Growth of β-NaYF4:Eu3+ Crystals by the Solvothermal Method with the Aid of Oleic Acid and Their Photoluminescence Properties
Abstract
:1. Introduction
2. Method
2.1. Materials
2.2. Synthesis of β-NaYF4:Eu3+ Crystals without Surfactant
2.3. Synthesis of β-NaYF4:Eu3+ Crystals with the Aid of an OA Surfactant
2.4. Characterization
3. Results and Discussion
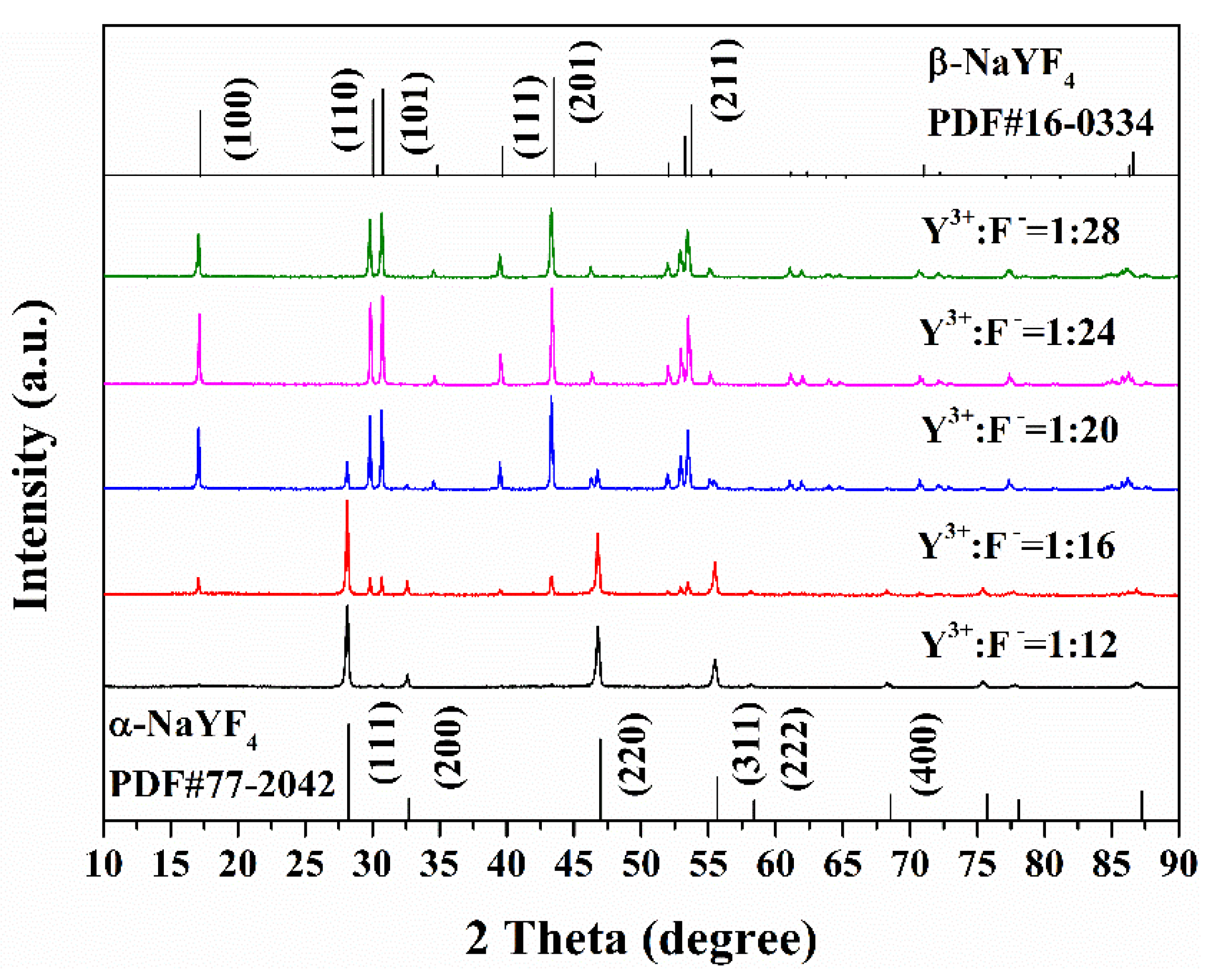
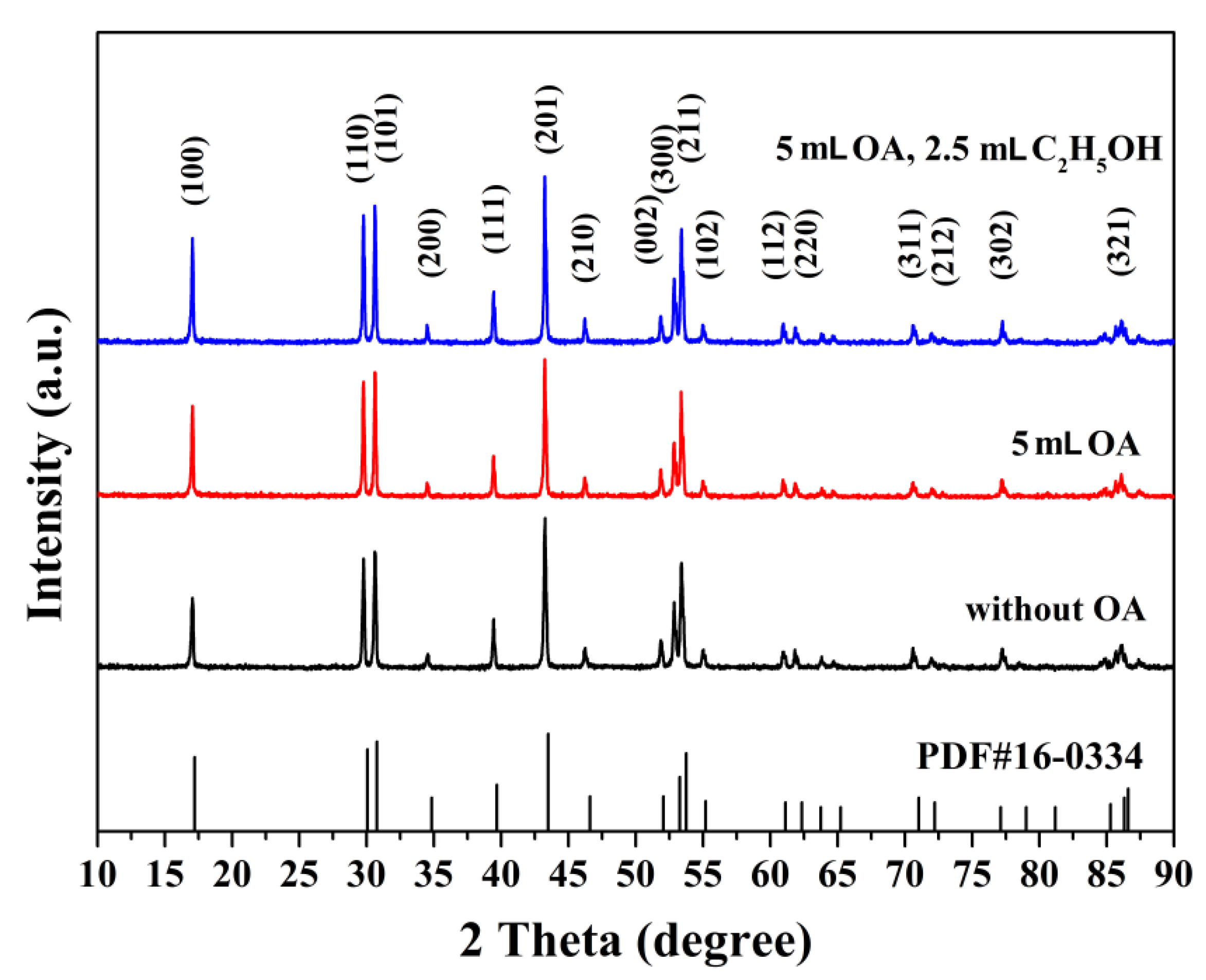
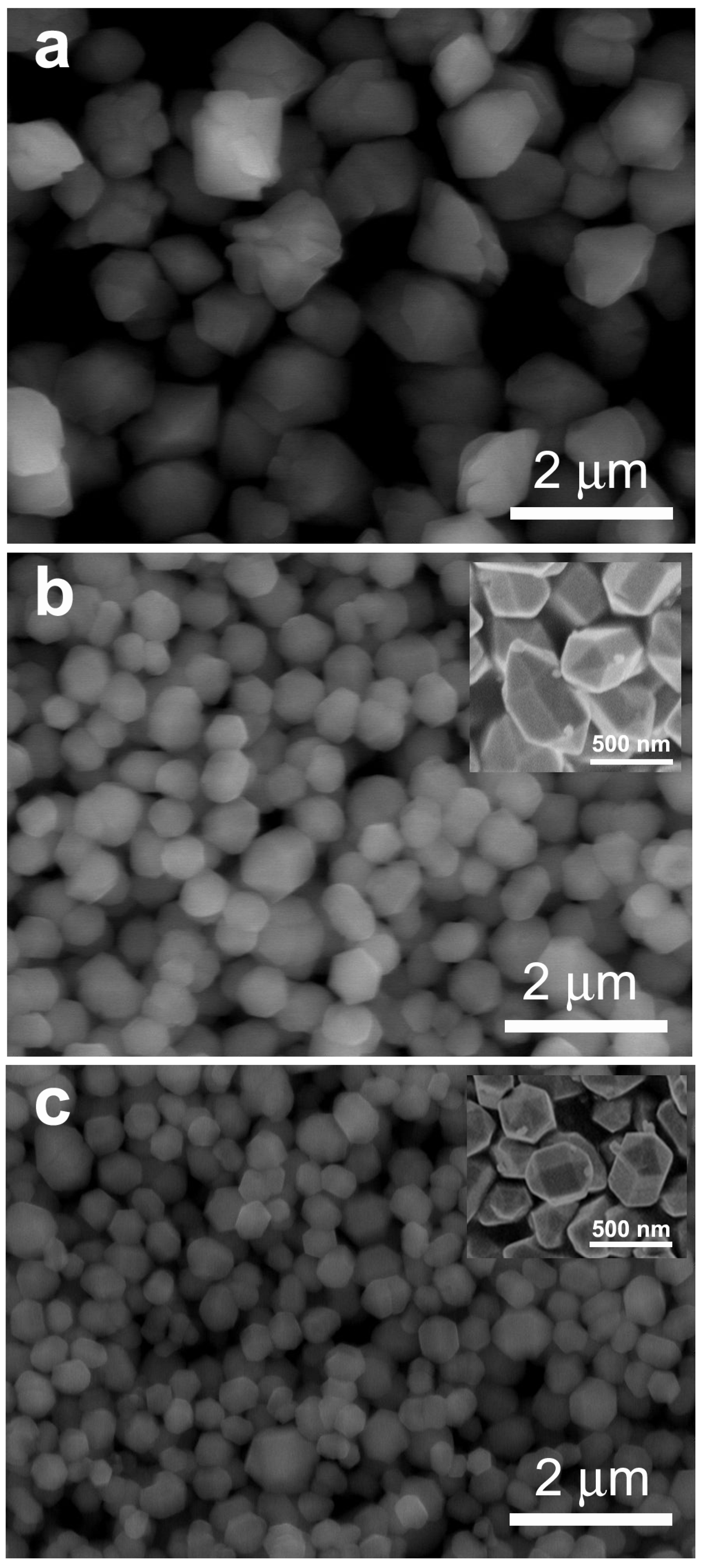
3.1. Phase Structures and Microstructural Characteristics
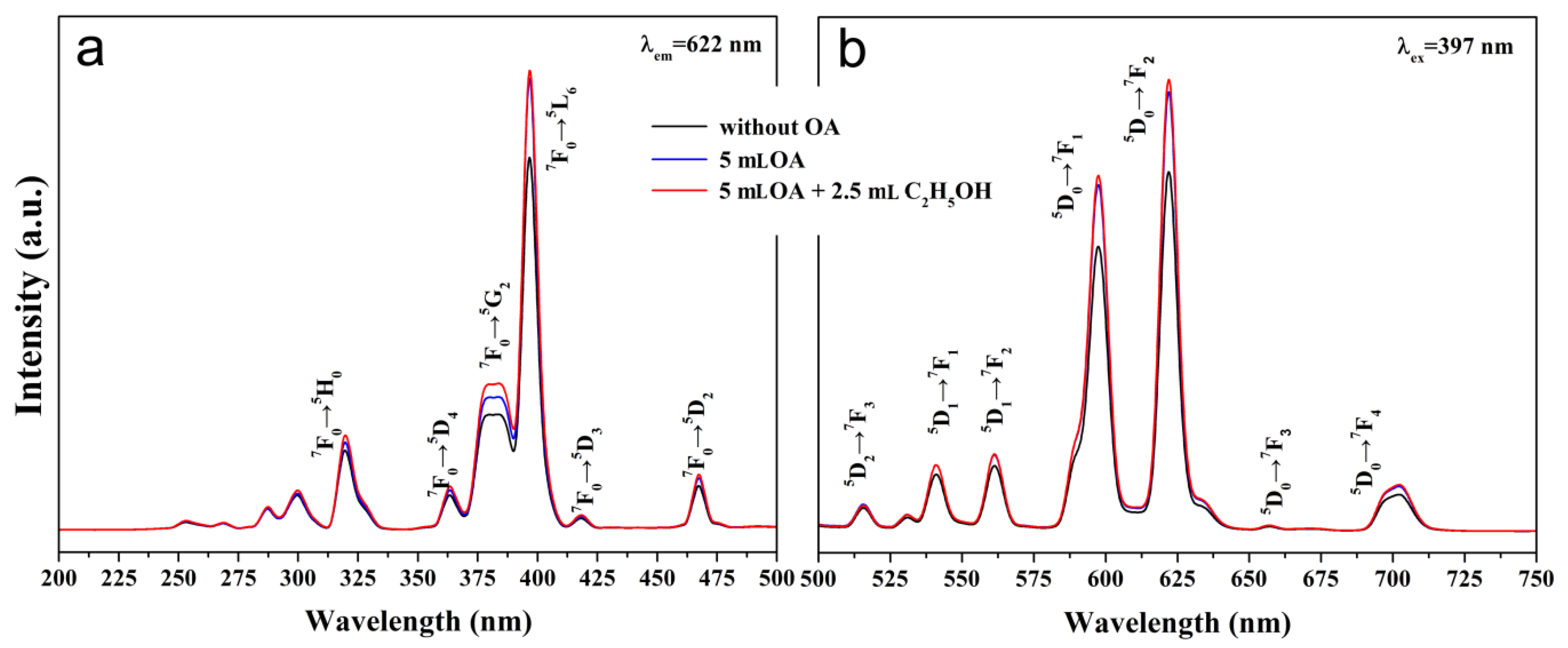
3.2. Photoluminescence Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, D.Q.; Huang, P.; Yu, Y.L.; Huang, F.; Yang, A.P.; Wang, Y.S. Dopant-induced phase transition: A new strategy of synthesizing hexagonal upconversion NaYF4 at low temperature. Chem. Commun. (Camb. Engl.) 2011, 47, 5801–5803. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, X.; Zhuang, J.; Peng, Q.; Li, Y. Synthesis of NaYF4 nanocrystals with predictable phase and shape. Adv. Funct. Mater. 2007, 17, 2757–2765. [Google Scholar] [CrossRef]
- Reddy, K.N.; Jafaruddin, M.D. Decay behaviour of NaYF4: Gd3+ phosphors. J. Mater. Sci. Lett. 1983, 2, 296–298. [Google Scholar] [CrossRef]
- Tu, D.; Liu, Y.; Zhu, H.; Li, R.; Liu, L.; Chen, X. Breakdown of crystallographic site symmetry in lanthanide-doped NaYF4 crystals. Angew. Chem. Int. Ed. 2013, 52, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, L. Controlled synthesis and luminescence of lanthanide doped NaYF4 nanocrystals. Chem. Mater. 2007, 19, 727–734. [Google Scholar] [CrossRef]
