Mechanism Understanding of the Role of Rare Earth Inclusions in the Initial Marine Corrosion Process of Microalloyed Steels
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials Preparation
2.2. Corrosion Rate Measurements
2.2.1. Potentiodynamic Polarization Tests
2.2.2. Weight Loss Experiments
2.3. Microstructure Characterization
2.4. First Principle Modeling
2.5. SVET Measurements
3. Results and Discussion
3.1. Corrosion Rate
3.2. Effect of RE Inclusions on Corrosion Nucleation Process
3.2.1. Statistical Identification of Inclusions
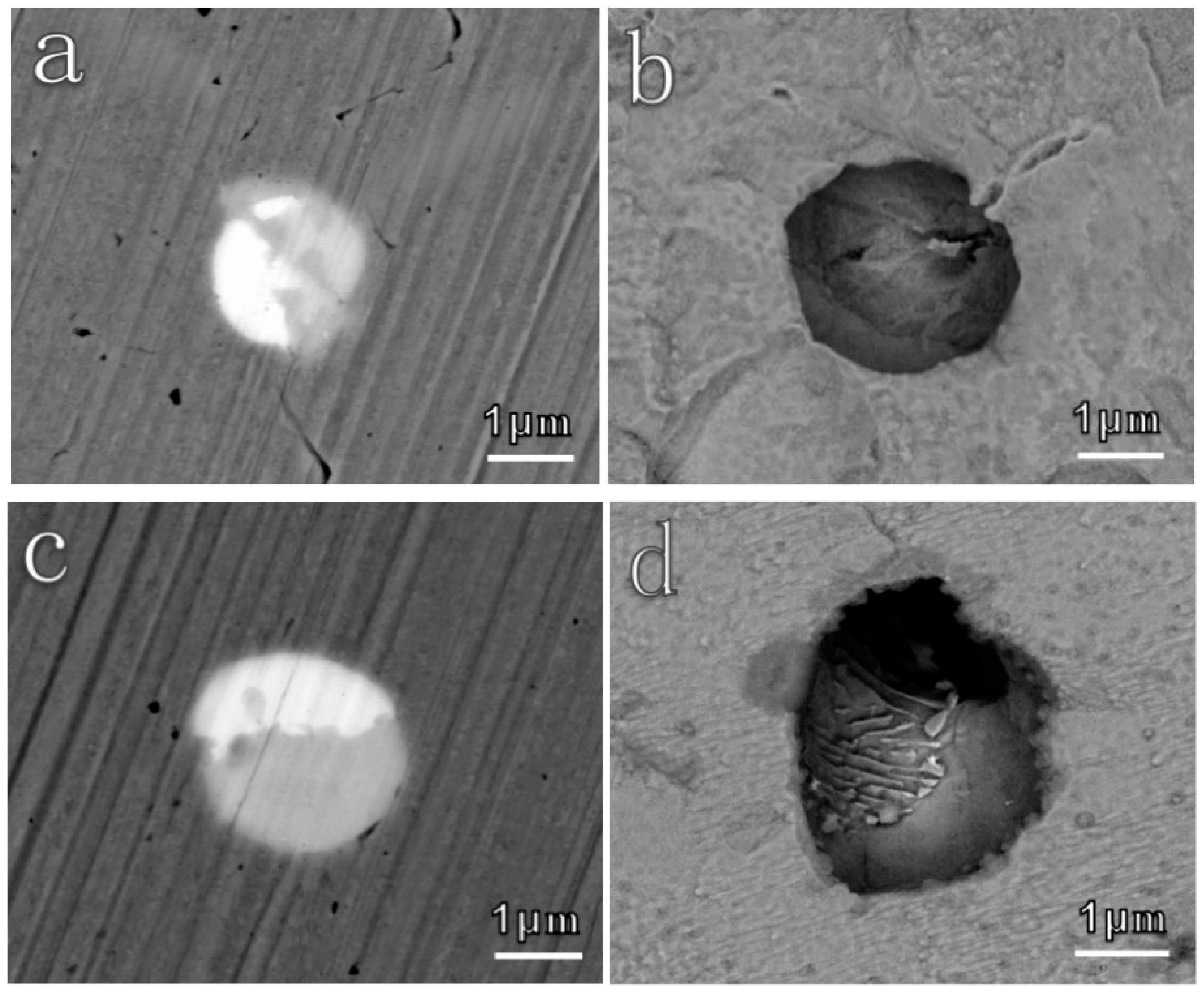
3.2.2. Corrosion Behavior of Inclusions
3.2.3. Theoretical Explanation for the Corrosion Behavior of Inclusions
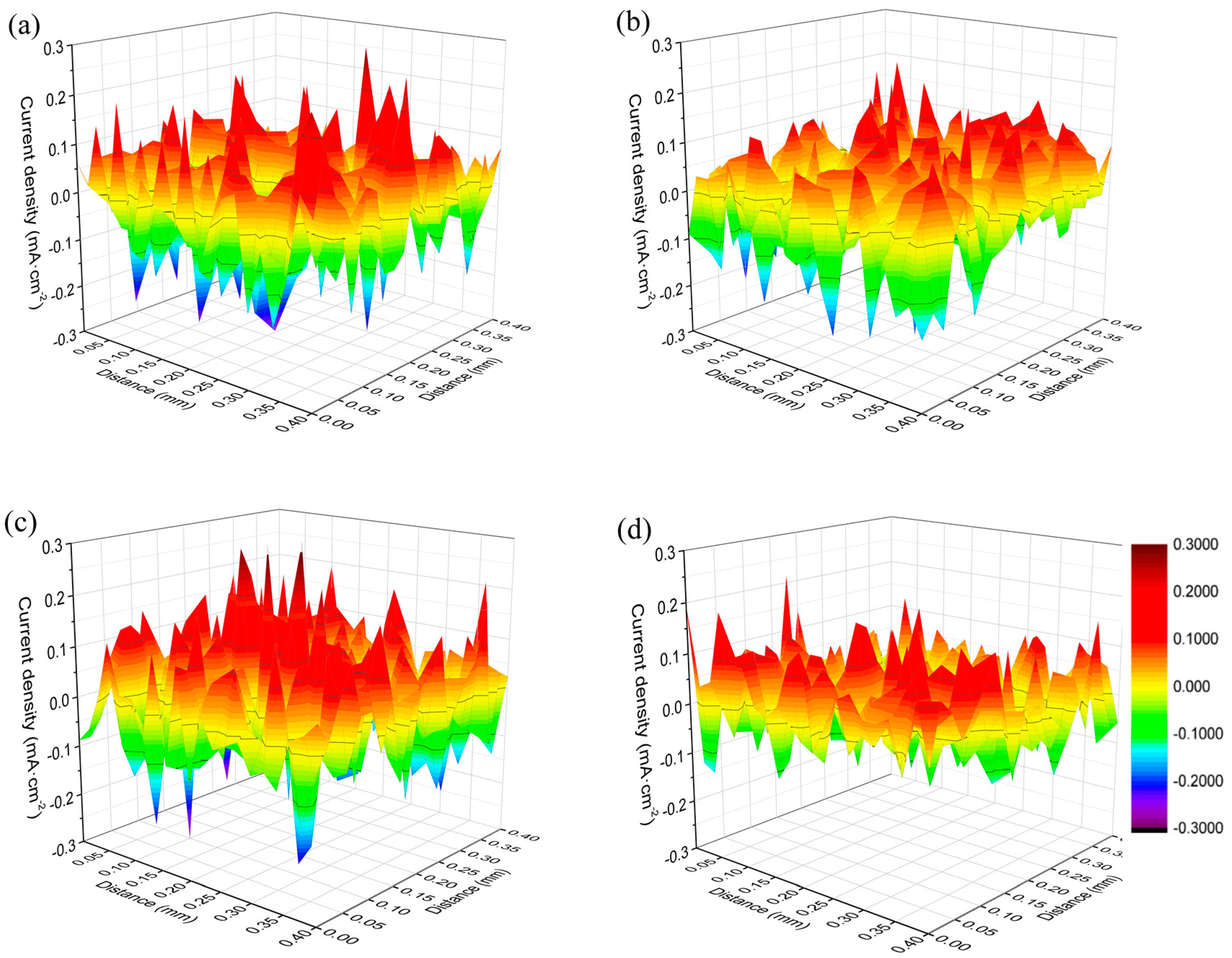
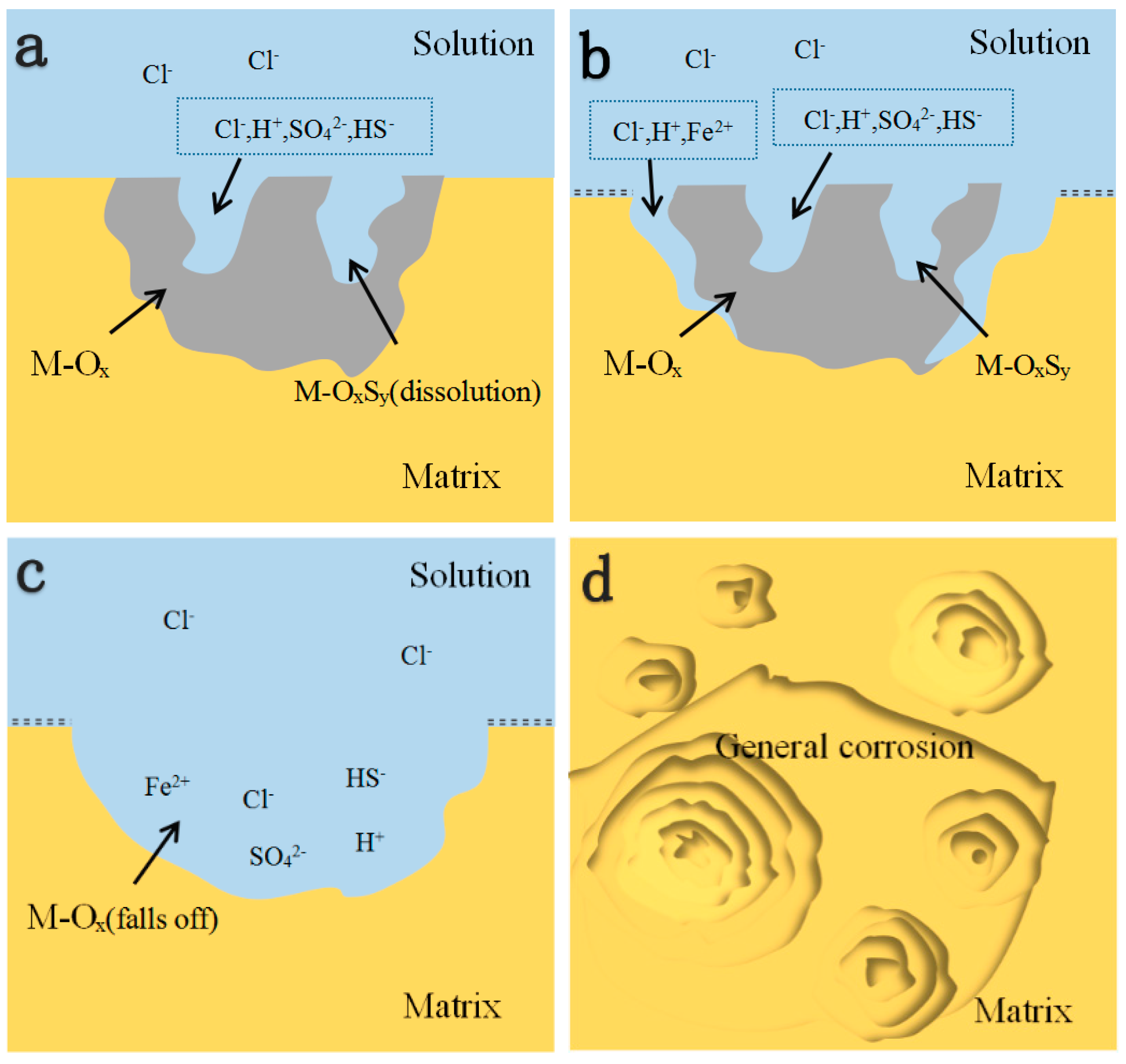
3.3. Effect of RE Inclusions on the Corrosion Propagation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Z.; Li, X.; Cheng, Y. In-situ characterization of the electrochemistry of grain and grain boundary of an X70 steel in a near-neutral pH solution. Electrochem. Commun. 2010, 12, 936–938. [Google Scholar] [CrossRef]
- Kong, D.C.; Dong, C.F.; Ni, X.Q.; Zhang, L.; Yao, J.Z.; Man, C.; Cheng, X.Q.; Xiao, K.; Li, X.G. Mechanical properties and corrosion behavior of selective lasermelted 316L stainless steel after different heat treatment processes. J. Mater. Sci. Tech. 2019, 35, 1499–1507. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, Y.; Zhang, B.; Zhu, Y.; Liu, C.; Hu, P.; Ma, X. Identification of MnCr2O4 nano-octahedron in catalysing pitting corrosion of austenitic stainless steels. Acta Mater. 2010, 58, 5070–5085. [Google Scholar] [CrossRef]
- Zheng, S.; Li, C.; Qi, Y.; Chen, L.; Chen, C. Mechanism of (Mg,Al,Ca)-oxide inclusion-induced pitting corrosion in 316L stainless steel exposed to sulphur environments containing chloride ion. Corros. Sci. 2013, 67, 20–31. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Meng, G.; Shao, Y.; Wang, F. Effect of pitting nucleation on critical pitting temperature of 316L stainless steel by nitric acid passivation. Corros. Sci. 2015, 91, 232–244. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Li, H.; Zhao, Y.; Wang, F.; Zhang, X.; Cheng, L.; Wu, K. Modeling of the Critical Pitting Temperature between the Laboratory-Scale Specimen and the Large-Scale Specimen. J. Electrochem. Soc. 2018, 165, C328–C333. [Google Scholar] [CrossRef] [Green Version]
- Ha, H.Y.; Park, C.J.; Kwon, H.S. Effects of non-metallic inclusions on the initiation of pitting corrosion in 11% Cr ferritic stainless steel examined by micro-droplet cell. Corros. Sci. 2007, 49, 1266–1275. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, G.; Wu, W.; Li, Y. Role of inclusions in the pitting initiation of pipeline steel and the effect of electron irradiation in SEM. Corros. Sci. 2018, 130, 252–260. [Google Scholar] [CrossRef]
- Avci, R.; Davis, B.; Wolfenden, M.; Beech, I.; Lucas, K.; Paul, D. Mechanism of MnS-mediated pit initiation and propagation in carbon steel in an anaerobic sulfidogenic media. Corros. Sci. 2013, 76, 267–274. [Google Scholar] [CrossRef]
- Liu, C.; Revilla, R.I.; Zhang, D.; Liu, Z.; Lutz, A.; Zhang, F.; Zhao, T.; Ma, H.; Li, X.; Terryn, H. Role of Al2O3 inclusions on the localized corrosion of Q460NH weathering steel in marine environment. Corros. Sci. 2018, 138, 96–104. [Google Scholar] [CrossRef]
- Wranglen, G. Pitting and sulphide inclusions in steel. Corros. Sci. 1974, 14, 331–349. [Google Scholar] [CrossRef]
- Stewart, J.; Williams, D. The initiation of pitting corrosion on austenitic stainless steel: On the role and importance of sulphide inclusions. Corros. Sci. 1992, 33, 457–474. [Google Scholar] [CrossRef]
- Suter, T.; Böhni, H. A new microelectrochemical method to study pit initiation on stainless steels. Electrochim. Acta 1997, 42, 3275–3280. [Google Scholar] [CrossRef]
