] [CrossRef]
- De Faria, E.H.; Lima, O.J.; Ciuffi, K.J.; Nassar, E.J.; Vicente, M.A.; Trujillano, R.; Calefi, P.S. Hybrid materials prepared by interlayer functionalization of kaolinite with pyridine-carboxylic acids. J. Colloid Interface Sci. 2009, 335, 210–215. [Google Scholar] [CrossRef]
- Elbokl, T.A.; Detellier, C. Intercalation of cyclic imides in kaolinite. J. Colloid Interface Sci. 2008, 323, 338–348. [Google Scholar] [CrossRef]
- Da Silva, T.; Ribeiro, A.; Nassar, E.; Trujillano, R.; Rives, V.; Vicente, M.; de Faria, E.; Ciuffi, K.J. Kaolinite/TiO2/cobalt(II) tetracarboxymetallophthalocyanine nanocomposites as heterogeneous photocatalysts for decomposition of organic pollutants trimethoprim, caffeine and prometryn. J. Braz. Chem. Soc. 2019, 30, 2610–2623. [Google Scholar] [CrossRef]
- Reinosa, J.J.; García-Baños, B.; Catalá-Civera, J.M.; Fernández, J.F. A step ahead on efficient microwave heating for kaolinite. Appl. Clay Sci. 2019, 168, 237–243. [Google Scholar] [CrossRef]
- Tonle, I.K.; Letaief, S.; Ngameni, E.; Detellier, C. Nanohybrid materials from the grafting of imidazolium cations on the interlayer surfaces of kaolinite. Application as electrode modifier. J. Mater. Chem. 2009, 19, 5996–6003. [Google Scholar] [CrossRef]
- Mamulová Kutlákova, K.; Tokarský, J.; Kovář, P.; Vojtěšková, S.; Kovářová, A.; Smetana, B.; Kukutschová, J.; Čapková, P.; Matějka, V. Preparation and characterization of photoactive composite kaolinite/TiO2. J. Hazard. Mater. 2011, 188, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Moreira, M.A.; Ciuffi, K.J.; Rives, V.; Vicente, M.A.; Trujillano, R.; Gil, A.; Korili, S.A.; de Faria, E.H. Effect of chemical modification of palygorskite and sepiolite by 3-aminopropyltriethoxisilane on adsorption of cationic and anionic dyes. Appl. Clay Sci. 2016, 135, 394–404. [Google Scholar] [CrossRef]
- Marçal, L.; de Faria, E.H.; Nassar, E.J.; Trujillano, R.; Martín, N.; Vicente, M.A.; Rives, V.; Gil, A.; Korili, S.A.; Ciuffi, K.J. Organically modified saponites: SAXS study of swelling and application in caffeine removal. ACS Appl. Mater. Interfaces 2015, 7, 10853–10862. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.M.; Ferreira, B.F.; Oliveira, N.P.; Nassar, E.J.; Ciuffi, K.J.; Vicente, M.A.; Trujillano, R.; Rives, V.; Gil, A.; Korili, S.; et al. Synthesis of zeolite A from metakaolin and its application in the adsorption of cationic dyes. Appl. Sci. 2018, 8, 608. [Google Scholar] [CrossRef]