- Wang, G.; Qin, W.; Zhang, J.; Wang, L.; Wei, G.; Zhu, P. Controlled synthesis and luminescence properties from cubic to hexagonal NaYF4:Ln3+ (Ln=Eu and Yb/Tm) microcrystals. J. Alloys Compd. 2009, 475, 452–455. [Google Scholar] [CrossRef]
- Podhorodecki, A.; Banski, M.; Misiewicz, J.; Afzaal, M.; O'Brien, P.; Cha, D. Multicolor light emitters based on energy exchange between Tb and Eu ions co-doped into ultrasmall β-NaYF4 nanocrystals. J. Mater. Chem. 2012, 22, 5356–5361. [Google Scholar] [CrossRef]
- Yu, W.; Wang, X.; Chen, N.; Du, G.; Gui, W. A strategy to prepare highly redispersible and strongly luminescent α-NaYF4:Eu3+ hybrid nanostructures with multi-channel excitation. CrystEngComm 2014, 16, 3214–3221. [Google Scholar] [CrossRef]
- Li, C.; Zhang, C.; Hou, Z.; Wang, L.; Quan, Z.; Lian, H.; Lin, J. β-NaYF4 and β-NaYF4:Eu3+ microstructures: Morphology control and tunable luminescence properties. J. Phys. Chem. C 2009, 113, 2332–2339. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Designing lanthanide-doped nanocrystals with both up- and down-conversion luminescence for anti-counterfeiting. Nanoscale 2011, 3, 4804–4810. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Zhang, D.; Chen, Q.H.; Xi, J.H.; Ji, Z. Color-tunable luminescence, energy transfer and temperature sensing behavior of hexagonal NaYF4:Ce3+/Tb3+/Eu3+ microcrystals. J. Alloys Compd. 