The Relationship between Residual Amount of Sr and Morphology of Eutectic Si Phase in A356 Alloy
Abstract
1. Introduction
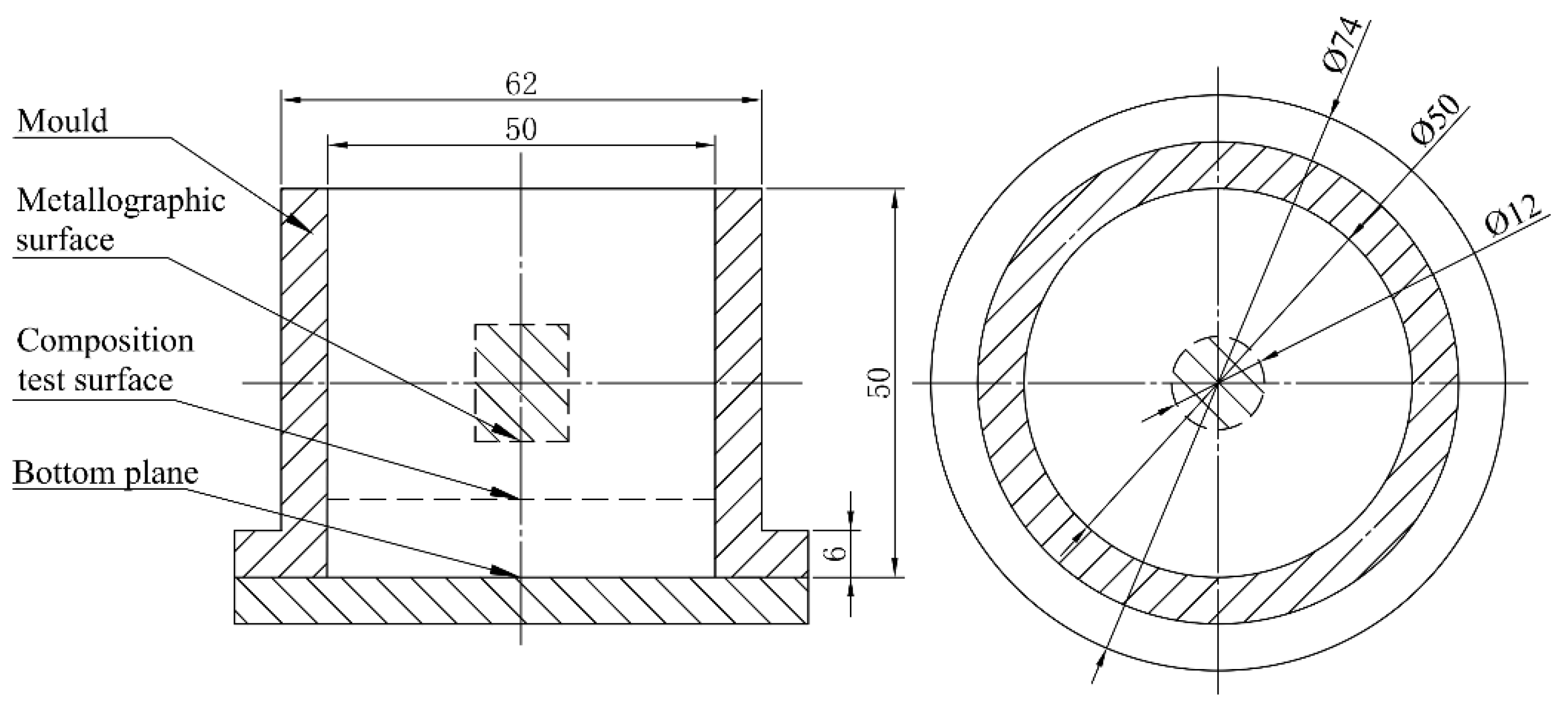
2. Materials and Methods
3. Results and Discussion
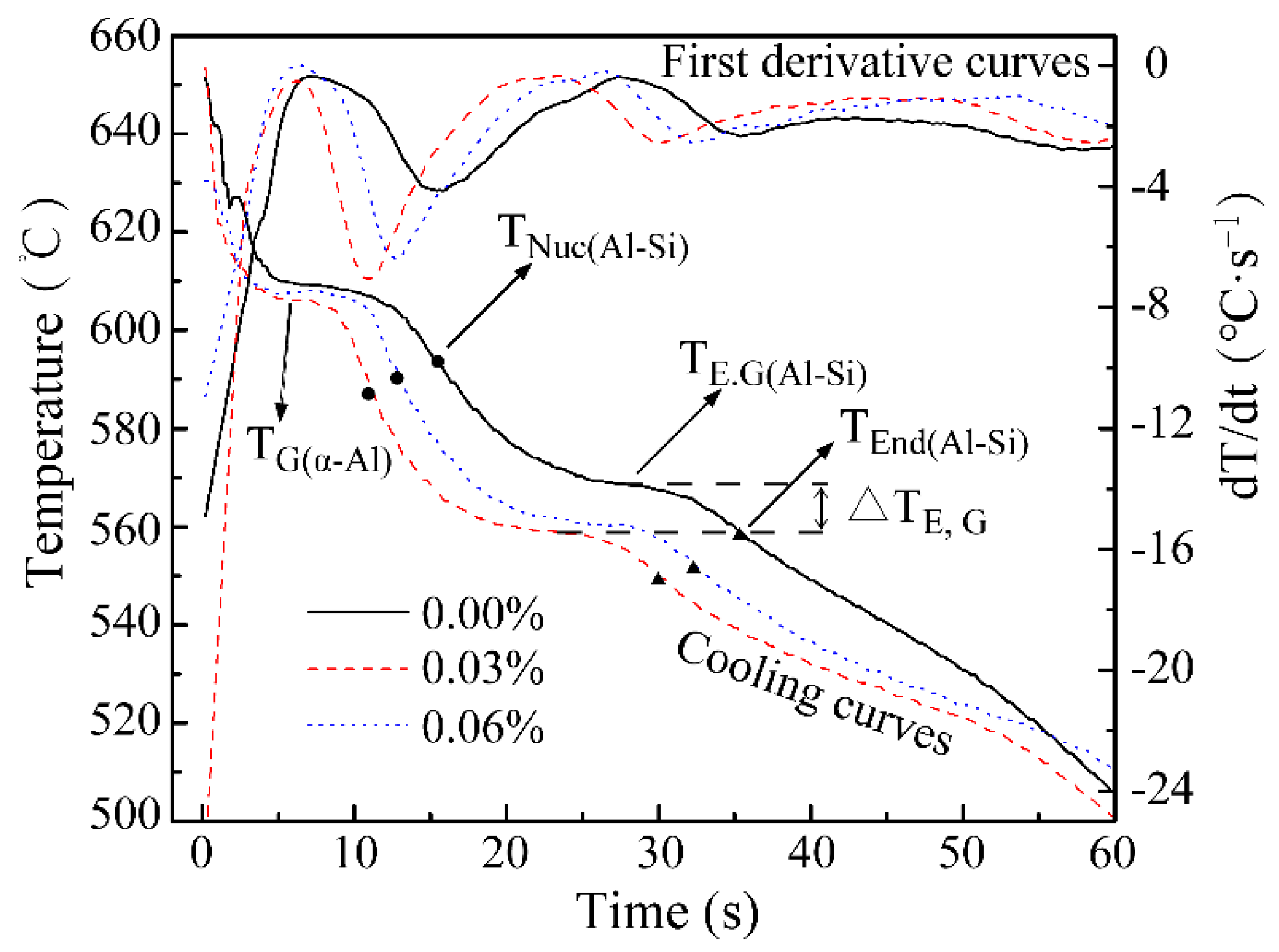
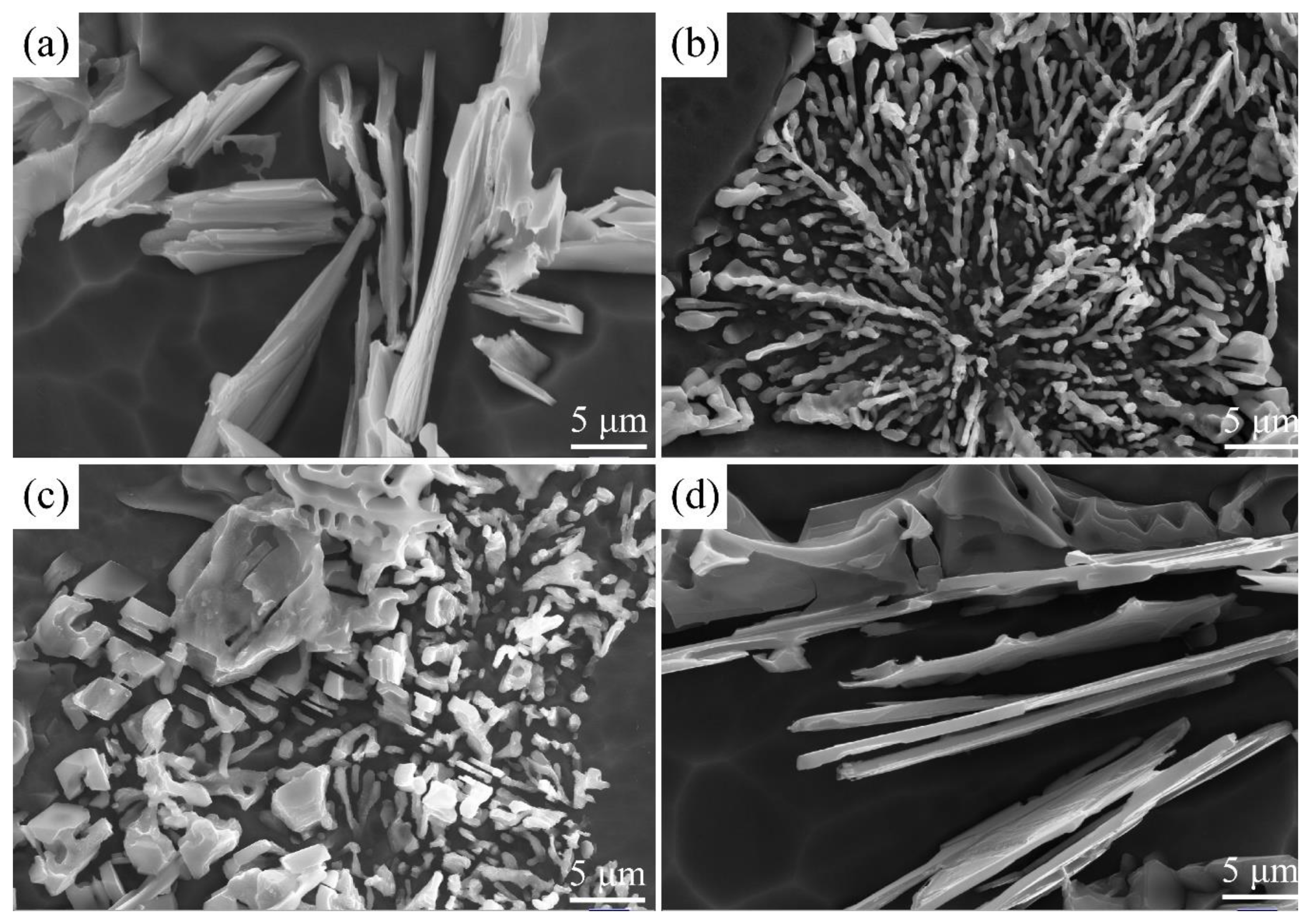
3.1. Influence of the Sr Additive Amount on Modification Effect
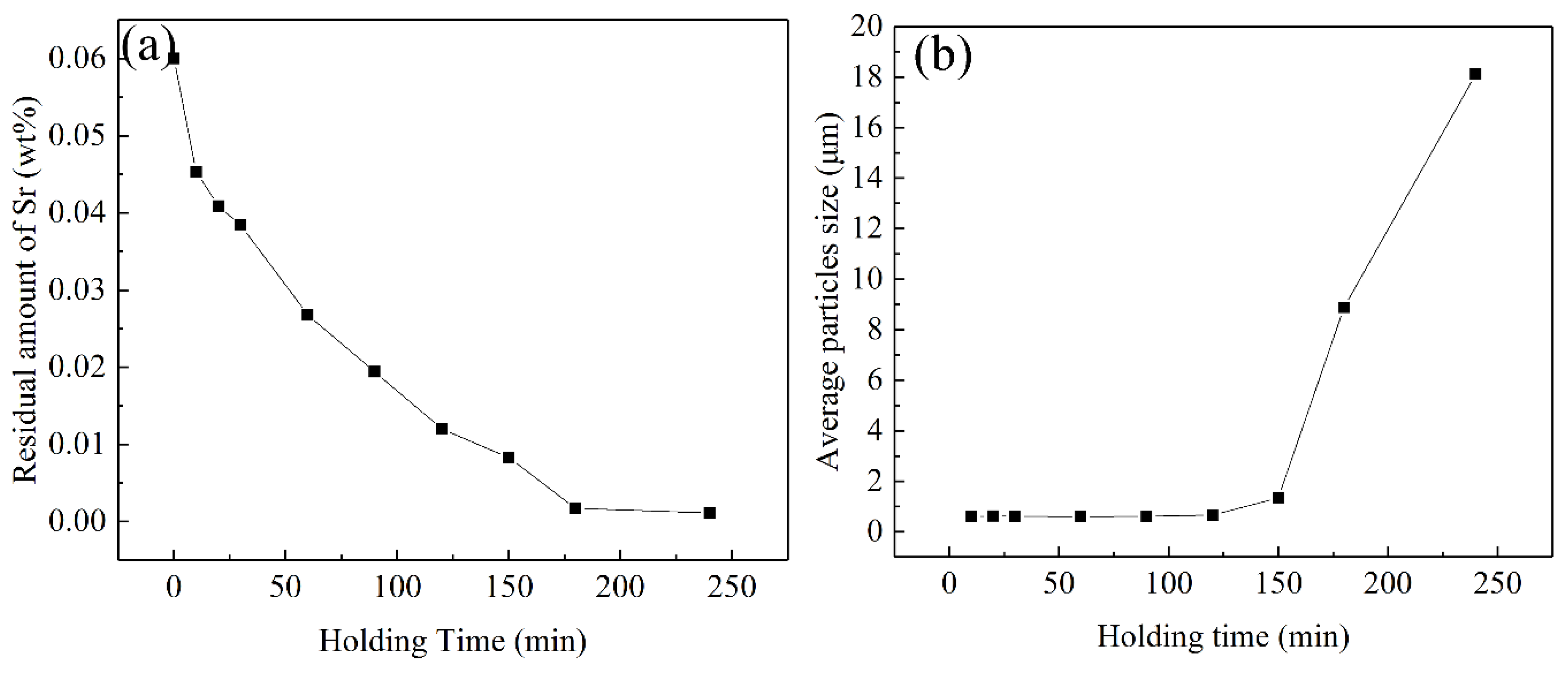
3.2. Influence of the Holding Time of Melt Modification Treatment on Modification Effect
3.3. Influence of Cooling Rate on Modification Effect
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Guo, F.X.; Wang, W.; Yu, W.H.; Zhang, Y.; Pan, S.P.; Zhou, Z.H.; Liu, D.; Qin, J.Y.; Wang, Y.; Tian, X.L. Enhanced nucleation and refinement of eutectic Si by high number-density nano-particles in Al–10Si–0.5Sb alloys. Mater. Des. 2017, 117, 382–389. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, W.; Li, M.; Qiao, H.; Wu, Y.; Qin, J.; Liu, X. The Effect of Mg Adding Order on the Liquid Structure and Solidified Microstructure of the Al-Si-Mg-P Alloy: An Experiment and ab Initio Study. Metals 2015, 5, 40–51. [Google Scholar] [CrossRef]
