Facile Preparative Access to Bioactive Silicon Oxycarbides with Tunable Porosity
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials Preparation
2.2. Simulated Body Fluid Assessment
2.3. Materials Characterization
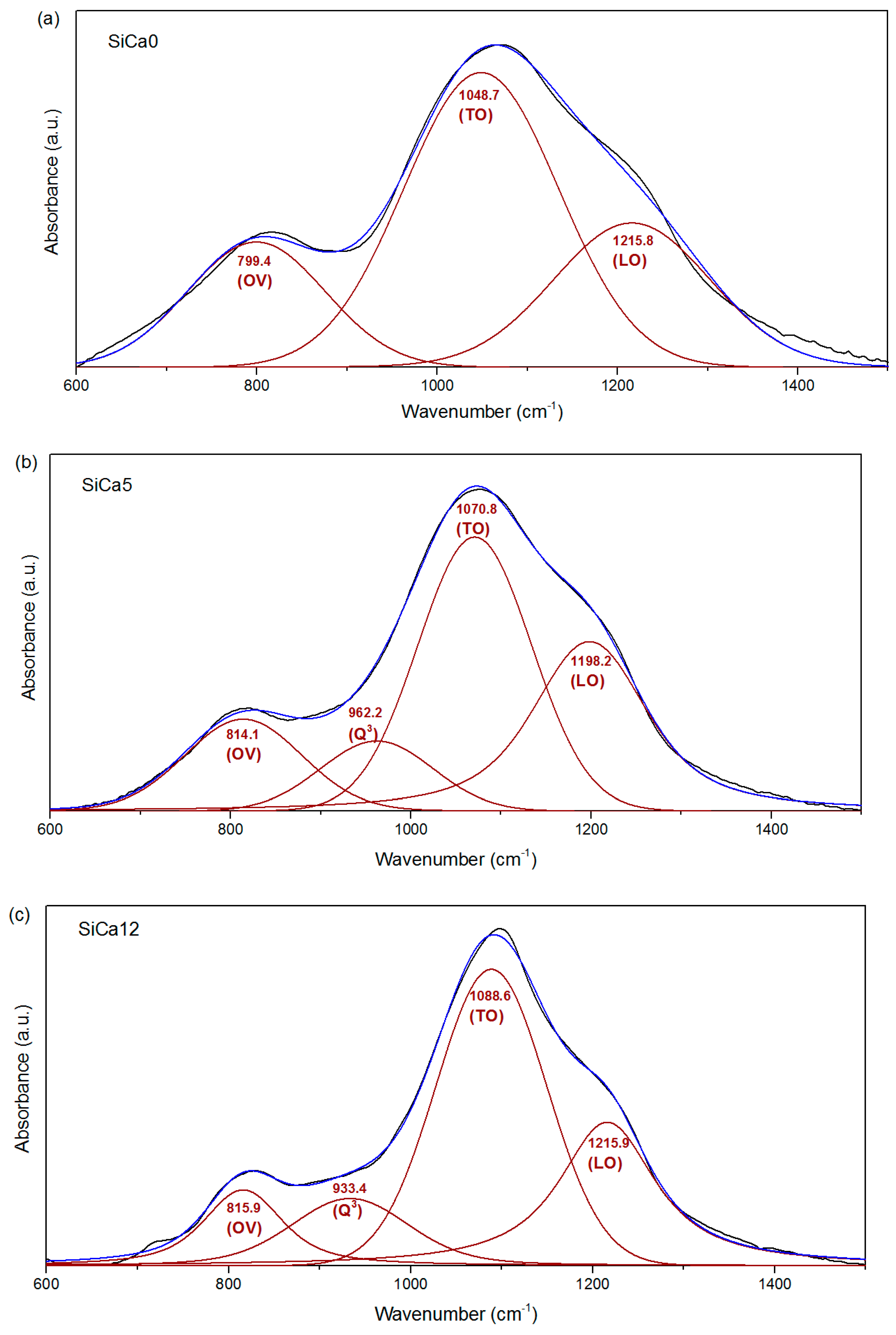
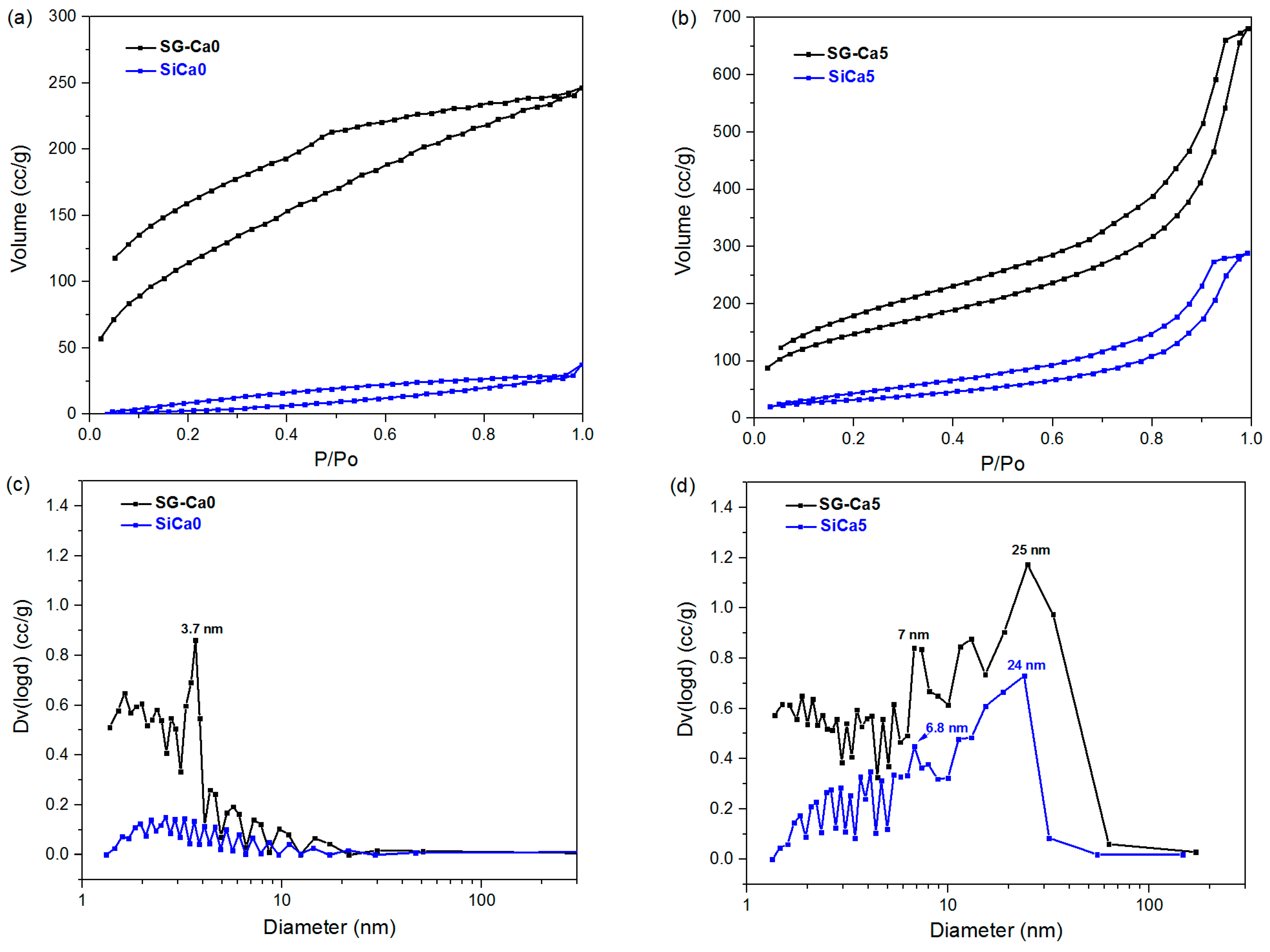
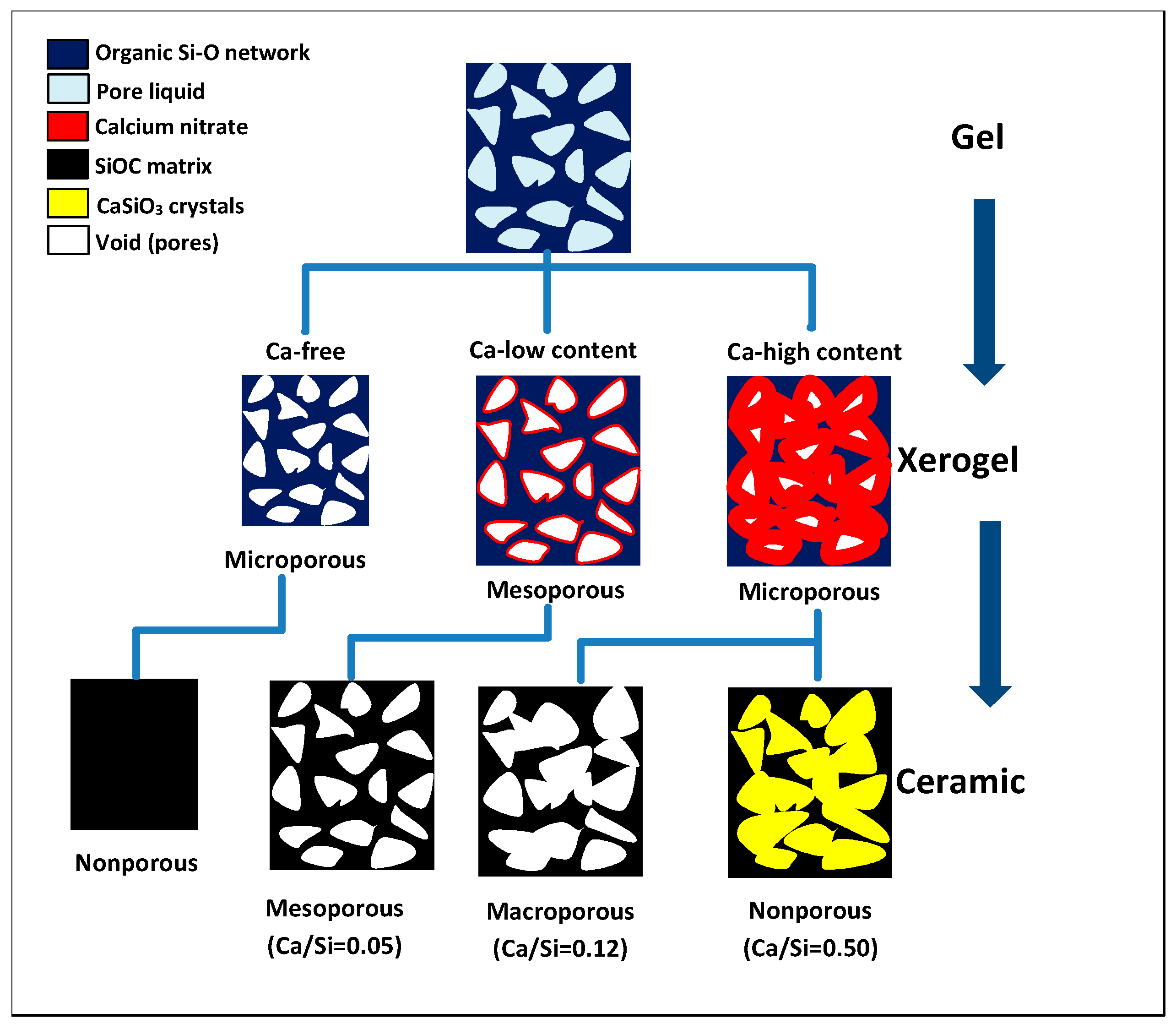
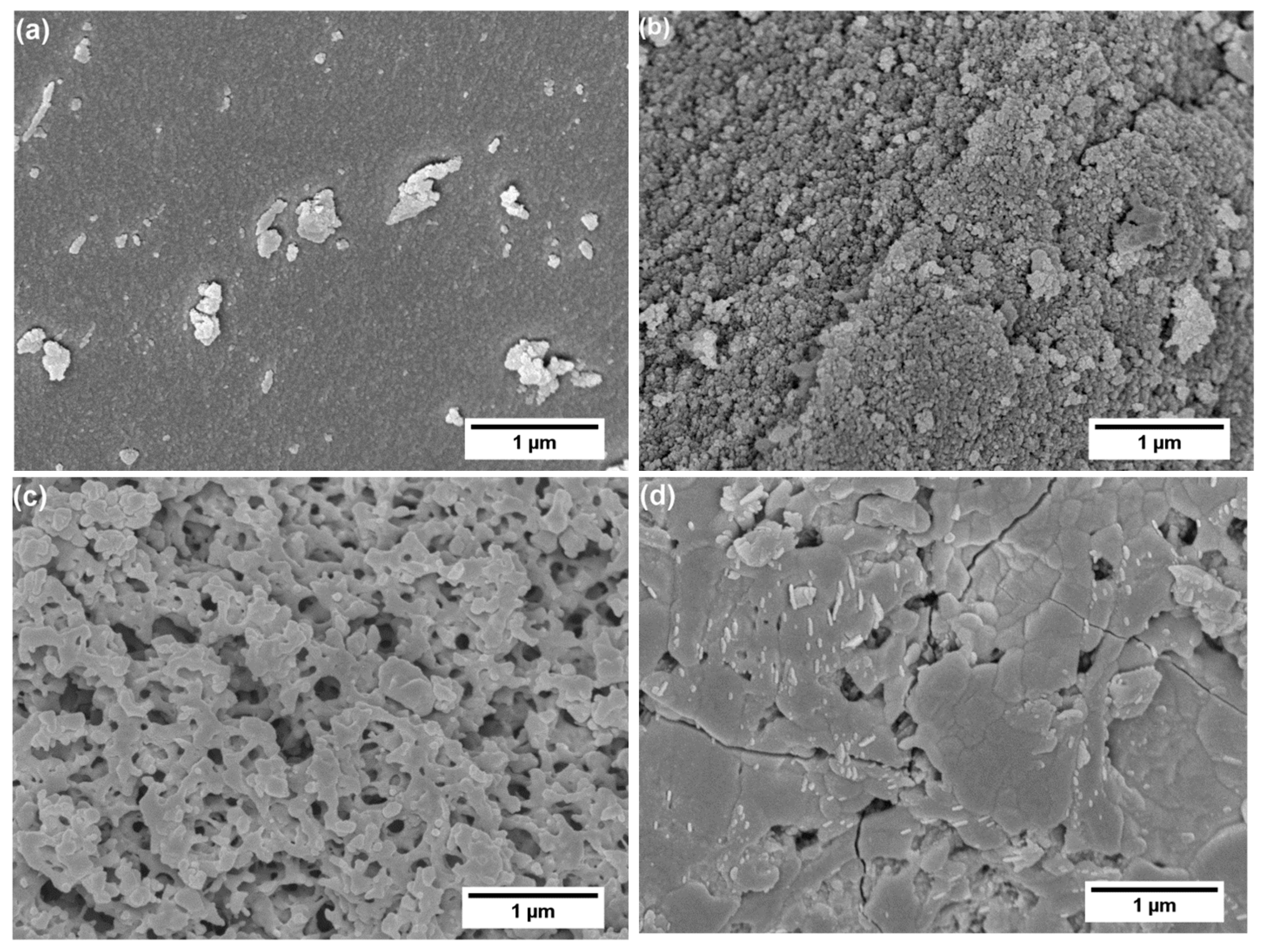
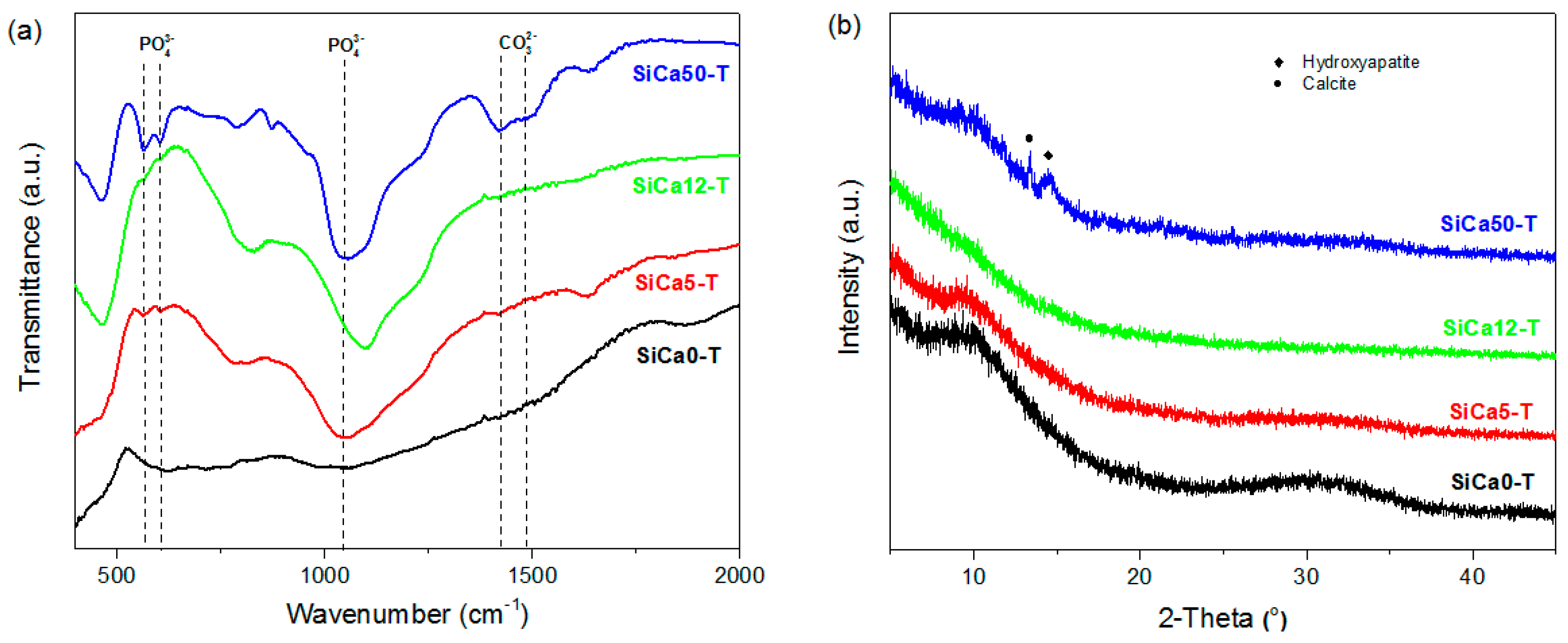
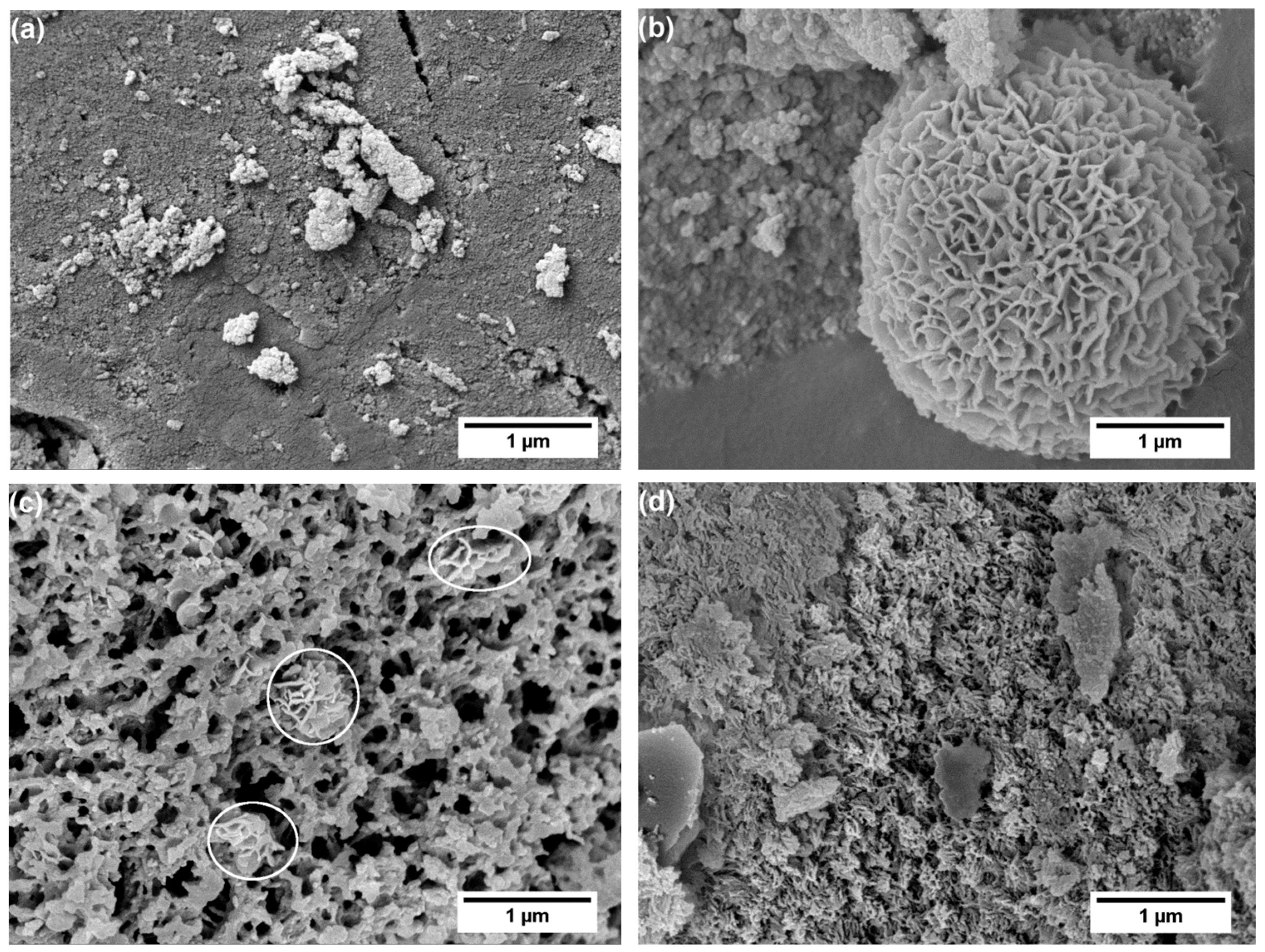
3. Results and Discussion
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhuo, R.; Colombo, P.; Pantano, C.; Vogler, E.A. Silicon oxycarbide glasses for blood-contact applications. Acta Biomater. 2005, 1, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Lagonegro, P.; Rossi, F.; Galli, C.; Smerieri, A.; Alinovi, R.; Pinelli, S.; Rimoldi, T.; Attolini, G.; Macaluso, G.; Macaluso, C.; et al. A cytotoxicity study of silicon oxycarbide nanowires as cell scaffold for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, A.; Mazo, M.A.; Ruiz-Caro, R.; Martín-Illana, A.; Bedoya, L.M.; Veiga-Ochoa, M.D.; Rubio, J. Mesoporous silicon oxycarbide materials for controlled drug delivery systems. Chem. Eng. J. 2015, 280, 165–174. [Google Scholar] [CrossRef]
- Gawęda, M.; Jeleń, P.; Długoń, E.; Wajda, A.; Leśniak, M.; Simka, W.; Sowa, M.; Detsch, R.; Boccaccini, A.R.; Sitarz, M. Bioactive layers based on black glasses on titanium substrates. J. Am. Ceram. Soc. 2018, 101, 590–601. [Google Scholar] [CrossRef]
- Arango-Ospina, M.; Xie, F.; Gonzalo-Juan, I.; Riedel, R.; Ionescu, E.; Boccaccini, A.R. Review: Silicon oxycarbide based materials for biomedical applications. Appl. Mater. Today 2019, 100482. [Google Scholar] [CrossRef]
