Influence of Effective Water-to-Cement Ratios on Internal Damage and Salt Scaling of Concrete with Superabsorbent Polymer
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Preparation of Mortar and Concrete Samples
2.2. Characterization of SAPs
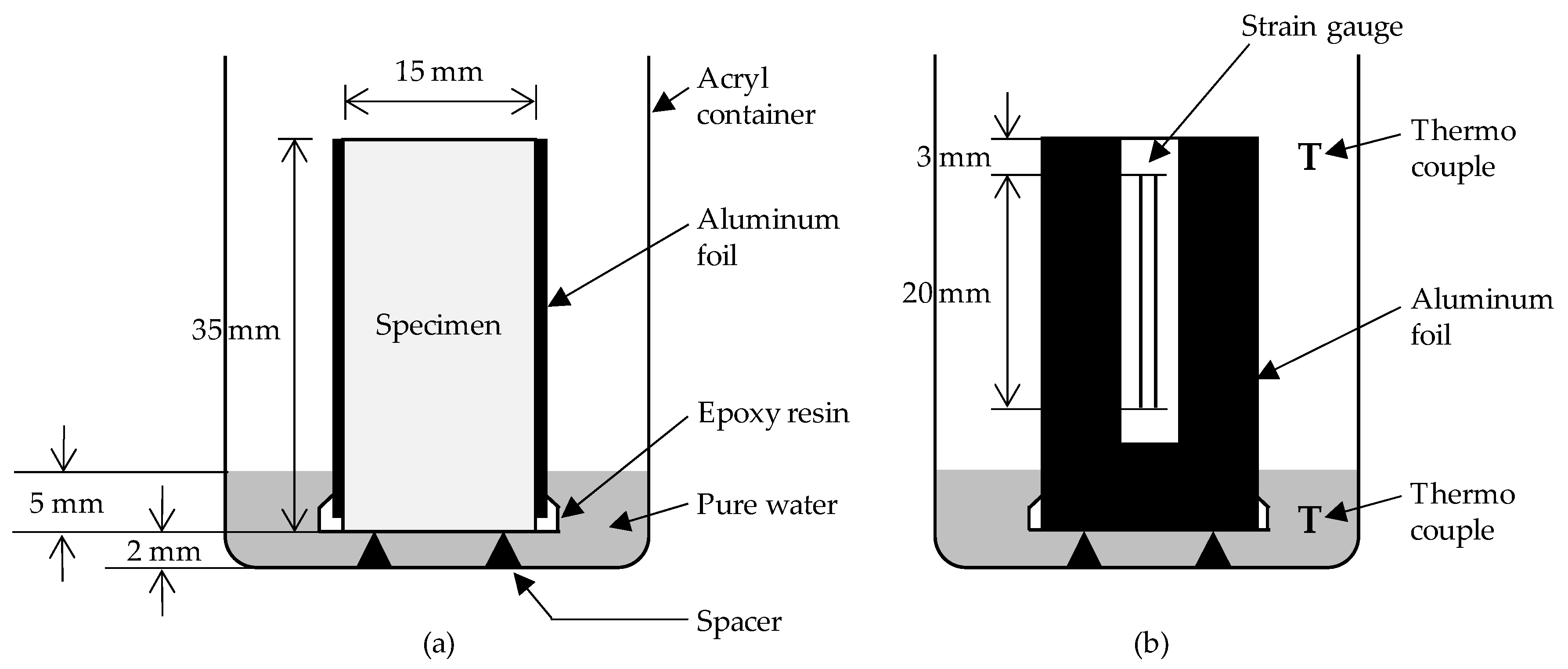
2.3. Experimental Methods
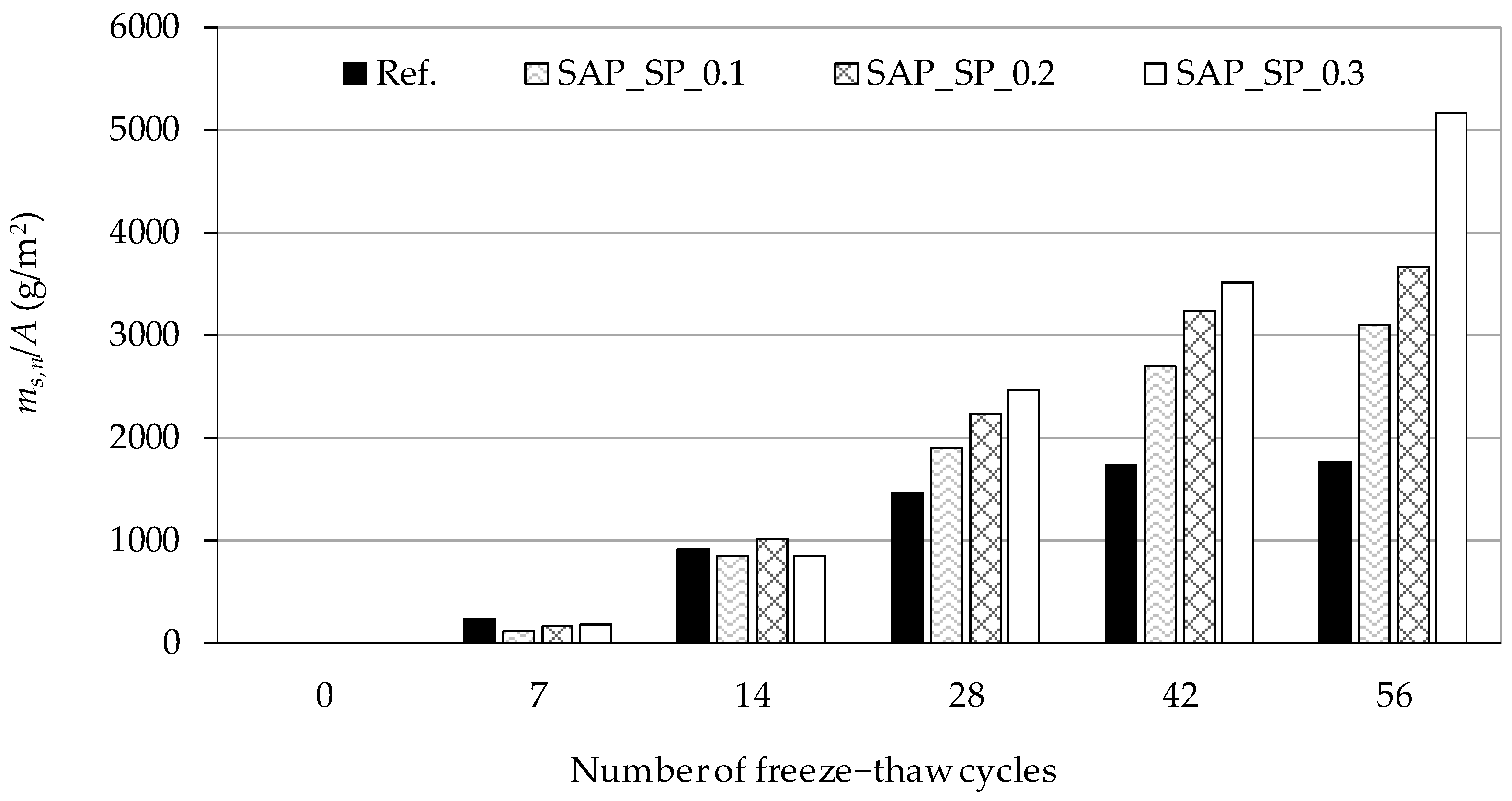
3. Results
3.1. Absorption Rate of SAPs in Concrete
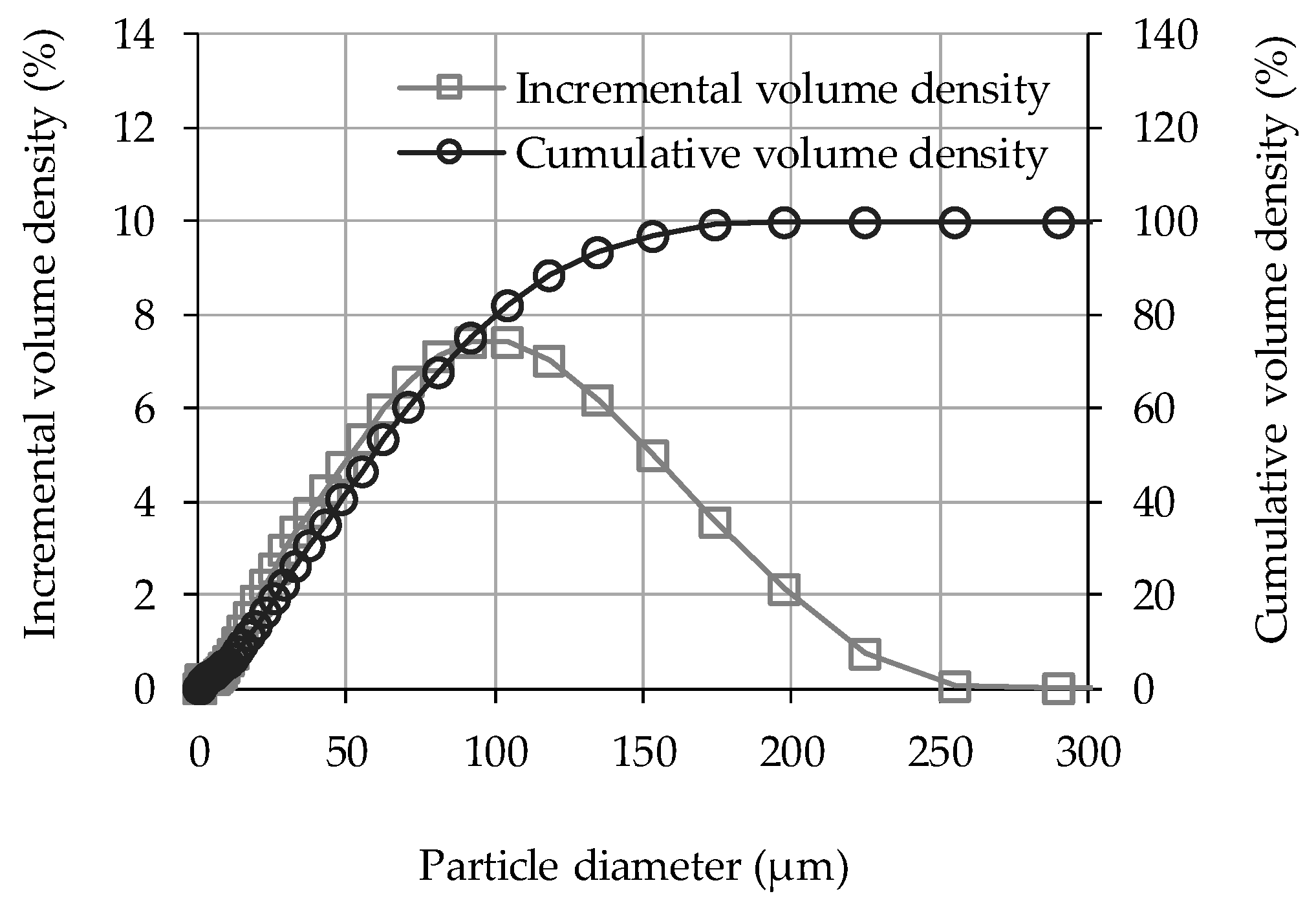
3.2. Pore Size Distribution
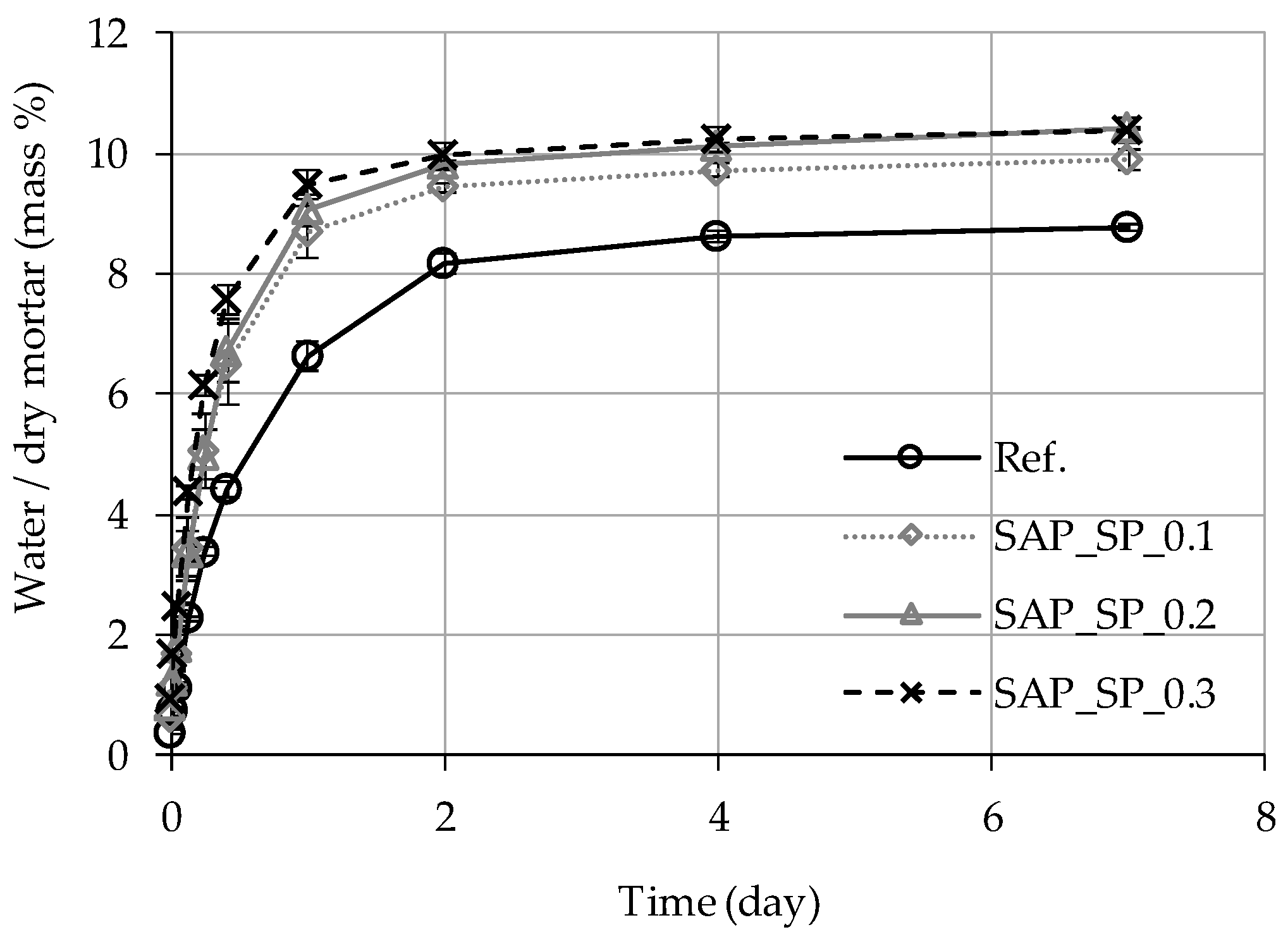
3.3. Capillary Water Absorption
3.4. Strain on Mortar During Freeze–Thaw Cycles
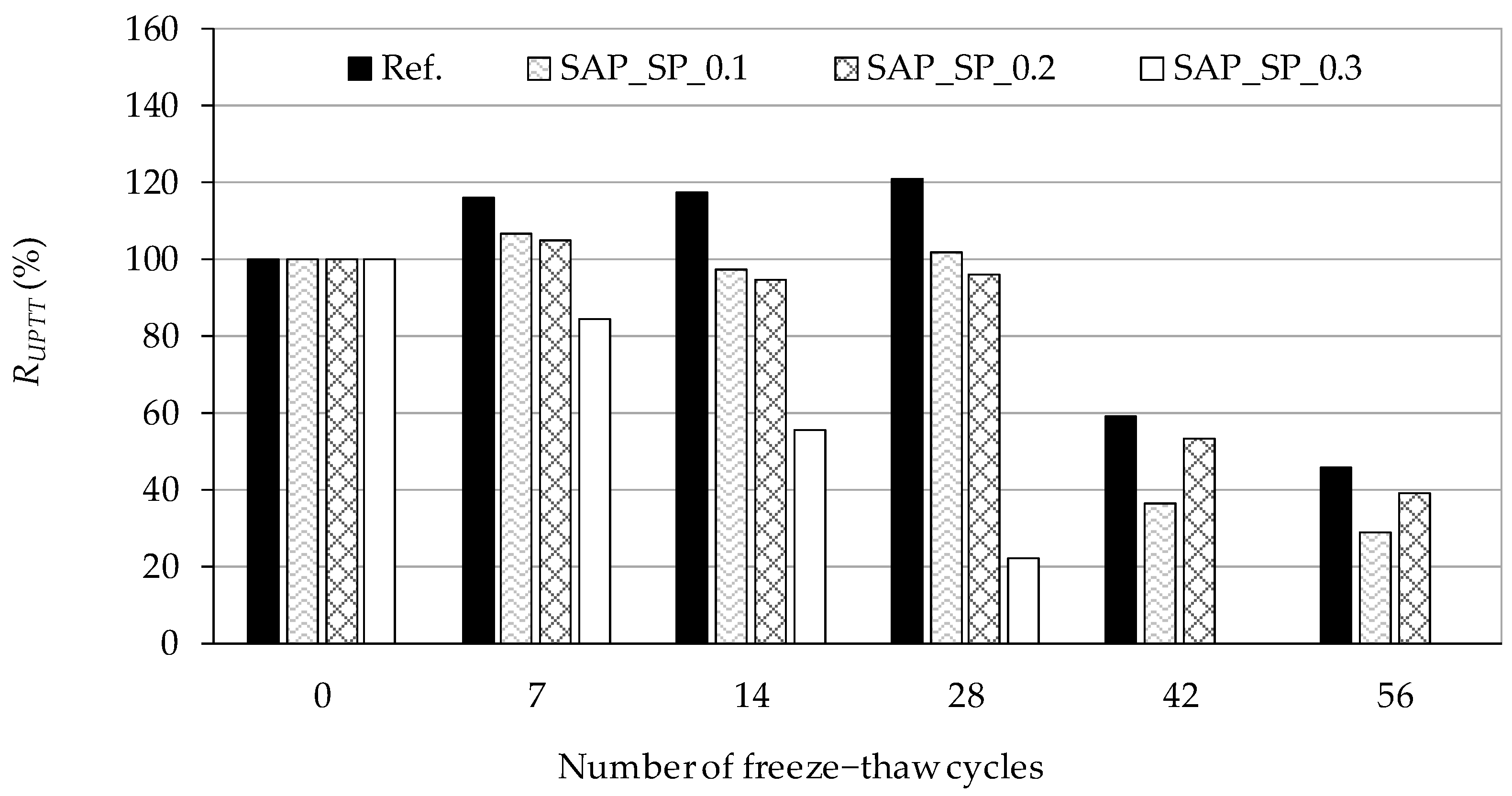
3.5. Freeze–Thaw Resistance of Concrete
4. Discussion
4.1. Air Voids Formed by SAPs
4.2. Water Absorption in Capillary Pores and Air Voids
4.3. Internal Damage
4.4. Salt Scaling Damage
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jensen, O.M.; Hansen, P.F. Water-entrained cement-based materials: I. Principles and theoretical background. Cem. Concr. Res. 2001, 31, 647–654. [Google Scholar] [CrossRef]
- Jensen, O.M.; Hansen, P.F. Water-entrained cement-based materials: II. Experimental observations. Cem. Concr. Res. 2002, 32, 973–978. [Google Scholar] [CrossRef]
