Advanced Open-Celled Structures from Low-Temperature Sintering of a Crystallization-Resistant Bioactive Glass
Abstract
:1. Introduction
2. Materials and Methods
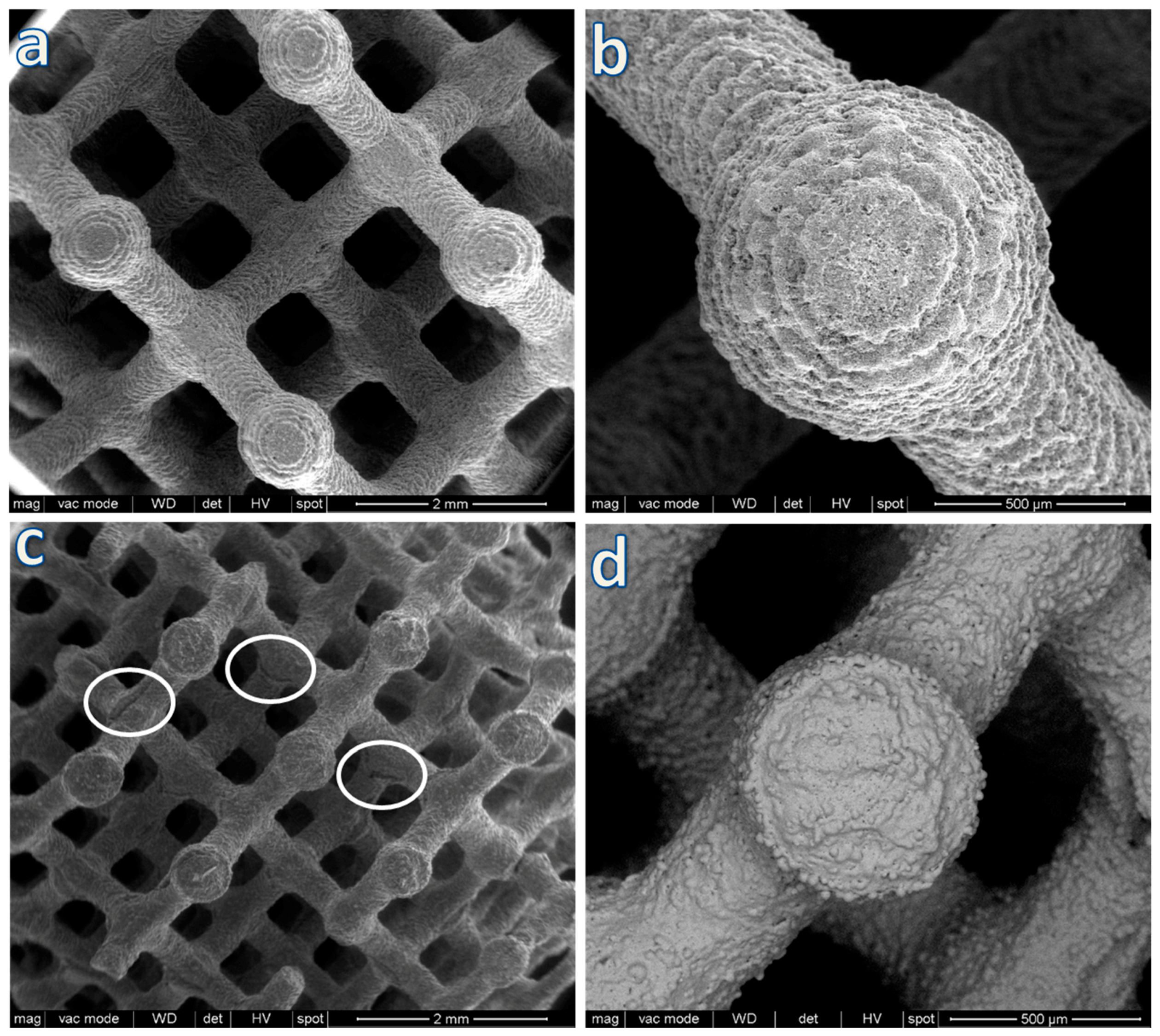
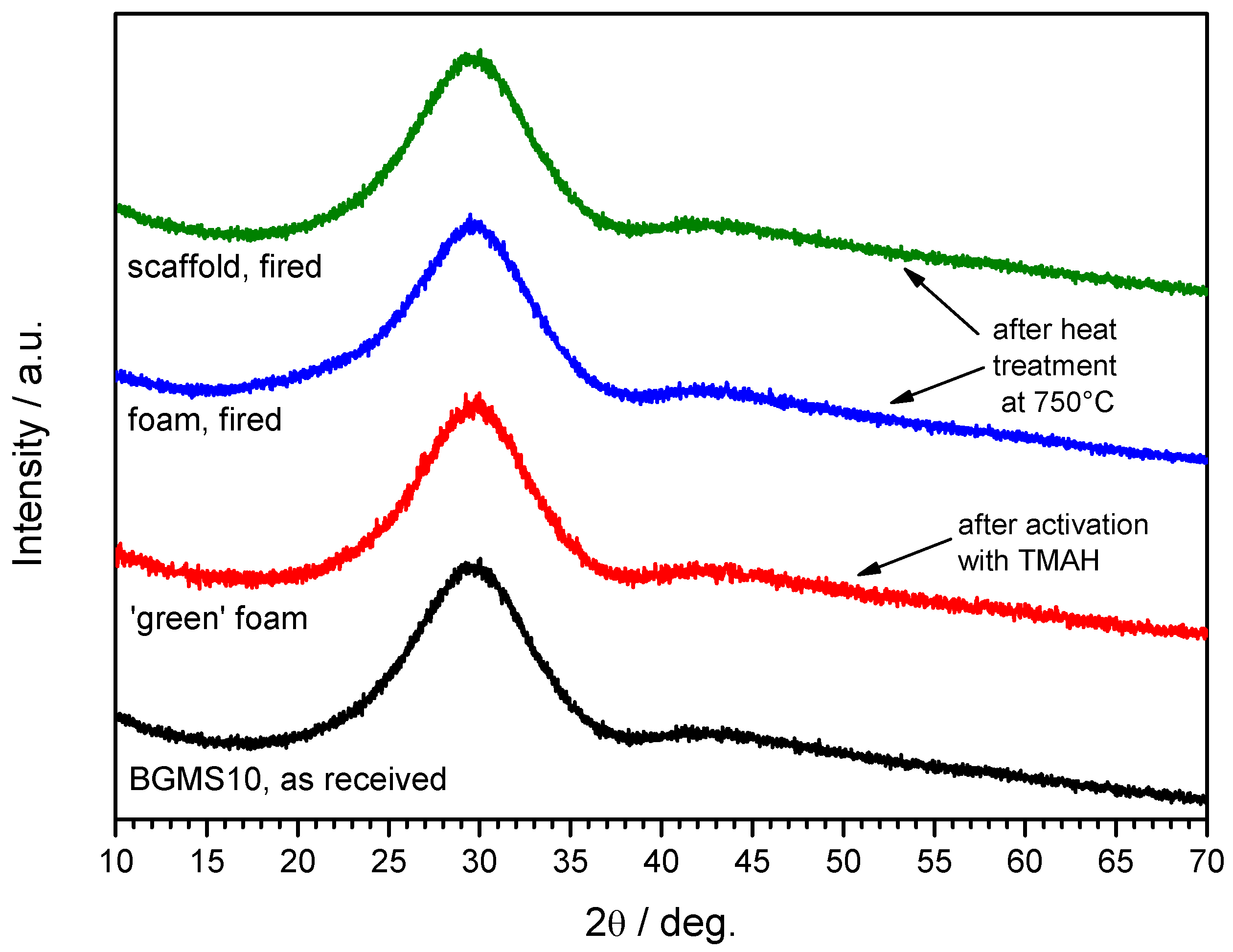
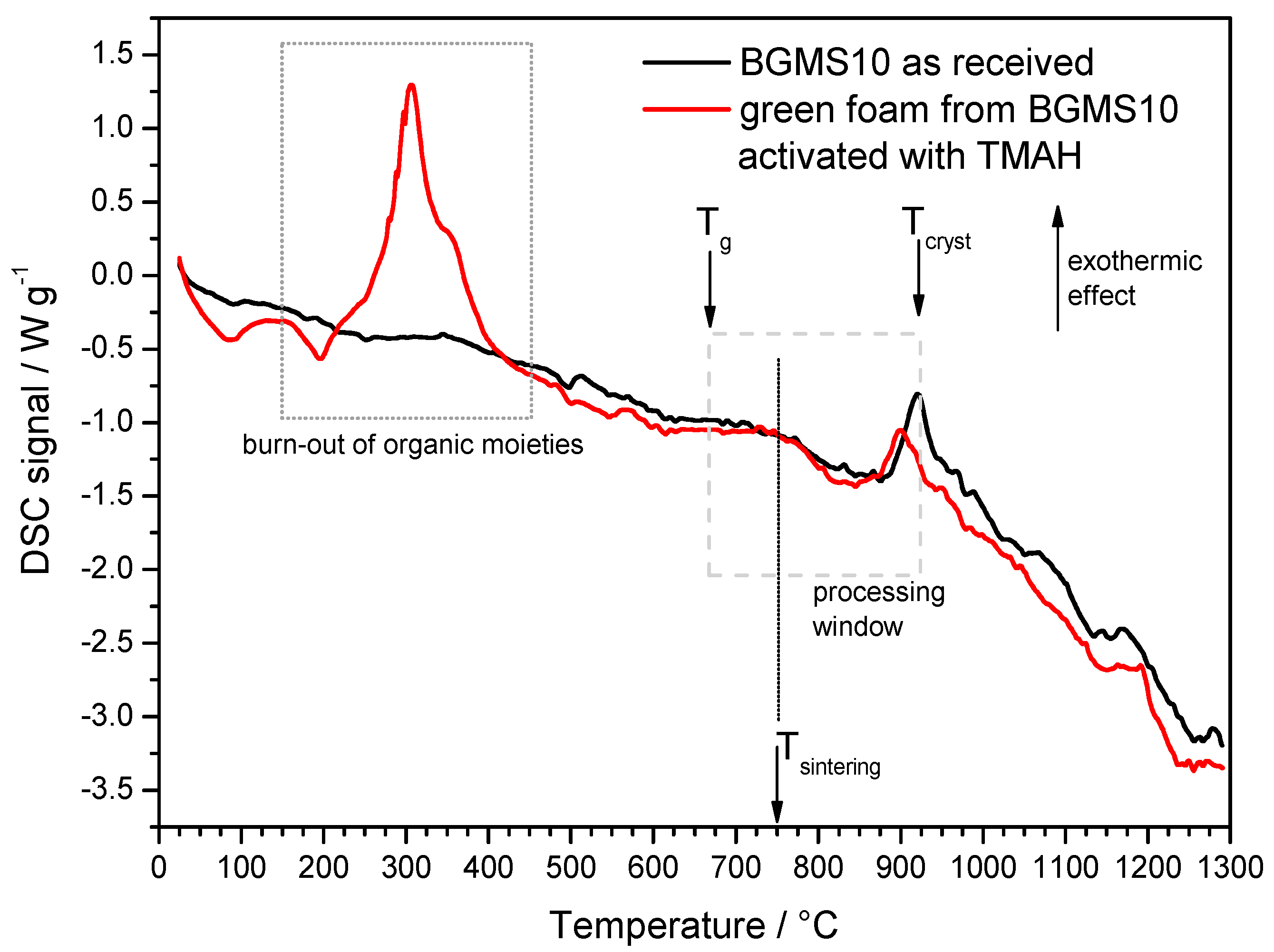
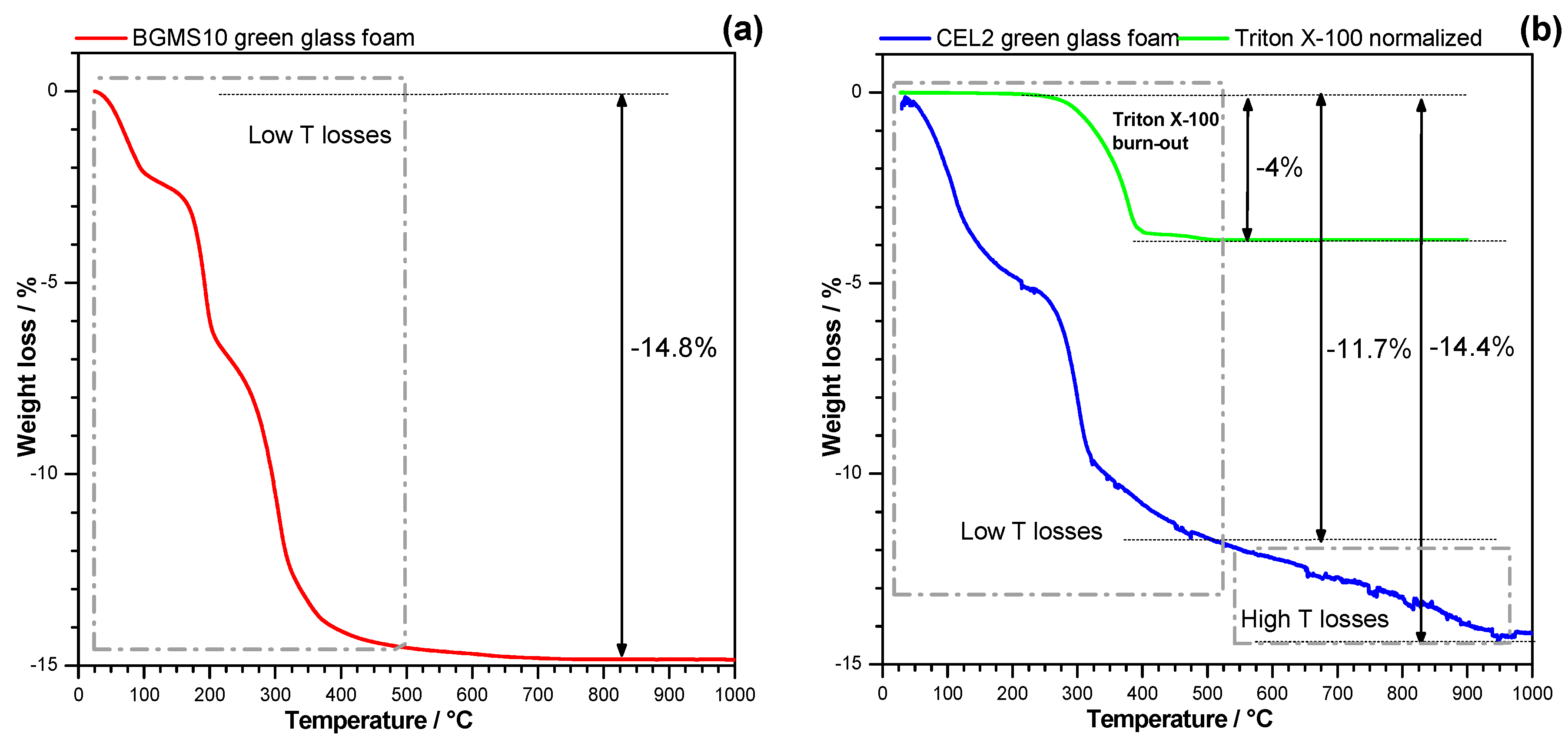
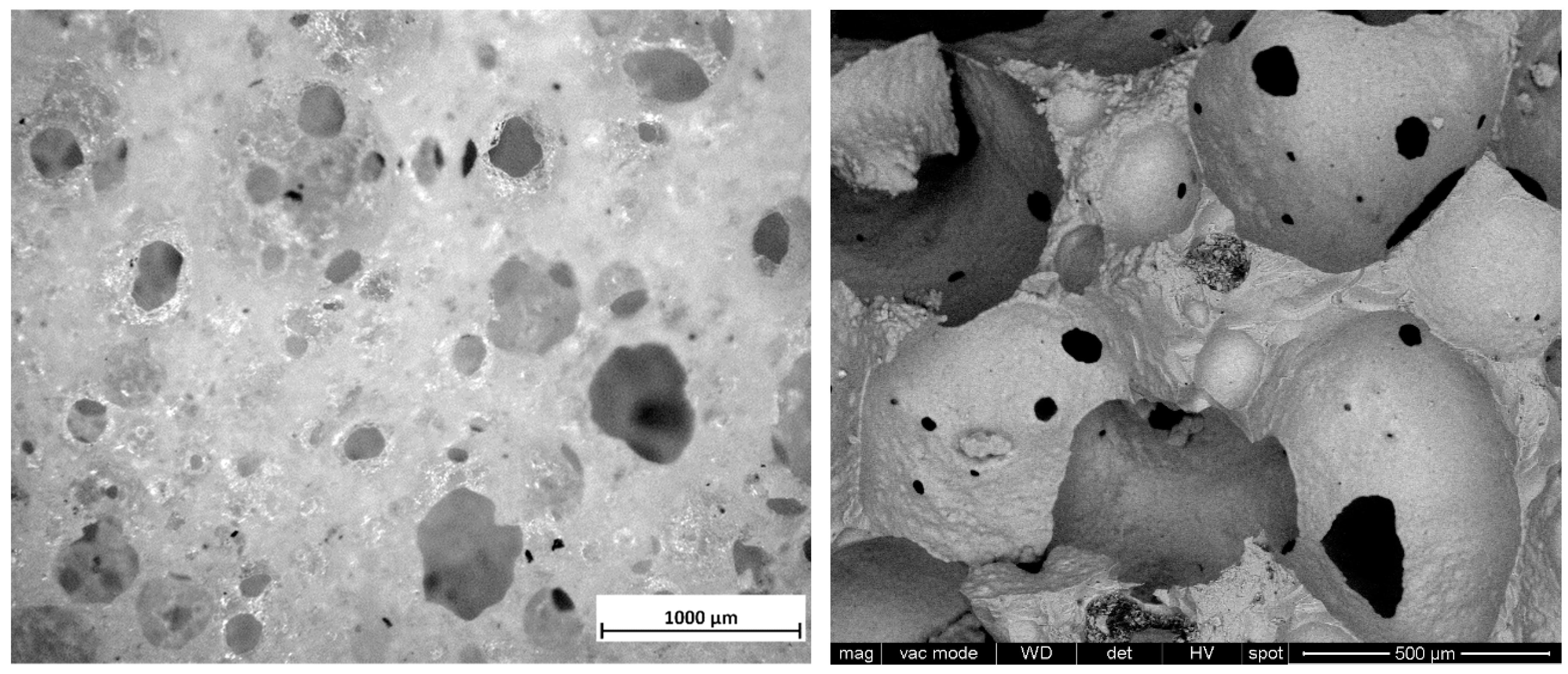
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rezwan, K.; Chen, Q.; Blaker, J.; Boccaccini, A.R.; Blaker, J. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.R.; Erol, M.; Stark, W.J.; Mohn, D.; Hong, Z.; Mano, J.F. Polymer/bioactive glass nanocomposites for biomedical applications: A review. Compos. Sci. Technol. 2010, 70, 1764–1776. [Google Scholar] [CrossRef] [Green Version]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef] [Green Version]
- Ray, A.; Tiwari, A. Compaction and sintering behaviour of glass–alumina composites. Mater. Chem. Phys. 2001, 67, 220–225. [Google Scholar] [CrossRef]
- Rawson, H. Properties and Applications of Glass; Elsevier: Amsterdam, The Netherlands, 1980. [Google Scholar]