- Gonzalo-Juan, I.; Detsch, R.; Mathur, S.; Ionescu, E.; Boccaccini, A.R.; Riedel, R. Synthesis and In Vitro Activity Assessment of Novel Silicon Oxycarbide-Based Bioactive Glasses. Materials 2016, 9, 959. [Google Scholar] [CrossRef]
- Ionescu, E.; Sen, S.; Mera, G.; Navrotsky, A. Structure, energetics and bioactivity of silicon oxycarbide-based amorphous ceramics with highly connected networks. J. Eur. Ceram. Soc. 2018, 38, 1311–1319. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Brauer, D.S.; Wilson, R.M.; Law, R.V.; Hill, R.G.; Karpukhina, N. Sodium is not essential for high bioactivity of glasses. Int. J. Appl. Glass Sci. 2017, 8, 428–437. [Google Scholar] [CrossRef]
- Brito, A.F.; Antunes, B.; dos Santos, F.; Fernandes, H.R.; Ferreira, J.M.F. Osteogenic capacity of alkali-free bioactive glasses. In vitro studies. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 2360–2365. [Google Scholar] [CrossRef]
- Kansal, I.; Reddy, A.; Muñoz, F.; Choi, S.-J.; Kim, H.-W.; Tulyaganov, D.U.; Ferreira, J.M.F. Structure, biodegradation behavior and cytotoxicity of alkali-containing alkaline-earth phosphosilicate glasses. Mater. Sci. Eng. C 2014, 44, 159–165. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous Materials for Drug Delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Gonzalo-Juan, I.; Breitzke, H.; Fasel, C.; Trapp, M.; Buntkowsky, G.; Kleebe, H.-J.; Riedel, R.; Boccaccini, A.R.; Ionescu, E. Effect of Ca and B incorporation into silicon oxycarbide on its microstructure and phase composition. J. Am. Ceram. Soc. 2019, 102, 7645–7655. [Google Scholar] [CrossRef]
- Ionescu, E.; Papendorf, B.; Kleebe, H.-J.; Poli, F.; Müller, K.; Riedel, R. Polymer-Derived Silicon Oxycarbide/Hafnia Ceramic Nanocomposites. Part I: Phase and Microstructure Evolution during the Ceramization Process. J. Am. Ceram. Soc. 2010, 93, 1774–1782. [Google Scholar] [CrossRef]
