Different Influences of Rare Earth Eu Addition on Primary Si Refinement in Hypereutectic Al–Si Alloys with Varied Purity
Abstract
:1. Introduction
2. Experimental Details
3. Results and Discussion
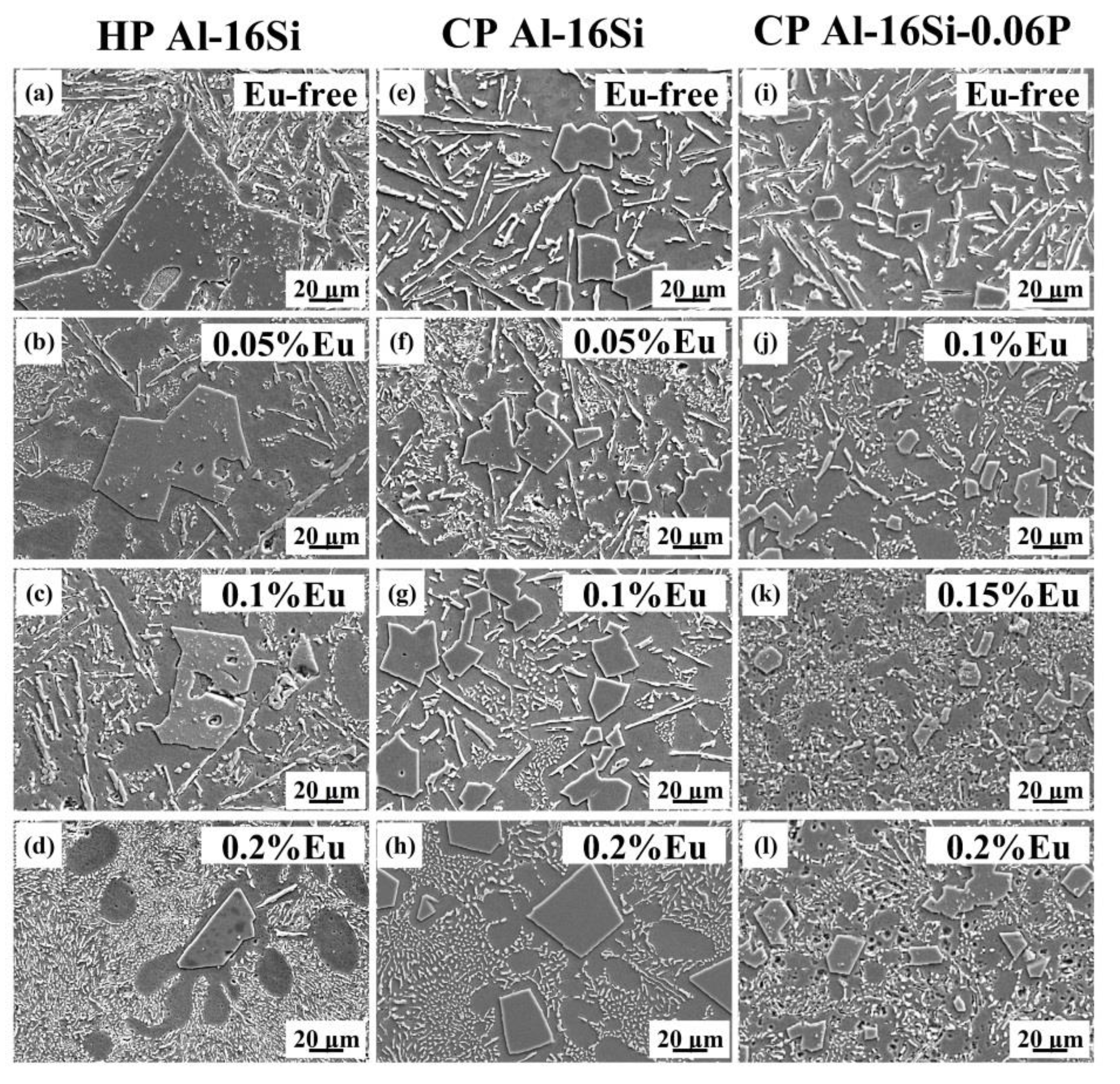
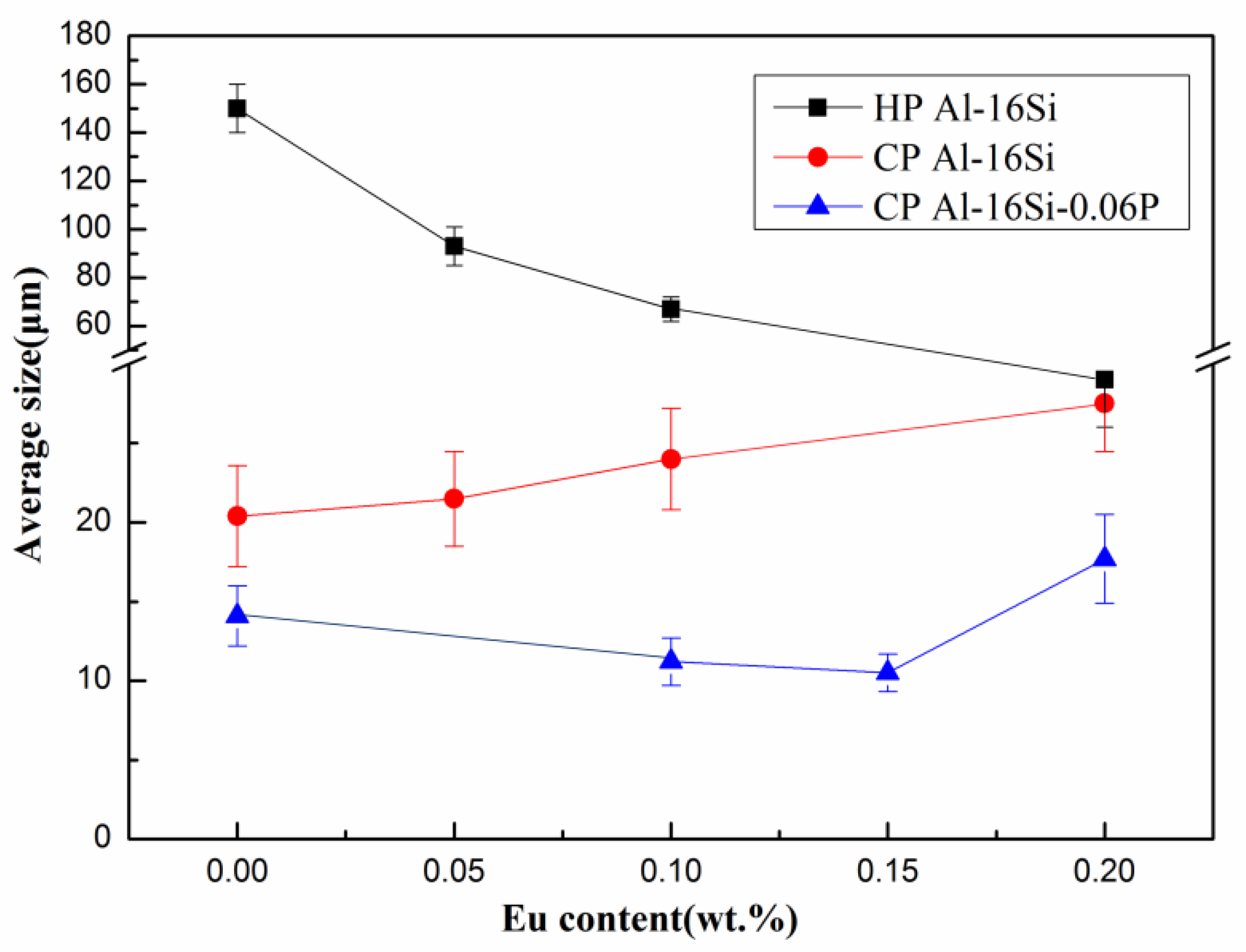
3.1. Microstructure Evaluation
3.2. Refinement Mechanism of Primary Si in the Eu–Modified HP Al–16Si Alloy
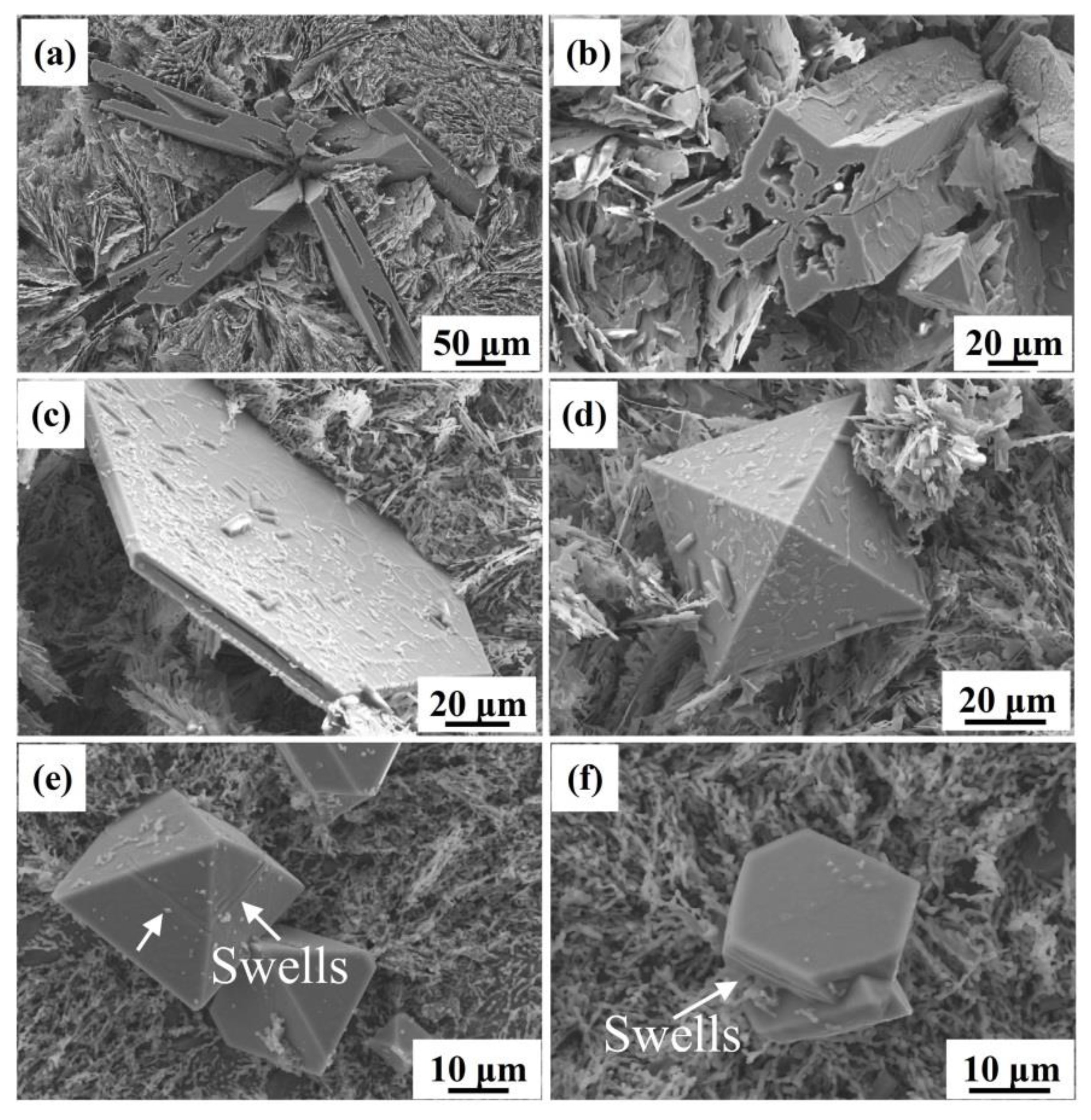
3.2.1. 3D Morphologies of Primary Si
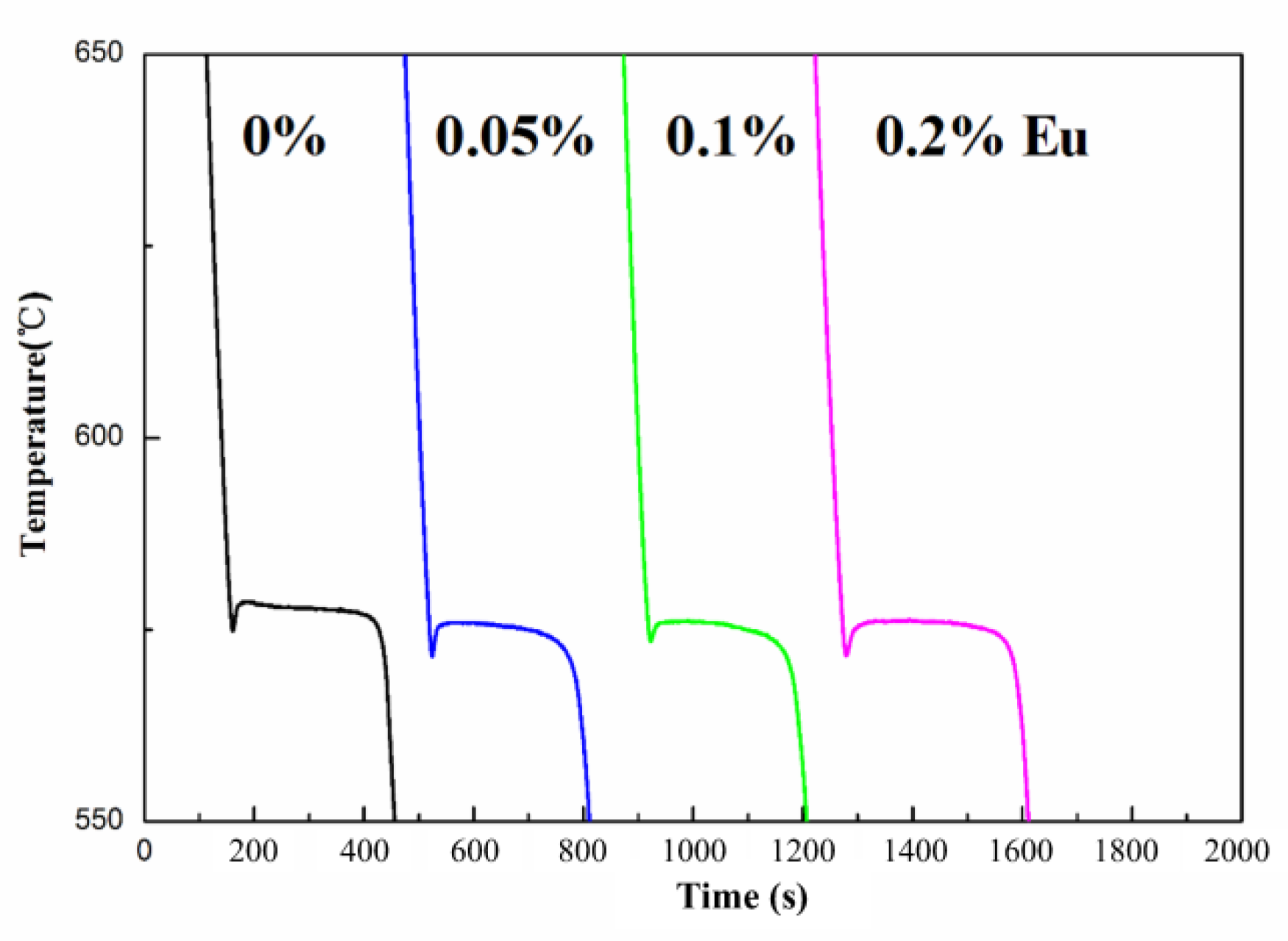
3.2.2. Thermal Analysis
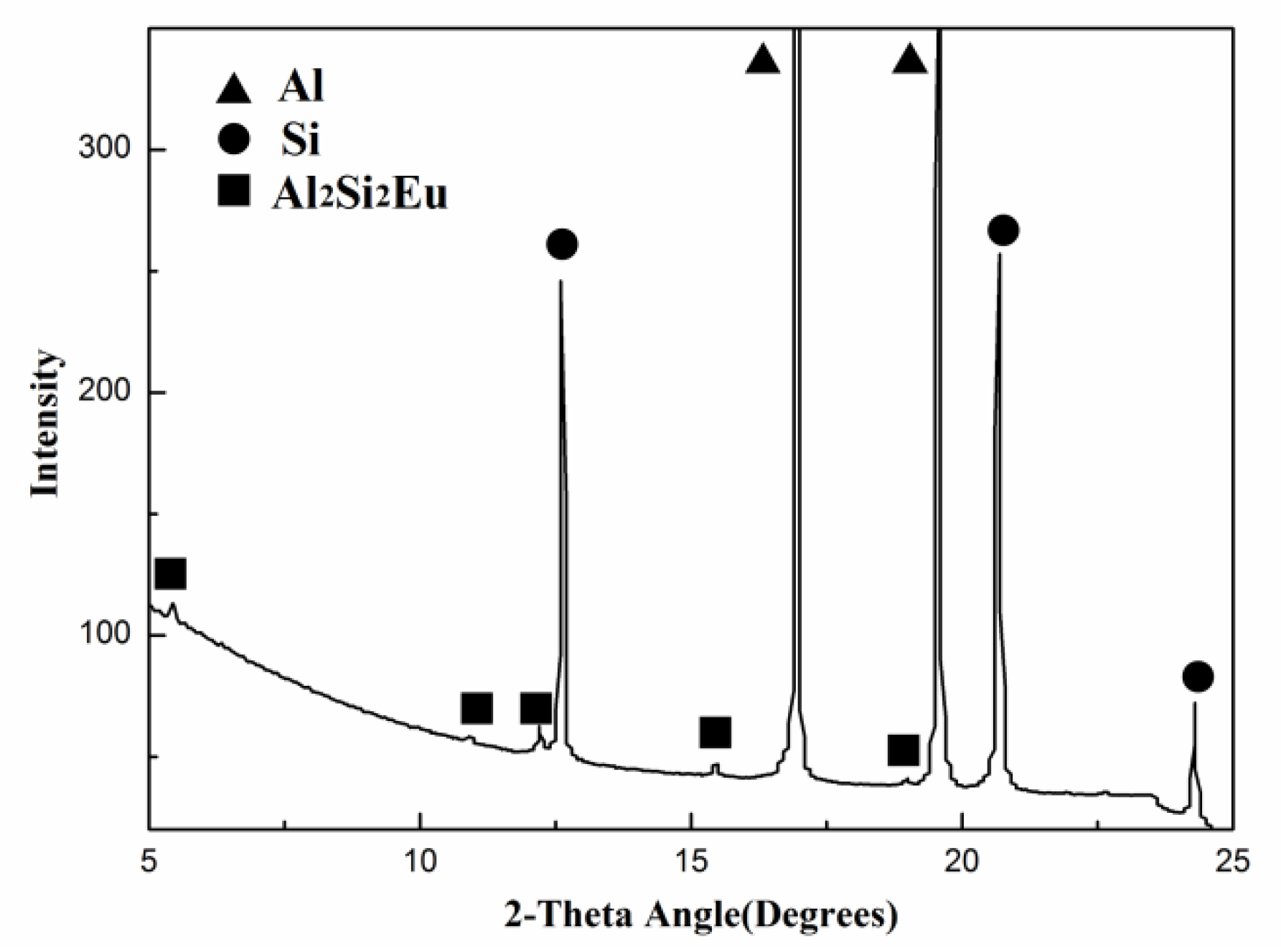
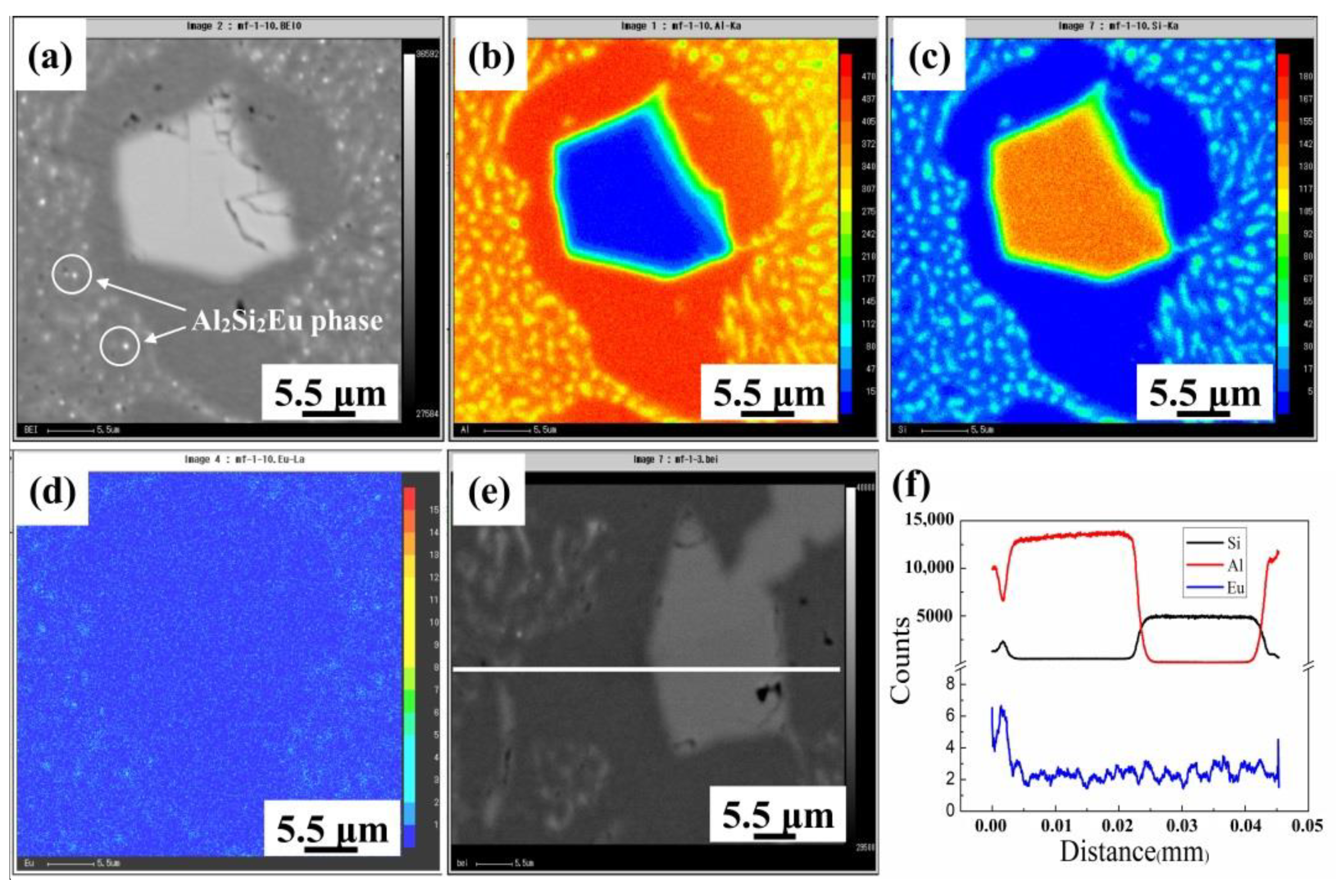
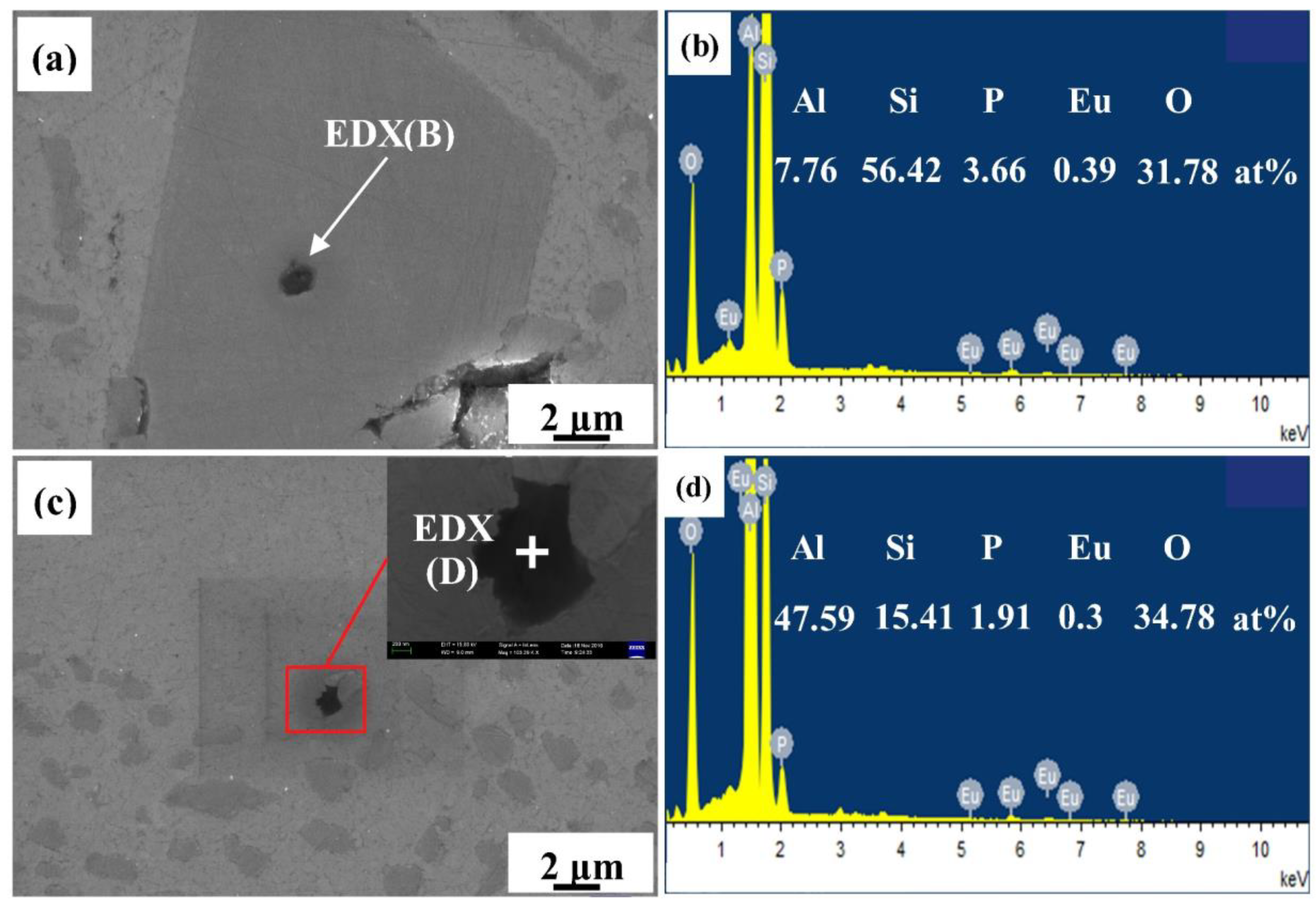
3.2.3. Eu Distribution in HP Al–16Si Alloy
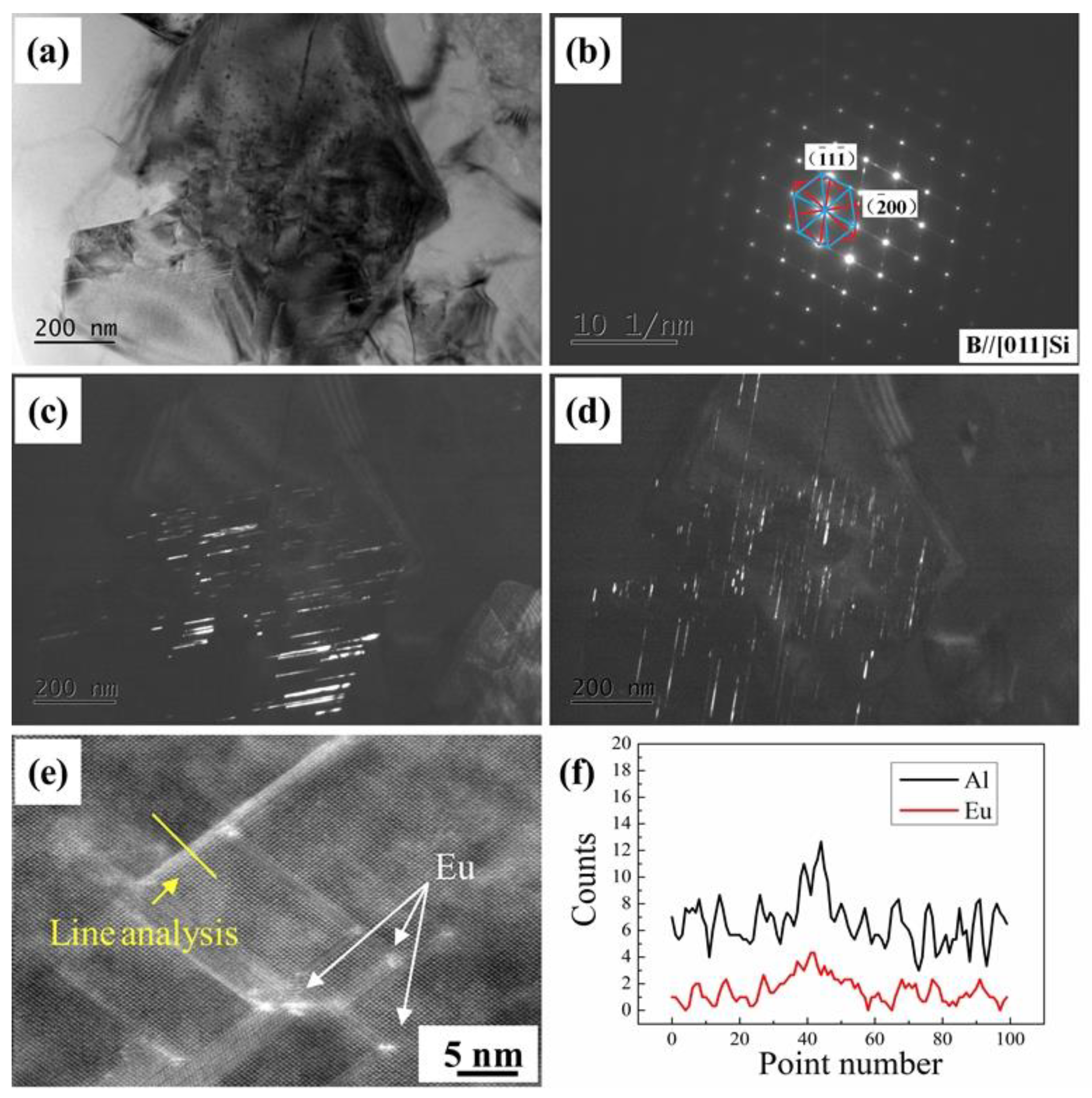
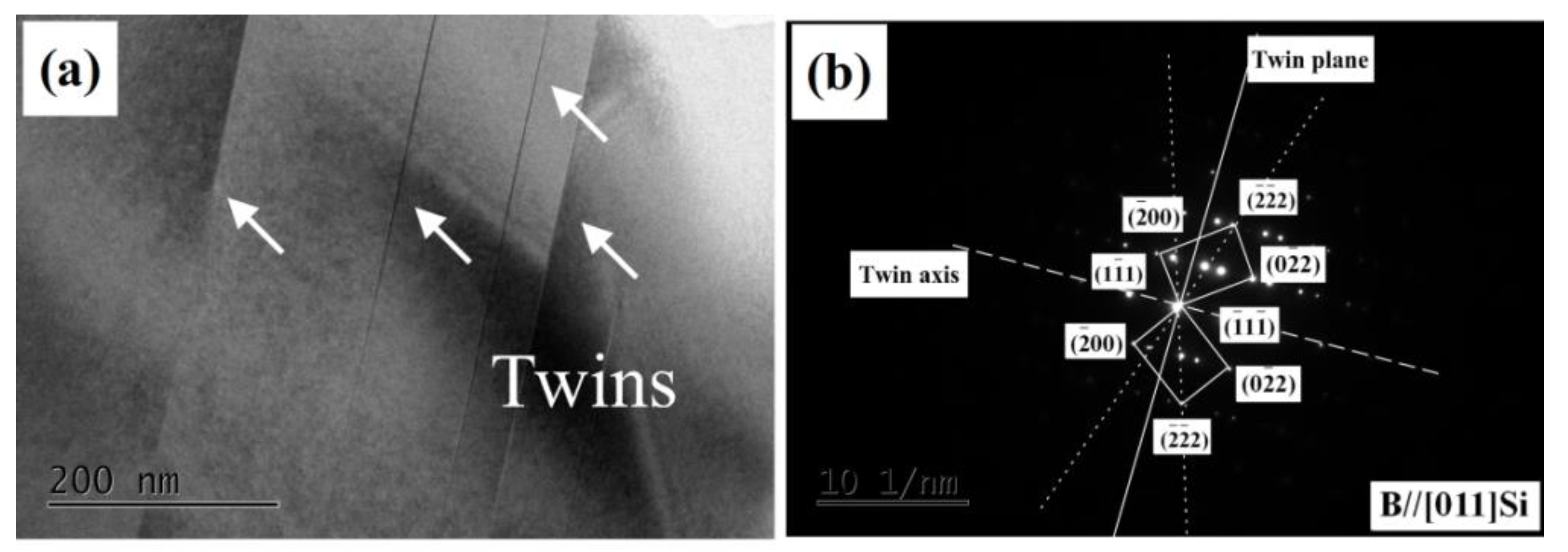
3.2.4. Transmission Electron Microscopy Observation
3.3. Refinement Mechanism of Primary Si in the Eu–Modified CP Al–16Si–0.06P Alloy
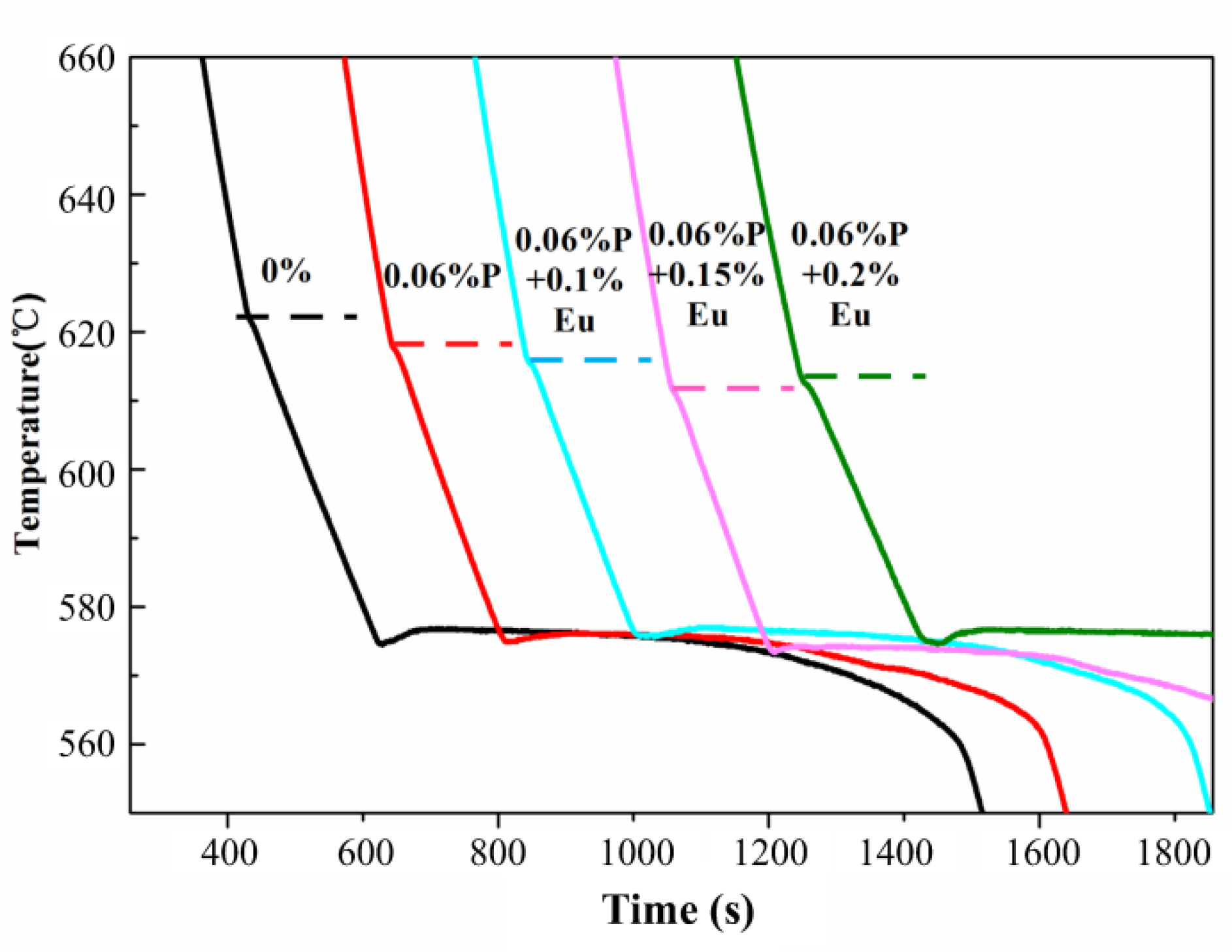
3.3.1. Thermal Analysis
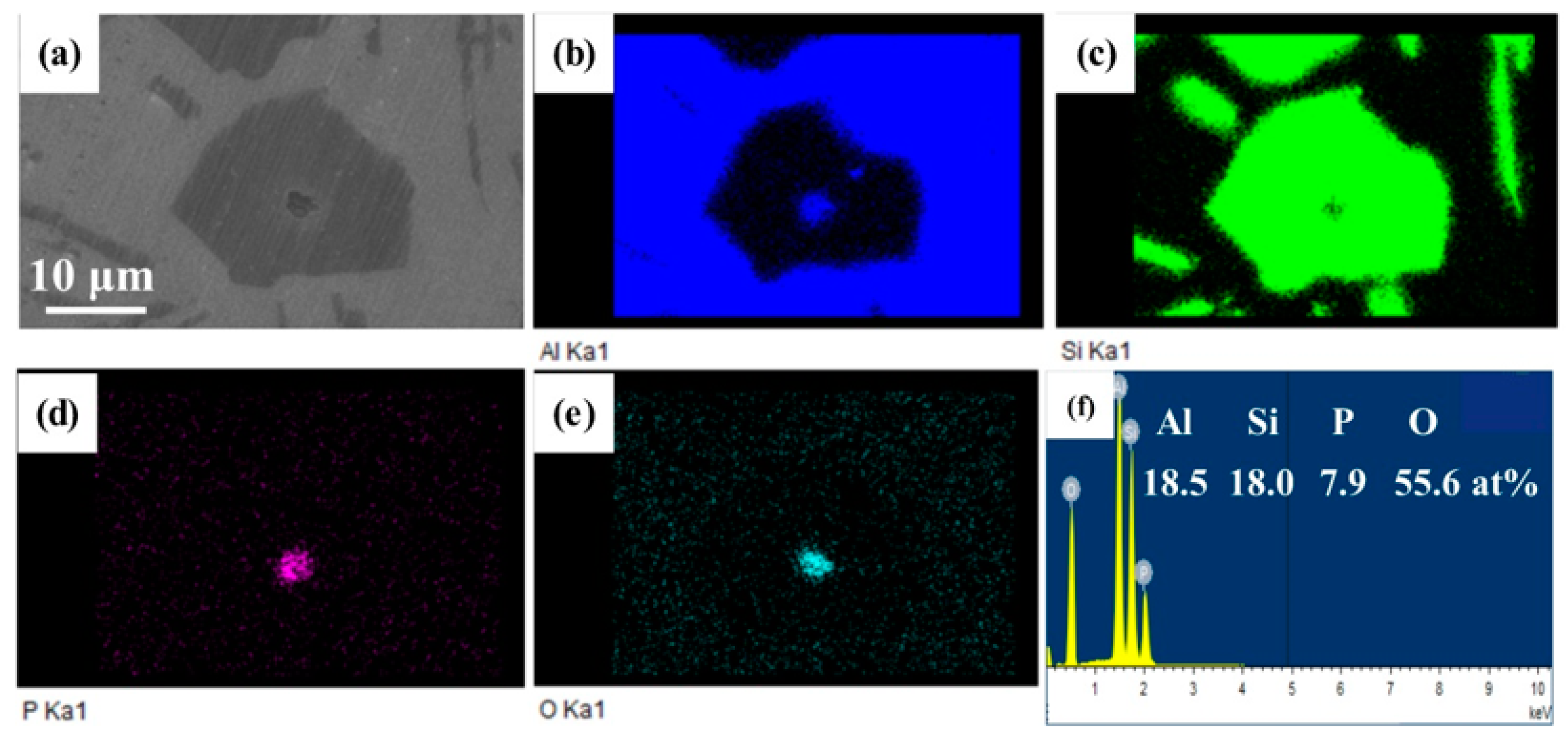
3.3.2. Primary Si Nucleus in the CP Al–16Si–0.06P Alloy
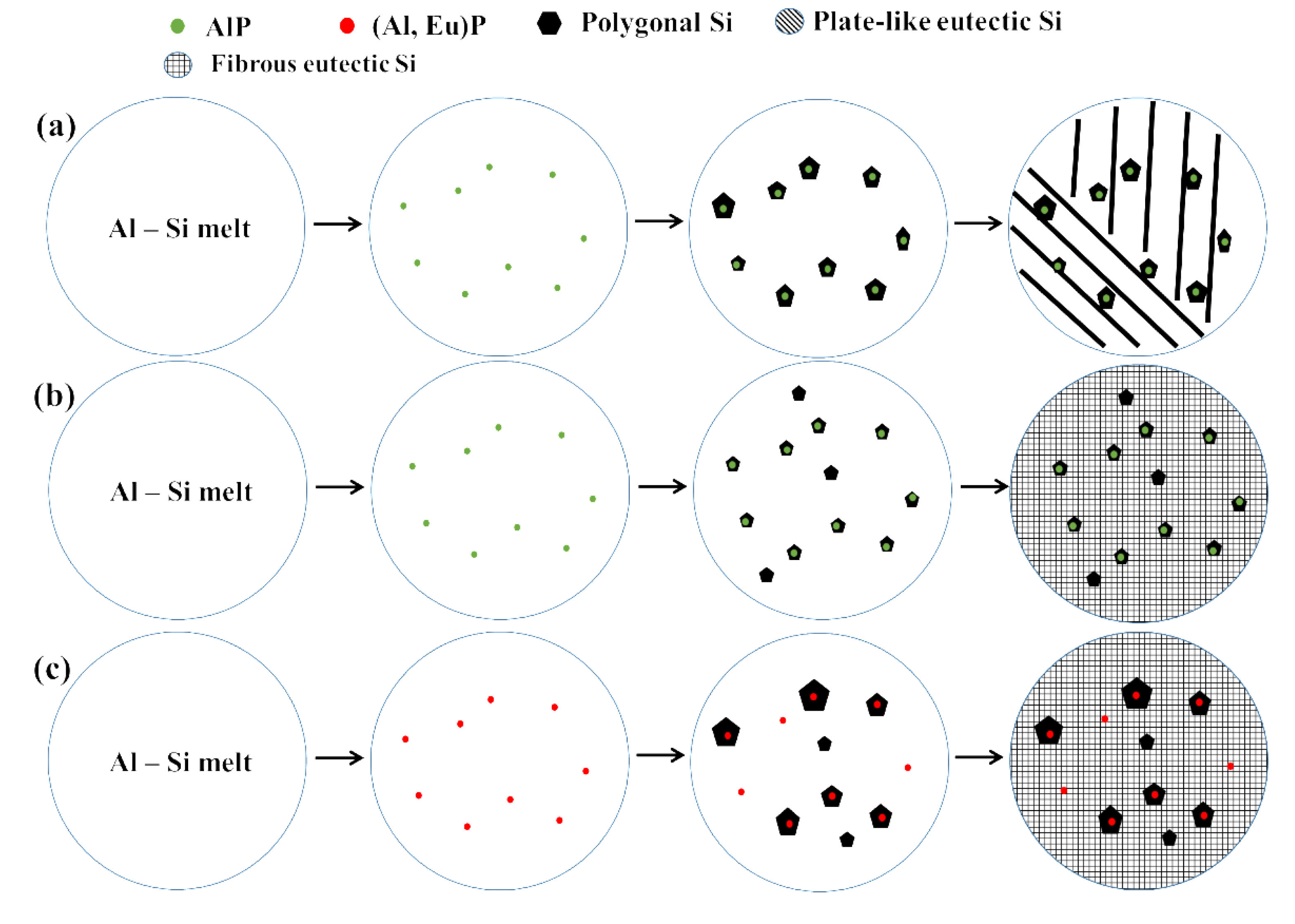
3.3.3. Solidification of the CP Al–16Si–0.06P Alloy
3.4. Mechanical Properties of the Eu–Modified CP Al–16Si–0.06P Alloy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Haque, M.; Sharif, A. Study on wear properties of aluminum–silicon piston alloy. J. Mater. Process. Technol. 2001, 118, 69–73. [Google Scholar] [CrossRef]
- Li, J.; Elmadagli, M.; Gertsman, V.; Lo, J.; Alpas, A. FIB and TEM characterization of subsurfaces of an Al–Si alloy (A390) subjected to sliding wear. Mater. Sci. Eng. A 2006, 421, 317–327. [Google Scholar] [CrossRef]
