Analysis of Carbon-Based Microelectrodes for Neurochemical Sensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Carbon Fibers
2.2. Scanning Electron Microscopy System
2.3. Confocal Raman System
2.4. Fast Scan Cyclic Voltammetry System
2.5. Boron-Doped Diamond Deposition System
3. Results and Discussion
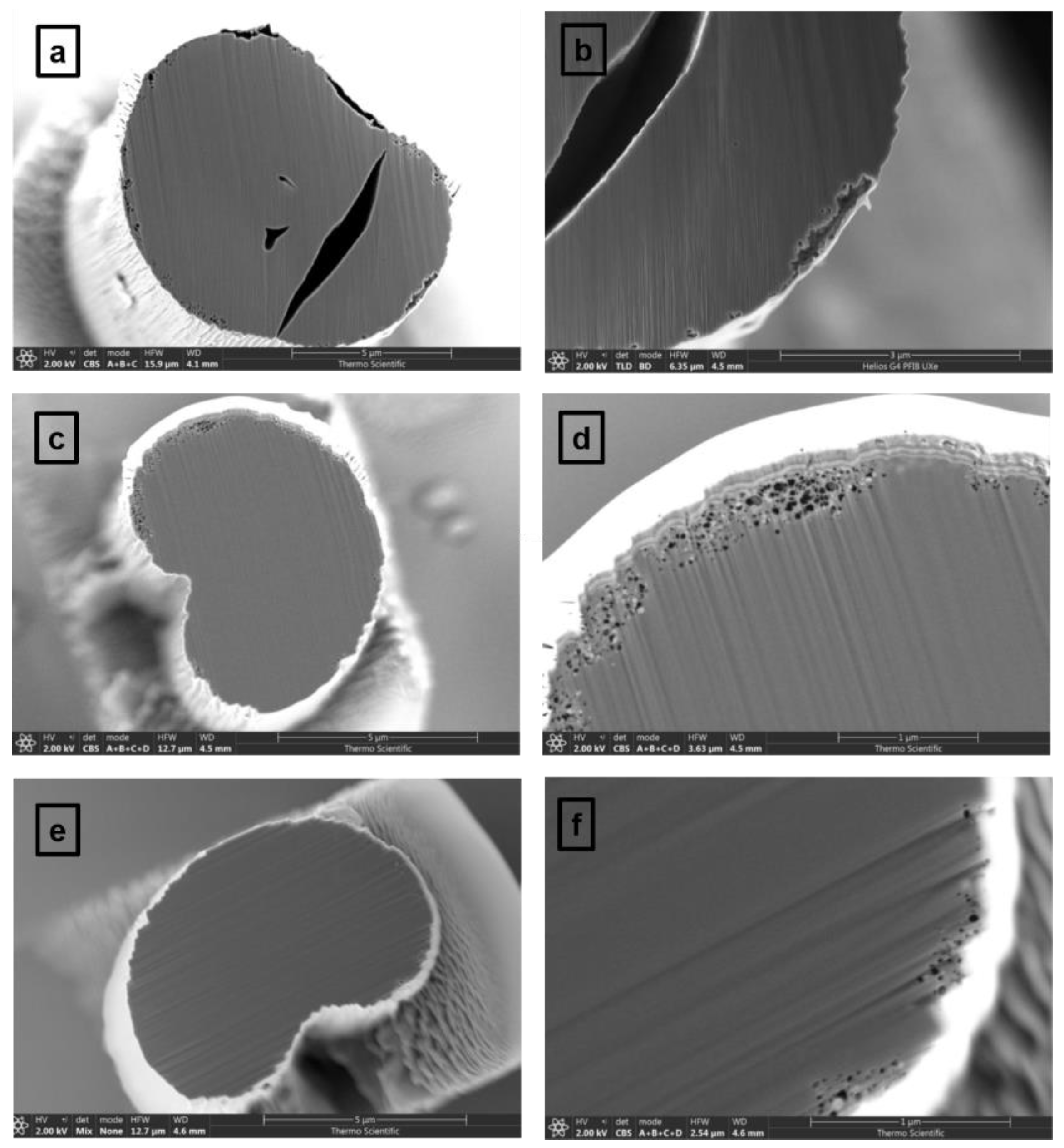
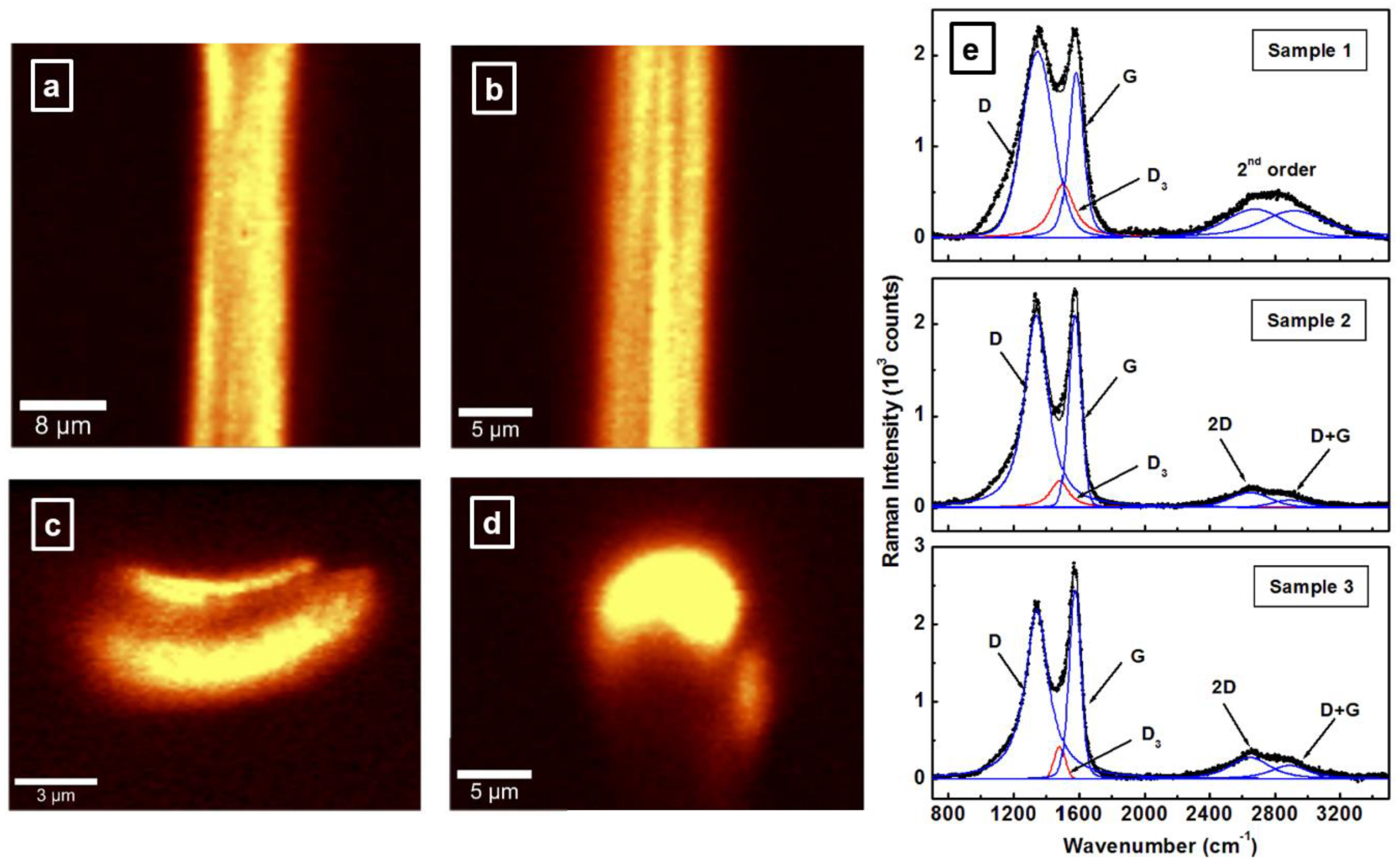
3.1. Microscopic and Spectroscopic Characterizations of Carbon Fibers
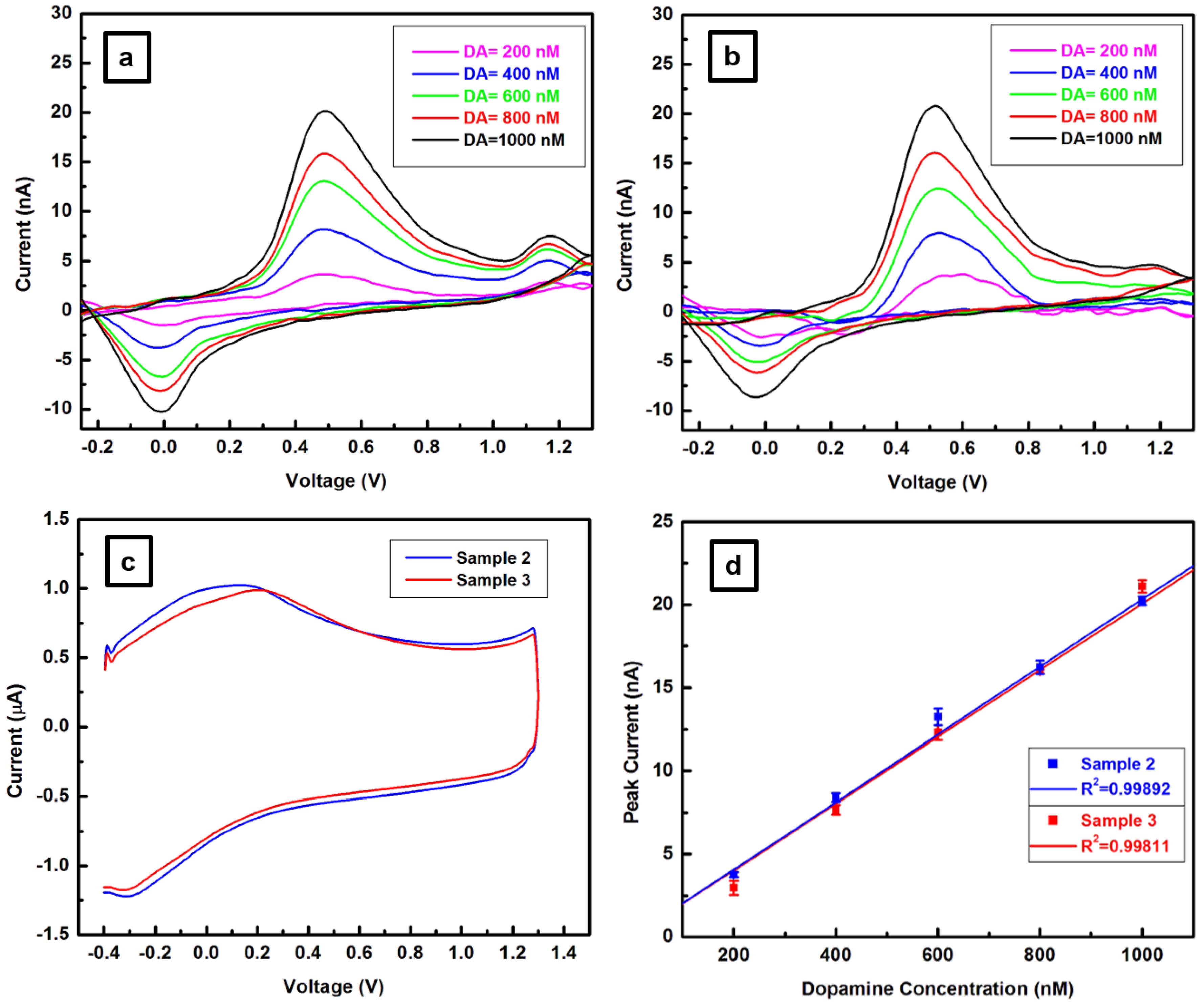
3.2. Fast Scan Cyclic Voltammetry Analysis
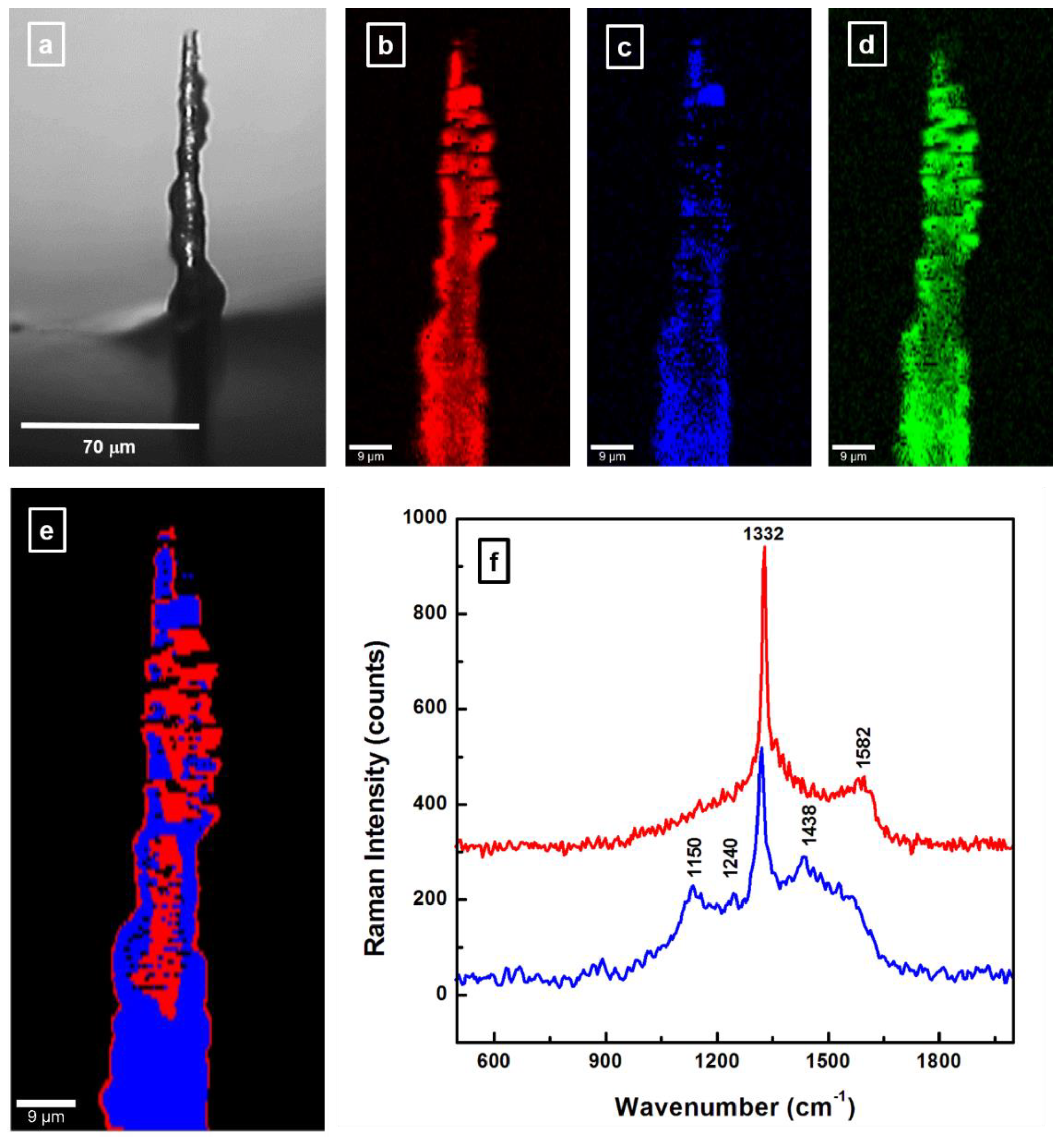
3.3. Confocal Raman Microscopy Investigation of BDD Coated Carbon Fiber
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huffman, M.L.; Venton, B.J. Carbon-Fiber Microelectrodes for In Vivo Applications. Analyst 2009, 134, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Huang, X. Fabrication and Properties of Carbon Fibers. Materials 2009, 2, 2369–2403. [Google Scholar] [CrossRef]
- Choi, D.; Kil, H.-S.; Lee, S. Fabrication of low-cost carbon fibers using economical precursors and advanced processing technologies. Carbon 2019, 142, 610–649. [Google Scholar] [CrossRef]
- Vomero, M.; Oliveira, A.; Ashouri, D.; Eickenscheidt, M.; Stieglitz, T. Graphitic Carbon Electrodes on Flexible Substrate for Neural Applications Entirely Fabricated Using Infrared Nanosecond Laser Technology. Sci. Rep. 2018, 8, 14749–14762. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.G.; Moody, B.P.; McCarty, G.S.; Sombers, L.A. Specific Oxygen-Containing Functional Groups on the Carbon Surface Underlie an Enhanced Sensitivity to Dopamine at Electrochemically Pretreated Carbon Fiber Microelectrodes. Langmuir 2010, 26, 9116–9122. [Google Scholar] [CrossRef] [PubMed]