- Singh, A.K.; Pantano, C.G. Porous Silicon Oxycarbide Glasses. J. Am. Ceram. Soc. 1996, 79, 2696–2704. [Google Scholar] [CrossRef]
- Yuan, X.; Jin, H.; Yan, X.; Cheng, L.; Hu, L.; Xue, Q. Synthesis of ordered mesoporous silicon oxycarbide monoliths via preceramic polymer nanocasting. Microporous Mesoporous Mater. 2012, 147, 252–258. [Google Scholar] [CrossRef]
- Duan, L.; Ma, Q.; Chen, Z. Preparation and characterization of mesoporous silicon oxycarbide ceramics without free carbon from polysiloxane. J. Eur. Ceram. Soc. 2013, 33, 841–846. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, H.; Komarneni, S.; Pantano, C.G. Porous silicon oxycarbide glasses from organically modified silica gels of high surface area. J. Sol.-Gel. Sci. Technol. 1994, 1, 141–151. [Google Scholar] [CrossRef]
- Aravind, P.R.; Soraru, G.D. Porous silicon oxycarbide glasses from hybrid ambigels. Microporous Mesoporous Mater. 2011, 142, 511–517. [Google Scholar] [CrossRef]
- Yan, X.; Su, D.; Duan, H.; Zhang, F. Preparation of SiOC/HfO2 fibers from silicon alkoxides and tetrachloride hafnium by a sol–gel process. Mater. Lett. 2015, 148, 196–199. [Google Scholar] [CrossRef]
- Wootton, A.M.; Rappensberger, M.; Lewis, M.H.; Kitchin, S.; Howes, A.P.; Dupree, R. Structural properties of multi-component silicon oxycarbide glasses derived from metal alkoxide precursors. J. Non-Cryst. Solids 1996, 204, 217–227. [Google Scholar] [CrossRef]
- Pereira, M.M.; Clark, A.E.; Hench, L.L. Calcium phosphate formation on sol-gel-derived bioactive glasses in vitro. J. Biomed. Mater. Res. 1994, 28, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-Z.; Li, Y.; Jin, L.-Y.; Quinn, J.M.W.; Komesaroff, P.A. A new sol–gel process for producing Na2O-containing bioactive glass ceramics. Acta Biomater. 2010, 6, 4143–4153. [Google Scholar] [CrossRef] [PubMed]