- Tauc, J. Absorption edge and internal electric fields in amorphous semiconductors. Mater. Res. Bull. 1970, 5, 721–729. [Google Scholar] [CrossRef]
- Manova, E.; Aranda, P.; Martín-Luengo, M.A.; Letaïef, S.; Ruiz-Hitzky, E. New titania-clay nanostructured porous materials. Microporous Mesoporous Mater. 2010, 131, 252–260. [Google Scholar] [CrossRef]
- Chong, M.N.; Vimonses, V.; Lei, S.; Jin, B.; Chow, C.; Saint, C. Synthesis and characterisation of novel titania impregnated kaolinite nano-photocatalyst. Microporous Mesoporous Mater. 2009, 117, 233–242. [Google Scholar] [CrossRef]
- Kočí, K.; Matějka, V.; Kovář, P.; Lacný, Z.; Obalová, L. Comparison of the pure TiO2 and kaolinite/TiO2 composite as catalyst for CO2 photocatalytic reduction. Catal. Today 2011, 161, 105–109. [Google Scholar] [CrossRef]
- González, B.; Muñoz, B.; Vicente, M.; Trujillano, R.; Rives, V.; Gil, A.; Korili, S. Photodegradation of 1,2,4-trichlorobenzene on montmorillonite–TiO2 nanocomposites. ChemEngineering 2018, 2, 22. [Google Scholar] [CrossRef]
- Papoulis, D.; Komarneni, S.; Nikolopoulou, A.; Tsolis-Katagas, P.; Panagiotaras, D.; Kacandes, H.G.; Zhang, P.; Yin, S.; Sato, T.; Katsuki, H. Palygorskite- and Halloysite-TiO2 nanocomposites: Synthesis and photocatalytic activity. Appl. Clay Sci. 2010, 50, 118–124. [Google Scholar] [CrossRef]
- Kim, S.H.; Ngo, H.H.; Shon, H.K.; Vigneswaran, S. Adsorption and photocatalysis kinetics of herbicide onto titanium oxide and powdered activated carbon. Sep. Purif. Technol. 2008, 58, 335–342. [Google Scholar] [CrossRef]
- Huang, H.H.; Tseng, D.H.; Juang, L.C. Heterogeneous photocatalytic degradation of monochlorobenzene in water. J. Hazard. Mater. 2008, 156, 186–193. [Google Scholar] [CrossRef]
