Low-Melting Manganese(II)-Based Ionic Liquids: Syntheses, Structures, Properties and Influence of Trace Impurities
Abstract
1. Introduction
2. Experimental
2.1. Instrumentation
2.2. Materials
2.3. Synthesis of Anx+[Mn(NCS)4]2−
2.4. Synthesis of Anx+[MnCl4]2−
2.5. Synthesis of A2+[Mn(NCO)4]2−
3. Results and Discussion
3.1. Syntheses
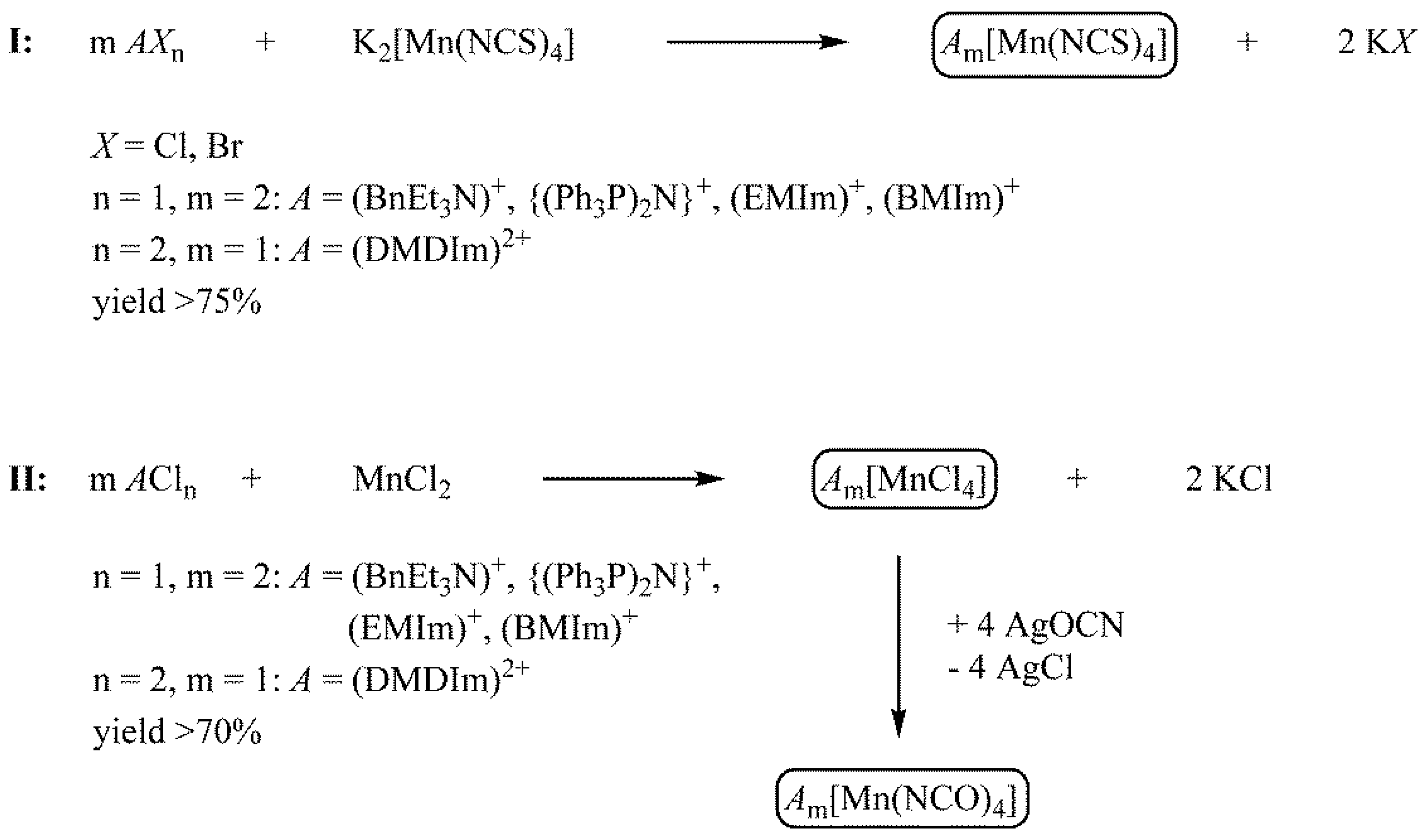
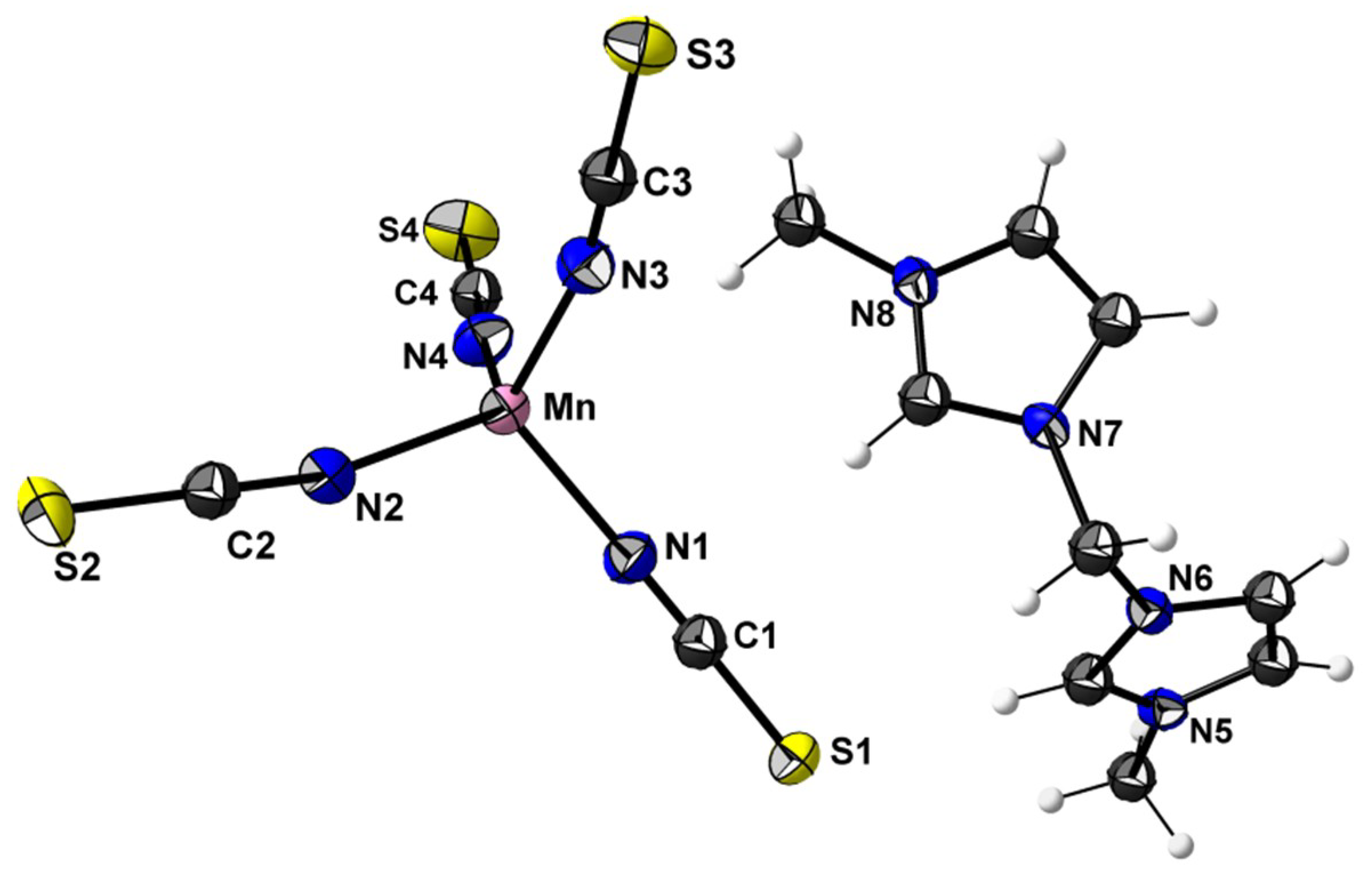
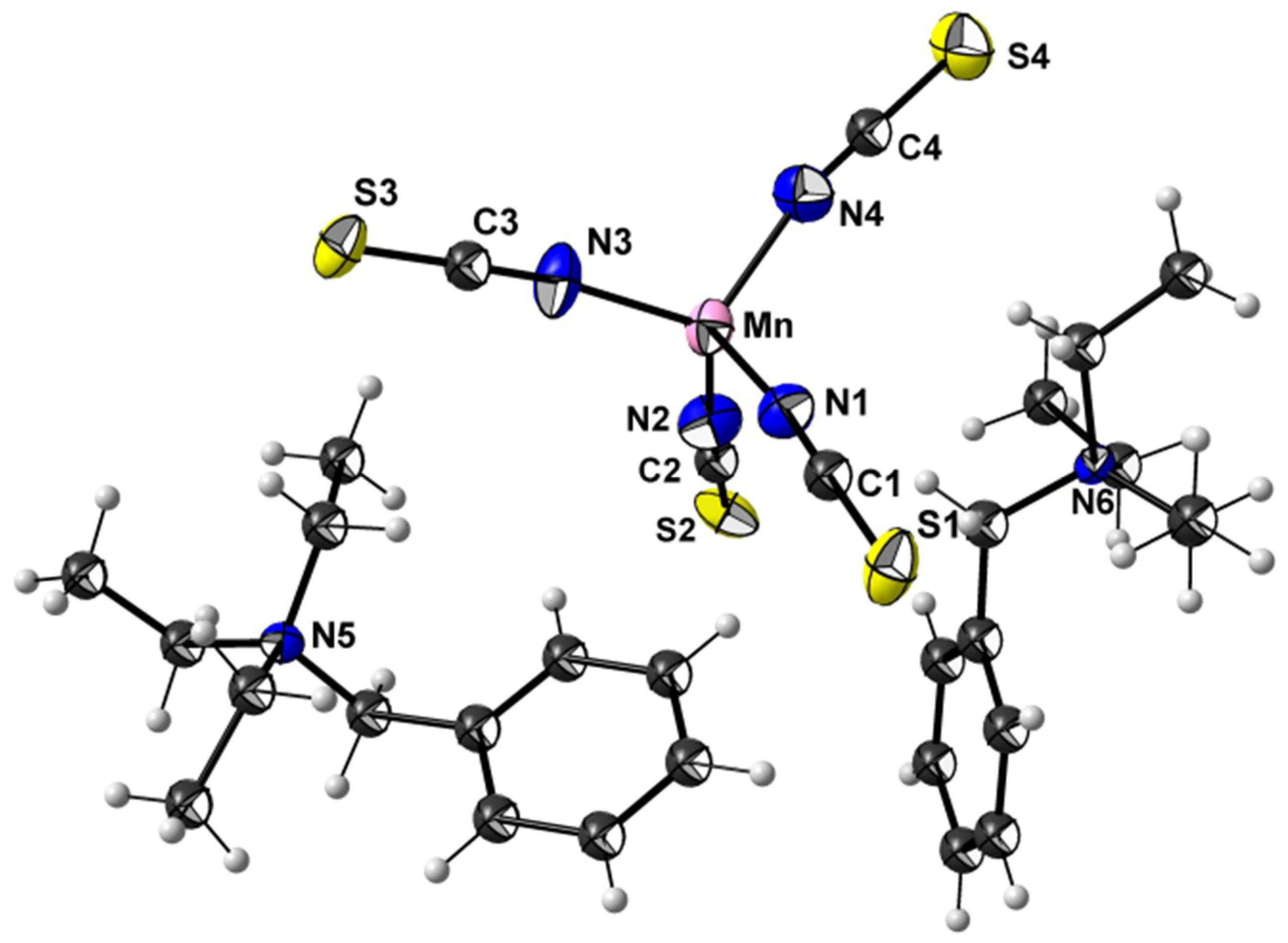
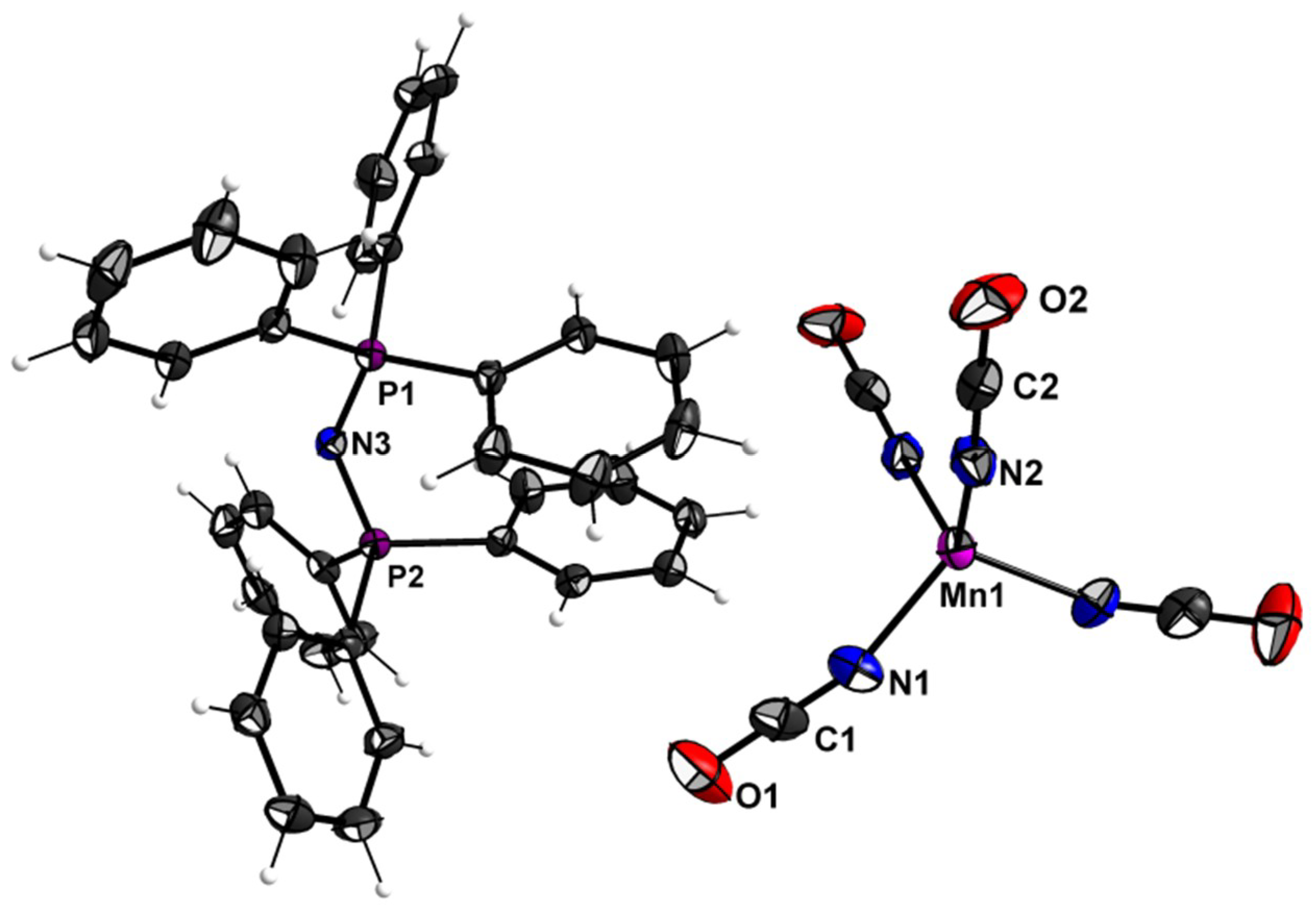
3.2. Crystal Structures
3.3. Thermal and Magnetic Properties
3.4. Physicochemical Properties and Influence of Trace Impurities
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Wasserscheid, P.; Keim, W. Ionic liquids—New “solutions” for transition metal catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Earle, M.J.; Seddon, K.R. Ionic liquids. Green solvents for the future. Pure Appl. Chem. 2000, 72, 1391–1398. [Google Scholar] [CrossRef]
- Sheldon, R. Catalytic reactions in ionic liquids. Chem. Commun. 2001, 2399–2407. [Google Scholar] [CrossRef]
- Galinski, M.; Lewandowski, A.; Stepniak, I. Ionic liquids as electrolytes. Electrochim. Acta 2006, 51, 5567–5580. [Google Scholar] [CrossRef]
- Parvulescu, V.I.; Hardacre, C. Catalysis in ionic liquids. Chem. Rev. 2007, 107, 2615–2665. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Tachikawa, N.; Forsyth, M.; Pringle, J.M.; Howlett, P.C.; Elliott, G.D.; Davis, J.H.; Watanabe, M.; Simon, P.; Angell, C.A. Energy applications of ionic liquids. Energy Environ. Sci. 2014, 7, 232–250. [Google Scholar] [CrossRef]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and nanostructure in ionic liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X. Imidazolium ionic liquids, imidazolylidene heterocyclic carbenes, and zeolitic imidazolate frameworks for CO2 capture and photochemical reduction. Angew. Chem. Int. Ed. 2016, 55, 2308–2320. [Google Scholar] [CrossRef] [PubMed]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Zhang, J.; Huang, C.; Lei, Z. Ionic liquids in selective oxidation: Catalysts and solvents. Chem. Rev. 2017, 117, 6929–6983. [Google Scholar] [CrossRef]