- Saravanapavan, P.; Hench, L.L. Mesoporous calcium silicate glasses. I. Synthesis. J. Non-Cryst. Solids 2003, 318, 1–13. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Maçon, A.L.B.; Kim, T.B.; Valliant, E.M.; Goetschius, K.; Brow, R.K.; Day, D.E.; Hoppe, A.; Boccaccini, A.R.; Kim, I.Y.; Ohtsuki, C.; et al. A unified in vitro evaluation for apatite-forming ability of bioactive glasses and their variants. J. Mater. Sci. Mater. Med. 2015, 26, 115. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Hurwitz, F.I.; Heimann, P.; Farmer, S.C.; Hembree, D.M. Characterization of the pyrolytic conversion of polysilsesquioxanes to silicon oxycarbides. J. Mater. Sci. 1993, 28, 6622–6630. [Google Scholar] [CrossRef]
- Ionescu, E.; Terzioglu, C.; Linck, C.; Kaspar, J.; Navrotsky, A.; Riedel, R. Thermodynamic Control of Phase Composition and Crystallization of Metal-Modified Silicon Oxycarbides. J. Am. Ceram. Soc. 2013, 96, 1899–1903. [Google Scholar] [CrossRef]
- Schiavon, M.A.; Armelin, N.A.; Yoshida, I.V.P. Novel poly(borosiloxane) precursors to amorphous SiBCO ceramics. Mater. Chem. Phys. 2008, 112, 1047–1054. [Google Scholar] [CrossRef]
- Lin, S.; Ionescu, C.; Pike, K.J.; Smith, M.E.; Jones, J.R. Nanostructure evolution and calcium distribution in sol–gel derived bioactive glass. J. Mater. Chem. 2009, 19, 1276–1282. [Google Scholar] [CrossRef]
- Rokita, M.; Mozgawa, W.; Adamczyk, A. Transformation of silicate gels during heat treatment in air and in argon – Spectroscopic studies. J. Mol. Struct. 2014, 1070, 125–130. [Google Scholar] [CrossRef]
