Study on the Fabrication of Super-Hydrophobic Surface on Inconel Alloy via Nanosecond Laser Ablation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Laser Processing
2.3. Measurement and Characterization
3. Results and Discussion
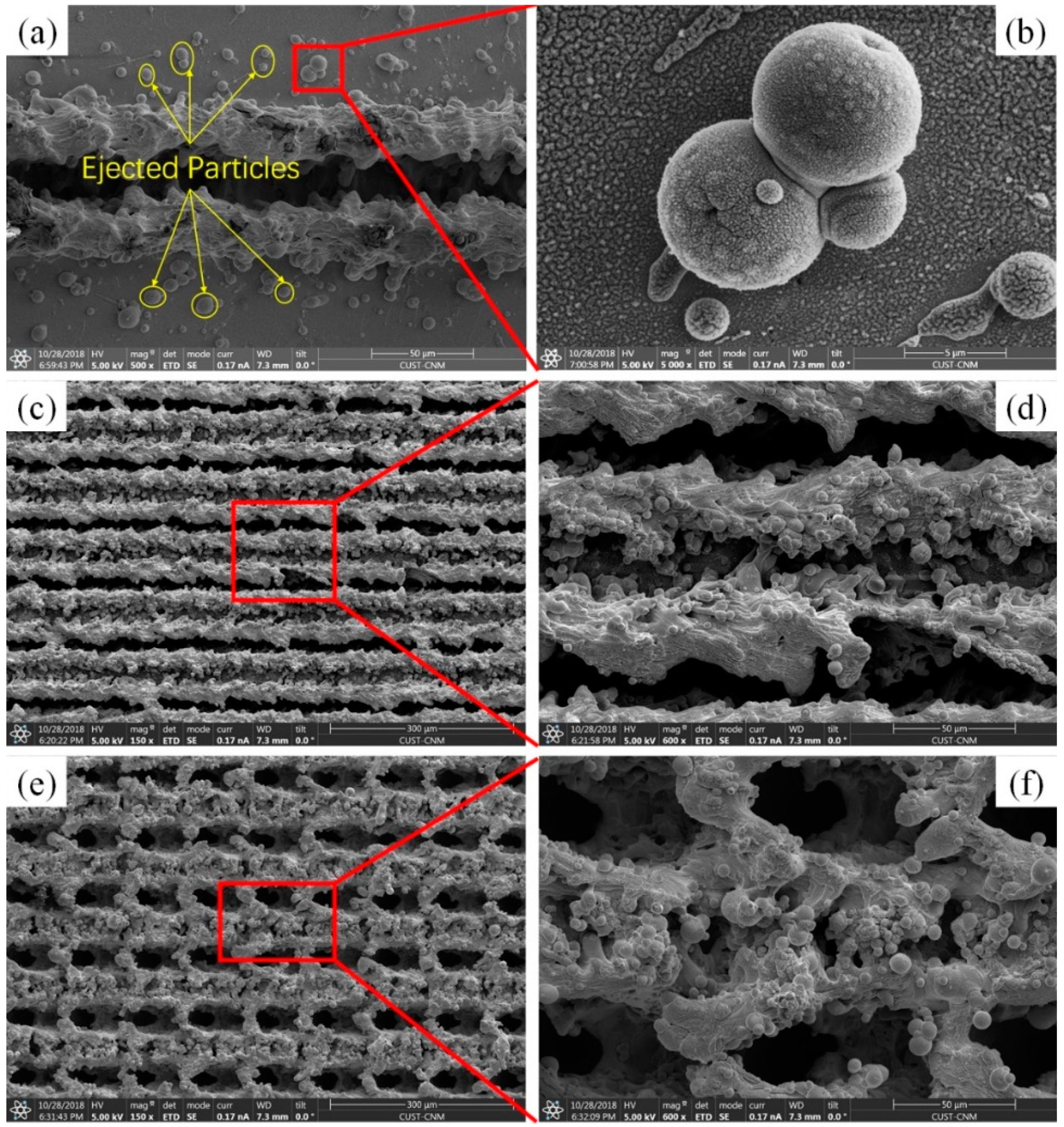
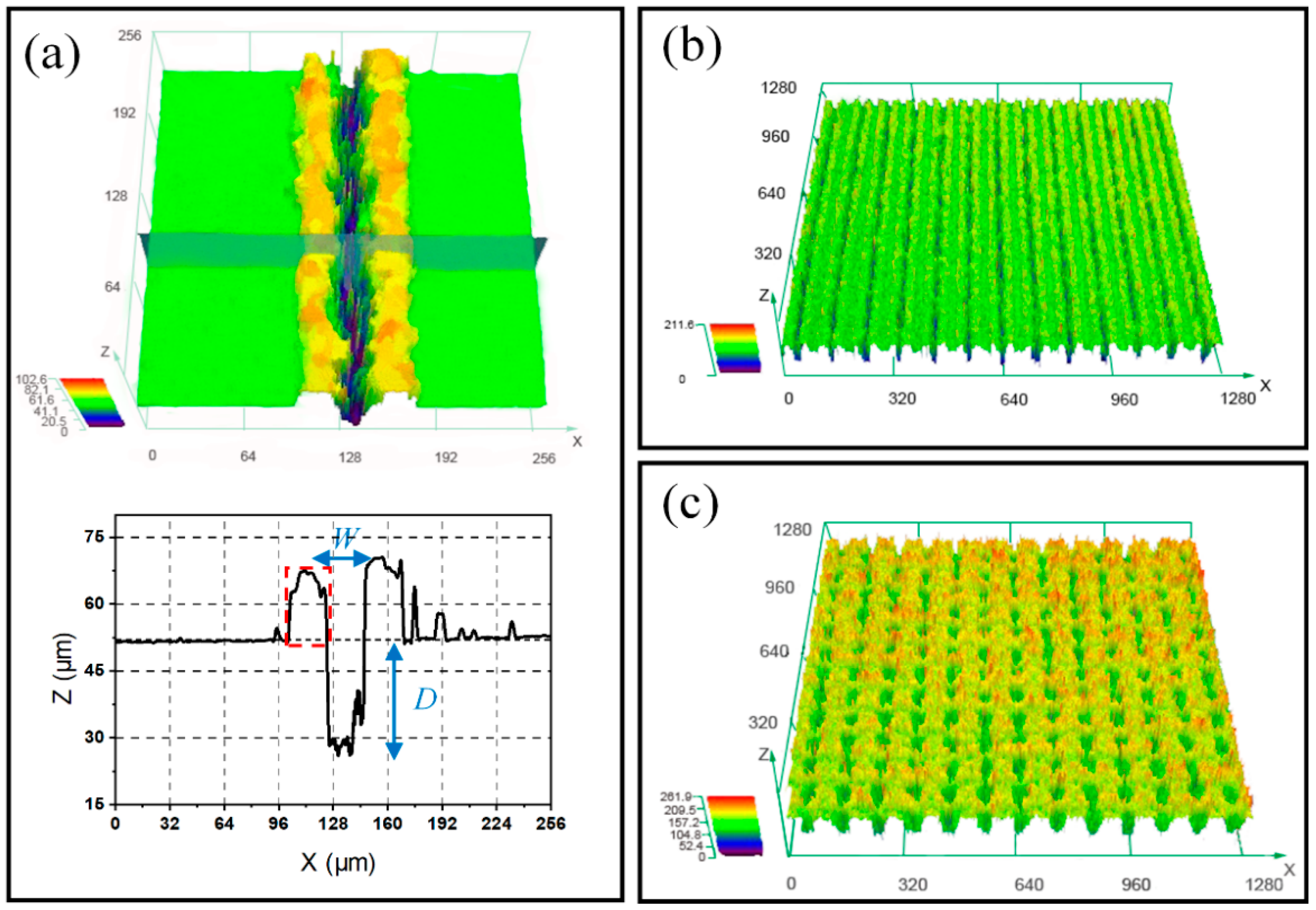
3.1. Surface Morphology
3.2. Wettability
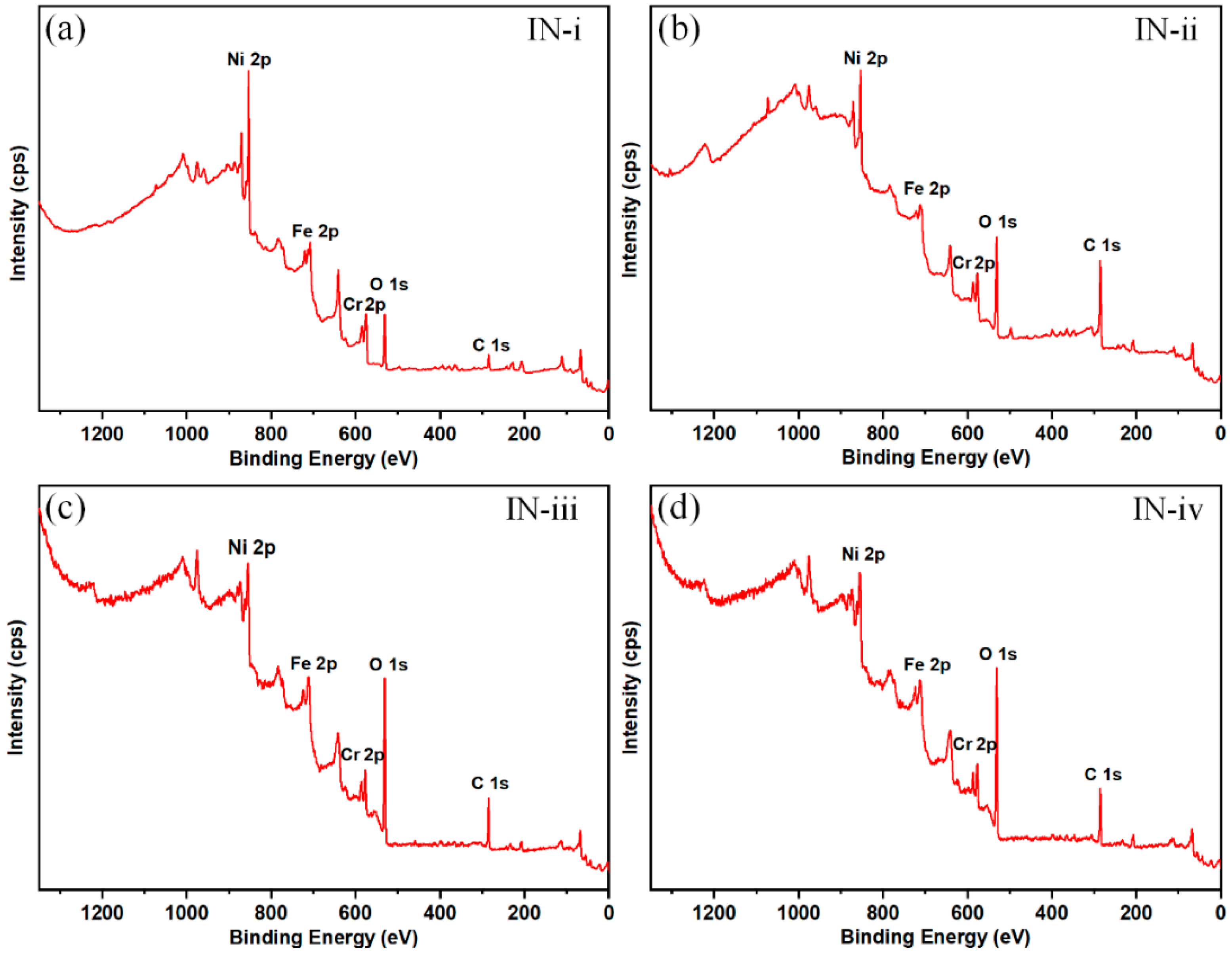
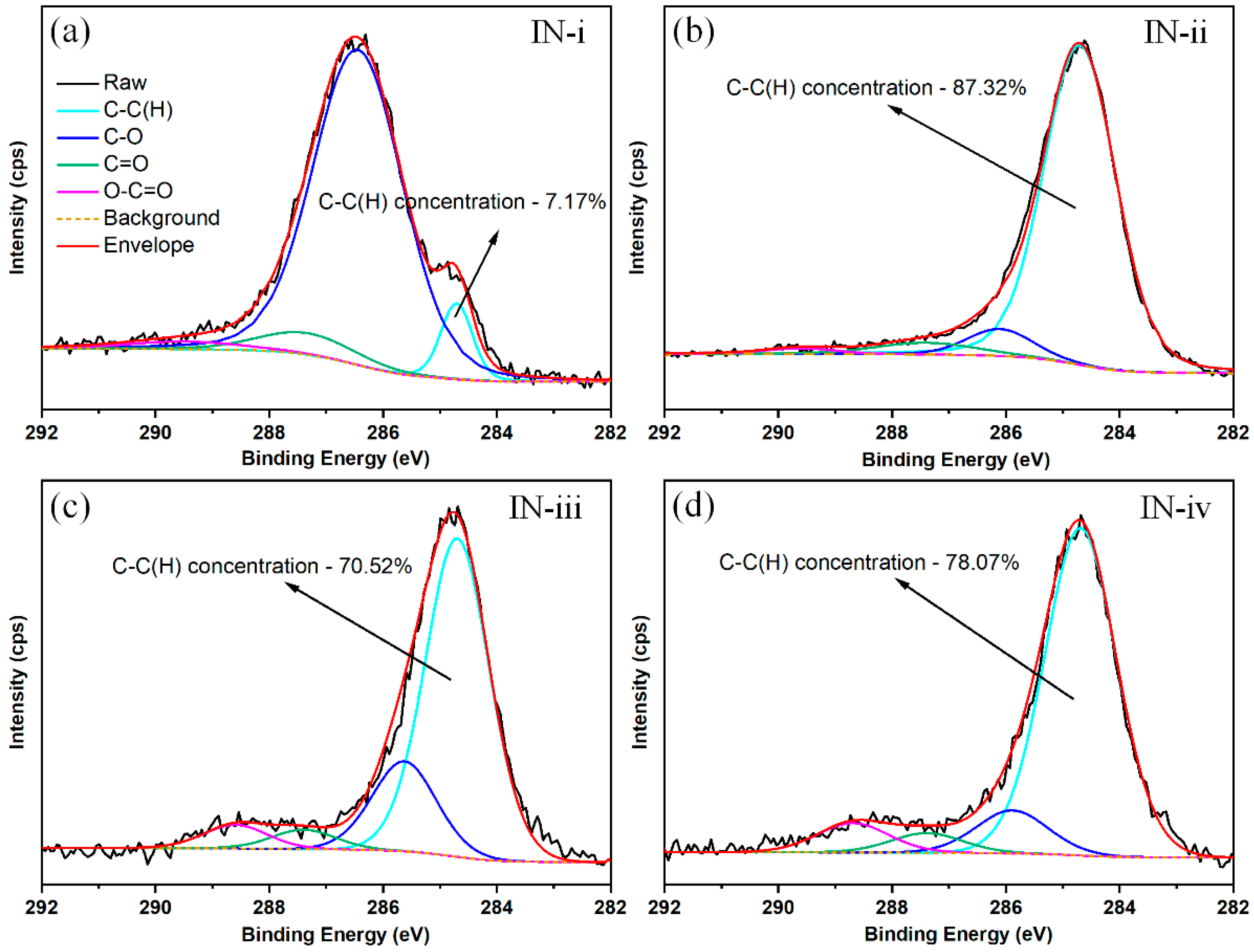
3.3. Surface Chemistry
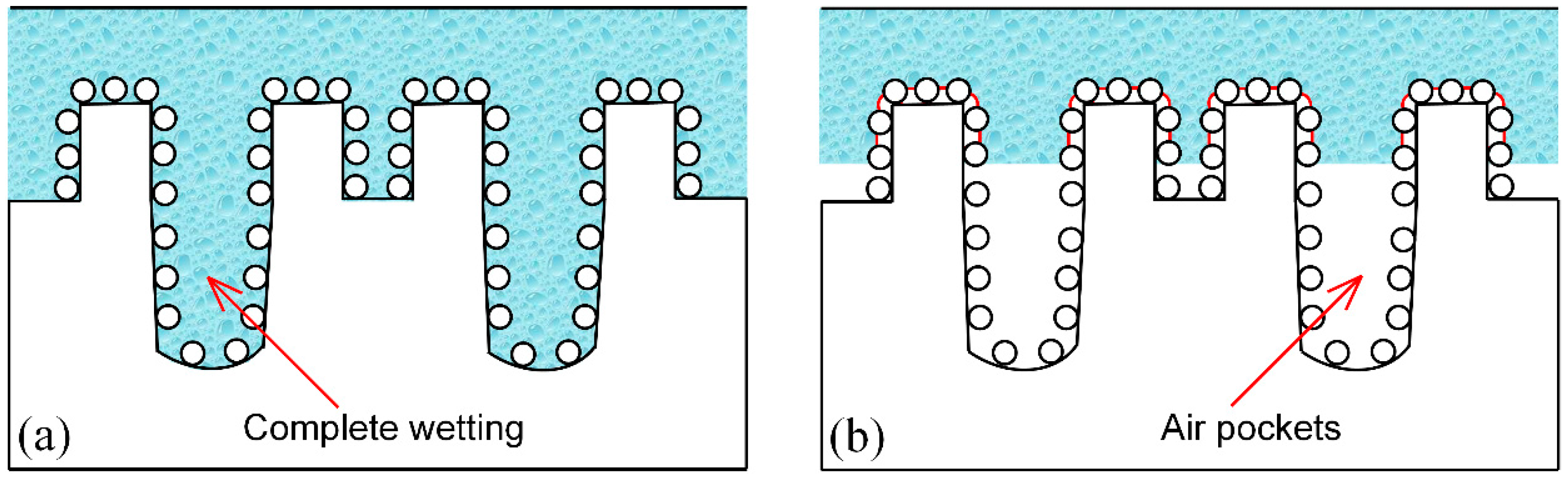
3.4. Wettability Conversion Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Venkatesan, K.; Ramanujam, K.; Kuppan, P. Parametric modeling and optimization of laser scanning parameters during laser assisted machining of Inconel 718. Opt. Laser Technol. 2016, 78, 10–18. [Google Scholar] [CrossRef]
- Keshavarzkermani, A.; Sadowski, M.; Ladani, M. Direct metal laser melting of Inconel 718: Process impact on grain formation and orientation. J. Alloy Compd. 2018, 736, 297–305. [Google Scholar] [CrossRef]
- Wang, Z.; Guan, K.; Gao, M.; Li, X.; Chen, X.; Zeng, X. The microstructure and mechanical properties of deposited-IN718 by selective laser melting. J. Alloy Compd. 2018, 513, 518–523. [Google Scholar] [CrossRef]
- Xiao, L.J.; Zeng, W.G.; Liao, G.F.; Yi, C.F.; Xu, Z.S. Thermally and chemically stable candle soot superhydrophobic surface with excellent self-cleaning properties in air and oil. ACS Appl. Nano Mater. 2018, 1, 1204–1211. [Google Scholar] [CrossRef]
- Xiao, L.J.; Deng, M.; Zeng, W.G.; Zhang, B.X.; Xu, Z.S.; Yi, C.F.; Liao, G.F. Novel robust super-hydrophobic coating with self-cleaning properties in air and oil based on rare earth metal oxide. Ind. Eng. Chem. Res. 2017, 56, 12354–12361. [Google Scholar] [CrossRef]