- Li, Q.L.; Li, B.Q.; Li, J.B.; Zhu, Y.Q.; Xia, T.D. Effect of yttrium addition on the microstructures and mechanical properties of hypereutectic Al–20Si alloy. Mater. Sci. Eng. A. 2018, 722, 47–57. [Google Scholar] [CrossRef]
- Shi, W.; Gao, B.; Tu, G.; Li, S. Effect of Nd on microstructure and wear resistance of hypereutectic Al–20%Si alloy. J. Alloy. Compd. 2010, 508, 480–485. [Google Scholar] [CrossRef]
- Li, Q.; Li, F.; Xia, T.; Lan, Y.; Jian, Y.; Tao, F. Effects of in–situ γ–Al2O3 particles and heat treatment on the microstructure and mechanical properties of A356 aluminium alloy. J. Alloy. Compd. 2015, 627, 352–358. [Google Scholar] [CrossRef]
- Roehling, J.D.; Coughlin, D.R.; Gibbs, J.W.; Baldwin, J.K.; Mertens, J.C.; Campbell, G.H.; Clarke, A.J.; McKeown, J.T. Rapid solidification growth mode transitions in Al–Si alloys by dynamic transmission electron microscopy. Acta Mater. 2017, 131, 22–30. [Google Scholar] [CrossRef]
- Xu, C.; Jiang, Q. Morphologies of primary silicon in hypereutectic Al–Si alloys with melt overheating temperature and cooling rate. Mater. Sci. Eng. A 2006, 437, 451–455. [Google Scholar] [CrossRef]
- Jung, J.-G.; Ahn, T.-Y.; Cho, Y.-H.; Kim, S.-H.; Lee, J.-M. Synergistic effect of ultrasonic melt treatment and fast cooling on the refinement of primary Si in a hypereutectic Al–Si alloy. Acta Mater. 2018, 144, 31–40. [Google Scholar] [CrossRef]
- Zou, Q.; Jie, J.; Sun, J.; Wang, T.; Cao, Z.; Li, T. Effect of Si content on separation and purification of the primary Si phase from hypereutectic Al–Si alloy using rotating magnetic field. Sep. Purif. Technol. 2015, 142, 101–107. [Google Scholar] [CrossRef]