- Mazahery, A.; Shabani, M.O. Modification Mechanism and Microstructural Characteristics of Eutectic Si in Casting Al-Si Alloys: A Review on Experimental and Numerical Studies. JOM 2014, 66, 726–738. [Google Scholar] [CrossRef]
- Sigworth, G.; Campbell, J.; Jorstad, J. The Modification of Al-Si Casting Alloys: Important Practical and Theoretical Aspects. Int. J. Metal Cast. 2009, 3, 65–78. [Google Scholar] [CrossRef]
- Samuel, A.M.; Doty, H.W.; Valtierra, S.; Samuel, F.H. New Method of Eutectic Silicon Modification in Cast Al–Si Alloys. Int. J. Metal Cast. 2017, 11, 475–493. [Google Scholar] [CrossRef]
- Liu, T.; Morales, S.; Karkkainen, M.; Brewer, L.N.; Nastac, L.; Arvikar, V.; Levin, I. The combined effects of Sr additions and heat treatment on the microstructure and mechanical properties of high pressure die cast A383 alloy. Mater. Sci. Eng. A 2019, 756, 373–380. [Google Scholar] [CrossRef]
- Öztürk, İ.; Hapçı Ağaoğlu, G.; Erzi, E.; Dispinar, D.; Orhan, G. Effects of strontium addition on the microstructure and corrosion behavior of A356 aluminum alloy. J. Alloys Comp. 2018, 763, 384–391. [Google Scholar] [CrossRef]
- Uludağ, M.; Çetin, R.; Dispinar, D.; Tiryakioğlu, M. The effects of degassing, grain refinement & Sr-addition on melt quality-hot tear sensitivity relationships in cast A380 aluminum alloy. Eng. Fail. Anal. 2018, 90, 90–102. [Google Scholar]