- Basile, A.; Hilder, M.; Makhlooghiazad, F.; Pozo-Gonzalo, C.; MacFarlane, D.R.; Howlett, P.C.; Forsyth, M. Ionic liquids and organic ionic plastic crystals: Advanced electrolytes for safer high performance sodium energy storage technologies. Adv. Energy Mater. 2018, 8, 1703491. [Google Scholar] [CrossRef]
- Gomes, J.M.; Silva, S.S.; Reis, R.L. Biocompatible ionic liquids: Fundamental behaviours and applications. Chem. Soc. Rev. 2019, 48, 4317–4335. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Saito, G. Design of functional ionic liquids using magneto- and luminescent-active anions. Phys. Chem. Chem. Phys. 2010, 12, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.; Albo, J.; Irabien, A. Magnetic ionic liquids: Synthesis, properties and applications. RSC Adv. 2014, 4, 40008–40018. [Google Scholar] [CrossRef]
- Estager, J.; Holbrey, J.D.; Swadzba-Kwasny, M. Halometallate ionic liquids–revisited. Chem. Soc. Rev. 2014, 43, 847–886. [Google Scholar] [CrossRef]
- Clark, K.D.; Nacham, O.; Purslow, J.A.; Pierson, S.A.; Anderson, J.L. Magnetic ionic liquids in analytical chemistry: A review. Anal. Chim. Acta 2016, 934, 9–21. [Google Scholar] [CrossRef]
- Joseph, A.; Zyla, G.; Thomas, V.I.; Nair, P.R.; Padmanabhan, A.S.; Mathew, S. Paramagnetic ionic liquids for advanced applications: A review. J. Mol. Liq. 2016, 218, 319–331. [Google Scholar] [CrossRef]
- Guerrero-Sanchez, C.; Lara-Ceniceros, T.; Jimenez-Regalado, E.; Raşa, M.; Schubert, U.S. Magnetorheological fluids based on ionic liquids. Adv. Mater. 2007, 19, 1740–1747. [Google Scholar] [CrossRef]
- Dodbiba, G.; Park, H.S.; Okaya, K.; Fujita, T. Investigating magnetorheological properties of a mixture of two types of carbonyl iron powders suspended in an ionic liquid. J. Magn. Magn. Mater. 2008, 320, 1322–1327. [Google Scholar] [CrossRef]
- De Vicente, J.; Klingenberg, D.J.; Hidalgo-Alvarez, R. Magnetorheological fluids: A review. Soft Matter 2011, 7, 3701–3710. [Google Scholar] [CrossRef]
- Kozlova, S.A.; Verevkin, S.P.; Heintz, A.; Peppel, T.; Köckerling, M. Paramagnetic ionic liquid 1-Butyl-3-methylimidazolium tetrabromidocobaltate(II): Activity coefficients at infinite dilution of organic solutes and crystal structure. J. Chem. Eng. Data 2009, 54, 1524–1528. [Google Scholar] [CrossRef]