- Li, J.; Hage, F.S.; Liu, X.; Ramasse, Q.; Schumacher, P. Revealing heterogeneous nucleation of primary Si and eutectic Si by AlP in hypereutectic Al–Si alloys. Sci. Rep. 2016, 6, 25244. [Google Scholar] [CrossRef]
- Ho, C.; Cantor, B. Heterogeneous nucleation of solidification of Si in Al–Si and Al–Si–P alloys. Acta Met. et Mater. 1995, 43, 3231–3246. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.; Wu, Y. Refinement and modification performance of Al–P master alloy on primary Mg2Si in Al–Mg–Si alloys. J. Alloy. Compd. 2008, 465, 145–150. [Google Scholar] [CrossRef]
- McDonald, S.D.; Dahle, A.K.; Nogita, K.; Tsujimoto, K.; Yasuda, K. Aluminium phosphide as a eutectic grain nucleus in hypoeutectic Al–Si alloys. Qjm: Int. J. Med. 2004, 53, 361–369. [Google Scholar]
- Liang, S.-M.; Schmid-Fetzer, R. Phosphorus in Al–Si cast alloys: Thermodynamic prediction of the AlP and eutectic (Si) solidification sequence validated by microstructure and nucleation undercooling data. Acta Mater. 2014, 72, 41–56. [Google Scholar] [CrossRef]
- Zuo, M.; Liu, X.; Sun, Q.; Jiang, K. Effect of rapid solidification on the microstructure and refining performance of an Al–Si–P master alloy. J. Mater. Process. Technol. 2009, 209, 5504–5508. [Google Scholar] [CrossRef]
- Bao, G.; Zuo, M.; Li, D.; Li, Y.; Liu, X. The improvement of microstructures and mechanical properties of near eutectic Al–Si multicomponent alloy by an Al–8Zr–2P master alloy. Mater. Sci. Eng. A 2012, 531, 55–60. [Google Scholar] [CrossRef]