2016, 672, 117–124. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y. Na(Y1.5Na0.5)F6 single-crystal nanorods as muticolor luminescent materials. Nano Lett. 2006, 6, 1645–1649. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y. An efficient and user-friendly method for the synthesis of hexagonal-phase NaYF(4):Yb, Er/Tm nanocrystals with controllable shape and upconversion fluorescence. Nanotechnology 2008, 19, 345606. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Jiang, S. Multicolor core/shell-structured upconversion fluorescent nanoparticles. Adv. Mater. 2008, 20, 4765–4769. [Google Scholar] [CrossRef]
- He, M.; Huang, P.; Zhang, C.; Ma, J.; He, R.; Cui, D. Phase- and size-controllable synthesis of hexagonal upconversion rare-earth fluoride nanocrystals through an oleic acid/ionic liquid two-phase system. Chem. Eur. J. 2012, 18, 5954–5969. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.M.; Yu, H.; Ma, E.; Zhang, X.Q.; Cao, W.B.; Yang, C.G.; Yu, J.C. Upconversion effective enhancement by producing various coordination surroundings of rare-earth ions. Inorg. Chem. 2015, 54, 2643–2651. [Google Scholar] [CrossRef] [PubMed]
- Santana-Alonso, A.; Yanes, A.C.; Méndez-Ramos, J.; del-Castillo, J.; Rodríguez, V.D. Sol–gel transparent nano-glass–ceramics containing Eu3+-doped NaYF4 nanocrystals. J. Non-Cryst. Solids 2010, 356, 933–936. [Google Scholar] [CrossRef]
- Reddy, K.N.; Shareef, M.A.H.; Pandaraiah, N. Growth and X-ray study of NaYF4 crystals. J. Mater. Sci. Lett. 1983, 2, 83–84. [Google Scholar] [CrossRef]