- Dong, X.G.; Zhou, J.; Jia, Y.J.; Liu, B.; Lei, Z.Z.; Wang, W.H.; Bi, H. Influence of Sr on microstructure and mechanical properties of ZL114 cast alloy. China Foundry 2011, 8, 401–406. [Google Scholar]
- Liao, H.; Sun, G. Mutual poisoning effect between Sr and B in Al–Si casting alloys. Scr. Mater. 2003, 48, 1035–1039. [Google Scholar] [CrossRef]
- Hadad, S.E.; Samuel, A.M.; Samuel, F.H.; Doty, H.W.; Valtierra, S. Role of Bi and Ca Additions in Controlling the Microstructure of Sr-Modified 319 Alloys. Mater. Sci. Forum 2006, 519–521, 1257–1264. [Google Scholar] [CrossRef]
- Samuel, A.M.; Doty, H.W.; Valtierra, S.; Samuel, F. The Effect of Bi-Sr and Ca-Sr Interactions on the Microstructure and Tensile Properties of Al-Si-Based Alloys. Materials 2016, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Seifeddine, S. Determination of Optimum Sr Level for Eutectic Si Modification in Al–Si Cast Alloys Using Thermal analysis and Tensile Properties. Int. J. Metal Cast. 2016, 10, 457–465. [Google Scholar] [CrossRef]
