Novel Therapeutic Strategies Applied to Pseudomonas aeruginosa Infections in Cystic Fibrosis
Abstract
1. Introduction
2. Inorganic Nanoparticles
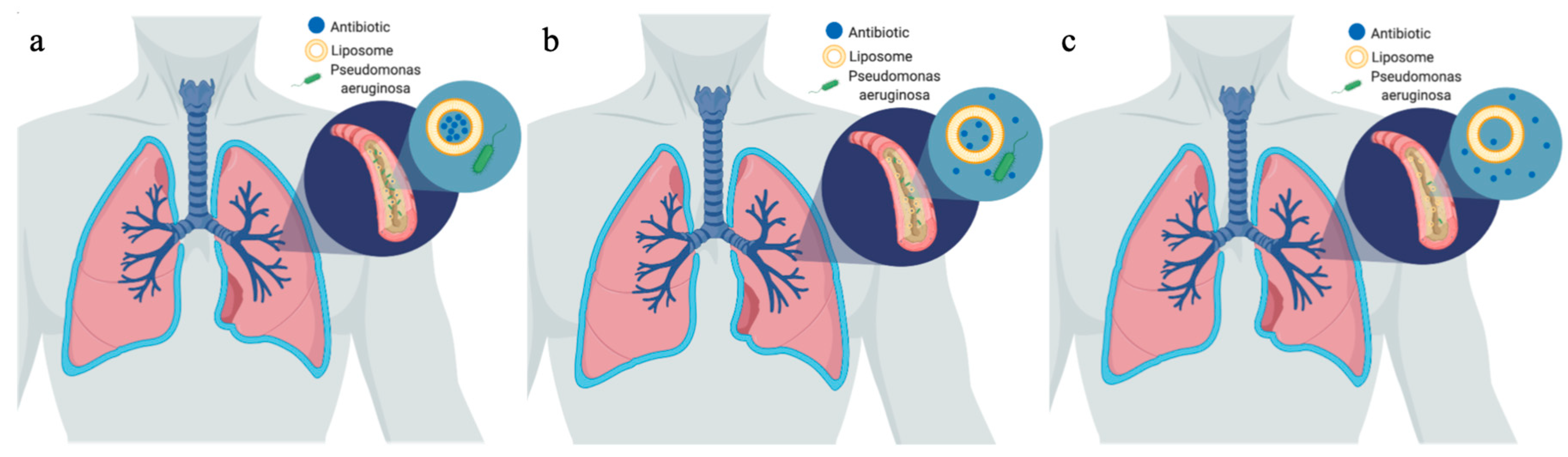
3. Liposomes
4. Solid Lipid Nanoparticles
5. Polymeric Nanoparticles
6. Bacteriophages
Clinical Trials
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bethesda, C.F.F. 4550 M.A.S. 1100 N.; Md 20814301-951-4422 800-344-4823 about Cystic Fibrosis. Available online: http://what-is-cf/about-cystic-fibrosis/ (accessed on 14 July 2019).
- Cystic Fibrosis—Symptoms and Causes. Available online: https://www.mayoclinic.org/diseases-conditions/cystic-fibrosis/symptoms-causes/syc-20353700 (accessed on 14 July 2019).
- Elborn, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Pseudomonas. Available online: http://textbookofbacteriology.net/pseudomonas_2.html (accessed on 14 July 2019).
- Centers for Disease Control and Prevention (US). Antibiotic Resistance Threats in the United States; U.S. Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019.