- Kakhki, R.M. A review to recent developments in modification of carbon fiber electrodes. Arabian J. Chem. 2014. [Google Scholar] [CrossRef]
- Li, D.; Liu, H.; Chen, B.; Niu, D.; Lei, B.; Ye, G.; Jiang, W.; Shi, Y.; Yin, L.; Lai, G. Amorphous Carbon-Induced Surface Defect Repair for Reinforcing the Mechanical Properties of Carbon Fiber. Materials 2019, 12, 1244. [Google Scholar] [CrossRef]
- Qiu, C.; Bennet, K.E.; Khan, T.; Ciubuc, J.D.; Manciu, F.S. Raman and Conductivity Analysis of Graphene for Biomedical Applications. Materials 2016, 9, 897. [Google Scholar] [CrossRef]
- Bennet, K.E.; Lee, K.H.; Kruchowski, J.N.; Chang, S.Y.; Marsh, M.P.; Van Orsow, A.A.; Paez, A.; Manciu, F.S. Development of Conductive Boron-Doped Diamond Electrode: A Microscopic, Spectroscopic, and Voltammetric Study. Materials 2013, 6, 5726–5741. [Google Scholar] [CrossRef]
- Bucher, E.S.; Wightman, M.R. Electrochemical Analysis of Neurotransmitters. Annu. Rev. Anal. Chem. 2015, 8, 239–261. [Google Scholar] [CrossRef]
- Rodeberg, N.T.; Sandberg, S.G.; Johnson, J.A.; Phillips, P.E.; Wightman, R.M. Hitchhiker’s Guide to Voltammetry: Acute and Chronic Electrodes for in Vivo Fast-Scan Cyclic Voltammetry. ACS Chem. Neurosci. 2017, 8, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Park, C.; Kim, D.H.; Shin, H.; Kang, Y.M.; DeWaele, M.; Lee, J.; Min, H.-K.; Blaha, C.D.; Bennet, K.E.; et al. Monitoring In Vivo Changes in Tonic Extracellular Dopamine Level by Charge-Balancing Multiple Waveform Fast-Scan Cyclic Voltammetry. Anal. Chem. 2016, 88, 10962–10970. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.J.; Sandberg, S.G.; Wanat, M.J.; Gan, J.O.; Horne, E.A.; Hart, A.S.; Akers, C.A.; Parker, J.G.; Willuhn, I.; Martinez, V.; et al. Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nat. Methods 2010, 7, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Y.; Kim, I.; Marsh, M.P.; Jang, D.P.; Hwang, S.C.; Van Gompel, J.J.; Goerss, S.J.; Kimble, C.J.; Bennet, K.E.; Garris, P.A.; et al. Wireless fast-scan cyclic voltammetry to monitor adenosine in patients with essential tremor during deep brain stimulation. Mayo Clin. Proc. 2012, 87, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Bennet, K.E.; Tomshine, J.R.; Min, H.-K.; Manciu, F.S.; Marsh, M.P.; Paek, S.B.; Settell, M.L.; Nicolai, E.N.; Blaha, C.D.; Kouzani, A.Z.; et al. A Diamond-Based Electrode for Detection of Neurochemicals in the Human Brain. Front. Hum. Neurosci. 2016, 10, 1–12. [Google Scholar] [CrossRef]
- Hendrickson, K.E., III. The Encyclopedia of the Industrial Revolution in World History, 3rd ed.; The Rowman & Littlefield Publishing Group, Inc.: Lanham, MD, USA, 2014; Volume 3, p. 564. [Google Scholar]
- Kim, M.-A.; Jang, D.; Tejima, S.; Cruz-Silva, R.; Joh, H.-I.; Kim, H.C.; Lee, S.; Endo, M. Strengthened PAN-based carbon fibers obtained by slow heating rate carbonization. Sci. Rep. 2016, 6, 22988–22995. [Google Scholar] [CrossRef]
- Song, Z.; Hou, X.; Zhang, L.; Wu, S. Enhancing Crystallinity and Orientation by Hot-Stretching to Improve the Mechanical Properties of Electrospun Partially Aligned Polyacrylonitrile (PAN) Nanocomposites. Materials 2011, 4, 621–632. [Google Scholar] [CrossRef]