- Hong, G.; Choi, S. Rapid self-sealing of cracks in cementitious materials incorporating superabsorbent polymers. Constr. Build. Mater. 2017, 143, 366–375. [Google Scholar] [CrossRef]
- Rodríguez, C.R.; Figueiredo, S.C.; Deprez, M.; Snoeck, D.; Schlangen, E.; Šavija, B. Numerical investigation of crack self-sealing in cement-based composites with superabsorbent polymers. Cem. Concr. Compos. 2019, 104, 103395. [Google Scholar] [CrossRef]
- Lee, H.X.D.; Wong, H.S.; Buenfeld, N. Self-sealing cement-based materials using superabsorbent polymers. In Proceedings of the International RILEM Conference on Use of Superabsorbent Polymers and Other New Additives in Concrete, Lyngby, Denmark, 15–18 August 2010; pp. 15–18. [Google Scholar]
- Snoeck, D.; Steuperaert, S.; Van Tittelboom, K.; Dubruel, P.; De Belie, N. Visualization of water penetration in cementitious materials with superabsorbent polymers by means of neutron radiography. Cem. Concr. Res. 2012, 42, 1113–1121. [Google Scholar] [CrossRef]
- Deng, H.; Liao, G. Assessment of influence of self-healing behavior on water permeability and mechanical performance of ECC incorporating superabsorbent polymer (SAP) particles. Constr. Build. Mater. 2018, 170, 455–465. [Google Scholar] [CrossRef]