- Marshall, B. Highlights of the 2014 Patient Registry; Cystic Fibrosis Foundation: Bethesda, MD, USA, 2016. [Google Scholar]
- Ciofu, O.; Rojo-Molinero, E.; Macià, M.D.; Oliver, A. Antibiotic treatment of biofilm infections. APMIS 2017, 125, 304–319. [Google Scholar] [CrossRef] [PubMed]
- Mihai, M.M.; Giurcaneanu, C.; Popa, L.G.; Nitipir, C.; Popa, M.I. Controversies and challenges of chronic wound infection diagnosis and treatment. Mod. Med. 2015, 22, 375–381. [Google Scholar]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for Wound Healing and Infection Control. Materials 2019, 12, 2176. [Google Scholar] [CrossRef]
- Ciobanu, S.; Mihai, M.M.; Popa, L.G.; Giurcaneanu, C.; Popa, M.I. Considerations on the pathogenesis of chronic venous ulcers—Review/Consideratii asupra patogenezei ulcerelor venoase cronice—Review. Infectio ro 2015, 44, 19–24. [Google Scholar]
- Grassi, L.; Di Luca, M.; Maisetta, G.; Rinaldi, A.C.; Esin, S.; Trampuz, A.; Batoni, G. Generation of Persister Cells of Pseudomonas aeruginosa and Staphylococcus aureus by Chemical Treatment and Evaluation of Their Susceptibility to Membrane-Targeting Agents. Front. Microbiol. 2017, 8, 1917. [Google Scholar] [CrossRef]
- Ong, H.X.; Traini, D.; Cipolla, D.; Gonda, I.; Bebawy, M.; Agus, H.; Young, P.M. Liposomal Nanoparticles Control the Uptake of Ciprofloxacin Across Respiratory Epithelia. Pharm. Res. 2012, 29, 3335–3346. [Google Scholar] [CrossRef]
- Pseudomonas Aeruginosa. Available online: http://www.antimicrobe.org/b112.asp (accessed on 14 July 2019).
- Podgoreanu, P.; Negrea, S.M.; Buia, R.; Delcaru, C.; Trusca, S.B.; Lazar, V.; Chifiriuc, M.C. Alternative strategies for fighting multidrug resistant bacterial infections. Biointerface Res. Appl. Chem. 2019, 9, 3834–3841. [Google Scholar]
- Soto-Chilaca, G.A.; Mejia-Garibay, B.; Navarro-Amador, R.; Ramirez-Corona, N.; Palou, E.; Lopez-Malo, A. Cinnamaldehyde-loaded chitosan nanoparticles: Characterization and antimicrobial activity. Biointerface Res. Appl. Chem. 2019, 9, 4060–4065. [Google Scholar]
- Pompilio, A.; Geminiani, C.; Bosco, D.; Rana, R.; Aceto, A.; Bucciarelli, T.; Scotti, L.; Di Bonaventura, G. Electrochemically Synthesized Silver Nanoparticles Are Active Against Planktonic and Biofilm Cells of Pseudomonas aeruginosa and Other Cystic Fibrosis-Associated Bacterial Pathogens. Front. Microbiol. 2018, 9, 1349. [Google Scholar] [CrossRef]
- Liao, S.; Zhang, Y.; Pan, X.; Zhu, F.; Jiang, C.; Liu, Q.; Cheng, Z.; Dai, G.; Wu, G.; Wang, L.; et al. Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa. Int. J. Nanomed. 2019, 14, 1469–1487. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shah, K.N.; Zhang, F.; Salazar, A.J.; Shah, P.N.; Li, R.; Sacchettini, J.C.; Wooley, K.L.; Cannon, C.L. Minocycline and Silver Dual-Loaded Polyphosphoester-Based Nanoparticles for Treatment of Resistant Pseudomonas aeruginosa. Mol. Pharm. 2019, 16, 1606–1619. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, A.M.; Gestal, M.C.; Holban, A.M.; Grumezescu, V.; Vasile, B.Ș.; Mogoantă, L.; Iordache, F.; Bleotu, C.; Mogoșanu, G.D. Biocompatible Fe3O4 Increases the Efficacy of Amoxicillin Delivery against Gram-Positive and Gram-Negative Bacteria. Molecules 2014, 19, 5013–5027. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, A.M.; Andronescu, E.; Holban, A.M.; Ficai, A.; Ficai, D.; Voicu, G.; Grumezescu, V.; Balaure, P.C.; Chifiriuc, C.M. Water dispersible cross-linked magnetic chitosan beads for increasing the antimicrobial efficiency of aminoglycoside antibiotics. Int. J. Pharm. 2013, 454, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, V.; Holban, A.; Iordache, F.; Socol, G.; Mogoşanu, G.; Grumezescu, A.; Ficai, A.; Vasile, B.; Chifiriuc, M.; Maniu, H. MAPLE fabricated magnetite@eugenol and (3-hidroxybutyric acid-co-3-hidroxyvaleric acid)—Polyvinyl alcohol microspheres coated surfaces with anti-microbial properties. Appl. Surf. Sci. 2014, 306, 16–22. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Bleotu, C.; Mihaiescu, D.E.; Chifiriuc, M.C. Synthesis, characterization and in vitro assessment of the magnetic chitosan–carboxymethylcellulose biocomposite interactions with the prokaryotic and eukaryotic cells. Int. J. Pharm. 2012, 436, 771–777. [Google Scholar] [CrossRef]