- Burns, J.H. Crystal structure of hexagonal sodium neodymium fluoride and related compounds. Inorg. Chem. 1965, 4, 881–886. [Google Scholar] [CrossRef]
- Grzechnik, A.; Bouvier, P.; Mezouar, M.; Mathews, M.D.; Tyagi, A.K.; Köhler, J. Hexagonal Na1.5Y1.5F6 at high pressures. J. Solid State Chem. 2002, 165, 159–164. [Google Scholar] [CrossRef]
- Velasco, D.S.; Moura, A.P.D.; Medina, A.N.; Baesso, M.L.; Rubora, A.F.; Cremona, M. Preparation, characterization, and spectroscopic properties of PC/PMMA doped blends: Study of the effect of rare-earth doping on luminescence, quenching rate, and lifetime enhancement. J. Phys. Chem. B 2010, 114, 5657–5660. [Google Scholar] [CrossRef] [PubMed]
- Heer, S.; Kömpe, K.; Güdel, H.U.; Haase, M. Highly efficient multicolour upconversion emission in transparent colloids of Lanthanide-doped NaYF4 nanocrystals. Adv. Mater. 2004, 16, 2102–2105. [Google Scholar] [CrossRef]
- Yao, Y.H.; Xu, C.; Zheng, Y.; Yang, C.S.; Liu, P.; Ding, J.X.; Jia, T.Q.; Qiu, J.R.; Zhang, S.; Sun, Z.R. Improving upconversion luminescence efficiency in Er3+-doped NaYF4 nanocrystals by two-color laser field. J. Mater. Sci. 2016, 51, 5460–5468. [Google Scholar] [CrossRef]
- Luoshan, M.D.; Bai, L.H.; Bu, C.H.; Liu, X.L.; Zhu, Y.D.; Guo, K.M.; Jiang, R.H.; Li, M.Y.; Zhao, X.Z. Surface plasmon resonance enhanced multi-shell-modified upconversion NaYF4:Yb3+, Er3+@SiO2@Au@TiO2 crystallites for dye-sensitized solar cells. J. Power Sour. 2016, 307, 468–473. [Google Scholar] [CrossRef]