- Peppel, T.; Köckerling, M.; Geppert-Rybczyńska, M.; Ralys, R.V.; Lehmann, J.K.; Verevkin, S.P.; Heintz, A. Low-viscosity paramagnetic ionic liquids with doubly charged [Co(NCS)4]2− ions. Angew. Chem. Int. Ed. 2010, 49, 7116–7119. [Google Scholar] [CrossRef]
- Geppert-Rybczyńska, M.; Lehmann, J.K.; Peppel, T.; Köckerling, M.; Heintz, A. Studies of physicochemical and thermodynamic properties of the paramagnetic 1-Alkyl-3-methylimidazolium ionic liquids (EMIm)2[Co(NCS)4] and (BMIm)2[Co(NCS)4]. J. Chem. Eng. Data 2010, 55, 5534–5538. [Google Scholar] [CrossRef]
- Peppel, T.; Köckerling, M. Salts with the 1,3-Dibutyl-2,4,5-trimethyl-imidazolium cation: (DBTMIm)X (X = Br, PF6) and (DBTMIm)2[MBr4] (M = Co, Ni). Z. Anorg. Allg. Chem. 2010, 636, 2439–2446. [Google Scholar] [CrossRef]
- Peppel, T.; Köckerling, M. Investigations on a series of ionic liquids containing the [CoIIBr3quin]− anion (quin = quinoline). Cryst. Growth Des. 2011, 11, 5461–5468. [Google Scholar] [CrossRef]
- Peppel, T.; Schmidt, C.; Köckerling, M. Synthesis, properties, and structures of salts with the reineckate anion, [CrIII(NCS)4(NH3)2]−, and large organic cations. Z. Anorg. Allg. Chem. 2011, 637, 1314–1321. [Google Scholar] [CrossRef]
- Peppel, T.; Thiele, P.; Köckerling, M. Low-melting salts with the [CrIII(NCS)4(1,10-Phenanthroline)]− complex anion: Syntheses, properties, and structures. Russ. J. Coord. Chem. 2012, 38, 207–218. [Google Scholar] [CrossRef]
- Peppel, T.; Hinz, A.; Köckerling, M. Salts with the [NiBr3(L)]− complex anion (L = 1-methylimidazole, 1-methylbenzimidazole, quinoline, and triphenylphosphane) and low melting points: A comparative study. Polyhedron 2013, 52, 482–490. [Google Scholar] [CrossRef]
- Peppel, T.; Thiele, P.; Tang, M.-B.; Zhao, J.-T.; Köckerling, M. Low-melting imidazolium-based salts with the paramagnetic Reineckate-analogue anion [Cr(NCS)4(bipy)]− (bipy = 2,2′-Bipyridine): Syntheses, properties, and structures. Inorg. Chem. 2015, 54, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Hensel-Bielowka, S.; Wojnarowska, Z.; Dzida, M.; Zorębski, E.; Zorębski, M.; Geppert-Rybczyńska, M.; Peppel, T.; Grzybowska, K.; Wang, Y.; Sokolov, A.P.; et al. Heterogeneous nature of relaxation dynamics of room-temperature ionic liquids (EMIm)2[Co(NCS)4] and (BMIm)2[Co(NCS)4]. J. Phys. Chem. C 2015, 119, 20363–20368. [Google Scholar] [CrossRef]