- Kyffin, W.J.; Rainforth, W.; Jones, H. Effect of phosphorus additions on the spacing between primary silicon particles in a Bridgman solidified hypereutectic Al–Si alloy. J. Mater. Sci. 2001, 36, 2667–2672. [Google Scholar] [CrossRef]
- Ludwig, T.H.; Schaffer, P.L.; Arnberg, L. Influence of Phosphorus on the Nucleation of Eutectic Silicon in Al–Si Alloys. Met. Mater. Trans. A 2013, 44, 5796–5805. [Google Scholar] [CrossRef]
- Li, J.; Albu, M.; Hofer, F.; Schumacher, P. Solute adsorption and entrapment during eutectic Si growth in A–Si–based alloys. Acta Mater. 2015, 83, 187–202. [Google Scholar] [CrossRef]
- Li, J.; Zarif, M.; Albu, M.; McKay, B.; Hofer, F.; Schumacher, P. Nucleation kinetics of entrained eutectic Si in Al–5Si alloys. Acta Mater. 2014, 72, 80–98. [Google Scholar] [CrossRef]
- Day, M.G. Primary Silicon Spherulites in Aluminium–Silicon Alloys. Nat. 1968, 219, 1357–1358. [Google Scholar] [CrossRef]
- Ylimaz, F.; Atasoy, O.A.; Elliott, R. Growth structures in aluminium–silicon alloys II. The influence of strontium. J. Cryst. Growth. 1992, 118, 377–384. [Google Scholar] [CrossRef]
- Crosley, P.B.; Mondolfo, L.F. The modification of aluminum silicon alloys. Modern Casting. 1966, 49, 53–64. [Google Scholar]
- Cho, Y.; Lee, H.-C.; Oh, K.; Dahle, A. Effect of Strontium and Phosphorus on Eutectic Al–Si Nucleation and Formation of β–Al5FeSi in Hypoeutectic Al–Si Foundry Alloys. Met. Mater. Trans. A 2008, 39, 2435–2448. [Google Scholar] [CrossRef]
- Li, Q.; Xia, T.; Lan, Y.; Zhao, W.; Fan, L.; Li, P. Effect of rare earth cerium addition on the microstructure and tensile properties of hypereutectic Al–20%Si alloy. J. Alloy. Compd. 2013, 562, 25–32. [Google Scholar] [CrossRef]
