The Use of Biodrying to Prevent Self-Heating of Alternative Fuel
Abstract
1. Introduction
- particle size (degree of material fineness);
- organic matter content;
- moisture content;
- prism size and height; and
- pressure in the heap.
- Ozonation. Mechanically, ozone reacts with polysaccharides, proteins, and lipids, transforming them into low molecular weight compounds as a result of cell membrane rupture. If the ozone dose is high enough, mineralization of released cellular compounds may also occur [24]. The effectiveness of ozonation depends on the type of waste, the dose of ozone, and the pH. Ozone treatment is a very effective method of waste hygienization, but expensive because of the need for an ozone generator.
- Interaction with ultrasound or microwave radiation. Microwave radiation is a type of electromagnetic radiation with a wavelength ranging from 1 m to about 1 mm. The wave spectrum is between IR and ultra-short wave. As in the case of ozonation, these processes destroy the cell membranes of microorganisms and are, unfortunately, cost-intensive [25]. Paradoxically, as a result of the use of ultrasound and microwaves, an organic substrate is released, which can be a source of easily absorbable organic carbon for microorganisms [26,27,28].
- Change in pH due to the addition of basic (e.g., pile lime) or acidic compounds. Bacteria require neutral conditions, so their fastest growth occurs at an environmental pH of 6.8 to 7.2 [29]. At pH below 6.6, the rate of bacterial growth is rapidly reduced [30]. Increasing the pH may, in turn, lead to an increase in the concentration of ammonia in the reactor and, as a result, to inhibition of the process [22]. The addition of quicklime to waste by several percentage points causes an increase in pH above 10 and complete and permanent deactivation of microorganisms.
- Biodrying. One of the processes leading to a decrease in the activity of microorganisms in the waste pile that uses the heat they release is biological drying. The process aims to reduce moisture while maintaining high heat of waste combustion. The waste is heated due to the decomposition of an easily biodegradable part of organic matter [15]. Then, fans are started to extract warm and humid air from the pile, e.g., using drainage pipes and bioreactor systems integrated with biofilters. The maximum temperature achieved in this process is 70 °C, which contributes to the destruction of microorganisms and the disappearance of the biological degradation process [31]. Biodrying is usually used in the biological transformation processes of mixed municipal waste and organic waste [16,17,18,32,33,34]. As a result of the process, a stable, manageable product for cement can be produced [17,18,35,36].
2. Materials and Methods
2.1. Materials
- Variant A—fuel produced from mixed MSW.
- Variant B—fuel produced from bulky waste and derived from mechanical plastic sorting (mainly HDPE, LDPE, PP, PS, PET) and paper—unsuitable for the material recycling process due to the level of organic residue contamination.
- Variant C—fuel produced from car tires and residues from the mechanical sorting process of plastic (mainly HDPE, LDPE, PP, PS, PET) and paper—unsuitable for the material recycling process due to the level of organic residue contamination.
2.2. Sampling and Laboratory Tests
2.3. Biodrying Process
2.4. Thermographic Analysis
3. Results
3.1. Characteristics of Raw Materials
3.2. Impact of Biodrying on Refuse-Derived Fuel (RDF) Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Georgiopoulou, M.; Lyberatos, G. Life cycle assessment of the use of alternative fuels in cement kilns: A case study. J. Environ. Manag. 2018, 216, 224–243. [Google Scholar] [CrossRef] [PubMed]
- GTZ-Holcim. Guidelines on Co-Processing, Waste Materials in Cement Production; Holcim Group Support Ltd.: Zurich, Switzerland; Deutsche Gesellschaft for Technische Zusammenarbeit (GTZ) GmbH: Eschborn, Germany, 2006. [Google Scholar]
- Vasconcelos, C.; Silva, B. Insight on the Self-Ignition Behaviour of RDF Components; Energy and Environmental Engineering Research Group (ENVERG) of IBB/CERENA: Lisboa, Portugal, 2014; pp. 1–10. [Google Scholar]