- Peppel, T.; Hinz, A.; Thiele, P.; Geppert-Rybczyńska, M.; Lehmann, J.K.; Köckerling, M. Synthesis, properties, and structures of low-melting tetraisocyanatocobaltate(II)-based ionic liquids. Eur. J. Inorg. Chem. 2017, 885–893. [Google Scholar] [CrossRef]
- Pincussohn, L. Über die metallverbindungen des pyridins und die elektrolyse des pyridins. Z. Anorg. Allg. Chem. 1897, 14, 379–403. [Google Scholar] [CrossRef]
- Reitzenstein, F. Ammoniak-pyridinsalze und hydrate bivalenter metalle. Z. Anorg. Allg. Chem. 1898, 18, 253–304. [Google Scholar] [CrossRef]
- Taylor, F.S. The manganohalides of pyridine and quinoline. J. Chem. Soc. 1934, 699–701. [Google Scholar] [CrossRef]
- Jørgensen, C.K. Comparative ligand field studies. III. The decrease of the integral Fk for manganese(II) complexes as evidence for partly covalent bonding. Acta Chem. Scand. 1957, 11, 53–72. [Google Scholar] [CrossRef][Green Version]
- Gill, N.S.; Nyholm, R.S.; Pauling, P. Stereochemistry of complex halides of the transition metals. Nature 1958, 182, 168–170. [Google Scholar] [CrossRef]
- Buffagni, S.; Dunn, T.M. Spectra of tetrahedral inorganic complexes. Nature 1960, 188, 937–938. [Google Scholar] [CrossRef]
- Cotton, F.A.; Goodgame, D.M.L.; Goodgame, M. Absorption spectra and electronic structures of some tetrahedral manganese(II) complexes. J. Am. Chem. Soc. 1962, 84, 167–172. [Google Scholar] [CrossRef]
- Forster, D.; Goodgame, D.M.L. Preparation, electronic spectra, and magnetic properties of some transition-metal Isocyanato-complexes. J. Chem. Soc. 1964, 2790–2798. [Google Scholar] [CrossRef]
- Cotton, F.A.; Daniels, L.M.; Huang, P. Correlation of structure and triboluminescence for tetrahedral manganese(II) compounds. Inorg. Chem. 2001, 40, 3576–3578. [Google Scholar] [CrossRef] [PubMed]