- Marková, Z.; Siskova, K.; Filip, J.; Safarova, K.; Prucek, R.; Panacek, A.; Kolář, M.; Zboril, R. Chitosan-based synthesis of magnetically-driven nanocomposites with biogenic magnetite core, controlled silver size, and high antimicrobial activity. Green Chem. 2012, 14, 2550–2558. [Google Scholar] [CrossRef]
- Omri, A.; Suntres, Z.E.; Shek, P.N. Enhanced activity of liposomal polymyxin B against Pseudomonas aeruginosa in a rat model of lung infection. Biochem. Pharmacol. 2002, 64, 1407–1413. [Google Scholar] [CrossRef]
- Wong, J.P.; Yang, H.; Blasetti, K.L.; Schnell, G.; Conley, J.; Schofield, L.N. Liposome delivery of ciprofloxacin against intracellular Francisella tularensis infection. J. Control. Release 2003, 92, 265–273. [Google Scholar] [CrossRef]
- Saari, S.M.; Vidgren, M.T.; Herrala, J.; Turjanmaa, V.M.H.; Koskinen, M.O.; Nieminen, M.M. Possibilities of formoterol to enhance the peripheral lung deposition of the inhaled liposome corticosteroids. Respir. Med. 2002, 96, 999–1005. [Google Scholar] [CrossRef][Green Version]
- Weers, J.; Metzheiser, B.; Taylor, G.; Warren, S.; Meers, P.; Perkins, W.R. A gamma scintigraphy study to investigate lung deposition and clearance of inhaled amikacin-loaded liposomes in healthy male volunteers. J. Aerosol Med. Pulm. Drug Deliv. 2009, 22, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Okusanya, Ó.O.; Bhavnani, S.M.; Hammel, J.; Minic, P.; Dupont, L.J.; Forrest, A.; Mulder, G.-J.; Mackinson, C.; Ambrose, P.G.; Gupta, R. Pharmacokinetic and Pharmacodynamic Evaluation of Liposomal Amikacin for Inhalation in Cystic Fibrosis Patients with Chronic Pseudomonal Infection. Antimicrob. Agents Chemother. 2009, 53, 3847–3854. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.D.; Sorensen, K.N.; Neial, M.J.; Durrant, C.; Proffit, R.T. Prophylactic efficacy of aerosolized liposomal (AmBisome) and non-lipsomal (Fungizone) amphotericin B in murine pulmonary aspergillosis. J. Antimicrob. Chemother. 1994, 34, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Slobbe, L.; Boersma, E.; Rijnders, B.J.A. Tolerability of prophylactic aerosolized liposomal amphotericin-B and impact on pulmonary function: Data from a randomized placebo-controlled trial. Pulm. Pharmacol. Ther. 2008, 21, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Vij, N.; Min, T.; Marasigan, R.; Belcher, C.N.; Mazur, S.; Ding, H.; Yong, K.-T.; Roy, I. Development of PEGylated PLGA nanoparticle for controlled and sustained drug delivery in cystic fibrosis. J. Nanobiotechnol. 2010, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Bruinenberg, P.; Blanchard, J.D.; Cipolla, D.C.; Dayton, F.; Mudumba, S.; Gonda, I. Inhaled Liposomal Ciprofloxacin: Once a Day Management of Respiratory Infections. Respir. Drug Deliv. 2010, 1, 73–82. [Google Scholar]
- Meers, P.; Neville, M.; Malinin, V.; Scotto, A.W.; Sardaryan, G.; Kurumunda, R.; Mackinson, C.; James, G.; Fisher, S.; Perkins, W.R. Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J. Antimicrob. Chemother. 2008, 61, 859–868. [Google Scholar] [CrossRef]
- Omri, A.; Beaulac, C.; Bouhajib, M.; Montplaisir, S.; Sharkawi, M.; Lagace, J. Pulmonary retention of free and liposome-encapsulated tobramycin after intratracheal administration in uninfected rats and rats infected with Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1994, 38, 1090–1095. [Google Scholar] [CrossRef]