- Hogland, W.; Marques, M. Physical, biological and chemical processes during storage and spontaneous combustion of waste fuel. Resour. Conserv Recycl. 2003, 40, 53–69. [Google Scholar] [CrossRef]
- Yasuhara, A. Chemical consideration on spontaneous incineration accidents of refuse-derived fuels and exothermic reaction mechanism. J. Jpn. Soc. Saf. Eng. 2006, 45, 117–124. [Google Scholar]
- Rahman, A.; Rasul, M.G.; Khan, M.M.K.; Sharma, S. Recent development on the uses of alternative fuels in cement manufacturing process. Fuel 2015, 145, 84–99. [Google Scholar] [CrossRef]
- Ariyaratne, H.W.K. Alternative Fuels in Cement Kilns–Characterization and Experiments. Master’s Thesis, Telemark University College, Porsgrunn, Norway, 23 January 2009. [Google Scholar]
- Yasuhara, A.; Amano, Y.; Shibamoto, T. Investigation of the self-heating and spontaneous ignition of refuse-derived fuel (RDF) during storage. Waste Manag. 2010, 30, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Hirano, T. Process of accidental explosions at a refuse derived fuel storage. J. Loss Prev. Process Ind. 2006, 19, 288–291. [Google Scholar] [CrossRef]
- Malinowski, M.; Sikora, G. Wpływ zawartości odpadów ulegających biodegradacji na właściwości paliwa alternatywnego z odpadów. Proc. Ecopole 2014, 8, 223–230. [Google Scholar]
- Malinowski, M.; Wolny-Koładka, K. Badania procesu samonagrzewania się paliwa alternatywnego wytwarzanego ze zmieszanych odpadów komunalnych. Proc. Ecopole 2015, 9, 256–261. [Google Scholar]
- CSO—Central Statistical Office. Ochrona Środowiska (Environment); Central Statistical Office: Warszawa, Poland, 2018. Available online: https://api.dane.gov.pl/media/resources/20190124/ochrona_srodowiska_2018.pdf (accessed on 2 July 2019).
- Polski Komitet Normalizacyjny. Charakteryzowanie Odpadów—Pobieranie Próbek Materiałów—Struktura Przygotowania i Zastosowania Planu Pobierania Próbek; PN-EN 14899:2006; Polski Komitet Normalizacyjny: Warshaw, Poland, 2006. [Google Scholar]
- Vaverková, M.D.; Adamcová, D.; Zloch, J.; Radziemska, M.; Boas Berg, A.; Voběrková, S.; Maxianová, A. Impact of Municipal Solid Waste Landfill on Environment—A Case Study. J. Ecol. Eng. 2018, 19, 55–68. [Google Scholar] [CrossRef]
- Velis, C.A.; Longhurst, H.; Drew, R.; Smith, R.; Pollard, S.J.T. Biodrying for mechanical-biological treatment of wastes: A review of process science and engineering. Bioresour. Technol. 2009, 100, 2747–2761. [Google Scholar] [CrossRef]
- Yang, B.; Hao, Z.; Jahng, D. Advances in biodrying technologies for converting organic wastes into solid fuel. Dry. Technol. 2017, 35, 1950–1969. [Google Scholar] [CrossRef]
- Adani, F.; Baido, D.; Calcatera, E.; Genevini, P. The influence of biomass temperature on biostabilization-biodrying of municipal solid waste. Bioresour. Technol. 2002, 83, 173–179. [Google Scholar] [CrossRef]
- Sugni, M.; Calcatera, E.; Adani, F. Biostabilization-biodrying of municipal solid waste by inverting air-flow. Bioresour. Technol. 2005, 96, 1331–1337. [Google Scholar] [CrossRef]
- Gajewska, T.; Grigoroudis, E. Estimating the performance of the logistics services attributes influencing customer satisfaction in the field of refrigerated transport. Int. J. Shipp. Transp. Logist. 2017, 9, 540–561. [Google Scholar] [CrossRef]
- Chwał, M.; Muc, A. Design of reinforcement in nanoand microcomposites. Materials 2019, 12, 1474. [Google Scholar] [CrossRef]
- Vaverková, M.D. Evaluation of the addition of immobilizing agents on selected physicochemical properties of soil contaminated with heavy metals. Pol. J. Soil Sci. 2018, 51, 59–69. [Google Scholar] [CrossRef]
- Jędrczak, A. Biologiczne Przetwarzanie Odpadów; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2008. [Google Scholar]
- Vaverková, M.D.; Elbl, J.; Radziemska, M.; Adamcová, D.; Kintl, A.; Baláková, L.; Bartoň, S.; Hladký, J.; Kynický, J.; Brtnický, M. Environmental risk assessment and consequences of municipal solid waste disposal. Chemosphere 2018, 208, 569–578. [Google Scholar] [CrossRef]