- Li, S.; Hou, Z.; Cheng, Z.; Lian, H.; Ma, P.A.; Li, C.; Lin, J. Enhanced near-infrared quantum cutting luminescence in 1,2,4,5-benzenetetracarboxylic acid/NaYF4:Tb3+, Yb3+ hybrid nanoparticles. RSC Adv. 2013, 3, 5491–5497. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, H.; Cui, L.; An, N.; Tong, R.; Chen, Y.; Li, X.; Qu, F.Y. NIR-sensitive UCNP@mSiO2 nanovehicles for on-demand drug release and photodynamic therapy. RSC Adv. 2016, 6, 26479–26489. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, P.; Li, C.; Wang, D.; Xu, J.; Gai, S.; Lin, J. Facile and mass production synthesis of beta-NaYF4:Yb3+, Er3+/Tm3+ 1D microstructures with multicolor up-conversion luminescence. Chem. Commun. (Camb.) 2011, 47, 12143–12145. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.S.; Chow, G.M. Synthesis of hexagonal-phase NaYF4:Yb,Er and NaYF4:Yb,Tm nanocrystals with efficient up-conversion fluorescence. Adv. Funct. Mater. 2006, 16, 2324–2329. [Google Scholar] [CrossRef]
- Lochhead, M.J.; Bray, K.L. Rare-earth clustering and Aluminum codoping in sol-gel silica: Investigation using Europium(III) fluorescence spectroscopy. Chem. Mater. 1995, 7, 572–577. [Google Scholar] [CrossRef]