- Bernardo, E.; Scarinci, G.; Edme, E.; Michon, U.; Planty, N. Fast-Sintered Gehlenite Glass-Ceramics from Plasma-Vitrified Municipal Solid Waste Incinerator Fly Ashes. J. Am. Ceram. Soc. 2009, 92, 528–530. [Google Scholar] [CrossRef]
- Boccaccini, A. On the viscosity of glass composites containing rigid inclusions. Mater. Lett. 1998, 34, 285–289. [Google Scholar] [CrossRef]
- Li, P.; Yang, Q.; Zhang, F.; Kokubo, T. The effect of residual glassy phase in a bioactive glass-ceramic on the formation of its surface apatite layer in vitro. J. Mater. Sci. Mater. Med. 1992, 3, 452–456. [Google Scholar] [CrossRef]
- Montazerian, M.; Zanotto, E.D. History and trends of bioactive glass-ceramics. J. Biomed. Mater. Res. Part A 2016, 104, 1231–1249. [Google Scholar] [CrossRef]
- Crovace, M.C.; Souza, M.T.; Chinaglia, C.R.; Peitl, O.; Zanotto, E.D. Biosilicate®—A multipurpose, highly bioactive glass-ceramic. In vitro, in vivo and clinical trials. J. Non-Cryst. Solids. 2016, 432, 90–110. [Google Scholar] [CrossRef]