- Wang, L.; Lin, Q.; Ji, J.; Lan, D. New study concerning development of application of rare earth metals in steels. J. Alloy. Compd. 2006, 408, 384–386. [Google Scholar] [CrossRef]
- Adabavazeh, Z.; Hwang, W.S.; Su, Y.H. Effect of adding cerium on microstructure and morphology of Ce-based inclusions formed in low-carbon steel. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Wang, L.M.; Lin, Q.; Yue, L.J.; Liu, L.; Guo, F.; Wang, F.M. Study of application of rare earth elements in advanced low alloy steels. J. Alloy. Compd. 2008, 451, 534–537. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Wang, J.F.; Zhou, S.Z.; Wang, X.D. Effects of rare earth addition on microstructure and mechanical properties of a Fe–15Mn–1.5Al–0.6C TWIP steel. Mater. Sci. Eng. A 2014, 608, 106–113. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.C.; Yang, L.; Gao, X.Z. Effect of Ce on inclusions and impact property of 2Cr13 stainless steel. J. Iron Steel Res. Int. 2010, 17, 59–64. [Google Scholar] [CrossRef]
- Jeon, S.H.; Hur, D.H.; Kim, H.J.; Park, Y.S. Effect of Ce addition on the precipitation of deleterious phases and the associated intergranular corrosion resistance of 27Cr–7Ni hyper duplex stainless steels. Corros. Sci. 2015, 90, 313–322. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Qiu, S.; Xiang, L. Effect of Ce on the Evolution of Recrystallization Texture in a 1.2%Si-0.4%Al Non-oriented Electrical Steel. ISIJ Int. 2016, 56, 1256–1261. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.T.; Jeon, S.H.; Lee, I.S.; Park, Y.S. Effects of rare earth metals addition on the resistance to pitting corrosion of super duplex stainless steel—Part 1. Corros. Sci. 2010, 52, 1897–1904. [Google Scholar] [CrossRef]
- Yang, J.; Yu, H.; Wang, X. Effect of the Ce on behavior of corrosion resistance and mechanical properties of A36 plate steel for shipbuilding. In Recent Advances in Structural Integrity Analysis of the International Congress (APCF/SIF-2014); Elsevier BV: Baotou, China, 2014; pp. 380–385. [Google Scholar]