- Wang, X.; Zhuang, J.; Peng, Q.; Li, Y. A general strategy for nanocrystal synthesis. Nature 2005, 437, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, X.; Zhuang, J.; Peng, Q.; Li, Y. Branched NaYF4 nanocrystals with luminescent properties. Inorg. Chem. 2007, 46, 6050–6055. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Pan, G.; Yang, H.K.; Chen, Y.; Chung, J.W.; Moon, B.K.; Jeong, J.H. Solvothermal synthesis and luminescence properties of NaYF4:Ln3+ (Eu3+, Tb3+, Yb3+/Er3+) nano- and microstructures. Opt. Mater. 2012, 34, 1007–1012. [Google Scholar] [CrossRef]
- Zhang, F.; Wan, Y.; Yu, T.; Zhang, F.; Shi, Y.; Xie, S.; Li, Y.G.; Xu, L.; Tu, B.; Zhao, D.Y. Uniform nanostructured arrays of sodium rare-earth fluorides for highly efficient multicolor upconversion luminescence. Angew. Chem. Int. Ed. Engl. 2007, 46, 7976–7979. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Lu, C.; Cao, L.; Ni, Y.; Xu, Z. Controllable synthesis, formation mechanism and upconversion luminescence of β-NaYF4 : Yb3+/Er3+ microcrystals by hydrothermal process. CrystEngComm 2013, 15, 8366–8373. [Google Scholar] [CrossRef]
- Huignard, A.; Buissette, V.R.; Franville, A.C.; Gacoin, T.; Boilot, J.P. Emission processes in YVO4:Eu nanoparticles. J. Phys. Chem. B 2003, 107, 6754–6759. [Google Scholar] [CrossRef]
- Stouwdam, J.W.; van Veggel, F.C.J.M. Near-infrared emission of redispersible Er3+, Nd3+, and Ho3+ doped LaF3 nanoparticles. Nano Lett. 2002, 2, 733–737. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Chen, N.; Liu, B.; Liu, G. Preparation of LaF3:Eu3+ based inorganic–organic hybrid nanostructures via an ion exchange method and their strong luminescence. J. Nanosci. Nanotechnol. 2016, 16, 3729–3734. [Google Scholar] [CrossRef] [PubMed]