- Elliott, A.; Mahmood, T. Pretreatment technologies for advancing anaerobic digestion of pulp and paper biotreatment residues. Water Res. 2007, 41, 4273–4286. [Google Scholar] [CrossRef]
- Dehghani, H.M. Effectiveness of ultrasound on the destruction of Escherichia coli. Am. J. Environ. Sci. 2005, 1, 187–189. [Google Scholar] [CrossRef][Green Version]
- Hu, Z.H.; Wen, Z.Y. Ehnancing enzymatic digestability of switchgrass by microwave-assisted alkali treatment. Biochem. Eng. J. 2008, 38, 369–378. [Google Scholar] [CrossRef]
- Kwiatowska, B.; Bennett, J.; Akunna, J.; Walker, G.M.; Bremner, D.H. Stimulation of bioprocess by ultrasound. Biotech. Adv. 2011, 29, 768–780. [Google Scholar] [CrossRef]
- Mason, T.J.; Peters, D. Practical Sonochemistry: Power Ultrasound and Applications, 2nd ed.; Woodhead Publishing: Chichester, UK, 2002; pp. 150–166. [Google Scholar]
- Thomé-Kozmiensky, K.J. Biologische Abfallbehandlung; EF—Verlag für Energie-und Umwelttechnik GmbH: Berlin, Germany, 1995. [Google Scholar]
- Mosey, F.E.; Fernandes, X.A. Patterns of hydrogen in biogas from the anaerobic diction of milk—Sugars. In Proceedings of the Fourteenth Biennial Conference of the International Association on Water Pollution Research and Control, Brighton, UK, 18–21 July 1988; Pergamon: Oxford, UK, 1988; pp. 187–196. [Google Scholar]
- Malinowski, M.; Wolny-Koładka, K. Microbiological and energetic assessment of the effects of the biodrying of fuel produced from waste. Ecol. Chem. Eng. 2017, 24, 551–564. [Google Scholar] [CrossRef][Green Version]
- Dębicka, M.; Żygadło, M.; Latosińska, J. Investigations of bio-drying process of municipal solid waste. Ecol. Chem. Eng. Ser. A 2013, 20, 1461–1470. [Google Scholar]
- Bilgin, M.; Tulun, S. Biodrying for municipal solid waste: Volume and weight reduction. Environ. Technol. 2015, 36, 1691–1697. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, D.; Li, Y.; Chadwick, D.; Li, G.; Li, Y.; Du, L. Effects of adding bulking agents on biostabilization and drying of municipal solid waste. Waste Manag. 2017, 62, 52–60. [Google Scholar] [CrossRef]
- Domińczyk, A.; Krzystek, L.; Ledakowicz, S. Biologiczne suszenie mieszaniny stałych odpadów przemysłu papierniczego oraz organicznej frakcji stałych odpadów komunalnych. Inżynieria Aparatura Chemiczna 2012, 51, 115–116. [Google Scholar]
- Trezza, M.A.; Scian, A.N. Waste fuels: Their effect on Portland cement clinker. Cem. Concr. Res. 2005, 35, 438–444. [Google Scholar] [CrossRef]
- European Committee for Standardization. Solid Recovered Fuels. Determination of Moisture Content Using the Oven Dry Method. Determination of Total Moisture by a Reference Method; DD CEN/TS 15414-1:2006; European Committee for Standardization: Brussels, Belgium, 2010. [Google Scholar]
- Polski Komitet Normalizacyjny. Stałe Paliwa Wtórne—Metody Oznaczania Zawartości Węgla (C), Wodoru (H), Azotu (N); PN-EN 15407: 2011; Polski Komitet Normalizacyjny: Warshaw, Poland, 2011. [Google Scholar]
- Polski Komitet Normalizacyjny. Stałe Paliwa Wtórne—Metody Oznaczania Zawartości Siarki (S), Chloru (Cl), Fluoru (F) i Bromu (Br); PN-EN 15408: 2011; Polski Komitet Normalizacyjny: Warshaw, Poland, 2011. [Google Scholar]
- Polski Komitet Normalizacyjny. Stałe Paliwa Wtórne—Oznaczanie Wartości Opałowej; PN-EN 15400: 2011; Polski Komitet Normalizacyjny: Warshaw, Poland, 2011. [Google Scholar]
- Hurka, M.; Malinowski, M. Assessment of the use of EVA bioreactor in the process of bio-drying of undersize fraction manufactured from mixed municipal solid waste. Infrastruct. Ecol. Rural Areas 2014, 4, 1127–1136. [Google Scholar]
- Tom, A.; Pawels, R.; Haridas, A. Biodrying process: A sustainable technology for treatment of municipal solid waste with high moisture content. Waste Manag. 2016, 49, 64–71. [Google Scholar] [CrossRef]
- Hao, Z.; Jahng, D. Variations of organic matters and extracellular enzyme activities during biodrying of dewatered sludge with different bulking agents. Biochem. Eng. J. 2019, 147, 126–135. [Google Scholar] [CrossRef]
- Agro Eko. Available online: https://www.agro-eko.cz/en/ (accessed on 19 July 2019).