- Li, Q.; Xia, T.; Lan, Y.; Li, P.; Fan, L. Effects of rare earth Er addition on microstructure and mechanical properties of hypereutectic Al–20% Si alloy. Mater. Sci. Eng. A 2013, 588, 97–102. [Google Scholar] [CrossRef]
- Li, Q.L.; Li, J.B.; Li, B.Q.; Lan, Y.F.; Xia, T.D. Effect of samarium (sm) addition on the microstructure and tensile properties of Al–20% Si casting alloy. Int. J. Metalcast. 2018, 12, 554–564. [Google Scholar] [CrossRef]
- Li, Q.L.; Li, J.B.; Li, B.Q. Mechanical properties and microstructural evolution of Yb–modified Al–20%Si alloy. J. Mater Eng. Perform. 2018, 27, 3498–3507. [Google Scholar] [CrossRef]
- Weiss, J.C.; Loper, C.R. Primary silicon in hypereutectic aluminum–silicon casting alloys. AFS Trans. 1987, 32, 51. [Google Scholar]
- Shafei, M.; Arab, N.; Madar, K.Z. On the modification of hypereutectic Al–15Si alloy using rare Earth Ce. Appl. Mech. Mater. 2014, 467, 16–19. [Google Scholar] [CrossRef]
- Sun, B.D.; Li, K.; Wang, J.; Zhou, X.H. Effects of La and Y on hypereutectic Al–Si alloy. J. Shanghai Jiaotong U. 1999, 37, 795–798. [Google Scholar]
- Kiliçaslan, M.F.; Lee, W.-R.; Lee, T.-H.; Sohn, Y.; Hong, S.-J. Effect of Sc addition on the microstructure and mechanical properties of as–atomized and extruded Al–20Si alloys. Mater. Lett. 2012, 71, 164–167. [Google Scholar]
- Chokemorh, P.; Pandee, P.; Limmaneevichitr, C. Role of scandium additions in primary silicon refinement of hypereutectic Al–20Si alloys. Int. J. Cast Met. Res. 2018, 31, 1–10. [Google Scholar] [CrossRef]