- Wang, C.; Bu, Y.; Guo, S.; Lu, Y.; Sun, B.; Shen, Z. Self-healing cement composite: Amine-and ammonium-based pH-sensitive superabsorbent polymers. Cem. Concr. Compos. 2019, 96, 154–162. [Google Scholar] [CrossRef]
- Shim, Y.; Hong, G.; Choi, S. Autogenous Healing of Early-Age Cementitious Materials Incorporating Superabsorbent Polymers Exposed to Wet/Dry Cycles. Materials 2018, 11, 2476. [Google Scholar] [CrossRef] [PubMed]
- Snoeck, D.; Van Tittelboom, K.; Steuperaert, S.; Dubruel, P.; De Belie, N. Self-healing cementitious materials by the combination of microfibres and superabsorbent polymers. J. Intel. Mat. Syst. Str. 2014, 25, 13–24. [Google Scholar] [CrossRef]
- Assmann, A. Physical properties of concrete modified with superabsorbent polymers. Ph.D. Thesis, University of Stuttgart, Stuttgart, Germany, 2013. [Google Scholar]
- Hasholt, M.T.; Jensen, O.M.; Laustsen, S. Superabsorbent polymers as a means of improving frost resistance of concrete. Adv. Civ. Eng. Mater. 2015, 4, 237–256. [Google Scholar] [CrossRef]
- Mechtcherine, V.; Schroefl, C.; Wyrzykowski, M.; Gorges, M.; Lura, P.; Cusson, D.; Margeson, J.; De Belie, N.; Snoeck, D.; Ichimiya, K. Effect of superabsorbent polymers (SAP) on the freeze–thaw resistance of concrete: Results of a RILEM interlaboratory study. Mater. Struct. 2017, 50, 14. [Google Scholar] [CrossRef]