- Beaulac, C.; Clément-Major, S.; Hawari, J.; Lagacé, J. Eradication of mucoid Pseudomonas aeruginosa with fluid liposome-encapsulated tobramycin in an animal model of chronic pulmonary infection. Antimicrob. Agents Chemother. 1996, 40, 665–669. [Google Scholar] [CrossRef]
- Bakker-Woudenberg, I.A.J.M.; ten Kate, M.T.; Guo, L.; Working, P.; Mouton, J.W. Ciprofloxacin in Polyethylene Glycol-Coated Liposomes: Efficacy in Rat Models of Acute or Chronic Pseudomonas aeruginosa Infection. Antimicrob. Agents Chemother. 2002, 46, 2575–2581. [Google Scholar] [CrossRef]
- Ghaffari, S.; Varshosaz, J.; Saadat, A.; Atyabi, F. Stability and antimicrobial effect of amikacin-loaded solid lipid nanoparticles. Int. J. Nanomed. 2010, 6, 35–43. [Google Scholar]
- Nafee, N.; Husari, A.; Maurer, C.K.; Lu, C.; de Rossi, C.; Steinbach, A.; Hartmann, R.W.; Lehr, C.-M.; Schneider, M. Antibiotic-free nanotherapeutics: Ultra-small, mucus-penetrating solid lipid nanoparticles enhance the pulmonary delivery and anti-virulence efficacy of novel quorum sensing inhibitors. J. Control Release 2014, 192, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Ghaffari, S.; Mirshojaei, S.F.; Jafarian, A.; Atyabi, F.; Kobarfard, F.; Azarmi, S. Biodistribution of amikacin solid lipid nanoparticles after pulmonary delivery. BioMed Res. Int. 2013, 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, R.; Zara, G.P.; Caputo, O.; Bargoni, A.; Fundarò, A.; Gasco, M.R. Transmucosal transport of tobramycin incorporated in SLN after duodenal administration to rats. Part I—A pharmacokinetic study. Pharmacol. Res. 2000, 42, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Bargoni, A.; Cavalli, R.; Zara, G.P.; Fundarò, A.; Caputo, O.; Gasco, M.R. Transmucosal transport of tobramycin incorporated in solid lipid nanoparticles (sln) after duodenal administration to rats. Part II—Tissue distribution. Pharmacol. Res. 2001, 43, 497–502. [Google Scholar] [CrossRef]
- Pastor, M.; Moreno-Sastre, M.; Esquisabel, A.; Sans, E.; Viñas, M.; Bachiller, D.; Asensio, V.J.; Pozo, Á.D.; Gainza, E.; Pedraz, J.L. Sodium colistimethate loaded lipid nanocarriers for the treatment of Pseudomonas aeruginosa infections associated with cystic fibrosis. Int. J. Pharm. 2014, 477, 485–494. [Google Scholar] [CrossRef]
- Ryan, G.M.; Kaminskas, L.M.; Kelly, B.D.; Owen, D.J.; McIntosh, M.P.; Porter, C.J.H. Pulmonary Administration of PEGylated Polylysine Dendrimers: Absorption from the Lung versus Retention within the Lung Is Highly Size-Dependent. Mol. Pharm. 2013, 10, 2986–2995. [Google Scholar] [CrossRef]
- Ungaro, F.; d’Angelo, I.; Coletta, C.; d’Emmanuele di Villa Bianca, R.; Sorrentino, R.; Perfetto, B.; Tufano, M.A.; Miro, A.; La Rotonda, M.I.; Quaglia, F. Dry powders based on PLGA nanoparticles for pulmonary delivery of antibiotics: Modulation of encapsulation efficiency, release rate and lung deposition pattern by hydrophilic polymers. J. Control Release 2012, 157, 149–159. [Google Scholar] [CrossRef]
- Günday Türeli, N.; Torge, A.; Juntke, J.; Schwarz, B.C.; Schneider-Daum, N.; Türeli, A.E.; Lehr, C.-M.; Schneider, M. Ciprofloxacin-loaded PLGA nanoparticles against cystic fibrosis P. aeruginosa lung infections. Eur. J. Pharm. Biopharm. 2017, 117, 363–371. [Google Scholar] [CrossRef]
- Hua, X.; Tan, S.; Bandara, H.M.H.N.; Fu, Y.; Liu, S.; Smyth, H.D.C. Externally Controlled Triggered-Release of Drug from PLGA Micro and Nanoparticles. PLoS ONE 2014, 9, e114271. [Google Scholar] [CrossRef]
- Cheow, W.S.; Hadinoto, K. Lipid-polymer hybrid nanoparticles with rhamnolipid-triggered release capabilities as anti-biofilm drug delivery vehicles. Particuology 2012, 10, 327–333. [Google Scholar] [CrossRef]
- Abdelghany, S.M.; Quinn, D.J.; Ingram, R.J.; Gilmore, B.F.; Donnelly, R.F.; Taggart, C.C.; Scott, C.J. Gentamicin-loaded nanoparticles show improved antimicrobial effects towards Pseudomonas aeruginosa infection. Int. J. Nanomed. 2012, 7, 4053–4063. [Google Scholar]