- Pitula, S.; Mudring, A.-V. Synthesis, structure, and physico-optical properties of manganate(II)-based ionic liquids. Chem. Eur. J. 2010, 16, 3355–3365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, P.; Fang, L. Hexaimidazolium tetrachloromanganate(II) hexachloromanganate(II). Acta Cryst. 2006, E62, m658–m660. [Google Scholar] [CrossRef]
- Chang, J.-C.; Ho, W.-Y.; Sun, I.-W.; Chou, Y.-K.; Hsieh, H.-H.; Wu, T.-Y. Synthesis and properties of new tetrachlorocobaltate(II) and tetrachloromanganate(II) anion salts with dicationic counterions. Polyhedron 2011, 30, 497–507. [Google Scholar] [CrossRef]
- Zhong, C.; Sasaki, T.; Jimbo-Kobayashi, A.; Fujiwara, E.; Kobayashi, A.; Tada, M.; Iwasawa, Y. Syntheses, structures, and properties of a series of metal ion-containing dialkylimidazolium ionic Liquids. Bull. Chem. Soc. Jpn. 2007, 80, 2365–2374. [Google Scholar] [CrossRef]
- Zehbe, K.; Kollosche, M.; Lardong, S.; Kelling, A.; Schilde, U.; Taubert, A. Ionogels based on poly(methyl methacrylate) and metal-containing ionic liquids: Correlation between structure and mechanical and electrical properties. Int. J. Mol. Sci. 2016, 17, 391. [Google Scholar] [CrossRef]
- Del Sesto, R.E.; McCleskey, T.M.; Burrell, A.K.; Baker, G.A.; Thompson, J.D.; Scott, B.L.; Wilkes, J.S.; Williams, P. Structure and magnetic behavior of transition metal based ionic liquids. Chem. Commun. 2008, 447–449. [Google Scholar] [CrossRef]
- Lokanath, N.K.; Sridhar, M.A.; Shashidhara Prasad, J. Crystal structure of metoclopramide manganese isothiocyanate complex. Z. Krist. New. Cryst. Struct. 1997, 212, 19–20. [Google Scholar]
- Chen, H.-J.; Zhang, L.-Z.; Cai, Z.-G.; Yang, G.; Chen, X.-M. Organic–inorganic hybrid materials assembled through weak intermolecular interactions. Synthesis, structures and non-linear optical properties of [4,4′-bipyH2][M(NCS)4] (M = Mn2+, Co2+ or Zn2+; 4,4′-bipy = 4,4′-bipyridine). J. Chem. Soc. Dalton Trans. 2000, 2463–2466. [Google Scholar] [CrossRef]
- Kushch, N.D.; Bardin, A.A.; Buravov, L.I.; Glushakova, N.M.; Shilov, G.V.; Dmitriev, A.I.; Morgunov, R.B.; Kulikov, A.V. Synthesis particularities, structure and properties of the radical cation salts ω-(BEDT-TTF)5M(NCS)6·C2H5OH, M = Mn, Ni. Synth. Met. 2014, 195, 75–82. [Google Scholar] [CrossRef]