- Jeon, S.H.; Kim, S.T.; Lee, I.S.; Park, Y.S. Effects of sulfur addition on pitting corrosion and machinability behavior of super duplex stainless steel containing rare earth metals: Part 2. Corros. Sci. 2010, 52, 3537–3547. [Google Scholar] [CrossRef]
- Yang, C.Y.; Luan, Y.K.; Li, D.Z.; Li, Y.Y. Effects of rare earth elements on inclusions and impact toughness of high-carbon chromium bearing steel. J. Mater. Sci. Tech. 2019, 35, 1298–1308. [Google Scholar] [CrossRef]
- Yue, L.; Wang, L.; Han, J. Effects of rare earth on inclusions and corrosion resistance of 10PCuRE weathering steel. J. Rare Earths 2010, 28, 952–956. [Google Scholar] [CrossRef]
- Beranoagirre, A.; Urbikain, G.; Marticorena, R.; Bustillo, A.; De Lacalle, L.L. Sensitivity Analysis of Tool Wear in Drilling of Titanium Aluminides. Metals 2019, 9, 297. [Google Scholar] [CrossRef]
- Yoo, Y.H.; Choi, Y.S.; Kim, J.G.; Park, Y.S. Effects of Ce, La and Ba addition on the electrochemical behavior of super duplex stainless steels. Corros. Sci. 2010, 52, 1123–1129. [Google Scholar] [CrossRef]
- Li, T.; Qin, M. Effect of rare earth on the characterization of corrosion of low carbon steel in CSP. In Recent Advances in Structural Integrity Analysis of the International Congress (APCF/SIF-2014); Elsevier BV: Baotou, China, 2014; pp. 375–379. [Google Scholar]
- Rodríguez, J.S.; Hernández, F.S.; González, J.G. XRD and SEM studies of the layer of corrosion products for carbon steel in various different environments in the province of Las Palmas (The Canary Islands, Spain). Corros. Sci. 2002, 44, 2425–2438. [Google Scholar] [CrossRef]
- Yang, H.; Yang, K.; Zhang, B. Pitting corrosion resistance of La added 316L stainless steel in simulated body fluids. Mater. Lett. 2007, 61, 1154–1157. [Google Scholar] [CrossRef]