- Sun, K.; Yang, H.; Xue, W.; He, A.; Zhu, D.H.; Liu, W.W.; Adeyemi, K.; Cao, Y. Anti-biofouling superhydrophobic surface fabricated by picosecond laser texturing of stainless steel. Appl. Surf. Sci. 2018, 436, 263–267. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.P.; Tian, Y.L. Fabrication of super-hydrophobic nickel film on copper substrate with improved corrosion inhibition by electrodeposition process. Colloids Surf. A 2019, 560, 205–212. [Google Scholar] [CrossRef]
- Lu, M.C.; Lin, C.C.; Lo, C.W.; Huang, C.W.; Wang, C.C. Superhydrophobic Si nanowires for enhanced condensation heat transfer. Int. J. Heat Mass Trans. 2017, 111, 614–623. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y.C. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef]
- Wang, C.Z.; Tang, F.; Hao, P.F.; Li, Q.; Wang, X.H. Experimental study on the drag reduction effect of a rotating superhydrophobic surface in micro gap flow field. Microsyst. Technol. 2017, 23, 3033–3040. [Google Scholar] [CrossRef]
- Tian, X.L.; Verho, T.; Ras, R.H.A. Moving superhydrophobic surfaces towards real-world applications. Science 2016, 352, 142–143. [Google Scholar] [CrossRef] [PubMed]
- Toosi, S.F.; Moradi, S.; Ebrahimi, M.; Hatzikiriakos, S.G. Microfabrication of polymeric surfaces with extreme wettability using hot embossing. Appl. Surf. Sci. 2016, 378, 426–434. [Google Scholar] [CrossRef]
- Latthe, S.S.; Terashima, C.; Nakata, K.; Sakai, M.; Fujishima, A. Development of sol–gel processed semi-transparent and self-cleaning superhydrophobic coatings. J. Mater. Chem. A 2014, 2, 5548–5553. [Google Scholar] [CrossRef]
- He, Y.; Jiang, C.Y.; Yin, H.X.; Yuan, W.Z. Tailoring the wettability of patterned silicon surfaces with dual-scale pillars: From hydrophilicity to superhydrophobicity. Appl. Surf. Sci. 2011, 257, 7689–7692. [Google Scholar] [CrossRef]
- Yang, X.M.; Zhong, Z.W.; Diallo, E.M.; Wang, Z.H.; Yue, W.S. Silicon wafer wettability and aging behaviors: Impact on gold thin-film morphology. Mater. Sci. Semicond. Process. 2014, 26, 25–32. [Google Scholar] [CrossRef]