- Fidalgo, A.; Ilharco, L.M. Chemical Tailoring of Porous Silica Xerogels: Local Structure by Vibrational Spectroscopy. Chem.–Eur. J. 2004, 10, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, A.; Peña-Alonso, R.; Rubio, J.; Raj, R.; Sorarù, G.D.; Oteo, J.L. Surface Energy of Sol Gel-Derived Silicon Oxycarbide Glasses. J. Am. Ceram. Soc. 2011, 94, 4523–4533. [Google Scholar] [CrossRef]
- Aguiar, H.; Serra, J.; González, P.; León, B. Structural study of sol–gel silicate glasses by IR and Raman spectroscopies. J. Non-Cryst. Solids 2009, 355, 475–480. [Google Scholar] [CrossRef]
- Serra, J.; González, P.; Liste, S.; Serra, C.; Chiussi, S.; León, B.; Pérez-Amor, M.; Ylänen, H.O.; Hupa, M. FTIR and XPS studies of bioactive silica based glasses. J. Non-Cryst. Solids 2003, 332, 20–27. [Google Scholar] [CrossRef]
- Roth, F.; Waleska, P.; Hess, C.; Ionescu, E.; Nicoloso, N. UV Raman spectroscopy of segregated carbon in silicon oxycarbides. J. Ceram. Soc. Jpn. 2016, 124, 1042–1045. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Cambridge, MA, USA, 2013; ISBN 978-0-08-057103-4. [Google Scholar]
- Schitco, C.; Bazarjani, M.S.; Riedel, R.; Gurlo, A. NH3-assisted synthesis of microporous silicon oxycarbonitride ceramics from preceramic polymers: A combined N2 and CO2 adsorption and small angle X-ray scattering study. J. Mater. Chem. A 2014, 3, 805–818. [Google Scholar] [CrossRef]
- Seifollahi Bazarjani, M.; Kleebe, H.-J.; Müller, M.M.; Fasel, C.; Baghaie Yazdi, M.; Gurlo, A.; Riedel, R. Nanoporous Silicon Oxycarbonitride Ceramics Derived from Polysilazanes in situ Modified with Nickel Nanoparticles. Chem. Mater. 2011, 23, 4112–4123. [Google Scholar] [CrossRef]
- Ettarh, C.; Galwey, A.K. A kinetic and mechanistic study of the thermal decomposition of calcium nitrate. Thermochim. Acta 1996, 288, 203–219. [Google Scholar] [CrossRef]