- Craeye, B.; Cockaerts, G.; Kara De Maeijer, P.J.I. Improving Freeze–Thaw Resistance of Concrete Road Infrastructure by Means of Superabsorbent Polymers. Infrastructures 2018, 3, 4. [Google Scholar] [CrossRef]
- Reinhardt, H.-W.; Assmann, A. Effect of superabsorbent polymers on durability of concrete. In Application of Super Absorbent Polymers (SAP) in Concrete Construction; Mechtcherine, V., Reinhardt, H.-W., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 115–135. [Google Scholar]
- Jones, W.A.; Weiss, W.J. Freeze thaw durability of internally cured concrete made using superabsorbent polymers. In Proceedings of the 4th International Conference on the Durability of Concrete Structures, Purdue University, West Lafayette, IN, USA, 21 July 2014. [Google Scholar]
- Fagerlund, G. The Long Time Water Absorption in the Air-Pore Structure of Concrete; Lund University Citeseer: Lund, Sweden, 1993. [Google Scholar]
- Kang, S.-H.; Hong, S.-G.; Moon, J. Importance of drying to control internal curing effects on field casting ultra-high performance concrete. Cem. Concr. Res. 2018, 108, 20–30. [Google Scholar] [CrossRef]
- Kang, S.-H.; Hong, S.-G.; Moon, J. Absorption kinetics of superabsorbent polymers (SAP) in various cement-based solutions. Cem. Concr. Res. 2017, 97, 73–83. [Google Scholar] [CrossRef]
- Mönnig, S. Superabsorbing Additions in Concrete: Applications, Modelling and Comparison of Different Internal Water Sources. Ph.D. Thesis, University of Stuttgart, Stuttgart, Germany, 2009. [Google Scholar]
- Valentina, S.; Eleonora, P.; Vleria, C.; Marco, A.O.; Paola, F.; Davide, G.; Giuseppe, C. Towards novel fluorinated methacrylic coatings for cultural heritage: A combined polymers and surfaces chemistry study. Polymers 2019, 11, 1190. [Google Scholar]
- CEN/TS 12390–9 Testing Hardened Concrete-Part 9: Freeze-thaw Resistance-Scaling; European Committee for Standardization: Brussels, Belgium, 2006.
- Snoeck, D.; De Belie, N. The influence of superabsorbent polymers on the microstructure and permeability of cementitious materials. In Proceedings of the International Conference on Concrete under Severe Conditions-Environment and Loading, Nanjing, China, 23–25 September 2013; pp. 1–11. [Google Scholar]