- Deacon, J.; Abdelghany, S.M.; Quinn, D.J.; Schmid, D.; Megaw, J.; Donnelly, R.F.; Jones, D.S.; Kissenpfennig, A.; Elborn, J.S.; Gilmore, B.F.; et al. Antimicrobial efficacy of tobramycin polymeric nanoparticles for Pseudomonas aeruginosa infections in cystic fibrosis: Formulation, characterisation and functionalisation with dornase alfa (DNase). J. Control Release 2015, 198, 55–61. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, I.; Casciaro, B.; Miro, A.; Quaglia, F.; Mangoni, M.L.; Ungaro, F. Overcoming barriers in Pseudomonas aeruginosa lung infections: Engineered nanoparticles for local delivery of a cationic antimicrobial peptide. Colloids Surf. B Biointerfaces 2015, 135, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Flockton, T.R.; Schnorbus, L.; Araujo, A.; Adams, J.; Hammel, M.; Perez, L.J. Inhibition of Pseudomonas aeruginosa Biofilm Formation with Surface Modified Polymeric Nanoparticles. Pathogens 2019, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Ho, K. Bacteriophage Therapy for Bacterial Infections: Rekindling a Memory from the Pre-Antibiotics Era. Perspect. Biol. Med. 2001, 44, 1–16. [Google Scholar] [CrossRef]
- Golshahi, L.; Lynch, K.H.; Dennis, J.J.; Finlay, W.H. In vitro lung delivery of bacteriophages KS4-M and ΦKZ using dry powder inhalers for treatment of Burkholderia cepacia complex and Pseudomonas aeruginosa infections in cystic fibrosis. J. Appl. Microbiol. 2011, 110, 106–117. [Google Scholar] [CrossRef]
- Matinkhoo, S.; Lynch, K.H.; Dennis, J.J.; Finlay, W.H.; Vehring, R. Spray-dried Respirable Powders Containing Bacteriophages for the Treatment of Pulmonary Infections. J. Pharm. Sci. 2011, 100, 5197–5205. [Google Scholar] [CrossRef]
- Sahota, J.S.; Smith, C.M.; Radhakrishnan, P.; Winstanley, C.; Goderdzishvili, M.; Chanishvili, N.; Kadioglu, A.; O’Callaghan, C.; Clokie, M.R.J. Bacteriophage Delivery by Nebulization and Efficacy Against Phenotypically Diverse Pseudomonas aeruginosa from Cystic Fibrosis Patients. J. Aerosol Med. Pulm. Drug Deliv. 2015, 28, 353–360. [Google Scholar] [CrossRef]
- Westwater, C.; Kasman, L.M.; Schofield, D.A.; Werner, P.A.; Dolan, J.W.; Schmidt, M.G.; Norris, J.S. Use of Genetically Engineered Phage To Deliver Antimicrobial Agents to Bacteria: An Alternative Therapy for Treatment of Bacterial Infections. Antimicrob. Agents Chemother. 2003, 47, 1301–1307. [Google Scholar] [CrossRef]
- Molin, S.; Jensen, L.B.; Kristensen, C.S.; Givskov, M.; Ramos, J.L.; Bej, A.K. Suicidal Genetic Elements and Their Use In Biological Containment Of Bacteria. Annu. Rev. Microb. 1993, 47, 139–166. [Google Scholar] [CrossRef] [PubMed]
- Glonti, T.; Chanishvili, N.; Taylor, P.W. Bacteriophage-derived enzyme that depolymerizes the alginic acid capsule associated with cystic fibrosis isolates of Pseudomonas aeruginosa. J. Appl. Microbiol. 2010, 108, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Ciofu, O.; Bjarnsholt, T. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol. 2010, 5, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Rao, S.; Bansal, A.; Dang, S.; Gupta, S.; Gabrani, R. Pseudomonas aeruginosa biofilm: Potential therapeutic targets. Biologicals 2014, 42, 1–7. [Google Scholar] [CrossRef]
- McVay, C.S.; Velásquez, M.; Fralick, J.A. Phage Therapy of Pseudomonas aeruginosa Infection in a Mouse Burn Wound Model. Antimicrob. Agents Chemother. 2007, 51, 1934–1938. [Google Scholar] [CrossRef]
- Danelishvili, L.; Young, L.S.; Bermudez, L.E. In Vivo Efficacy of Phage Therapy for Mycobacterium avium Infection As Delivered by a Nonvirulent Mycobacterium. Microb. Drug Resist. 2006, 12, 1–6. [Google Scholar] [CrossRef]
- Safety/Tolerability Study of ArikayceTM in Cystic Fibrosis Patients with Chronic Infection Due to Pseudomonas Aeruginosa—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00558844 (accessed on 15 September 2019).