- Kwon, M.H.; Jee, W.Y.; Chu, C.N. Fabrication of hydrophobic surfaces using copper electrodeposition and oxidation. Microsyst. Technol. 2015, 16, 877–882. [Google Scholar] [CrossRef]
- Ye, Y.W.; Liu, Z.Y.; Liu, W.; Zhang, D.W.; Zhao, H.C.; Wang, L.P.; Li, X.G. Superhydrophobic oligoaniline-containing electroactive silica coating as pre-process coating for corrosion protection of carbon steel. Chem. Eng. J. 2018, 348, 940–951. [Google Scholar] [CrossRef]
- Crick, C.R.; Bear, J.C.; Southern, P.; Parkin, I.P. A general method for the incorporation of nanoparticlesinto superhydrophobic films by aerosol assisted chemical vapour deposition. J. Mater. Chem. A 2013, 1, 4336–4344. [Google Scholar] [CrossRef]
- Zhai, Z.Y.; Wang, W.J.; Mei, X.S.; Li, M.; Cui, J.L.; Wang, F.C.; Pan, A.F. Effect of the surface microstructure ablated by femtosecond laser on the bonding strength of EBCs for SiC/SiC composites. Opt. Commun. 2018, 424, 137–144. [Google Scholar] [CrossRef]
- Baron, C.F.; Mimidis, A.; Puerto, D.; Skoulas, E.; Stratakis, E.; Solis, J.; Siegel, J. Biomimetic surface structures in steel fabricated with femtosecond laser pulses: Influence of laser rescanning on morphology and wettability. Beilstein J. Nanotechnol. 2018, 9, 2802–2812. [Google Scholar] [CrossRef]
- Rung, S.; Schwarz, S.; Götzendorfer, B.; Esen, C.; Hellmann, R. Time dependence of wetting behavior upon applying hierarchic nano-micro periodic surface structures on brass using ultra short laser pulses. Appl. Sci. 2018, 8, 700. [Google Scholar] [CrossRef]
- Trdan, U.; Hočevar, M.; Gregorčič, P. Transition from superhydrophilic to superhydrophobic state of laser textured stainless steel surface and its effect on corrosion resistance. Corros. Sci. 2017, 123, 21–26. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, Y.L.; Yang, C.J.; Wang, F.J.; Liu, X.P. Modification of wetting property of Inconel 718 surface by nanosecond laser texturing. Appl. Surf. Sci. 2017, 414, 313–324. [Google Scholar] [CrossRef]