- Nunna, S.; Naebe, M.; Hameed, N.; Fox, B.L.; Creighton, C. Evolution of radial heterogeneity in polyacrylonitrile fibres during thermal stabilization: An overview. Polym. Degrad. Stabil. 2017, 136, 20–30. [Google Scholar] [CrossRef]
- Cao, Q.; Puthongkham, P.; Venton, B.J. Review: New insights into optimizing chemical and 3D surface structures of carbon electrodes for neurotransmitter detection. Anal. Methods 2019, 11, 247–261. [Google Scholar] [CrossRef]
- Du, J.; Yue, R.; Ren, F.; Yao, Z.; Jiang, F.; Yang, P.; Du, Y. Novel graphene flowers modified carbon fibers for simultaneous determination of ascorbic acid, dopamine and uric acid. Biosens. Bioelectron. 2014, 53, 220–224. [Google Scholar] [CrossRef]
- Hermans, A.; Seipel, A.T.; Miller, C.E.; Wightman, R.M. Carbon-fiber microelectrodes modified with 4-sulfobenzene have increased sensitivity and selectivity for catecholamines. Langmuir 2006, 22, 1964–1969. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Li, J.; Yuan, Z.; Zeng, H.; Chen, X. Surface Modification of Carbon Fibers for Interface Improvement in Textile Composites. Appl. Compos. Mater. 2018, 25, 853–860. [Google Scholar] [CrossRef]
- Barsan, M.M.; Ghica, M.E.; Brett, C.M.A. Electrochemical sensors and biosensors based on redox polymer/carbon nanotube modified electrodes: A review. Anal. Chim. Acta 2015, 881, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Pihel, K.; Walker, Q.D.; Wightman, R.M. Overoxidized Polypyrrole-Coated Carbon Fiber Microelectrodes for Dopamine Measurements with Fast-Scan Cyclic Voltammetry. Anal. Chem. 1996, 68, 2084–2089. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, G.A.; Oke, A.F.; Nagy, G.; Moghaddam, B.; Adams, R.N. Nafion-coated electrodes with high selectivity for CNS electrochemistry. Brain Res. 1984, 290, 390–395. [Google Scholar] [CrossRef]