- Opiela, M.; Grajcar, A. Modification of Non-Metallic Inclusions by Rare-Earth Elements in Microalloyed Steels. Arch. Foundry Eng. 2012, 12, 129–134. [Google Scholar] [CrossRef]
- Zhu, T.; Huang, F.; Liu, J.; Hu, Q.; Li, W. Effects of inclusion on corrosion resistance of weathering steel in simulated industrial atmosphere. Anti-Corros. Methods Mater. 2016, 63, 490–498. [Google Scholar] [CrossRef]
- Ma, Q.; Wu, C.; Cheng, G.; Li, F. Characteristic and Formation Mechanism of Inclusions in 2205 Duplex Stainless Steel Containing Rare Earth Elements. Mater. Today: Proc. 2015, 2, S300–S305. [Google Scholar] [CrossRef]
- Wilson, W.G.; Kay, D.A.R.; Vahed, A. The use of thermodynamics and phase equilibria to predict the behavior of the rare earth elements in steel. JOM 1974, 26, 14–23. [Google Scholar] [CrossRef]
- Jeon, S.H.; Kim, S.T.; Choi, M.S.; Kim, J.S.; Kim, K.T.; Park, Y.S. Effects of cerium on the compositional variations in and around inclusions and the initiation and propagation of pitting corrosion in hyperduplex stainless steels. Corros. Sci. 2013, 75, 367–375. [Google Scholar] [CrossRef]
- Ha, H.; Park, C.; Kwon, H. Effects of misch metal on the formation of non-metallic inclusions and the associated resistance to pitting corrosion in 25% Cr duplex stainless steels. Scr. Mater. 2006, 55, 991–994. [Google Scholar] [CrossRef]
- Liu, C.; Revilla, R.I.; Liu, Z.; Zhang, D.; Li, X.; Terryn, H. Effect of inclusions modified by rare earth elements (Ce, La) on localized marine corrosion in Q460NH weathering steel. Corros. Sci. 2017, 129, 82–90. [Google Scholar] [CrossRef]
- Fruehan, R.J. The free energy of formation of Ce2O2S and the nonstoichiometry of cerium oxides. Met. Mater. Trans. A 1979, 10, 143–148. [Google Scholar] [CrossRef]