- Tian, Y.L.; Zhao, Y.C.; Yang, C.J.; Wang, F.J.; Liu, X.P.; Jing, X.B. Fabrication of bio-inspired nitinol alloy surface with tunable anisotropic wetting and high adhesive ability. J. Colloid Interface Sci. 2018, 537, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, C.E.; Bellouard, Y. A monolithic micro-tensile tester for investigating silicon dioxide polymorph micromechanics, fabricated and operated using a femtosecond laser. Micromachines 2015, 6, 1365–1386. [Google Scholar] [CrossRef]
- Dadashi, S.; Dadashi, R.; Delavari, H. Optical and structural properties of Bi-based nanoparticles prepared via pulsed Nd:YAG laser ablation in organic liquids. Appl. Phys. A 2018, 124, 406. [Google Scholar] [CrossRef]
- Yang, W.J.; Gerke, S.A.; Ng, K.W.; Rao, Y.; Chase, C.; Chang-Hasnain, C.J. Laser optomechanics. Sci. Rep. 2015, 5, 13700. [Google Scholar] [CrossRef]
- Wang, Z.K.; Zhang, H.Y.; Lim, R.Y.H.; Wang, Z.F.; Lam, Y.C. Improving surface smoothness of laser-fabricated microchannels for microfluidic application. J. Micromech. Microeng. 2011, 21, 095008. [Google Scholar] [CrossRef]
- Wang, Z.K.; Zheng, H.Y.; Xia, H.M. Femtosecond laser-induced modification of surface wettability of PMMA for fluid separation in microchannels. Microfluid. Nanofluid. 2011, 10, 225–229. [Google Scholar] [CrossRef]
- Phillips, K.C.; Gandhi, H.H.; Mazur, E.; Sundaram, S.K. Ultrafast laser processing of materials: A review. Adv. Opt. Photonics 2015, 7, 684–712. [Google Scholar] [CrossRef]
- Yan, H.P.; Rashid, M.R.; Khew, S.Y.; Li, F.P.; Hong, M.H. Wettability transition of laser textured brass surfaces inside different mediums. Appl. Surf. Sci. 2018, 427, 369–375. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, X.W.; Han, Y.H.; Ze, Z. Control of water droplet motion by alteration of roughness gradient on silicon wafer by laser surface treatment. Thin Solid Films 2008, 516, 4059–4063. [Google Scholar] [CrossRef]