- Fu, Q.; Rahaman, M.N.; Bal, B.S.; Brown, R.F.; Day, D.E. Mechanical and in vitro performance of 13–93 bioactive glass scaffolds prepared by a polymer foam replication technique. Acta Biomater. 2008, 4, 1854–1864. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, D.; Cannillo, V.; Sola, A. A New Highly Bioactive Composite for Scaffold Applications: A Feasibility Study. Materials 2011, 4, 339–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, M.T.; Renno, A.M.; Peitl, O.; Zanotto, E. New highly bioactive crystallization-resistant glass for tissue engineering applications. Transl. Mater. Res. 2017, 4, 014002. [Google Scholar] [CrossRef]
- Bellucci, D.; Cannillo, V. A novel bioactive glass containing strontium and magnesium with ultra-high crystallization temperature. Mater. Lett. 2018, 213, 67–70. [Google Scholar] [CrossRef]
- Bellucci, D.; Salvatori, R.; Anesi, A.; Chiarini, L.; Cannillo, V. SBF assays, direct and indirect cell culture tests to evaluate the biological performance of bioglasses and bioglass-based composites: Three paradigmatic cases. Mater. Sci. Eng. C 2019, 96, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, D.; Cannillo, V.; Anesi, A.; Salvatori, R.; Chiarini, L.; Manfredini, T.; Zaffe, D. Bone Regeneration by Novel Bioactive Glasses Containing Strontium and/or Magnesium: A Preliminary In-Vivo Study. Materials 2018, 11, 2223. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R.; Hench, L.L. Factors affecting the structure and properties of bioactive foam scaffolds for tissue engineering. J. Biomed. Mater. Res. Part B: App. Biomater. 2004, 68, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Zocca, A.; Colombo, P.; Gomes, C.M.; Günster, J. Additive Manufacturing of Ceramics: Issues, Potentialities, and Opportunities. J. Am. Ceram. Soc. 2015, 98, 1983–2001. [Google Scholar] [CrossRef]
- Travitzky, N.; Bonet, A.; Dermeik, B.; Fey, T.; Filbert-Demut, I.; Schlier, L.; Schlordt, T.; Greil, P. Additive Manufacturing of Ceramic-Based Materials. Adv. Eng. Mater. 2014, 16, 729–754. [Google Scholar]
- Jang, J.H.; Wang, S.; Pilgrim, S.M.; Schulze, W.A. Preparation and characterization of barium titanate suspensions for stereolithography. J. Am. Ceram. Soc. 2000, 83, 1804–1806. [Google Scholar] [CrossRef]
- Halloran, J.W.; Tomeckova, V.; Gentry, S.; Das, S.; Cilino, P.; Yuan, D.; Guo, R.; Rudraraju, A.; Shao, P.; Wu, T.; et al. Photopolymerization of powder suspensions for shaping ceramics. J. Eur. Ceram. Soc. 2011, 31, 2613–2619. [Google Scholar] [CrossRef]