- Gao, J.; Fu, P.; Liu, H.; Li, D. Effects of Rare Earth on the Microstructure and Impact Toughness of H13 Steel. Metals 2015, 5, 383–394. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, L.; Chou, K. Effect of cerium on the cleanliness of spring steel used in fastener of high-speed railway. J. Rare Earths 2014, 32, 759–766. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, W.; Cheng, L.; Liu, J.; Wu, K.; Liu, M. Effects of niobium and rare earth elements on microstructure and initial marine corrosion behavior of low-alloy steels. Appl. Surf. Sci. 2019, 475, 83–93. [Google Scholar] [CrossRef]
- Waudby, P.E. Rare earth additions to steel. Int. Met. Rev. 1978, 2, 74–98. [Google Scholar]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- BlÖchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreto, J.; Marino, C.; Filho, W.B.; Rocha, L.; Fernandes, J.; Fernandes, J.C.S. SVET, SKP and EIS study of the corrosion behaviour of high strength Al and Al–Li alloys used in aircraft fabrication. Corros. Sci. 2014, 84, 30–41. [Google Scholar] [CrossRef]
- Tedim, J.; Bastos, A.; Kallip, S.; Zheludkevich, M.; Ferreira, M.; Zheludkevich, M. Corrosion protection of AA2024-T3 by LDH conversion films. Analysis of SVET results. Electrochim. Acta 2016, 210, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Marques, A.; Simões, A. EIS and SVET assessment of corrosion resistance of thin Zn-55% Al-rich primers: Effect of immersion and of controlled deformation. Electrochim. Acta 2014, 148, 153–163. [Google Scholar] [CrossRef]
- Marques, A.G.; Simões, A.M. Influence of the rare-earth elements on the morphology of non-metallic inclusions in microalloyed steels. J. Achiev. Mater. Manuf. Eng. 2011, 47, 149–156. [Google Scholar]
- Ånmark, N.; Karasev, A.; Jönsson, P.G. The Effect of Different Non-Metallic Inclusions on the Machinability of Steels. Materials 2015, 8, 751–783. [Google Scholar] [CrossRef]
- Bin, W.; Bo, S. In Situ Observation of the Evolution of Intragranular Acicular Ferrite at Ce-Containing Inclusions in 16Mn Steel. Steel Res. Int. 2012, 83, 487–495. [Google Scholar] [CrossRef]