- Kietzig, A.M.; Mirvakili, M.N.; Kamal, S.; Englezos, P.; Hatzikiriakos, S.G. Cassie to Wenzel wetting transitions on femtosecond laser-patterned pure metallic substrates. J. Adhes Sci. Technol. 2011, 25, 2789–2809. [Google Scholar]
- Yang, C.J.; Mei, X.S.; Tian, Y.L.; Zhang, D.W.; Li, Y.; Liu, X.P. Modification of wettability property of titanium by laser texturing. Int. J. Adv. Manuf. Technol. 2016, 87, 1–8. [Google Scholar] [CrossRef]
- Rung, S.; Schwarz, S.; Zettl, J.; Götzendorfer, B.; Esen, C.; Hellmann, R. Static and dynamic contact angle of water influenced by femtosecond laser based ripple structures on metals. J. Laser Micro Nanoen. 2018, 12, 100–104. [Google Scholar]
- Yang, Z.; Liu, X.P.; Tian, Y.L. Insights into the wettability transition of nanosecond laser ablated surface under ambient air exposure. J. Colloid Interface Sci. 2019, 533, 268–277. [Google Scholar] [CrossRef]
- Eral, H.B.; Oh, J.M. Contact angle hysteresis: A review of fundamentals and applications. Colloid. Polym. Sci. 2013, 291, 247–260. [Google Scholar] [CrossRef]
- Zeng, W.G.; Chen, J.; Yang, H.; Deng, L.D.; Liao, G.F.; Xu, Z.S. Robust coating with superhydrophobic and self-cleaning properties in either air and oil based on natural zeolite. Surf. Coat. Technol. 2017, 309, 1045–1051. [Google Scholar] [CrossRef]
- Long, J.Y.; Zhong, M.L.; Zhang, H.J.; Fan, P.X. Superhydrophilicity to superhydrophobicity transition of picosecond laser microstructured aluminum in ambient air. J. Colloid Interface Sci. 2015, 441, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rajab, F.H.; Liu, Z.; Li, L. Long term superhydrophobic and hybrid superhydrophobic/superhydrophilic surfaces produced by laser surface micro/nano surface structing. Appl. Surf. Sci. 2019, 466, 808–821. [Google Scholar] [CrossRef]
- Marmur, A.; Della, V.C.; Siboni, S.; Amirfazli, A.; Drelich, J.W. Contact angles and wettability: Towards common and accurate terminology. Surf. Innov. 2017, 5, 3–8. [Google Scholar] [CrossRef]