- Neumann, T.; Jess, I.; Nather, C. Crystal structures of bis-[4-(di-methyl-amino)-pyridinium] tetra-kis-(thio-cyanato-κN)manganate(II) and tris-[4-(di-methyl-amino)-pyridinium] penta-kis(thio-cyanato-κN)manganate(II). Acta Cryst. 2017, E73, 1786–1789. [Google Scholar]
- Thuyweang, N.; Koh, L.L.; Hor, T.S.A.; Leelasubcharoen, S. Synthesis, characterization, and catalytic activity of heterometallic ion-pair Ni/Mn and Ni/Zn complexes. J. Coord. Chem. 2014, 67, 1219–1235. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR chemical shifts of trace impurities: Common laboratory solvents, organics, and gases in deuterated solvents relevant to the organometallic chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Bruker AXS Inc. Apex-2, v. 1.6–8, Saint, v. 6.25a, SADABS—Software for the CCD Detector System; Bruker-Nonius Inc.: Madison, WI, USA, 2003. [Google Scholar]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Evans, D.F. The determination of the paramagnetic susceptibility of substances in solution by nuclear magnetic resonance. J. Chem. Soc. 1959, 2003–2005. [Google Scholar] [CrossRef]
- Sur, S.K. Measurement of magnetic susceptibility and magnetic moment of paramagnetic molecules in solution by high-field fourier transform NMR spectroscopy. J. Magn. Reson. 1989, 82, 169–173. [Google Scholar] [CrossRef]
- Pacault, A.; Hoarau, J.; Marchand, A. New views of diamagnetism. Adv. Chem. Phys. 1960, 3, 171–238. [Google Scholar]
- Langevin, P. Magnétisme et théorie des électrons. Ann. Chim. Phys. 1905, 8, 70–127. [Google Scholar]
- Wandschneider, A.; Lehmann, J.K.; Heintz, A. Surface tension and density of pure ionic liquids and some binary mixtures with 1-propanol and 1-butanol. J. Chem. Eng. Data 2008, 53, 596–599. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Padilla-Martinez, I.I.; Ariza-Castolo, A.; Contreras, R. NMR study of isolobal N-CH3+, N-BH3 and N-BF3 imidazole derivatives. Magn. Reson. Chem. 1993, 31, 189–193. [Google Scholar] [CrossRef]
- Forster, D.; Goodgame, D.M.L. Electronic spectra and magnetic properties of some isothiocyanate complexes of manganese and iron. J. Chem. Soc. 1965, 268–274. [Google Scholar] [CrossRef]