- Li, J.H.; Suetsugu, S.; Tsunekawa, Y.; Schumacher, P. Refinement of eutectic Si phase in Al–5Si alloys with Yb additions. Metall. Mater. Trans. A. 2013, 44, 669–681. [Google Scholar] [CrossRef]
- Li, J.H.; Schumacher, P. Effect of Y addition and cooling rate on refinement of eutectic Si in Al–5 wt–%Si alloys. Int. J. Cast Met. Res. 2012, 25, 347–357. [Google Scholar] [CrossRef]
- Pandee, P.; Gourlay, C.M.; Belyakov, S.A.; Ozaki, R.; Yasuda, H.; Limmaneevichitr, C.; Gourlay, C. Eutectic Morphology of Al–7Si–0.3Mg Alloys with Scandium Additions. Met. Mater. Trans. A 2014, 45, 4549–4560. [Google Scholar] [CrossRef]
- Nogita, K.; McDonald, S.D.; Dahle, A.K. Eutectic Modification of Al–Si Alloys with Rare Earth Metals. Mater. Trans. 2004, 45, 323–326. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Ludwig, T.; Tsunekawa, Y.; Arnberg, L.; Jiang, J.; Schumacher, P. Modification of eutectic Si in Al–Si alloys with Eu addition. Acta Mater. 2015, 84, 153–163. [Google Scholar] [CrossRef]
- Mao, F.; Yan, G.; Xuan, Z.; Cao, Z.; Wang, T. Effect of Eu addition on the microstructures and mechanical properties of A356 aluminum alloys. J. Alloy. Compd. 2015, 650, 896–906. [Google Scholar] [CrossRef]
- Mao, F.; Yan, G.; Li, J.; Wang, T.; Cao, Z. The interaction between Eu and P in high purity Al–7Si alloys. Mater. Charact. 2016, 120, 129–142. [Google Scholar] [CrossRef]
- Li, J.H.; Ludwig, T.H.; Oberdorfer, B.; Schumacher, P. Solidification behaviour of Al–Si based alloys with controlled additions of Eu and P. Int. J. Cast Met. Res. 2018, 31, 1–14. [Google Scholar] [CrossRef]
- Pei, Y.; De Hosson, J. Five–fold branched Si particles in laser clad AlSi functionally graded materials. Acta Mater. 2001, 49, 561–571. [Google Scholar] [CrossRef]