- Marmur, A. Soft contact: Measurement and interpretation of contact angles. Soft Matter 2006, 2, 12–17. [Google Scholar] [CrossRef]
- Huhtamäki, T.; Tian, X.L.; Korhonen, J.T.; Ras, R.H.A. Surface-wetting characterization using contact-angle measurements. Nat. Protoc. 2018, 13, 1521–1538. [Google Scholar]
- Cardoso, J.T.; Garcia-Girón, A.; Romano, J.M.; Huerta-Murillo, D.; Jagdheesh, R.; Walker, M.; Dimov, S.S.; Ocaña, J.L. Influence of ambient conditions on the evolution of wettability properties of an IR-, ns-laser textured aluminium alloy. RSC Adv. 2017, 7, 39617–39627. [Google Scholar] [CrossRef]
- Farid, N.; Harilal, S.S.; EI-Atwani, O.; Ding, H.; Hassanein, A. Experimental simulation of materials degradation of plasma-facing components using lasers. Nucl. Fusion 2014, 54, 012002. [Google Scholar] [CrossRef]
- Besozzi, E.; Maffini, A.; Dellasega, D.; Russo, V.; Facibeni, A.; Pazzaglia, A.; Beghi, M.G.; Passoni, M. Nanosecond laser pulses for mimicking thermal effects on nanostructured tungsten-based materials. Nucl. Fusion 2018, 58, 036019. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. J. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Bormashenko, E. Wetting of Real Surfaces; De Gruyter: Berlin, Germany, 2013. [Google Scholar]
- Fadeeva, E.; Truong, V.K.; Stiesch, M.; Chichkov, B.N.; Crawford, R.J.; Wang, J.; Ivanova, E.P. Bacterial retention on superhydrophobic titanium surfaces fabricated by femtosecond laser ablation. Langmuir 2011, 27, 3012–3019. [Google Scholar] [CrossRef] [PubMed]
- Long, J.Y.; Zhong, M.L.; Fan, P.X.; Gong, D.W.; Zhang, H.J. Wettability conversion of ultrafast laser structured copper surface. J. Laser Appl. 2015, 27, S29107. [Google Scholar] [CrossRef]
- Dabek-Zlotorzynska, E.; Celo, V. Capillary Electrophoresis; Humana Press: Totowa, NJ, USA, 2008; pp. 43–64. [Google Scholar]