- Kawagoe, K.T.; Jankowski, J.A.; Wightman, R.M. Etched carbon-fiber electrodes as amperometric detectors of catecholamine secretion from isolated biological cells. Anal. Chem. 1991, 63, 1589–1594. [Google Scholar] [CrossRef]
- Strand, A.M.; Venton, B.J. Flame Etching Enhances the Sensitivity of Carbon-Fiber Microelectrodes. Anal. Chem. 2008, 80, 3708–3715. [Google Scholar] [CrossRef]
- Liu, J.; Tian, Y.; Chen, Y.; Liang, J. Interfacial and mechanical properties of carbon fibers modified by electrochemical oxidation in NH4HCO3/(NH4)2C2O4_H2O aqueous compound solution. Appl. Surf. Sci. 2010, 256, 6199–6204. [Google Scholar] [CrossRef]
- Donnet, J.B.; Park, S.J.; Wang, W.D. The effect of electrochemical surface treatments of carbon fibers using scanning tunneling microscopy. Polym. Adv. Technol. 1994, 5, 395–399. [Google Scholar] [CrossRef]
- Chang, S.Y.; Jay, T.; Munoz, J.; Kim, I.; Lee, K.H. Wireless fast-scan cyclic voltammetry measurement of histamine using WINCS—A proof-of-principle study. Analyst 2012, 137, 2158–2165. [Google Scholar] [CrossRef]
- Bennet, K.E.; Lee, K.H.; Tomshine, J.R.; Sundin, E.M.; Kruchowski, J.N.; Durrer, W.G.; Manciu, B.M.; Kouzani, A.; Manciu, F.S. Raman microscopic analysis of internal stress in boron-doped diamond. Materials 2015, 8, 2782–2793. [Google Scholar] [CrossRef]
- Manciu, F.S.; Manciu, M.; Durrer, W.G.; Salazar, J.G.; Lee, K.H.; Bennet, K.E. A Drude model analysis of conductivity and free carriers in boron-doped diamond films and investigations of their internal stress and strain. J. Mater. Sci. 2014, 49, 5782–5789. [Google Scholar] [CrossRef] [PubMed]