- Cho, Y.H.; Lee, H.C.; Oh, K.H.; Dahle, A.K. Effect of Strontium and Phosphorus on Eutectic Al-Si Nucleation and Formation of β-Al5FeSi in Hypoeutectic Al-Si Foundry Alloys. Metall. Mater. Trans. A 2008, 39, 2435–2448. [Google Scholar] [CrossRef]
- Zarif, M.; Mckay, B.; Schumacher, P. Study of Heterogeneous Nucleation of Eutectic Si in High-Purity Al-Si Alloys with Sr Addition. Metall. Mater. Trans. A 2011, 42, 1684–1691. [Google Scholar] [CrossRef]
- Fortini, A.; Lattanzi, L.; Merlin, M.; Garagnani, G.L. Comprehensive Evaluation of Modification Level Assessment in Sr-Modified Aluminium Alloys. Int. J. Metal Cast. 2018, 12, 697–711. [Google Scholar] [CrossRef]
- Miresmaeili, S.M.; Campbell, J.; Shabestari, S.G.; Boutorabi, S.M.A. Precipitation of Sr-rich intermetallic particles and their influence on pore formation in Sr-modified A356 alloy. Metall. Mater. Trans. A 2005, 36, 2341–2349. [Google Scholar] [CrossRef]
- Miresmaeili, S.M.; Shabestari, S.G.; Boutorabi, S.M.A. The Effect of SR-Modification Treatment on Porosity Formation of Reduced Pressure 319 Al Alloy Castings. Can. Metall. Quart. 2014, 42, 245–251. [Google Scholar] [CrossRef]
- Malekan, M.; Dayani, D.; Mir, A. Thermal analysis study on the simultaneous grain refinement and modification of 380.3 aluminum alloy. J. Therm. Anal. Calorim. 2014, 115, 393–399. [Google Scholar]