- Mokrzycki, E.; Uliasz-Bocheńczyk, A.; Sarna, M. Use of alternative fuels in the Polish cement industry. Appl. Energy 2003, 74, 101–111. [Google Scholar] [CrossRef]
- Baran, D.; Famielec, S.; Koncewicz-Baran, M.; Malinowski, M.; Sobol, Z. The changes in exhaust gas and selected waste properties during biostabilization process. Proc. Ecopole 2016, 10, 11–18. [Google Scholar]
- Skourides, I.; Theophilou, C.; Loizides, M.; Hood, P.; Smith, S.R. Optimisation of advanced technology for production of consistent auxiliary fuels from biodegradable municipal waste for industrial purposes. In Proceedings of the Waste 2006–41 Sustainable Waste and Resource Management, Stratford-upon-Avon, UK, 19–21 September 2006. [Google Scholar]
- Winkler, M.K.H.; Bennenbroek, M.H.; Horstink, F.H.; van Loosdrecht, M.C.M.; van de Pol, G.J. The biodrying concept: An innovative technology creating energy from sewage sludge. Bioresour. Technol. 2013, 147, 124–129. [Google Scholar] [CrossRef]
- Sadaka, S.; Van Devender, K.; Costello, T.; Sharara, M. Composting for Biodrying Organic Materials. FSA1055. University of Arkansas Division of Agriculture, 2010. Available online: https://www.uaex.edu/publications/PDF/FSA-1055.pdf (accessed on 12 July 2019).
- Colomer-Mendoza, F.J.; Robles-Martinez, F.; Pina-Guzman, A.B.; Monserrat, P.V.; Gallardo, A. Influence of different airflows and the presence of bulking agent on biodrying of gardening wastes in reactors. Rev. Int. Contaminación Ambient. 