- Nishida, T.; Tashiro, Y.; Yamamoto, M. Physical and electrochemical properties of 1-alkyl-3-methylimidazolium tetrafluoroborate for electrolyte. J. Fluor. Chem. 2003, 120, 135–141. [Google Scholar] [CrossRef]
- Namboodiri, V.V.; Varma, R.S. Solvent-free sonochemical preparation of ionic liquids. Org. Lett. 2002, 4, 3161–3163. [Google Scholar] [CrossRef]
- Earle, M.; Seddon, K.R. Light Emitting Complex Salts. Patent WO 2006/043110 A1, 27 April 2006. [Google Scholar]
- Sabatini, A.; Bertini, I. Infrared spectra between 100 and 2500 Cm.-1 of some complex metal cyanates, thiocyanates, and selenocyanates. Inorg. Chem. 1965, 4, 959–961. [Google Scholar] [CrossRef]
- Kauzmann, W. The nature of the glassy state and the behavior of liquids at low temperatures. Chem. Rev. 1948, 43, 219–256. [Google Scholar] [CrossRef]
- Lee, W.A.; Knight, G.J. Ratio of the glass transition temperature to the melting point in polymers. Br. Polym. J. 1970, 2, 73–80. [Google Scholar] [CrossRef]
- Widegren, J.A.; Laesecke, A.; Magee, J.W. The effect of dissolved water on the viscosities of hydrophobic room-temperature ionic liquids. Chem. Commun. 2005, 1610–1612. [Google Scholar] [CrossRef]
- Jacquemin, J.; Husson, P.; Padua, A.A.H.; Majer, V. Density and viscosity of several pure and water-saturated ionic liquids. Green Chem. 2006, 8, 172–180. [Google Scholar] [CrossRef]
- Tariq, M.; Freire, M.G.; Saramago, B.; Coutinho, J.A.P.; Canongia Lopes, J.N.; Rebelo, L.P.N. Surface tension of ionic liquids and ionic liquid solutions. Chem. Soc. Rev. 2012, 41, 829–868. [Google Scholar] [CrossRef]
- Dzida, M.; Zorębski, E.; Zorębski, M.; Żarska, M.; Geppert-Rybczyńska, M.; Chorążewski, M.; Jacquemin, J.; Cibulka, I. Speed of sound und ultrasound absorption in ionic liquids. Chem. Rev. 2017, 117, 3883–3929. [Google Scholar] [CrossRef] [PubMed]
- Feder-Kubis, J.; Musiał, M.; Dizda, M.; Geppert-Rybczyńska, M. The new evolution of protic ionic liquids: Antielectrostatic activity correlated with their surface properties. J. Ind. Eng. Chem. 2016, 41, 40–49. [Google Scholar] [CrossRef]