- Shankar, S.; Riddle, Y.W.; Makhlouf, M.M. Eutectic solidification of aluminum-silicon alloys. Metall. Mater. Trans. A 2004, 35, 3038–3043. [Google Scholar] [CrossRef]
- Lan, T.; Li, J. Distributions of Sr and Fe and their influence on modification of hypoeutectic Al-Si alloy. China Foundry 2016, 13, 85–92. [Google Scholar] [CrossRef][Green Version]
- Vandersluis, E.; Sediako, D.; Ravindran, C.; Elsayed, A.; Byczynski, G. Analysis of eutectic silicon modification during solidification of Al-6Si using in-situ neutron diffraction. J. Alloys Comp. 2018, 736, 172–180. [Google Scholar] [CrossRef]
- Hu, H. Fundamentals of Solidification of Metals; China Machine Press: Beijing, China, 2000. [Google Scholar]
- Eguskiza, S.; Niklas, A.; Fernandez-Calvo, A.I.; Santos, F.; Djurdjevic, M. Study of Strontium Fading in Al-Si-Mg and Al-Si-Mg-Cu Alloy by Thermal Analysis. Int. J. Metal Cast. 2015, 9, 43–50. [Google Scholar] [CrossRef]
- Uuuda˘g, M.; Çetin, R.; Dispinar, D.; Tiryakio˘glu, M. Characterization of the Effect of Melt Treatments on Melt Quality in Al-7wt %Si-Mg Alloys. Metals 2017, 7, 157. [Google Scholar]