- Li, Z.T.; Wang, Y.J.; Kozbial, A.; Shenoy, G.; Zhou, F.; McGinley, R.; Ireland, P.; Morganstein, B.; Kunkel, A.; Surwade, S.P.; et al. Effect of airborne contaminants on the wettability of supported graphene and graphite. Nat. Mater. 2013, 12, 925–931. [Google Scholar] [CrossRef]
- Drzymala, J. Hydrophobicity and collectorless flotation of inorganic materials. Adv. Colloid Interface 1994, 50, 143–185. [Google Scholar] [CrossRef]
- Su, F.H.; Yao, K. Facile fabrication of superhydrophobic surface with excellent mechanical abrasion and corrosion resistance on copper substrate by a novel method. ACS Appl. Mater. Interfaces 2014, 6, 8762–8770. [Google Scholar] [CrossRef]
- Bryuzgin, E.V.; Klimov, V.V.; Repin, S.A.; Navrotskiy, A.V.; Novakov, I.A. Aluminum surface modification with fluoroalkyl methacrylate-based copolymers to attain superhydrophobic properties. Appl. Surf. Sci. 2017, 419, 454–459. [Google Scholar] [CrossRef]
- Baldacchini, T.; Carey, J.E.; Zhou, M.; Mazur, E. Superhydrophobic surfaces prepared by microstructuring of silicon using a femtosecond laser. Langmuir 2006, 22, 4917–4919. [Google Scholar] [CrossRef] [PubMed]
- Boinovich, L.B.; Emelyanenko, A.M.; Pashinin, A.S.; Lee, C.H.; Drelich, J.; Yap, Y.K. Origins of Thermodynamically stable superhydrophobicity of Boron Nitride nanotubes coatings. Langmuir 2012, 28, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.A.; Kunz, C.; Graf, S. Bio-inspired functional surfaces based on laser-induced periodic surface structures. Materials 2016, 9, 476. [Google Scholar] [CrossRef] [PubMed]
- Herminghaus, S. Roughness-induced non-wetting. Europhys. Lett. 2000, 52, 165–170. [Google Scholar] [CrossRef]
- Bormashenko, E.; Stein, T.; Whyman, G.; Bormashenko, Y.; Pogreb, R. Wetting properties of the multiscaled nanostructured polymer and metallic superhydrophobic surfaces. Langmuir 2006, 22, 9982–9985. [Google Scholar] [CrossRef]