- Elsayed, H.; Zocca, A.; Schmidt, J.; Günster, J.; Colombo, P.; Bernardo, E. Bioactive glass-ceramic scaffolds by additive manufacturing and sinter-crystallization of fine glass powders. J. Mater. Res. 2018, 33, 1960–1971. [Google Scholar] [CrossRef] [Green Version]
- Elsayed, H.; Schmidt, J.; Bernardo, E.; Colombo, P. Comparative Analysis of Wollastonite-Diopside Glass-Ceramic Structures Fabricated via Stereo-Lithography. Adv. Eng. Mater. 2019, 21, 1801160. [Google Scholar] [CrossRef]
- Tesavibul, P.; Felzmann, R.; Gruber, S.; Liska, R.; Thompson, I.; Boccaccini, A.R.; Stampfl, J. Processing of 45S5 Bioglass® by lithography-based additive manufacturing. Mater. Lett. 2012, 74, 81–84. [Google Scholar] [CrossRef]
- Gmeiner, R.; Deisinger, U.; Schönherr, J.; Lechner, B.; Detsch, R.; Boccaccini, A.R.; Stampfl, J. Additive Manufacturing of Bioactive Glasses and Silicate Bioceramics. J. Ceram. Sci. Tech. 2015, 06, 75–86. [Google Scholar]