- Valente, J.P.S.; Padilha, P.M.; Florentino, A.O. Studies on the adsorption and kinetics of photodegradation of a model compound for heterogeneous photocatalysis onto TiO2. Chemosphere 2006, 64, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Yurdakal, S.; Loddo, V.; Augugliaro, V.; Berber, H.; Palmisano, G.; Palmisano, L. Photodegradation of pharmaceutical drugs in aqueous TiO2 suspensions: Mechanism and kinetics. Catal. Today 2007, 129, 9–15. [Google Scholar] [CrossRef]
- Hidaka, H.; Zhao, J.; Pelizzetti, E.; Serpone, N. Photodegradation of surfactants. 8. Comparison of photocatalytic processes between anionic sodium dodecylbenzenesulfonate and cationic benzyldodecyldimethylammonium chloride on the TiO2 surface. J. Phys. Chem. 1992, 96, 2226–2230. [Google Scholar] [CrossRef]
- Rizzo, L.; Meric, S.; Kassinos, D.; Guida, M.; Russo, F.; Belgiorno, V. Degradation of diclofenac by TiO2 photocatalysis: UV absorbance kinetics and process evaluation through a set of toxicity bioassays. Water Res. 2009, 43, 979–988. [Google Scholar] [CrossRef]
- Rauf, M.A.; Meetani, M.A.; Khaleel, A.; Ahmed, A. Photocatalytic degradation of Methylene Blue using a mixed catalyst and product analysis by LC/MS. Chem. Eng. J. 2010, 157, 373–378. [Google Scholar] [CrossRef]

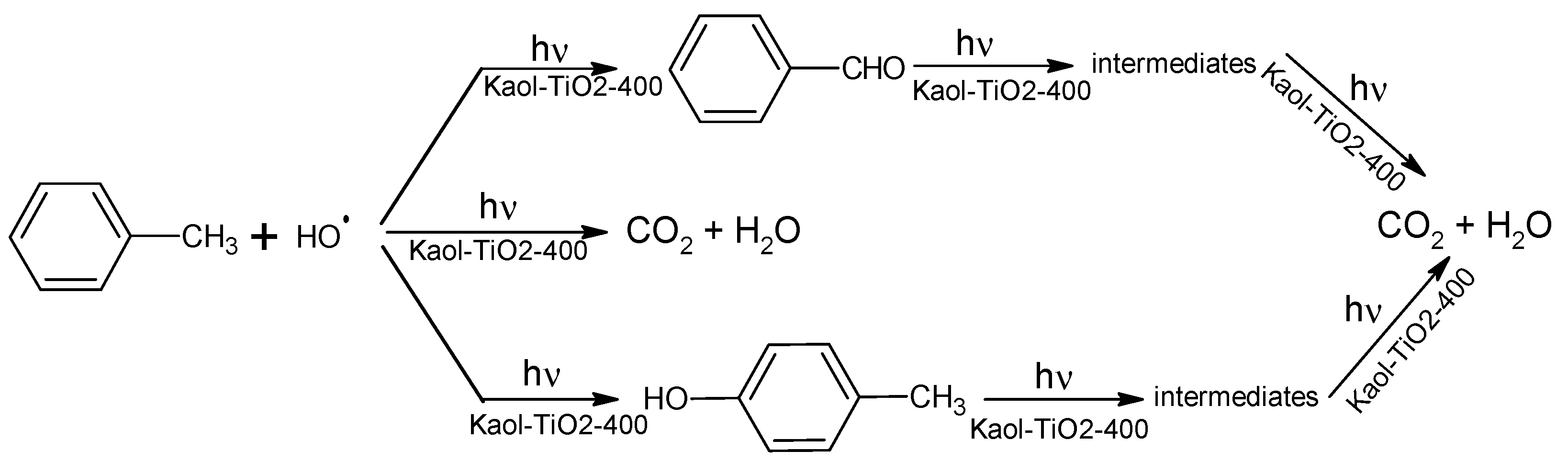
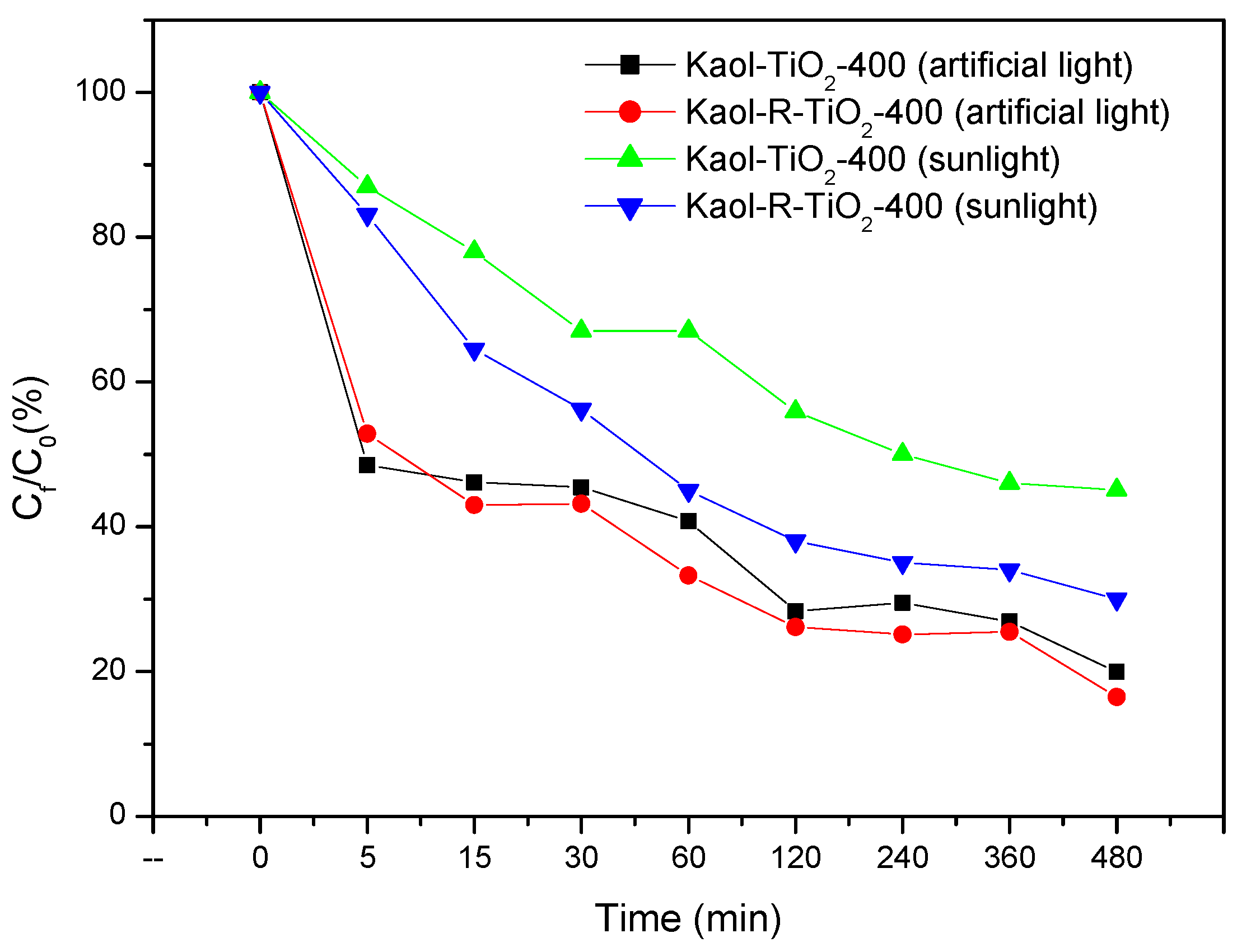
| Sample | SiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | TiO2 |
|---|---|---|---|---|---|---|---|---|---|
| Kaol | 55.90 | 43.72 | 1.12 | 0.007 | 0.27 | 0.09 | 0.06 | 0.44 | 1.14 |
| Kaol-R | 52.37 | 43.26 | 2.24 | 0,064 | 0,24 | 0.02 | 0.01 | 0.39 | 1.15 |
| Exposition Site | Sun Light | Photoreactor | ||
|---|---|---|---|---|
| UVA/UVB | Luminosity Intensity | UVA/UVB | Intensity of Luminosity | |
| Photoreactor (artificial ultraviolet radiation (λ = 365 nm, P = 30 W). Lowest and highest values measured | - | - | 7–20 | 1548–1650 |
| Indirect exposure (under shadow) | 530 | 13,470 | - | - |
| Direct exposure | 1628 | 501,000 | - | - |
| Laboratory, ambient quantification | - | - | 1 | 800–949 |
| Solid | SBET (m2/g) | SMB (m2/g) | VP (cm3/g) | Band Gap (eV) | ||||
|---|---|---|---|---|---|---|---|---|
| Kaol | Kaol-R | Kaol | Kaol-R | Kaol | Kaol-R | Kaol | Kaol-R | |
| Kaol | 15 | 18 | 78 | 62 | 0.110 | 0.113 | 4.51 | 4.49 |