- Snoeck, D.; Schaubroeck, D.; Dubruel, P.; De Belie, N.J.C.; Materials, B. Effect of high amounts of superabsorbent polymers and additional water on the workability, microstructure and strength of mortars with a water-to-cement ratio of 0.50. Constr. Build. Mater. 2014, 72, 148–157. [Google Scholar] [CrossRef]
- Abell, A.; Willis, K.; Lange, D. Mercury intrusion porosimetry and image analysis of cement-based materials. J. Colloid Interface Sci. 1999, 211, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Scherer, G.W. Pore size and shape in mortar by thermoporometry. Cem. Concr. Res. 2010, 40, 740–751. [Google Scholar] [CrossRef]
- Powers, T.C.; Willis, T. The air requirement of frost resistant concrete. In Proceedings of the Highway Research Board, Washington, DC, USA, 13–16 December 1950; Volume 29. [Google Scholar]
- Sun, Z.; Scherer, G.W. Effect of air voids on salt scaling and internal freezing. Cem. Concr. Res. 2010, 40, 260–270. [Google Scholar] [CrossRef]




| Sample | Water/Cement Ratio | SAP/Cement Ratio | Raw Materials/Cement Ratio | |||
|---|---|---|---|---|---|---|
| w/c(total) | w/c(add) | w/c(stored by SAPs) | w/c(effective) | |||
| Ref. | 0.45 | - | - | 0.45 | - | Sand (1.6) |
| SAP_SP_0.1 | 0.471 | 0.021 | 0.003 | 0.468 | 0.001 | PCE (0.007) |