2016, 32, 161–171. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, L.; Li, A. Energy-efficient co-biodrying of dewatered sludge and food waste: Synergistic enhancement and variables investigation. Waste Manag. 2016, 56, 411–422. [Google Scholar] [CrossRef]
- Mohammed, M.; Ozbay, I.; Durmusoglu, E. Bio-drying of green waste with high moisture content. Process Saf. Environ. Prot. 2017, 111, 420–427. [Google Scholar] [CrossRef]


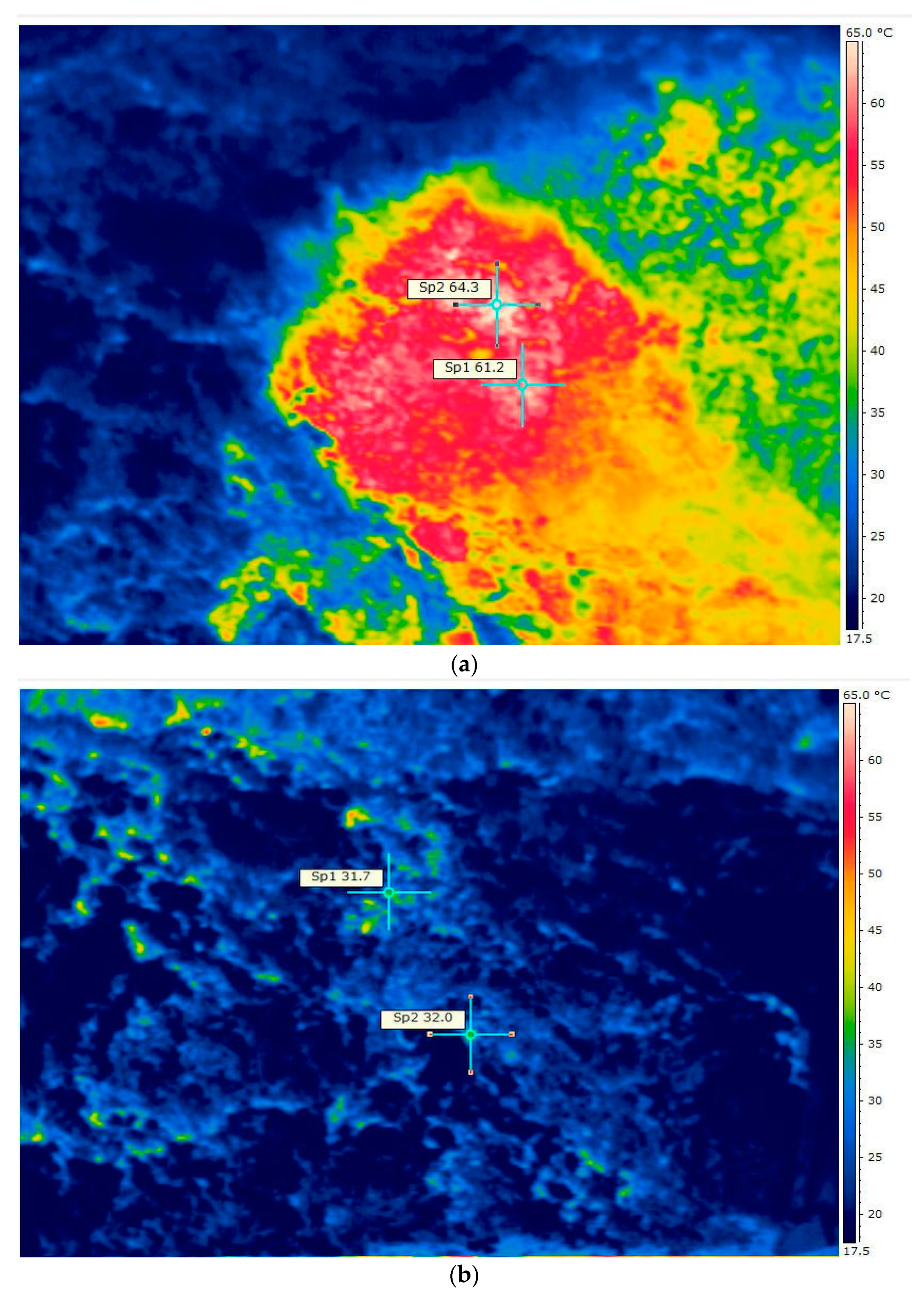
| Parameter | Unit | Variants | ||
|---|---|---|---|---|
| - | - | from Mixed Municipal Waste (A) | from Residues from Selective Collection Waste and Bulky Waste (B) | Plastic and Tires (C) |
| Moisture content | % w/w | 25.4 ± 3.2 | 13.4 ± 0.3 | 3.1 ± 0.3 |
| Ash content | % d.m. | 23.1 ± 0.9 | 22.6 ± 0.9 | 11.5 ± 0.7 |
| Sulphur content | % d.m. | 0.39 ± 0.08 | 0.45 ± 0.05 | 1.66 ± 0.11 |
| Total carbon content | % d.m. | 48.3 ± 3.0 | 53.6 ± 3.1 | 58.7 ± 4.2 |
| Hydrogen content | % d.m. | 6.56 ± 0.71 | 5.78 ± 0.82 | 8.76 ± 1.01 |
| Nitrogen content | % d.m. | 0.78 ± 0.08 | 0.70 ± 0.08 | 0.68 ± 0.08 |
| Heat of combustion | kJ·kg−1 d.m. | 19,979 ± 1019 | 23,366 ± 772 | 31266 ± 819 |
| Calorific value | kJ·kg−1 | 13,833 ± 883 | 18,762 ± 704 | 30975 ± 725 |
| Chlorine content | % d.m. | 0.65 ± 0.13 | 1.12 ± 0.22 | 1.43 ± 0.17 |
| Element | Unit | A | B | C |
|---|---|---|---|---|
| As | mg·kg−1 d.m. | 14 ± 2 | 11 ± 2 | 38 ± 4 |
| Ba | mg·kg−1 d.m. | 272 ± 11 | 258 ± 9 | 401 ± 6 |