- Sarmento, C.; Luklinska, Z.B.; Brown, L.; Anseau, M.; Aza, P.N.D.; Aza, S.D.; Hughes, F.J.; McKay, I.J. In vitro behavior of osteoblastic cells cultured in the presence of pseudowollastonite ceramic. J. Biomed. Mater. Res. A 2004, 69A, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pradas, J.M.; Serra, P.; Morenza, J.L.; De Aza, P.N. Pulsed laser deposition of pseudowollastonite coatings. Biomaterials 2002, 23, 2057–2061. [Google Scholar] [CrossRef]
- Almasri, K.A.; Sidek, H.A.A.; Matori, K.A.; Zaid, M.H.M. Effect of sintering temperature on physical, structural and optical properties of wollastonite based glass-ceramic derived from waste soda lime silica glasses. Results Phys. 2017, 7, 2242–2247. [Google Scholar] [CrossRef]
- Bernardo, E. Fast sinter-crystallization of a glass from waste materials. J. Non-Cryst. Solids 2008, 354, 3486–3490. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Massuyeau, F.; Constantin, L.V.; Predoi, D. Structural and physical properties of antibacterial Ag-doped nano-hydroxyapatite synthesized at 100 °C. Nanoscale Res. Lett. 2011, 6, 613. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, J.; Dong, X.; Zhang, L.; Zeng, H. A mesoporous bioactive glass/polycaprolactone composite scaffold and its bioactivity behavior. J. Biomed. Mater. Res. A 2008, 84A, 84–91. [Google Scholar] [CrossRef]
- Peitl, O.; Dutra Zanotto, E.; Hench, L.L. Highly bioactive P2O5–Na2O–CaO–SiO2 glass-ceramics. J. Non-Cryst. Solids 2001, 292, 115–126. [Google Scholar] [CrossRef]
- Lukito, D.; Xue, J.M.; Wang, J. In vitro bioactivity assessment of 70 (wt.)%SiO2–30 (wt.)%CaO bioactive glasses in simulated body fluid. Mater. Lett. 2005, 59, 3267–3271. [Google Scholar] [CrossRef]
- Iimori, Y.; Kameshima, Y.; Okada, K.; Hayashi, S. Comparative study of apatite formation on CaSiO3 ceramics in simulated body fluids with different carbonate concentrations. J. Mater. Sci. Mater. Med. 2005, 16, 73–79. [Google Scholar] [CrossRef]