- Almeida, E.C.; Diniz, A.V.; Trava-Airoldi, V.J.; Ferreira, N.G. Electrochemical characterization of doped diamond-coated carbon fibers at different boron concentrations. Thin Solid Films 2005, 485, 241–246. [Google Scholar] [CrossRef]
- Haubner, R.; Lessiak, M. Deposition of CVD diamond coatings on carbon fiber composite substrates. Key Eng. Mater. 2017, 742, 419–426. [Google Scholar] [CrossRef]
- Lee, K.H.; Lujan, J.L.; Trevathan, J.K.; Ross, E.K.; Bartoletta, J.J.; Park, J.O.; Paek, S.B.; Nicolai, E.N.; Lee, J.H.; Min, H.-K.; et al. WINCS Harmoni: Closed-loop dynamic neurochemical control of therapeutic interventions. Sci. Rep. 2017, 7, 46675–46687. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.R.; Hermans, A.; Seipel, A.T.; Wightman, R.M. Monitoring Rapid Chemical Communication in the Brain. Chem. Rev. 2008, 108, 2554–2584. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.G.; Lugo-Morales, L.Z.; Loziuk, P.L.; Sombers, L.A. Leading Methods in Dopamine Research; Kabbani, N., Ed.; Humana Press: New York, NY, USA, 2013; pp. 275–294. [Google Scholar]
- Robinson, D.L.; Wightman, R.M. Electrochemical Methods for Neuroscience; Michael, A.C., Borland, L.M., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 17–34. [Google Scholar]
- Nunna, S.; Creighton, C.; Fox, B.L.; Naebe, M.; Maghe, M.; Tobin, M.J.; Bambery, K.; Vongsvivut, J.; Hameed, N. The effect of thermalyy induced chemical transformations on the structure and properties of carbon fiber precursors. J. Mater. Chem. A 2017, 5, 7372–7382. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Sugihara, K.; Spain, I.L.; Goldberg, H.A. Graphite Fibers and Filaments; Cardona, M., Ed.; Springer: Berlin/Heidelberg, Germany, 1988; pp. 1–339. [Google Scholar]