| Cd | mg·kg−1 d.m. | 0.8 ± 0.2 | 3.1 ± 0.3 | 6.1 ± 0.2 |
| Co | mg·kg−1 d.m. | 12 ± 1 | 7 ± 1 | 11 ± 2 |
| Cu | mg·kg−1 d.m. | 114 ± 11 | 592 ± 32 | 710 ± 31 |
| Cr | mg·kg−1 d.m. | 136 ± 9 | 260 ± 12 | 600 ± 18 |
| Hg | mg·kg−1 d.m. | 0.9 ± 0.1 | 0.4 ± 0.1 | 0.8 ± 0.1 |
| Mo | mg·kg−1 d.m. | 20 ± 6 | 59 ± 7 | 171 ± 8 |
| Ni | mg·kg−1 d.m. | 9 ± 3 | 140 ± 3 | 315 ± 6 |
| Pb | mg·kg−1 d.m. | 3 ± 2 | 123 ± 7 | 25 ± 5 |
| Sb | mg·kg−1 d.m. | 32 ± 2 | 32 ± 2 | 12 ± 2 |
| Se | mg·kg−1 d.m. | 18 ± 3 | 39 ± 6 | 22 ± 6 |
| Sn | mg·kg−1 d.m. | 14 ± 4 | 45 ± 3 | 31 ± 3 |
| Sr | mg·kg−1 d.m. | 107 ± 4 | 177 ± 11 | 412 ± 17 |
| V | mg·kg−1 d.m. | 12 ± 1 | 12 ± 1 | 32 ± 1 |
| Zn | mg·kg−1 d.m. | 540 ± 17 | 600 ± 12 | 633 ± 10 |
| Parameter | Unit | Variants of Alternative Fuel Produced | ||
|---|---|---|---|---|
| - | - | from Mixed Municipal Waste (A) | from Residues from Selective Collection Waste and Bulky Waste (B) | Plastic and Tires (C) |
| Moisture content | % w/w | 11.9 ± 1.0 | 6.8 ± 0.8 | 4.7 ± 0.8 |
| Ashes content | % d.m. | 22.5 ± 0.2 | 21.3 ± 0.3 | 12.6 ± 0.2 |
| Sulphur content | % d.m. | 0.23 ± 0.04 | 0.70 ± 0.12 | 1.59 ± 0.05 |
| Total carbon content | % d.m. | 48.5 ± 1.5 | 53.7 ± 1.1 | 55.1 ± 1.8 |
| Hydrogen content | % d.m. | 6.1 ± 0.2 | 7.6 ± 0.3 | 8.8 ± 0.2 |
| Nitrogen content | % d.m. | 0.65 ± 0.17 | 0.47 ± 0.16 | 0.63 ± 0.21 |
| Heat of combustion | kJ·kg−1 d.m. | 20,848 ± 156 | 23,540 ± 334 | 30962 ± 152 |