- Kumar, G.S.; Girija, E.K. Flower-like hydroxyapatite nanostructure obtained from eggshell: A candidate for biomedical applications. Ceram. Int. 2013, 39, 8293–8299. [Google Scholar] [CrossRef]
- He, Q.; Huang, Z.; Liu, Y.; Chen, W.; Xu, T. Template-directed one-step synthesis of flowerlike porous carbonated hydroxyapatite spheres. Mater. Lett. 2007, 61, 141–143. [Google Scholar] [CrossRef]
- Xie, F.; Gonzalo Juan, I.; Arango-Ospina, M.; Riedel, R.; Boccaccini, A.R.; Ionescu, E. Apatite Forming Ability and Dissolution Behavior of Boron- and Calcium-Modified Silicon Oxycarbides in Comparison to Silicate Bioactive Glass. ACS Biomater. Sci. Eng. 2019, 5, 5337–5347. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- Arcos, D.; Greenspan, D.C.; Vallet-Regí, M. A new quantitative method to evaluate the in vitro bioactivity of melt and sol-gel-derived silicate glasses. J. Biomed. Mater. Res. A 2003, 65A, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
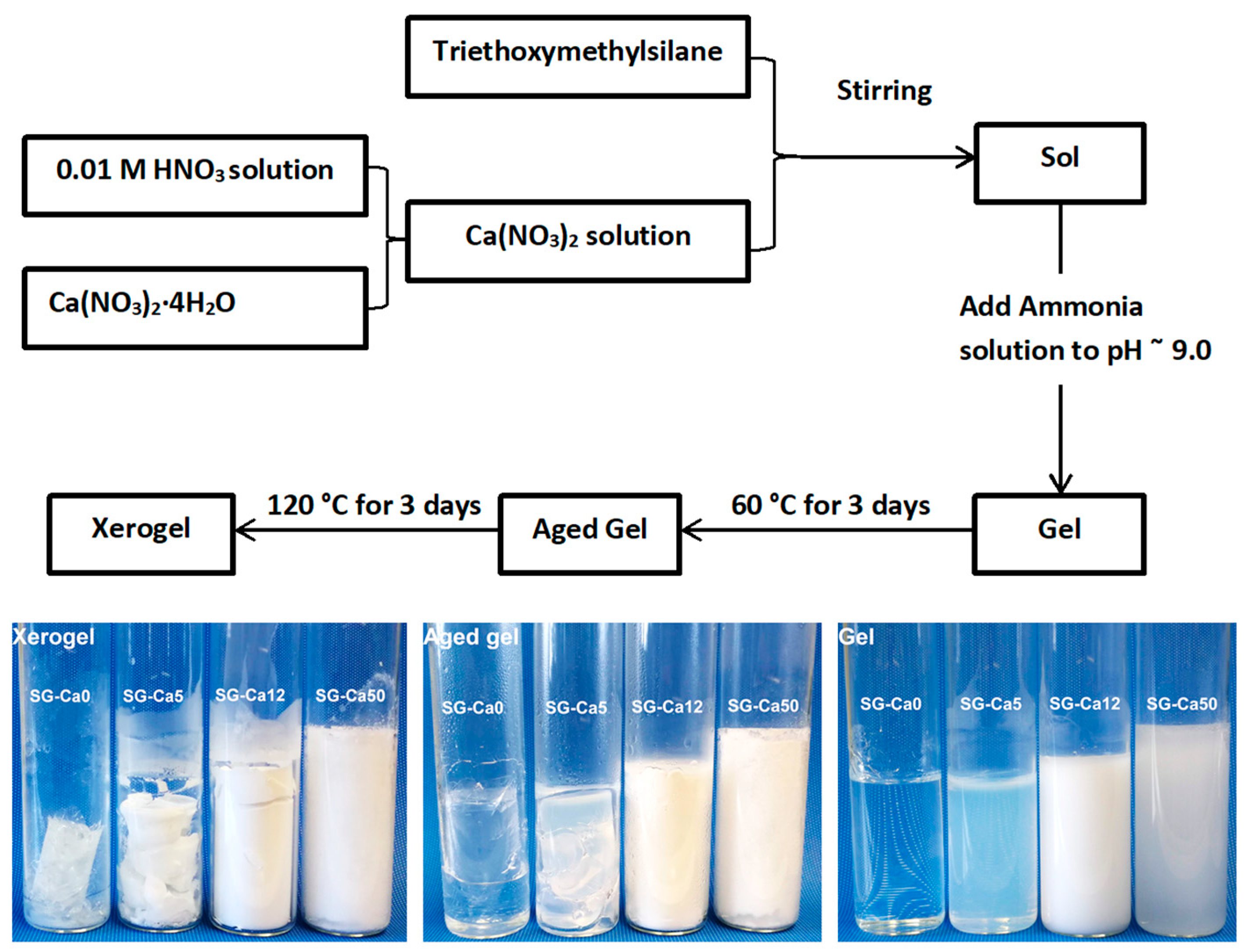
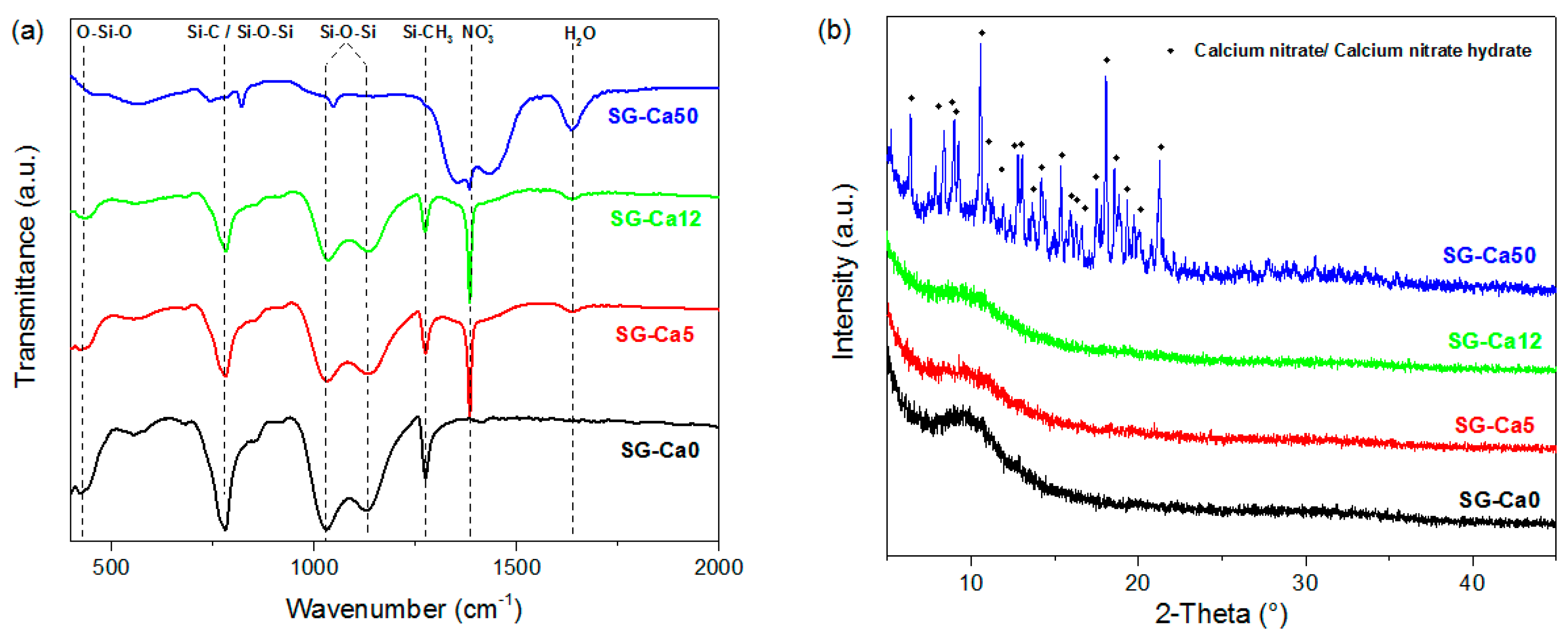
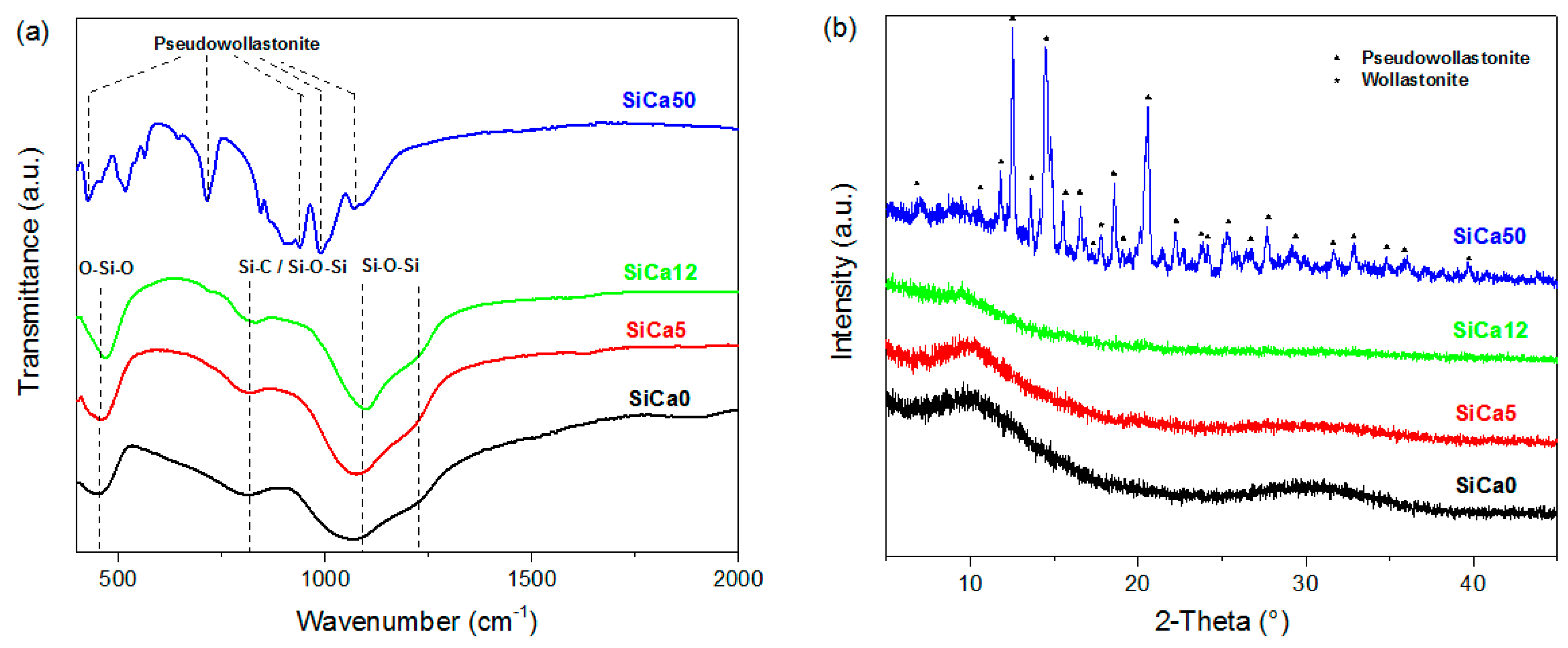
| Sample | Molar Ratio (Ca/Si) | Triethoxymethylsilane (g) | 0.01M HNO3 Solution (g) | Ca(NO3)2·4H2O (g) |
|---|---|---|---|---|
| SG-Ca0 | 0.00 | 10 | 3.0 | 0.00 |
| SG-Ca5 | 0.05 | 10 | 3.5 | 0.66 |
| SG-Ca12 | 0.12 | 10 | 4.0 | 1.59 |
| SG-Ca50 | 0.50 | 10 | 4.5 | 6.62 |
| Sample | LO Si–O–Si (%) | TO Si–O–Si (%) | OV Si–O–Si and Si–C (%) | Q3 (%) | Q3/OV | OV/(LO + TO) | LO-TO splitting (cm−1) |
|---|---|---|---|---|---|---|---|
| SiCa0 | 26.8 | 53.1 | 20.1 | - | - | 0.25 | 167.1 |
| SiCa5 | 33.3 | 41.6 | 14.6 | 10.5 | 0.7 | 0.20 | 127.4 |
| SiCa12 | 28.8 | 47.3 | 11.1 | 12.8 | 1.1 | 0.15 | 127.3 |