- Jones, J.; Hench, L.L. Effect of surfactant concentration and composition on the structure and properties of sol-gel-derived bioactive glass foam scaffolds for tissue engineering. J. Mater. Sci. 2003, 38, 3783–3790. [Google Scholar] [CrossRef]
- Poologasundarampillai, G.; Lee, P.D.; Lam, C.; Kourkouta, A.; Jones, J.R. Compressive Strength of Bioactive Sol-Gel Glass Foam Scaffolds. Int. J. Appl. Glas. Sci. 2016, 7, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.Y.; Hill, R.G.; Yue, S.; Nightingale, D.; Lee, P.D.; Jones, J.R. Melt-derived bioactive glass scaffolds produced by a gel-cast foaming technique. Acta Biomater. 2011, 7, 1807–1816. [Google Scholar] [CrossRef]
- Novajra, G.; Perdika, P.; Pisano, R.; Miola, M.; Bari, A.; Jones, J.; Detsch, R.; Boccaccini, A.R.; Vitale-Brovarone, C. Structure optimisation and biological evaluation of bone scaffolds prepared by co-sintering of silicate and phosphate glasses. Adv. Appl. Ceram. 2015, 114, S48–S55. [Google Scholar] [CrossRef] [Green Version]
- Rincón, A.; Giacomello, G.; Pasetto, M.; Bernardo, E. Novel ‘inorganic gel casting’ process for the manufacturing of glass foams. J. Eur. Ceram. Soc. 2017, 37, 2227–2234. [Google Scholar] [CrossRef]