| SAP_SP_0.2 | 0.492 | 0.042 | 0.006 | 0.486 | 0.002 | Gravel (2.5) 1 |
| SAP_SP_0.3 | 0.513 | 0.063 | 0.009 | 0.504 | 0.003 | - |
| Sample | Ref. | SAP_SP_0.1 | SAP_SP_0.2 | SAP_SP_0.3 |
|---|---|---|---|---|
| Air Content (%) | 2.2 | 1.9 | 1.6 | 1.4 |
| Sample | Ref. | SAP_SP_0.1 | SAP_SP_0.2 | SAP_SP_0.3 |
|---|---|---|---|---|
| Bulk Density (g/mL) | 2.05 | 2.04 | 2.01 | 2.04 |
| Skeletal Density (g/mL) | 2.55 | 2.57 | 2.54 | 2.59 |
| Porosity (vol. %) | 19.4 | 20.5 | 20.8 | 21.0 |
| Sample | Ref. | SAP_SP_0.1 | SAP_SP_0.2 | SAP_SP_0.3 |
|---|---|---|---|---|
| Strain × 106 | 193.7 | 87.4 | 312.3 | 466.7 |
| Sample | SAP_SP_0.1 | SAP_SP_0.2 | SAP_SP_0.3 |
|---|---|---|---|
| Mass of Dry SAPs in Mortar (mg/mL) | 0.36 | 0.70 | 1.08 |
| Volume of SAP Air Voids in Mortar (vol. %) | 0.11 | 0.22 | 0.34 |
| Sample | Ref. | SAP_SP_0.1 | SAP_SP_0.2 | SAP_SP_0.3 |
|---|---|---|---|---|
| (1) Capillary Porosity 1 (mL/mL) | 0.185 | 0.193 | 0.198 | 0.195 |
| (2) Water Volume in Mortar (Figure 9a) (mL/mL) | 0.178 | 0.184 | 0.191 | 0.186 |
| (3) Water Volume in Mortar (Figure 9b) (mL/mL) | 0.171 | 0.177 | 0.183 | 0.178 |
| (4) Water Volume in Mortar on the Seventh Day (mL/mL) | 0.180 | 0.202 | 0.209 | 0.212 |