| Sample | Si (wt.%) | O (wt.%) | C( wt.%) | Ca (wt.%) | Empirical Formulae | Estimated Phase Compositions |
|---|---|---|---|---|---|---|
| SiCa0 | 47.92a | 40.18 | 11.90 | - | Si1O1.47C0.58 | Si1O1.47C0.27 + 0.31 C |
| SiCa5 | 44.49a | 41.65 | 10.68 | 3.18a | Si1Ca0.05O1.64C0.56 | Si1Ca0.05O1.64C0.21 + 0.35 C |
| SiCa12 | 40.93a | 42.64 | 9.41 | 7.02a | Si1Ca0.12O1.82C0.54 | Si1Ca0.12O1.82C0.15 + 0.39 C |
| SiCa50 | 30.79a | 41.10 | 6.11 | 22.00a | Si1Ca0.50O2.34C0.46 | 0.50 Si1O1.68C0.16 + 0.50 CaSiO3 + 0.38 C |
| BET Specific Surface Area SSA (m2/g) | Ca/Si Molar Ratio = 0.00 | Ca/Si Molar Ratio = 0.05 | Ca/Si Molar Ratio = 0.12 | Ca/Si Molar Ratio = 0.50 |
|---|---|---|---|---|
| Xerogel | 435.4 | 534.6 | 493.0 | 157.8 |
| Silicon oxycarbide | 22.5 | 123.4 | 39.5 | 23.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, F.; Ionescu, E.; Arango-Ospina, M.; Riedel, R.; Boccaccini, A.R.; Gonzalo-Juan, I. Facile Preparative Access to Bioactive Silicon Oxycarbides with Tunable Porosity. Materials 2019, 12, 3862. https://doi.org/10.3390/ma12233862
Xie F, Ionescu E, Arango-Ospina M, Riedel R, Boccaccini AR, Gonzalo-Juan I. Facile Preparative Access to Bioactive Silicon Oxycarbides with Tunable Porosity. Materials. 2019; 12(23):3862. https://doi.org/10.3390/ma12233862
Chicago/Turabian StyleXie, Fangtong, Emanuel Ionescu, Marcela Arango-Ospina, Ralf Riedel, Aldo R. Boccaccini, and Isabel Gonzalo-Juan. 2019. "Facile Preparative Access to Bioactive Silicon Oxycarbides with Tunable Porosity" Materials 12, no. 23: 3862. https://doi.org/10.3390/ma12233862
APA StyleXie, F., Ionescu, E., Arango-Ospina, M., Riedel, R., Boccaccini, A. R., & Gonzalo-Juan, I. (2019). Facile Preparative Access to Bioactive Silicon Oxycarbides with Tunable Porosity. Materials, 12(23), 3862. https://doi.org/10.3390/ma12233862