- Elsayed, H.; Romero, A.R.; Molino, G.; Brovarone, C.V.; Bernardo, E. Bioactive Glass-Ceramic Foam Scaffolds from ‘Inorganic Gel Casting’ and Sinter-Crystallization. Materials 2018, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.R.; Tamburini, S.; Taveri, G.; Toušek, J.; Dlouhy, I.; Bernardo, E. Extension of the ‘Inorganic Gel Casting’ Process to the Manufacturing of Boro-Alumino-Silicate Glass Foams. Materials 2018, 11, 2545. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, H.; Romero, A.R.; Ferroni, L.; Gardin, C.; Zavan, B.; Bernardo, E. Bioactive Glass-Ceramic Scaffolds from Novel ‘Inorganic Gel Casting’ and Sinter-Crystallization. Materials 2017, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.J.; Ashby, M.F. Cellular Solids, Structure and Properties, 2nd ed.; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Mecholsky, J.J.; Rice, R.W.; Freiman, S.W. Prediction of Fracture Energy and Flaw Size in Glasses from Measurements of Mirror Size. J. Am. Ceram. Soc. 1974, 57, 440–443. [Google Scholar] [CrossRef]
- Tetramethylammonium Hydroxide. Available online: http://www.hanhonggroup.com/ir/ir_en/RF09010003.html (accessed on 20 September 2019).