- Wilson, W.; Wells, R. Identifying inclusions in rare earth treated steels. Met. Prog. 1973, 107, 75–77. [Google Scholar]
- Wilson, W.G.; Heaslip, L.J.; Sommerville, I.D. Rare Earth Additions in Continuously Cast Steel. JOM 1985, 37, 36–41. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, S.; Zhao, M.; Zhu, L.; Li, J. Pitting Corrosion of Steel Induced by Al2O3 Inclusions. Metals 2017, 7, 347. [Google Scholar] [CrossRef]
- Ma, H.; Chen, X.Q.; Li, R.; Wang, S.; Dong, J.; Ke, W. First-principles modeling of anisotropic anodic dissolution of metals and alloys in corrosive environments. Acta Mater. 2017, 130, 137–146. [Google Scholar] [CrossRef]


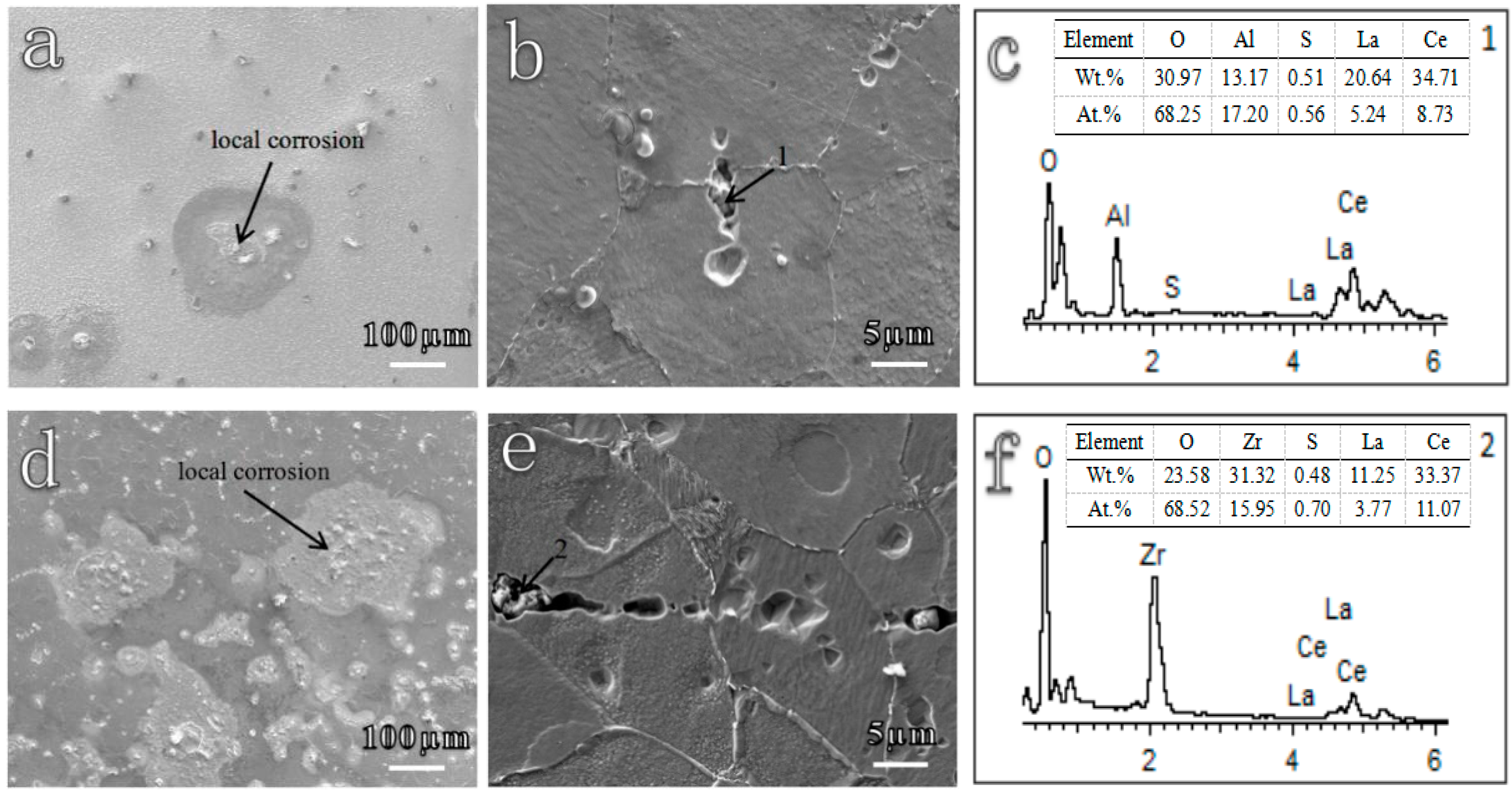
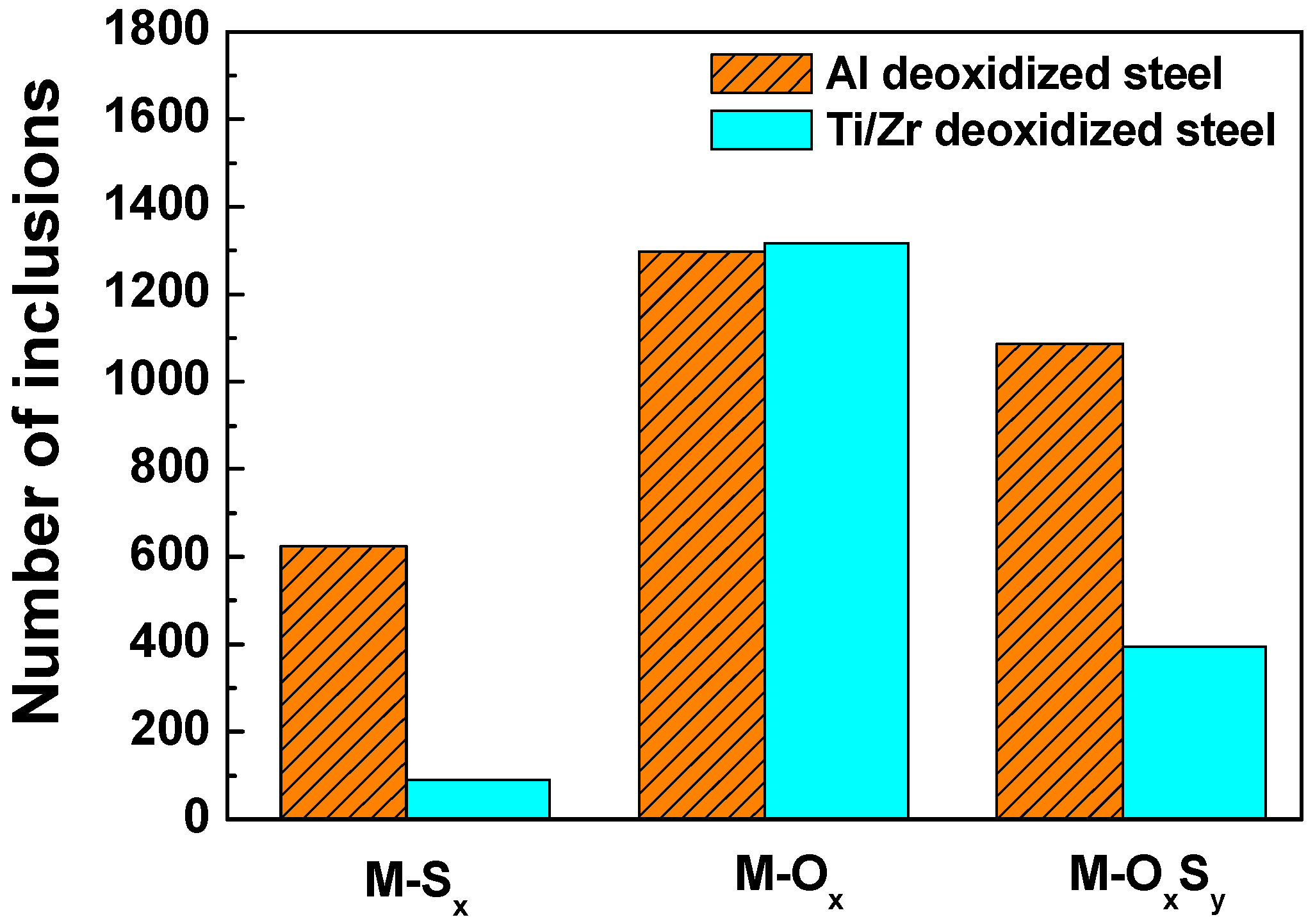


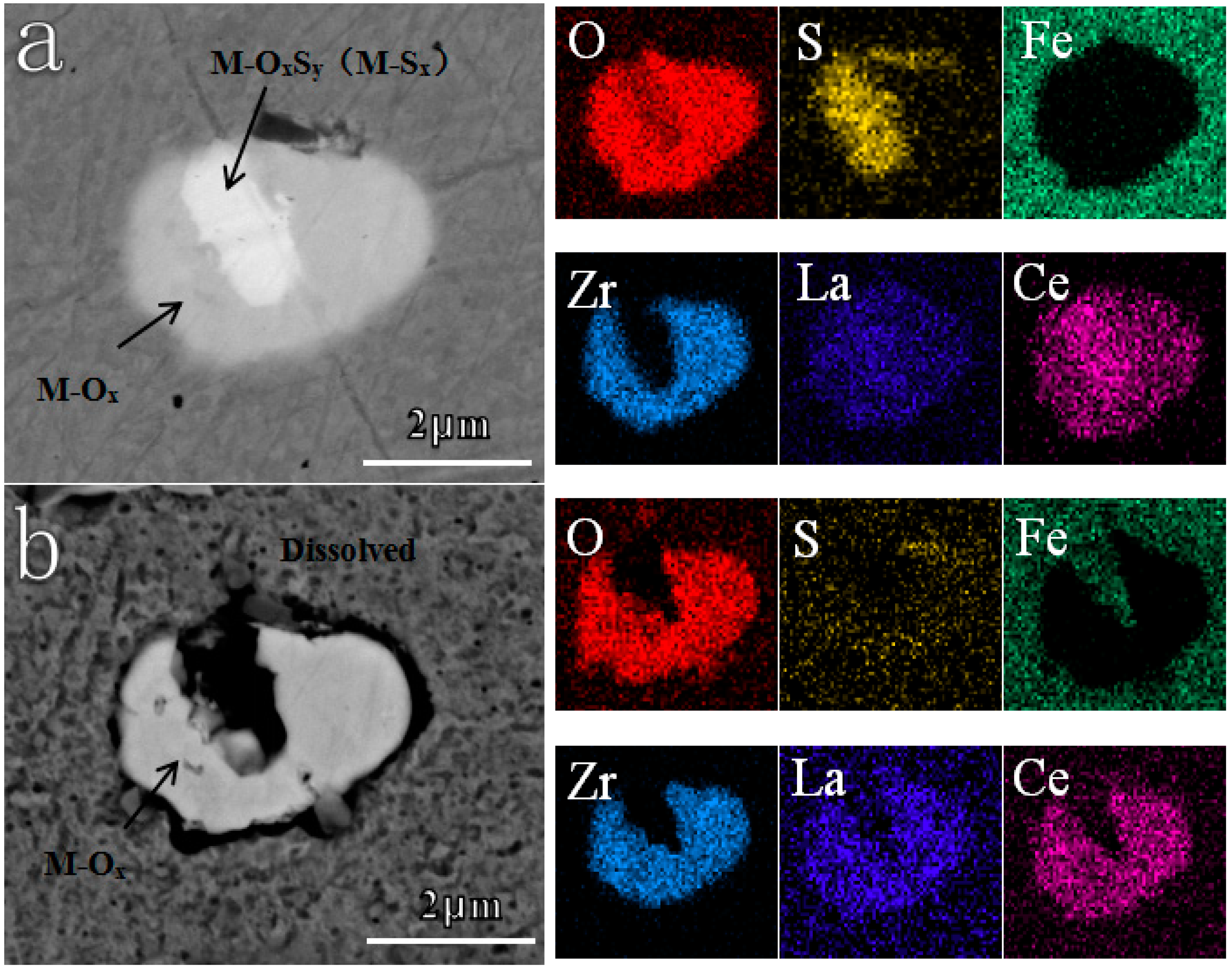
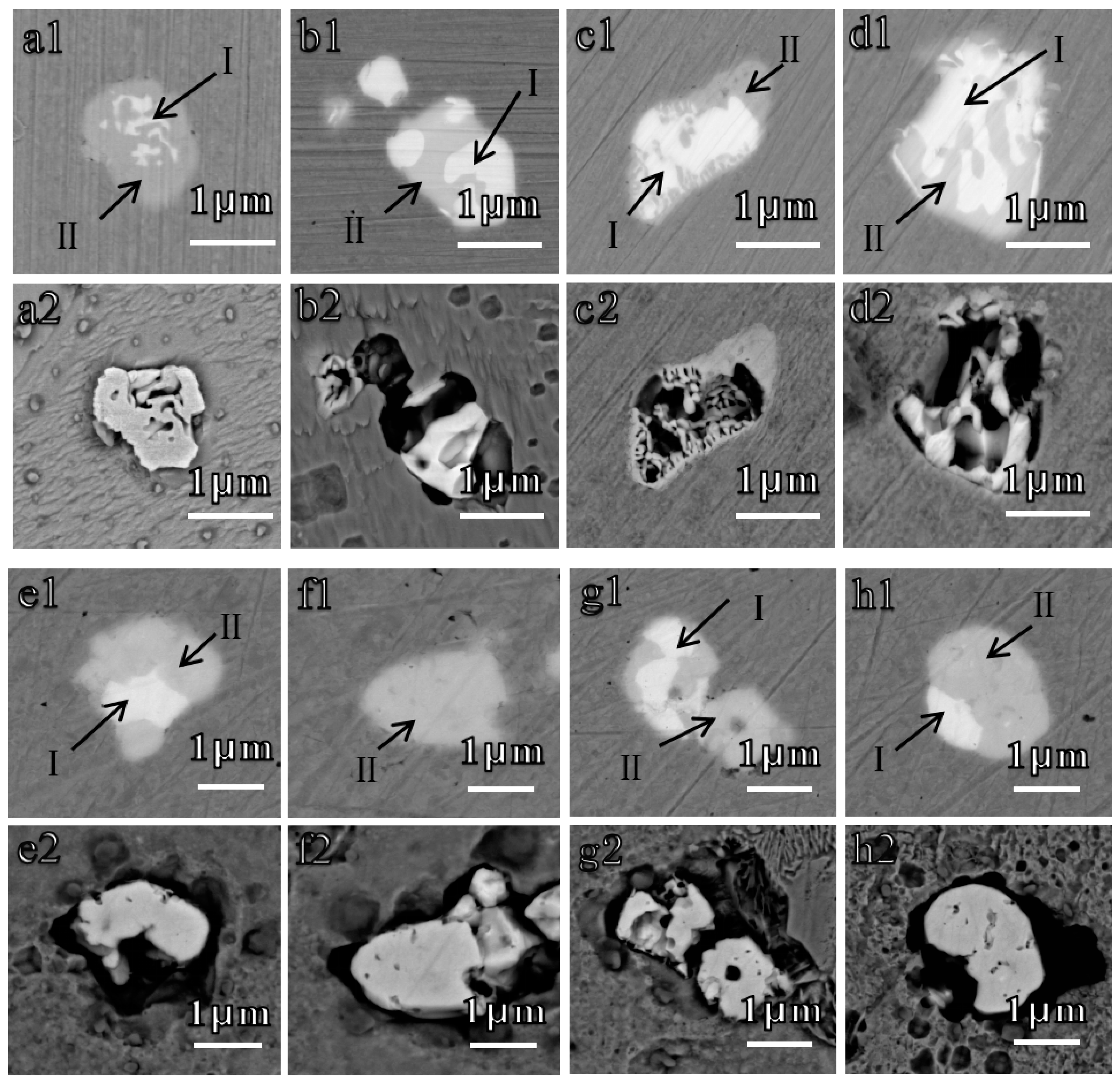

| Sample | C | Si | Mn | P | S | O | Cu | Cr | Ni | Al | Ti | Zr | La | Ce | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al deoxidized | 0.053 | 0.23 | 0.13 | 0.005 | 0.003 | 0.050 | 0.39 | 1.21 | 0.31 | 0.03 | - | - | 0.002 | 0.003 | balance |
| Ti/Zr deoxidized | 0.050 | 0.22 | 0.13 | 0.005 | 0.003 | 0.065 | 0.43 | 1.22 | 0.33 | - | 0.008 | 0.008 | 0.002 | 0.003 |
| Sample | icorr (×10−5 A·cm−2) | Ecorr (V) | ||
|---|---|---|---|---|
| Mean | Deviation | Mean | Deviation | |
| Normal steel without RE | 5.31 | ±0.46 | −0.122 | ±0.013 |
| Al deoxidized RE steel | 4.60 | ±0.33 | −0.137 | ±0.010 |
| Ti/Zr deoxidized RE steel | 2.93 | ±0.60 | −0.237 | ±0.023 |
| Sample | Types | Density (mm−2) | Average Size (µm2) | ||
|---|---|---|---|---|---|
| Individual | Overall | Individual | Overall | ||
| Al deoxidized | RE-Sx | 20.81 | 100.29 | 3.36 | 4.02 |
| RE-Ox | 14.95 | 3.81 | |||
| (Al,RE)-Ox | 28.31 | 4.15 | |||
| RE-OxSy | 18.34 | 3.98 | |||
| (Al,RE)-OxSy | 17.88 | 4.78 | |||
| Ti/Zr deoxidized | RE-Sx | 2.97 | 60.04 | 5.85 | 6.11 |
| RE-Ox | 3.52 | 5.13 | |||
| (Zr,RE)-Ox | 40.38 | 5.83 | |||
| RE-OxSy | 3.75 | 6.17 | |||
| (Zr,RE)-OxSy | 9.42 | 7.57 | |||