- Hamilton, D.R.; Seidensticker, R.G. Propagation Mechanism of Germanium Dendrites. J. Appl. Phys. 1960, 31, 1165. [Google Scholar] [CrossRef]
- Kobayashi, K.F.; Hogan, L.M. The crystal growth of silicon in Al–Si alloys. J. Mater. Sci. 1985, 20, 1961–1975. [Google Scholar] [CrossRef]
- Jackson, K. Current concepts in crystal growth from the melt. Prog. Solid State Chem. 1967, 4, 53–80. [Google Scholar] [CrossRef]
- Lu, S.-Z.; Hellawell, A. The mechanism of silicon modification in aluminum–silicon alloys: Impurity induced twinning. Met. Mater. Trans. A 1987, 18, 1721–1733. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Yu, W.; Wang, X.; Zheng, H.; Tian, X. Pre–nucleation clusters mediated crystallization in Al–Si melts. Scr. Mater. 2016, 110, 87–91. [Google Scholar] [CrossRef]
- Xu, C.; Wang, H.; Yang, Y.; Jiang, Q. Effect of Al–P–Ti–TiC–Nd2O3 modifier on the microstructure and mechanical properties of hypereutectic Al–20wt.%Si alloy. Mater. Sci. Eng. A 2007, 452, 341–346. [Google Scholar] [CrossRef]
- Tong, X.C.; Ghosh, A.K. Fabrication of in situ TiC reinforced aluminum matrix composites. J. Mater. Sci. 2001, 36, 4059–4069. [Google Scholar] [CrossRef]
- Zhou, J.; Duszczyk, J. Fracture features of a silicon–dispersed aluminium alloy extruded from rapidly solidified powder. J. Mater. Sci. 1990, 25, 4541–4548. [Google Scholar] [CrossRef]
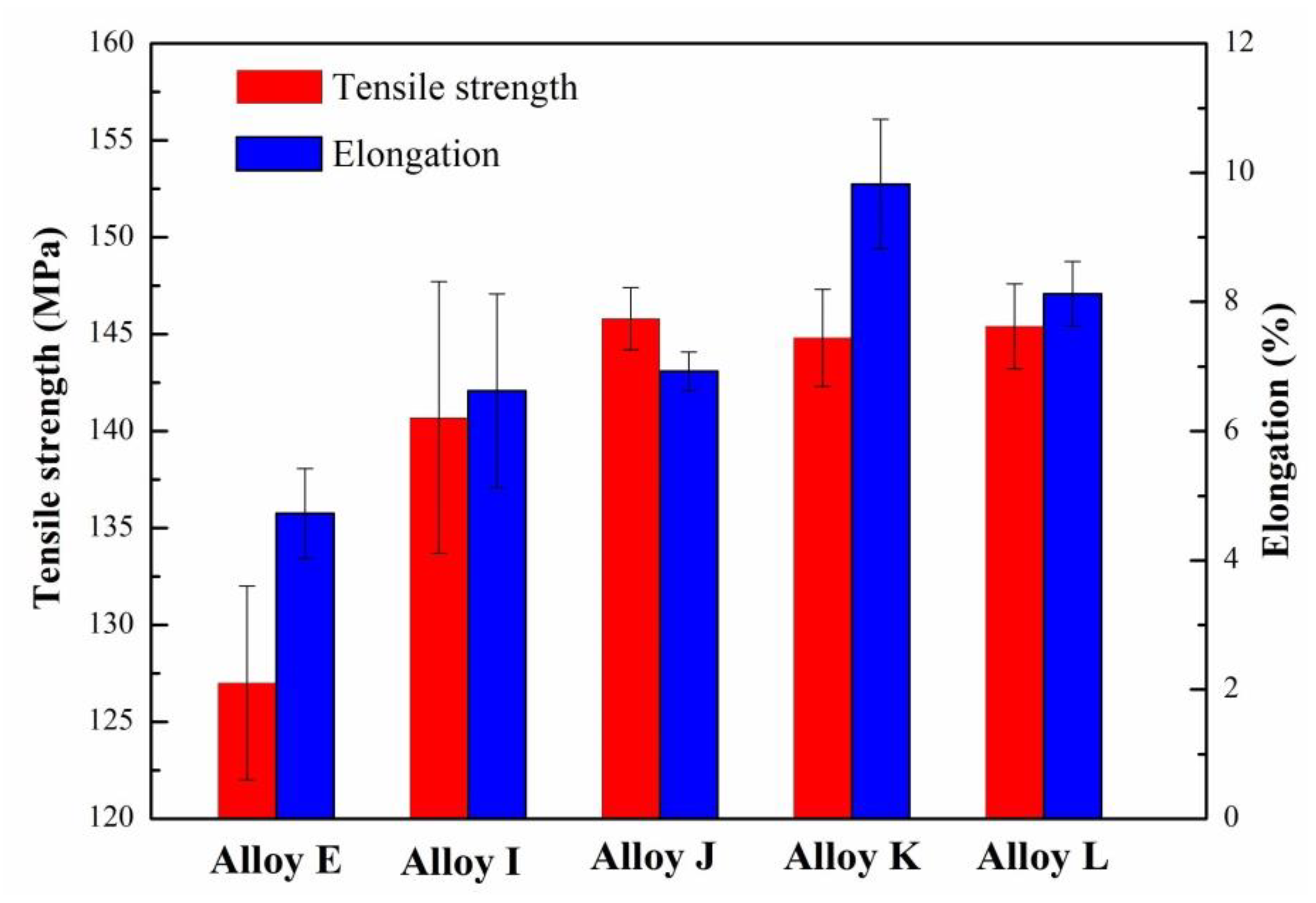
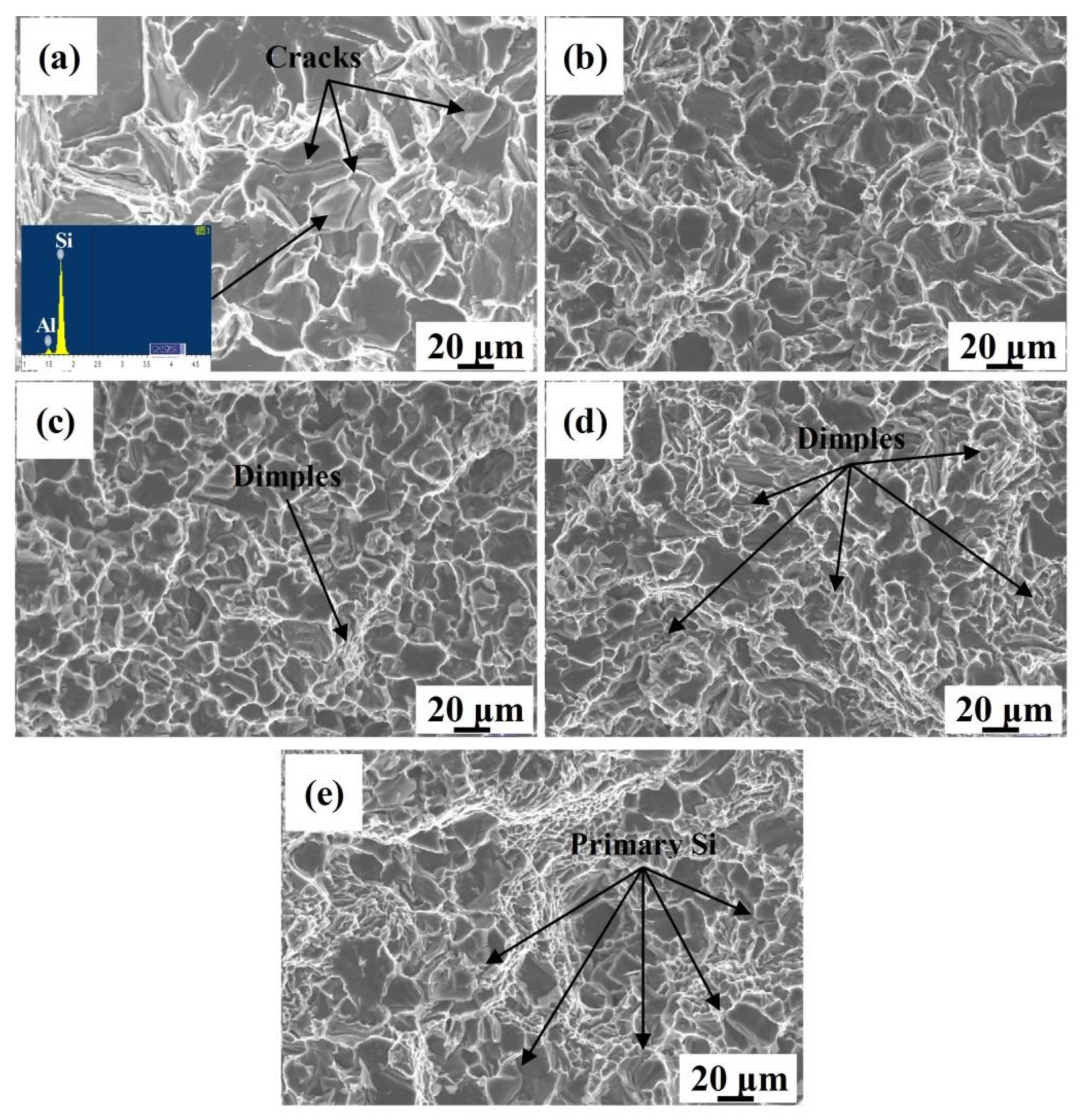
| Sample | Purity | Si (wt. %) | Fe (wt. %) | P (ppm) | Al | Eu Addition (wt. %) |
|---|---|---|---|---|---|---|