- Starostin, A.; Valtsifer, V.; Strelnikov, V.; Bormashenko, E.; Grynuov, R.; Bormashenko, Y.; Gladkikh, A. Robust technique allowing the manufacture of superoleophobic (omniphobic) metallic surfaces. Adv. Eng. Mater. 2014, 16, 1127–1132. [Google Scholar] [CrossRef]


| Element | Ni | Cr | Nb | Al | Mo | Si | C | Mn |
| wt. (%) | 53.16 | 18.03 | 5.48 | 0.66 | 3.11 | 0.16 | 0.07 | 0.08 |
| Element | P | Co | Ti | Cu | B | Ta | S | Fe |
| wt. (%) | 0.015 | 0.23 | 1.15 | 0.07 | 0.0028 | 0.008 | 0.002 | Balanced |
| Laser Parameters | Values |
|---|---|
| Wavelength | 1064 nm |
| Average laser power | 10 W |
| Repetition rate | 20 kHz |
| Pulse duration | 50 ns |
| Laser fluence | 17.69 J/cm2 |
| Spacing | 100 μm |
| Scanning speed | 50 mm/s |
| Sample No. | C (atomic %) | O (atomic %) | Ni (atomic %) | Fe (atomic %) | Cr (atomic %) |
|---|---|---|---|---|---|
| IN-i | 26.62 | 25.04 | 16.59 | 14.55 | 17.21 |
| IN-ii | 61.29 | 20.93 | 6.21 | 6.30 | 6.30 |
| IN-iii | 34.23 | 44.65 | 9.30 | 5.28 | 6.54 |
| IN-iv | 40.50 | 43.42 | 8.39 | 3.39 | 4.30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Tian, Y.; Zhao, Y.; Yang, C. Study on the Fabrication of Super-Hydrophobic Surface on Inconel Alloy via Nanosecond Laser Ablation. Materials 2019, 12, 278. https://doi.org/10.3390/ma12020278
Yang Z, Tian Y, Zhao Y, Yang C. Study on the Fabrication of Super-Hydrophobic Surface on Inconel Alloy via Nanosecond Laser Ablation. Materials. 2019; 12(2):278. https://doi.org/10.3390/ma12020278
Chicago/Turabian StyleYang, Zhen, Yanling Tian, Yuechao Zhao, and Chengjuan Yang. 2019. "Study on the Fabrication of Super-Hydrophobic Surface on Inconel Alloy via Nanosecond Laser Ablation" Materials 12, no. 2: 278. https://doi.org/10.3390/ma12020278
APA StyleYang, Z., Tian, Y., Zhao, Y., & Yang, C. (2019). Study on the Fabrication of Super-Hydrophobic Surface on Inconel Alloy via Nanosecond Laser Ablation. Materials, 12(2), 278. https://doi.org/10.3390/ma12020278