| Crystallographic Plane | La2S3 | La2O3 | LaAlO3 | La2Zr2O7 | La2O2S | Fe |
|---|---|---|---|---|---|---|
| (100) | 5.20 | 7.32 | 7.06 | 6.70 | 6.14 | 5.64 |
| (110) | 5.20 | 7.87 | 7.37 | 6.07 | 5.55 | 6.79 |
| (111) | 5.48 | 5.77 | 5.96 | 6.06 | 5.59 | 4.02 |
| Crystallographic Plane | La2S3 | La2O3 | LaAlO3 | La2Zr2O7 | La2O2S | Fe |
|---|---|---|---|---|---|---|
| (100) | 2.07 | 2.46 | 2.55 | 0.12 | −0.21 | 1.10 |
| (110) | 1.50 | 2.91 | 1.82 | 0.38 | 1.80 | 1.85 |
| (111) | 1.27 | 2.17 | 2.23 | −0.63 | 1.87 | −0.30 |
| Crystallographic Plane | La2S3 | La2O3 | LaAlO3 | La2Zr2O7 | La2O2S | Fe |
|---|---|---|---|---|---|---|
| (100) | 3.13 | 4.86 | 4.51 | 6.58 | 6.35 | 4.54 |
| (110) | 3.70 | 4.96 | 5.32 | 5.69 | 3.75 | 5.12 |
| (111) | 4.21 | 3.60 | 3.73 | 6.70 | 3.72 | 4.32 |
| Average | 3.68 | 4.47 | 4.52 | 6.32 | 4.61 | 4.66 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, M.; Wu, K.; Liu, J.; Cheng, L.; Zhang, X.; Chen, Y. Mechanism Understanding of the Role of Rare Earth Inclusions in the Initial Marine Corrosion Process of Microalloyed Steels. Materials 2019, 12, 3359. https://doi.org/10.3390/ma12203359
Tang M, Wu K, Liu J, Cheng L, Zhang X, Chen Y. Mechanism Understanding of the Role of Rare Earth Inclusions in the Initial Marine Corrosion Process of Microalloyed Steels. Materials. 2019; 12(20):3359. https://doi.org/10.3390/ma12203359
Chicago/Turabian StyleTang, Meng, Kaiming Wu, Jing Liu, Lin Cheng, Xian Zhang, and Yan Chen. 2019. "Mechanism Understanding of the Role of Rare Earth Inclusions in the Initial Marine Corrosion Process of Microalloyed Steels" Materials 12, no. 20: 3359. https://doi.org/10.3390/ma12203359
APA StyleTang, M., Wu, K., Liu, J., Cheng, L., Zhang, X., & Chen, Y. (2019). Mechanism Understanding of the Role of Rare Earth Inclusions in the Initial Marine Corrosion Process of Microalloyed Steels. Materials, 12(20), 3359. https://doi.org/10.3390/ma12203359