- Qian, X.; Wang, X.; Zhong, J.; Zhi, J.; Heng, F.; Zhang, Y.; Song, S. Effect of fiber microsctructure studied by Raman spectroscopy upon the mechanical properties of carbon fiber. J. Raman Spectrosc. 2019, 50, 665–673. [Google Scholar] [CrossRef]
- Pawalyta, M.; Rouzaud, J.N.; Duber, S. Raman microspectroscopy characterization of carbon black: Spectral analysis and structural information. Carbon 2015, 84, 479–490. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manciu, F.S.; Oh, Y.; Barath, A.; Rusheen, A.E.; Kouzani, A.Z.; Hodges, D.; Guerrero, J.; Tomshine, J.; Lee, K.H.; Bennet, K.E. Analysis of Carbon-Based Microelectrodes for Neurochemical Sensing. Materials 2019, 12, 3186. https://doi.org/10.3390/ma12193186
Manciu FS, Oh Y, Barath A, Rusheen AE, Kouzani AZ, Hodges D, Guerrero J, Tomshine J, Lee KH, Bennet KE. Analysis of Carbon-Based Microelectrodes for Neurochemical Sensing. Materials. 2019; 12(19):3186. https://doi.org/10.3390/ma12193186
Chicago/Turabian StyleManciu, Felicia S., Yoonbae Oh, Abhijeet Barath, Aaron E. Rusheen, Abbas Z. Kouzani, Deidra Hodges, Jose Guerrero, Jonathan Tomshine, Kendall H. Lee, and Kevin E. Bennet. 2019. "Analysis of Carbon-Based Microelectrodes for Neurochemical Sensing" Materials 12, no. 19: 3186. https://doi.org/10.3390/ma12193186
APA StyleManciu, F. S., Oh, Y., Barath, A., Rusheen, A. E., Kouzani, A. Z., Hodges, D., Guerrero, J., Tomshine, J., Lee, K. H., & Bennet, K. E. (2019). Analysis of Carbon-Based Microelectrodes for Neurochemical Sensing. Materials, 12(19), 3186. https://doi.org/10.3390/ma12193186