- Nogami, M.; Enomoto, T.; Hayakawa, T. Enhanced fluorescence of Eu3+ induced by energy transfer from nanosized SnO2 crystals in glass. J. Lumin. 2002, 97, 147–152. [Google Scholar] [CrossRef]
- Zhan, Y.; Du, G.; Chen, N.; Li, Y.; Liu, B.; Liu, G. Photoluminescence properties of YVO4:Eu3+,Ba2+ nanoparticles prepared by an ion exchange method. Mater. Sci. Semiconduct. Process. 2016, 41, 233–239. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Wang, X.; Shao, A.; Du, G.; Chen, N. Growth of β-NaYF4:Eu3+ Crystals by the Solvothermal Method with the Aid of Oleic Acid and Their Photoluminescence Properties. Materials 2019, 12, 3711. https://doi.org/10.3390/ma12223711
Huang J, Wang X, Shao A, Du G, Chen N. Growth of β-NaYF4:Eu3+ Crystals by the Solvothermal Method with the Aid of Oleic Acid and Their Photoluminescence Properties. Materials. 2019; 12(22):3711. https://doi.org/10.3390/ma12223711
Chicago/Turabian StyleHuang, Jianhua, Xiaojing Wang, An Shao, Guoping Du, and Nan Chen. 2019. "Growth of β-NaYF4:Eu3+ Crystals by the Solvothermal Method with the Aid of Oleic Acid and Their Photoluminescence Properties" Materials 12, no. 22: 3711. https://doi.org/10.3390/ma12223711
APA StyleHuang, J., Wang, X., Shao, A., Du, G., & Chen, N. (2019). Growth of β-NaYF4:Eu3+ Crystals by the Solvothermal Method with the Aid of Oleic Acid and Their Photoluminescence Properties. Materials, 12(22), 3711. https://doi.org/10.3390/ma12223711