| Alloy A | HP | 16 | <0.01 | 0.5 | Balance | 0 |
| Alloy B | 0.05 | |||||
| Alloy C | 0.1 | |||||
| Alloy D | 0.2 | |||||
| Alloy E | CP | 16 | 0.13 | 26 | Balance | 0 |
| Alloy F | 0.05 | |||||
| Alloy G | 0.1 | |||||
| Alloy H | 0.2 | |||||
| Alloy I | CP | 16 | 0.13 | 594 | Balance | 0 |
| Alloy J | 0.1 | |||||
| Alloy K | 0.15 | |||||
| Alloy L | 0.2 |
| Alloys | Alloy E | Alloy I | Alloy J | Alloy K | Alloy L |
|---|---|---|---|---|---|
| Primary Si reaction temperature (°C) | 621.7 | 617.9 | 615.2 | 611.5 | 612.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, F.; Wei, S.; Ou, L.; Zhang, C.; Chen, C.; Wang, X.; Cao, Z. Different Influences of Rare Earth Eu Addition on Primary Si Refinement in Hypereutectic Al–Si Alloys with Varied Purity. Materials 2019, 12, 3505. https://doi.org/10.3390/ma12213505
Mao F, Wei S, Ou L, Zhang C, Chen C, Wang X, Cao Z. Different Influences of Rare Earth Eu Addition on Primary Si Refinement in Hypereutectic Al–Si Alloys with Varied Purity. Materials. 2019; 12(21):3505. https://doi.org/10.3390/ma12213505
Chicago/Turabian StyleMao, Feng, Shizhong Wei, Liming Ou, Cheng Zhang, Chong Chen, Xiaodong Wang, and Zhiqiang Cao. 2019. "Different Influences of Rare Earth Eu Addition on Primary Si Refinement in Hypereutectic Al–Si Alloys with Varied Purity" Materials 12, no. 21: 3505. https://doi.org/10.3390/ma12213505
APA StyleMao, F., Wei, S., Ou, L., Zhang, C., Chen, C., Wang, X., & Cao, Z. (2019). Different Influences of Rare Earth Eu Addition on Primary Si Refinement in Hypereutectic Al–Si Alloys with Varied Purity. Materials, 12(21), 3505. https://doi.org/10.3390/ma12213505