| (4) − (3) (mL/mL) | 0.009 | 0.025 | 0.026 | 0.034 |
| Increased Water Volume Due to SAP_SPs 2 (mL/mL) | 0 | 0.016 | 0.017 | 0.025 |
| Water Absorbency of SAP_SPs 3 (g/g) | - | 44 | 24 | 23 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Kang, S.-H.; Hong, S.-G.; Moon, J. Influence of Effective Water-to-Cement Ratios on Internal Damage and Salt Scaling of Concrete with Superabsorbent Polymer. Materials 2019, 12, 3863. https://doi.org/10.3390/ma12233863
Kim M, Kang S-H, Hong S-G, Moon J. Influence of Effective Water-to-Cement Ratios on Internal Damage and Salt Scaling of Concrete with Superabsorbent Polymer. Materials. 2019; 12(23):3863. https://doi.org/10.3390/ma12233863
Chicago/Turabian StyleKim, Minsoo, Sung-Hoon Kang, Sung-Gul Hong, and Juhyuk Moon. 2019. "Influence of Effective Water-to-Cement Ratios on Internal Damage and Salt Scaling of Concrete with Superabsorbent Polymer" Materials 12, no. 23: 3863. https://doi.org/10.3390/ma12233863
APA StyleKim, M., Kang, S.-H., Hong, S.-G., & Moon, J. (2019). Influence of Effective Water-to-Cement Ratios on Internal Damage and Salt Scaling of Concrete with Superabsorbent Polymer. Materials, 12(23), 3863. https://doi.org/10.3390/ma12233863