| Kaol-DMSO | 13 | 18 | 78 | 62 | 0.088 | 0.136 | 4.71 | 4.52 |
| Kaol-TiO2 | 15 | 17 | 47 | 62 | 0.088 | 0.094 | 4.67 | 4.71 |
| Kaol-TiO2-400 | 13 | 16 | 78 | 62 | 0.080 | 0.090 | 4.92 | 4.72 |
| Kaol-TiO2-700 | 13 | 14 | 78 | 78 | 0.079 | 0.051 | 4.76 | 4.74 |
| Kaol-TiO2-1000 | 14 | 12 | 62 | 62 | 0.081 | 0.082 | 4.79 | 4.85 |
| Catalysts | k (min−1) | R2 | t1/2(min) | Х2 |
|---|---|---|---|---|
| Kaol-TiO2-400 | 0.017 | 0.81 | 40 | 0.190 |
| Kaol-400 | 0.0003 | 0.43 | 2074 | 0.080 |
| Kaol-R-TiO2-400 | 0.034 | 0.62 | 20 | 0.370 |
| Kaol-R-400 | 0.0004 | 0.62 | 1848 | 0.104 |
| TiO2 P25 (Degussa) | 0.0010 | 0.69 | 728 | 0.158 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora, L.D.; Bonfim, L.F.; Barbosa, L.V.; da Silva, T.H.; Nassar, E.J.; Ciuffi, K.J.; González, B.; Vicente, M.A.; Trujillano, R.; Rives, V.; et al. White and Red Brazilian São Simão’s Kaolinite–TiO2 Nanocomposites as Catalysts for Toluene Photodegradation from Aqueous Solutions. Materials 2019, 12, 3943. https://doi.org/10.3390/ma12233943
Mora LD, Bonfim LF, Barbosa LV, da Silva TH, Nassar EJ, Ciuffi KJ, González B, Vicente MA, Trujillano R, Rives V, et al. White and Red Brazilian São Simão’s Kaolinite–TiO2 Nanocomposites as Catalysts for Toluene Photodegradation from Aqueous Solutions. Materials. 2019; 12(23):3943. https://doi.org/10.3390/ma12233943
Chicago/Turabian StyleMora, Lucas D., Larissa F. Bonfim, Lorrana V. Barbosa, Tiago H. da Silva, Eduardo J. Nassar, Katia J. Ciuffi, Beatriz González, Miguel A. Vicente, Raquel Trujillano, Vicente Rives, and et al. 2019. "White and Red Brazilian São Simão’s Kaolinite–TiO2 Nanocomposites as Catalysts for Toluene Photodegradation from Aqueous Solutions" Materials 12, no. 23: 3943. https://doi.org/10.3390/ma12233943
APA StyleMora, L. D., Bonfim, L. F., Barbosa, L. V., da Silva, T. H., Nassar, E. J., Ciuffi, K. J., González, B., Vicente, M. A., Trujillano, R., Rives, V., Pérez-Bernal, M. E., Korili, S., Gil, A., & de Faria, E. H. (2019). White and Red Brazilian São Simão’s Kaolinite–TiO2 Nanocomposites as Catalysts for Toluene Photodegradation from Aqueous Solutions. Materials, 12(23), 3943. https://doi.org/10.3390/ma12233943