- Extension Study of Liposomal Amikacin for Inhalation in Cystic Fibrosis (CF) Patients with Chronic Pseudomonas Aeruginosa (Pa) Infection—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01316276 (accessed on 15 September 2019).
- Multidose Safety and Tolerability Study of Dose Escalation of Liposomal Amikacin for Inhalation (ARIKACETM)—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00777296 (accessed on 15 September 2019).
- Study of Dose Escalation of Liposomal Amikacin for Inhalation (ARIKAYCETM)—Extension Phase—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03905642 (accessed on 15 September 2019).
- Study to Evaluate ArikayceTM in CF Patients With Chronic Pseudomonas Aeruginosa Infections—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01315678 (accessed on 15 September 2019).
| Title of Clinical Trial | Phase | Participants | Location | Begin Date | End Date | Result |
|---|---|---|---|---|---|---|
| Safety/Tolerability Study of Arikayce™ in Cystic Fibrosis Patients With Chronic Infection Due to P. aeruginosa | 1 and 2 | 41 | USA | January 2008 | June 2009 | Safe for use |
| Extension Study of Liposomal Amikacin for Inhalation in Cystic Fibrosis (CF) Patients With Chronic P. aeruginosa (Pa) Infection | 3 | 206 | Austria, Belgium, Bulgaria, Canada, Denmark, France, Germany, Greece, Hungary, Ireland, Italy, Netherlands, Poland, Serbia, Slovakia, Spain, United Kingdom | 5 October 2012 | 16 July 2015 | Had Adverse Events throughout study |
| Multidose Safety and Tolerability Study of Dose Escalation of Liposomal Amikacin for Inhalation (ARIKACE™) | 1 and 2 | 66 | Belgium, Hungary, North Macedonia, Poland, Serbia, Slovakia, Ukraine | 22 February 2007 | 27 February 2008 | There were some clinically significant laboratory abnormalities |
| Study of Dose Escalation of Liposomal Amikacin for Inhalation (ARIKAYCE™)—Extension Phase | 2 | 49 | Belgium, Hungary, North Macedonia, Poland, Serbia, Slovakia, Ukraine | 8 January 2009 | 2 November 2010 | Adverse events of 560 mg dose of Arikayce administered for six cycles in eighteen months |
| Study to Evaluate Arikayce™ in CF Patients With Chronic P. aeruginosa Infections | 3 | 302 | Austria, Belgium, Bulgaria, Canada, Denmark, France, Germany, Greece, Hungary, Ireland, Italy, Netherlands, Poland, Serbia, Slovakia, Spain, Sweden, United Kingdom | 29 February 2012 | June 2013 | Adverse effects |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chirgwin, M.E.; Dedloff, M.R.; Holban, A.M.; Gestal, M.C. Novel Therapeutic Strategies Applied to Pseudomonas aeruginosa Infections in Cystic Fibrosis. Materials 2019, 12, 4093. https://doi.org/10.3390/ma12244093
Chirgwin ME, Dedloff MR, Holban AM, Gestal MC. Novel Therapeutic Strategies Applied to Pseudomonas aeruginosa Infections in Cystic Fibrosis. Materials. 2019; 12(24):4093. https://doi.org/10.3390/ma12244093
Chicago/Turabian StyleChirgwin, Michael E., Margaret R. Dedloff, Alina Maria Holban, and Monica C. Gestal. 2019. "Novel Therapeutic Strategies Applied to Pseudomonas aeruginosa Infections in Cystic Fibrosis" Materials 12, no. 24: 4093. https://doi.org/10.3390/ma12244093
APA StyleChirgwin, M. E., Dedloff, M. R., Holban, A. M., & Gestal, M. C. (2019). Novel Therapeutic Strategies Applied to Pseudomonas aeruginosa Infections in Cystic Fibrosis. Materials, 12(24), 4093. https://doi.org/10.3390/ma12244093