- Lv, X.; Liu, C.; Song, S.; Qiao, Y.; Hu, Y.; Li, P.; Lia, Z.; Sun, S. Construction of a quaternary ammonium salt platform with different alkyl groups for antibacterial and biosensor applications. RSC Adv. 2018, 8, 2941. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Liu, C.; Chang, S.-T.; Tsai, M.-S.; Feng, M.-S.; Tseng, W.-T. Chemical-mechanical polishing of low-dielectric-constant spin-on-glasses: Film chemistries, slurry formulation and polish selectivity. Thin Solid Films 1997, 308, 550–554. [Google Scholar] [CrossRef]
- Prasetyoko, D.; Ramli, Z.; Endud, S.; Hamdan, H.; Sulikowski, B. Conversion of rice husk ash to zeolite beta. Waste Manag. 2006, 26, 1173–1179. [Google Scholar] [CrossRef]
- Calabro, D.C.; Valyocsik, E.W.; Ryan, F.X. In situ ATR/FTIR study of mesoporous silicate syntheses. Microporous Mater. 1996, 7, 243–259. [Google Scholar] [CrossRef]
- Del Bosque, I.S.; Martinez-Ramirez, S.; Blanco-Varela, M. FTIR study of the effect of temperature and nanosilica on the nano structure of C–S–H gel formed by hydrating tricalcium silicate. Constr. Build. Mater. 2014, 52, 314–323. [Google Scholar] [CrossRef]
- Kooli, F. Recrystallization of a new layered silicate from Na-kanemite–tetramethylammonium hydroxide–water–1,4-dioxane mixture. J. Mater. Chem. 2002, 12, 1374–1380. [Google Scholar] [CrossRef]
- Sakai, N.; Nakano, T.; Yanaba, K.; Imazeki, S. Studies of the characteristic and reaction mechanism of silica xerogel by sol–gel method catalyzed by alkylbiguanide as a strong organic base. J. Sol-Gel Sci. Technol. 2018, 88, 379–385. [Google Scholar] [CrossRef]
- Zhang, Q.; Ye, G. Dehydration kinetics of Portland cement paste at high temperature. J. Therm. Anal. Calorim. 2012, 110, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, T.; Hashimoto, H.; Hayashi, M.; Nagata, K. Effect of Alkali Oxides on Crystallization in CaO–SiO2–CaF2 Glasses. ISIJ Int. 2008, 48, 925–933. [Google Scholar] [CrossRef]



| Oxide | BGMS10 Glass | |
|---|---|---|
| mol% | wt. % | |
| SiO2 | 47.2 | 44.6 |
| CaO | 25.6 | 22.6 |
| MgO | 10 | 6.3 |
| SrO | 10 | 16.3 |
| Na2O | 2.3 | 2.2 |
| K2O | 2.3 | 3.4 |
| P2O5 | 2.6 | 4.5 |
| Cellular Structure | Bulk Density (g/cm3) | Apparent Density (g/cm3) | True Density (g/cm3) | Total Porosity (%) | Open Porosity (%) | Compressive Strength (MPa) | |
|---|---|---|---|---|---|---|---|
| Reticulated scaffold | Diamond | 0.31 ± 0.05 | 2.93 ± 0.02 | 3.00 ± 0.02 | 90 | 89.4 | 0.49 ± 0.03 |
| Cubic, 4 × 4 | 1.12 ± 0.04 | 2.93 ± 0.02 | 3.02 ± 0.02 | 63 | 61.8 | 7.67 ± 0.61 | |
| Cubic, 6 × 6 | 1.39 ± 0.03 | 2.93 ± 0.02 | 2.96 ± 0.02 | 53 | 50.6 | 15.53 ± 1.30 | |
| Foam | 0.57 ± 0.03 | 2.94 ± 0.02 | 3.17 ± 0.02 | 82 | 80.5 | 1.92 ± 0.10 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsayed, H.; Rincon Romero, A.; Bellucci, D.; Cannillo, V.; Bernardo, E. Advanced Open-Celled Structures from Low-Temperature Sintering of a Crystallization-Resistant Bioactive Glass. Materials 2019, 12, 3653. https://doi.org/10.3390/ma12223653
Elsayed H, Rincon Romero A, Bellucci D, Cannillo V, Bernardo E. Advanced Open-Celled Structures from Low-Temperature Sintering of a Crystallization-Resistant Bioactive Glass. Materials. 2019; 12(22):3653. https://doi.org/10.3390/ma12223653
Chicago/Turabian StyleElsayed, Hamada, Acacio Rincon Romero, Devis Bellucci, Valeria Cannillo, and Enrico Bernardo. 2019. "Advanced Open-Celled Structures from Low-Temperature Sintering of a Crystallization-Resistant Bioactive Glass" Materials 12, no. 22: 3653. https://doi.org/10.3390/ma12223653
APA StyleElsayed, H., Rincon Romero, A., Bellucci, D., Cannillo, V., & Bernardo, E. (2019). Advanced Open-Celled Structures from Low-Temperature Sintering of a Crystallization-Resistant Bioactive Glass. Materials, 12(22), 3653. https://doi.org/10.3390/ma12223653