| Calorific value | kJ·kg−1 | 18,439 ± 155 | 20,686 ± 301 | 30556 ± 140 |
| Chloride content | % d.m. | 0.80 ± 0.11 | 1.14 ± 0.09 | 1.50 ± 0.08 |
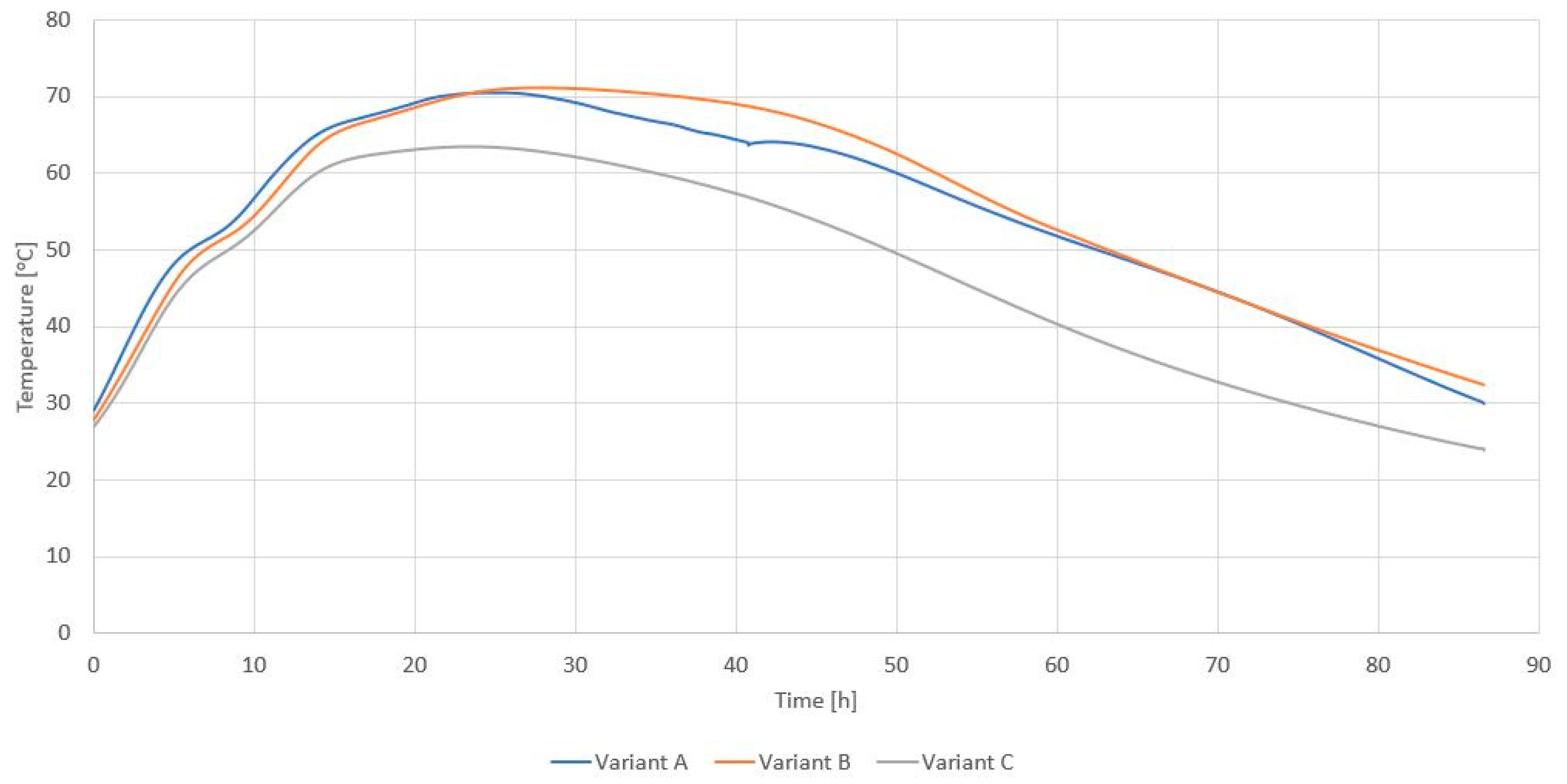
| Variant | Maximum RDF Temperature [°C] during Storage on the Pile after: | |||||
|---|---|---|---|---|---|---|
| 6 h | 12 h | 24 h | 48 h | 72 h | ||
| A | Before biodrying | 26.8 | 30.6 | 45.4 | 66.5 | 68.2 |
| After biodrying | 30.2 | 29.7 | 31.2 | 30.9 | 29.6 | |
| B | Before biodrying | 25.4 | 29.9 | 42.8 | 62.9 | 66.2 |
| After biodrying | 30.0 | 29.8 | 28.7 | 28.6 | 27.2 | |
| C | Before biodrying | 24.6 | 29.4 | 39.7 | 48.8 | 52.6 |
| After biodrying | 24.6 | 25.7 | 24.3 | 25.2 | 24.9 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajewska, T.; Malinowski, M.; Szkoda, M. The Use of Biodrying to Prevent Self-Heating of Alternative Fuel. Materials 2019, 12, 3039. https://doi.org/10.3390/ma12183039
Gajewska T, Malinowski M, Szkoda M. The Use of Biodrying to Prevent Self-Heating of Alternative Fuel. Materials. 2019; 12(18):3039. https://doi.org/10.3390/ma12183039
Chicago/Turabian StyleGajewska, Teresa, Mateusz Malinowski, and Maciej Szkoda. 2019. "The Use of Biodrying to Prevent Self-Heating of Alternative Fuel" Materials 12, no. 18: 3039. https://doi.org/10.3390/ma12183039
APA StyleGajewska, T., Malinowski, M., & Szkoda, M. (2019). The Use of Biodrying to Prevent Self-Heating of Alternative Fuel. Materials, 12(18), 3039. https://doi.org/10.3390/ma12183039