- Yaghini, N.; Nordstierna, L.; Martinelli, A. Effect of water on the transport properties of protic and aprotic imidazolium ionic liquids—An analysis of self-diffusivity, conductivity, and proton exchange mechanism. Phys. Chem. Chem. Phys. 2014, 16, 9266–9275. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H. Das temperaturabhängigkeitsgesetz der viskosität von flüssigkeiten. Phys. Z. 1921, 22, 645–646. [Google Scholar]
- Fulcher, G.S. Analysis of recent measurements of the viscosity of glasses. J. Am. Ceram. Soc. 1925, 8, 339–355. [Google Scholar] [CrossRef]
- Tammann, G.; Hesse, W. Die abhängigkeit der viscosität von der temperatur bei unterkühlten flüssigkeiten. Z. Anorg. Allg. Chem. 1926, 156, 245–257. [Google Scholar] [CrossRef]
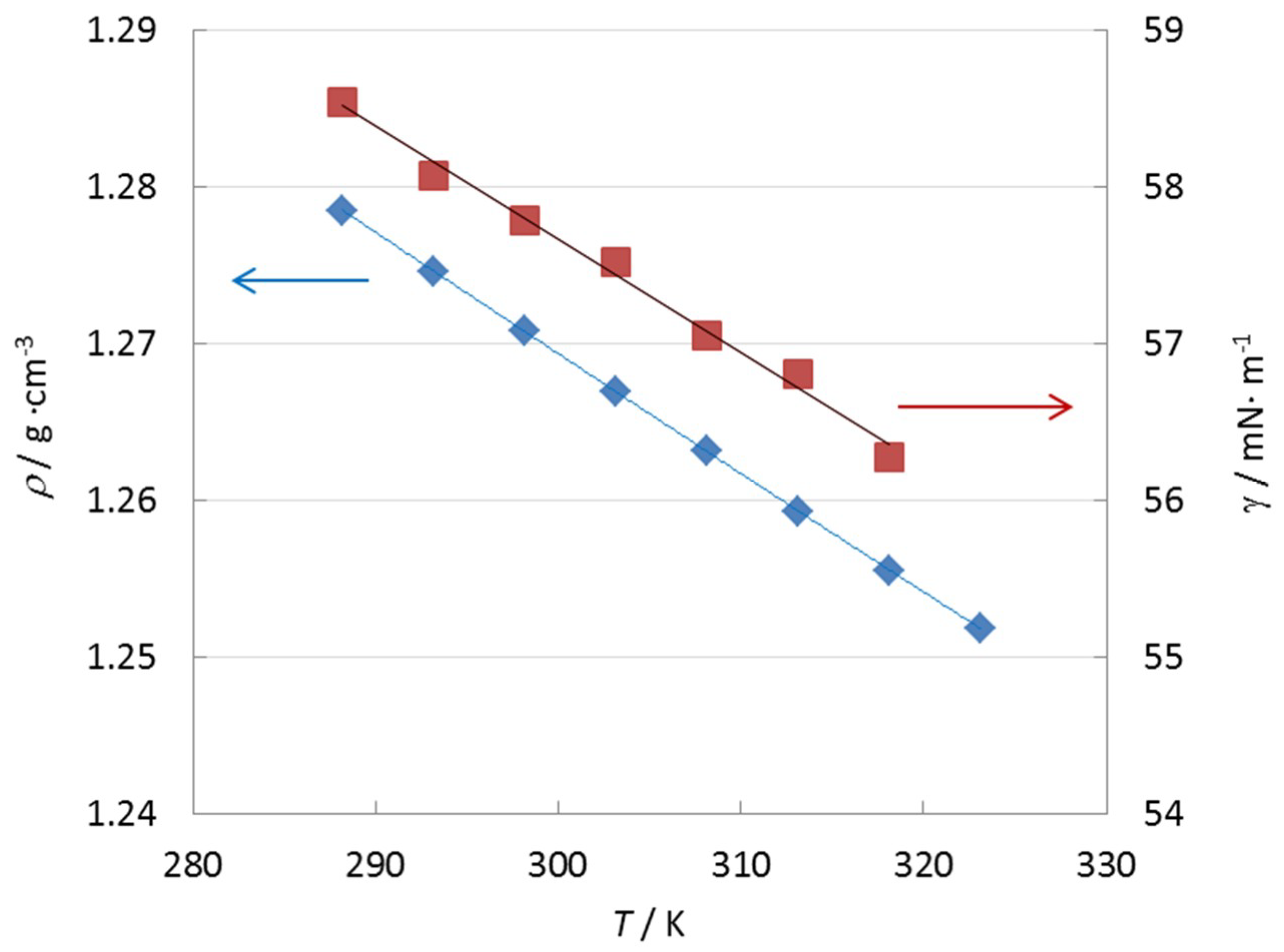
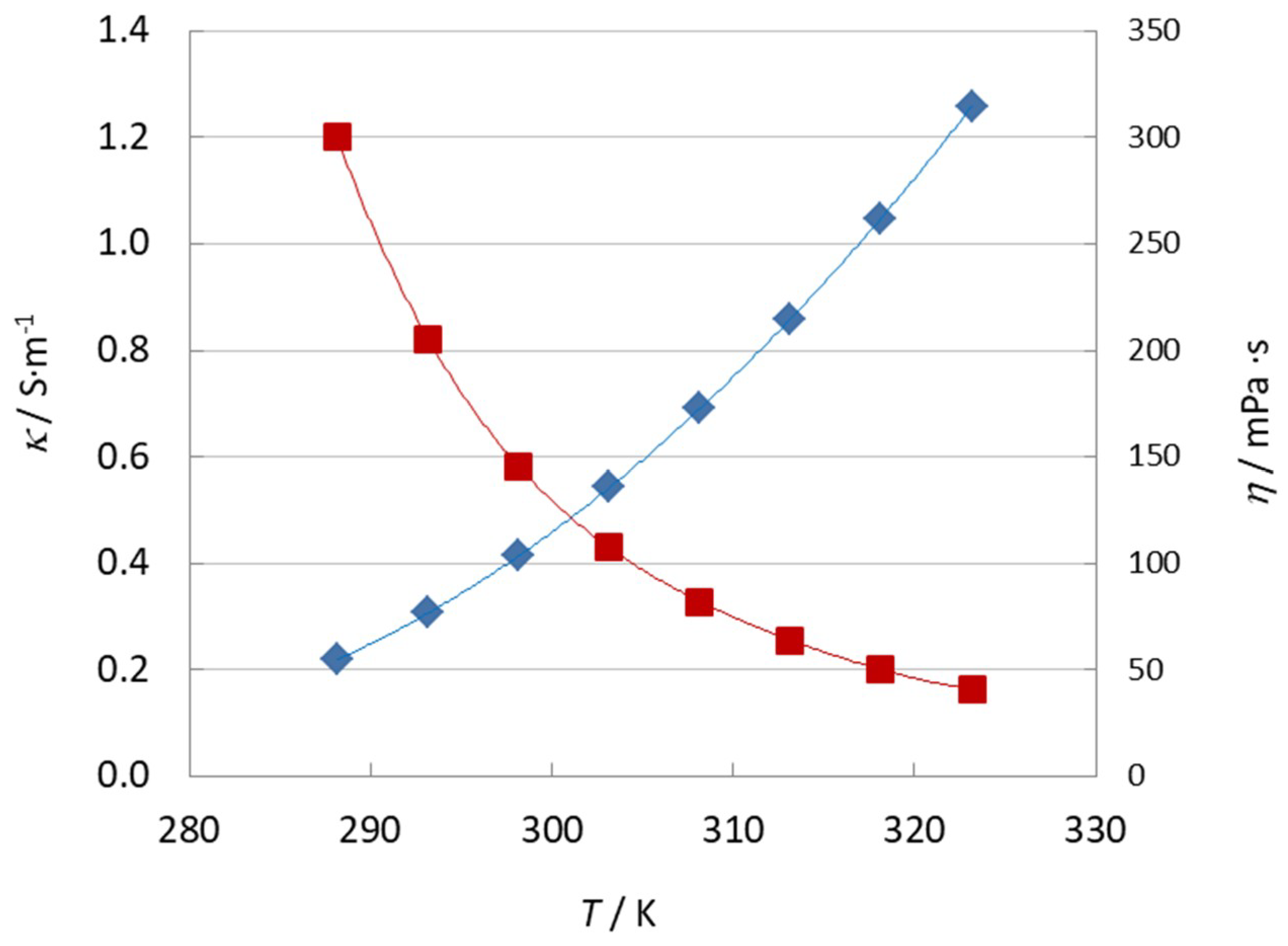
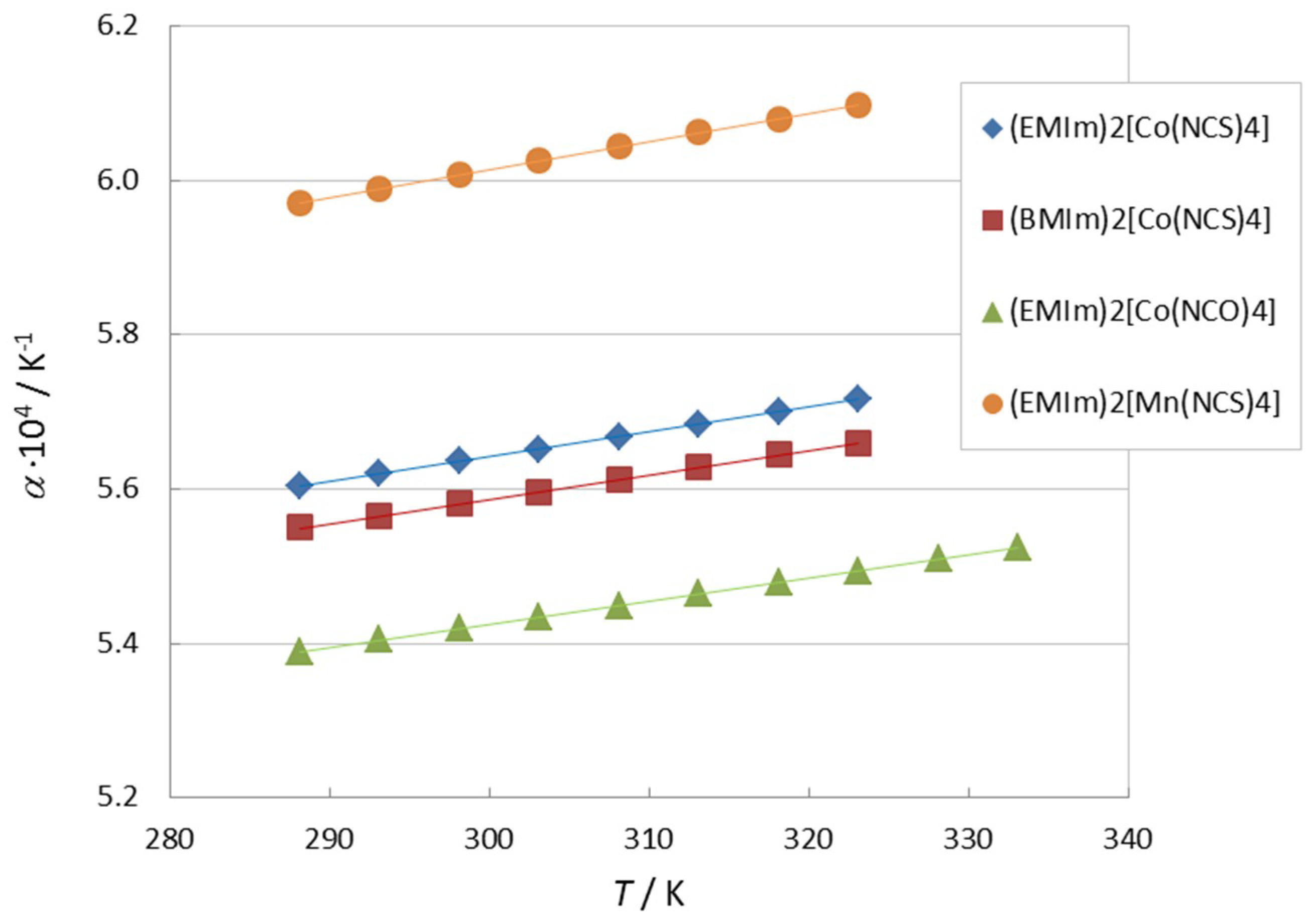
| η0/(mPa·s) | B/K | T0/K | r |
| 0.348 ± 0.034 | 570 ± 20 | 204 ± 2 | 0.999996 |
| κ0/(S·m−1) | B/K | T0/K | r |
| 72.4 ± 4.5 | −471 ± 13 | 206.9 ± 1.4 | 0.999996 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peppel, T.; Geppert-Rybczyńska, M.; Neise, C.; Kragl, U.; Köckerling, M. Low-Melting Manganese(II)-Based Ionic Liquids: Syntheses, Structures, Properties and Influence of Trace Impurities. Materials 2019, 12, 3764. https://doi.org/10.3390/ma12223764
Peppel T, Geppert-Rybczyńska M, Neise C, Kragl U, Köckerling M. Low-Melting Manganese(II)-Based Ionic Liquids: Syntheses, Structures, Properties and Influence of Trace Impurities. Materials. 2019; 12(22):3764. https://doi.org/10.3390/ma12223764
Chicago/Turabian StylePeppel, Tim, Monika Geppert-Rybczyńska, Christin Neise, Udo Kragl, and Martin Köckerling. 2019. "Low-Melting Manganese(II)-Based Ionic Liquids: Syntheses, Structures, Properties and Influence of Trace Impurities" Materials 12, no. 22: 3764. https://doi.org/10.3390/ma12223764
APA StylePeppel, T., Geppert-Rybczyńska, M., Neise, C., Kragl, U., & Köckerling, M. (2019). Low-Melting Manganese(II)-Based Ionic Liquids: Syntheses, Structures, Properties and Influence of Trace Impurities. Materials, 12(22), 3764. https://doi.org/10.3390/ma12223764