- Sun, S.; Yuan, B.; Liu, M. Effects of moulding sands and wall thickness on microstructure and mechanical properties of Sr-modified A356 aluminum casting alloy. Trans. Nonferrous Metals Soc. China 2012, 22, 1884–1890. [Google Scholar] [CrossRef]



| Alloy | Si | Mg | Fe | V | Al |
|---|---|---|---|---|---|
| A356 | 7.580 | 0.341 | 0.101 | 0.032 | Balance |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Ma, S.; Wei, Z.; Bai, P. The Relationship between Residual Amount of Sr and Morphology of Eutectic Si Phase in A356 Alloy. Materials 2019, 12, 3222. https://doi.org/10.3390/ma12193222
Zhang W, Ma S, Wei Z, Bai P. The Relationship between Residual Amount of Sr and Morphology of Eutectic Si Phase in A356 Alloy. Materials. 2019; 12(19):3222. https://doi.org/10.3390/ma12193222
Chicago/Turabian StyleZhang, Wenda, Shixuan Ma, Zhenhua Wei, and Peikang Bai. 2019. "The Relationship between Residual Amount of Sr and Morphology of Eutectic Si Phase in A356 Alloy" Materials 12, no. 19: 3222. https://doi.org/10.3390/ma12193222
APA StyleZhang, W., Ma, S., Wei, Z., & Bai, P. (2019). The Relationship between Residual Amount of Sr and Morphology of Eutectic Si Phase in A356 Alloy. Materials, 12(19), 3222. https://doi.org/10.3390/ma12193222





