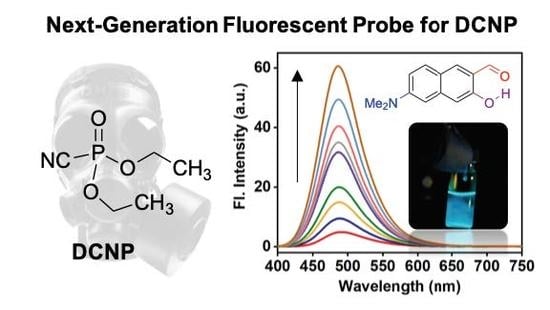
A Selective Fluorescence Turn-On Probe for the Detection of DCNP (Nerve Agent Tabun Simulant)
Abstract
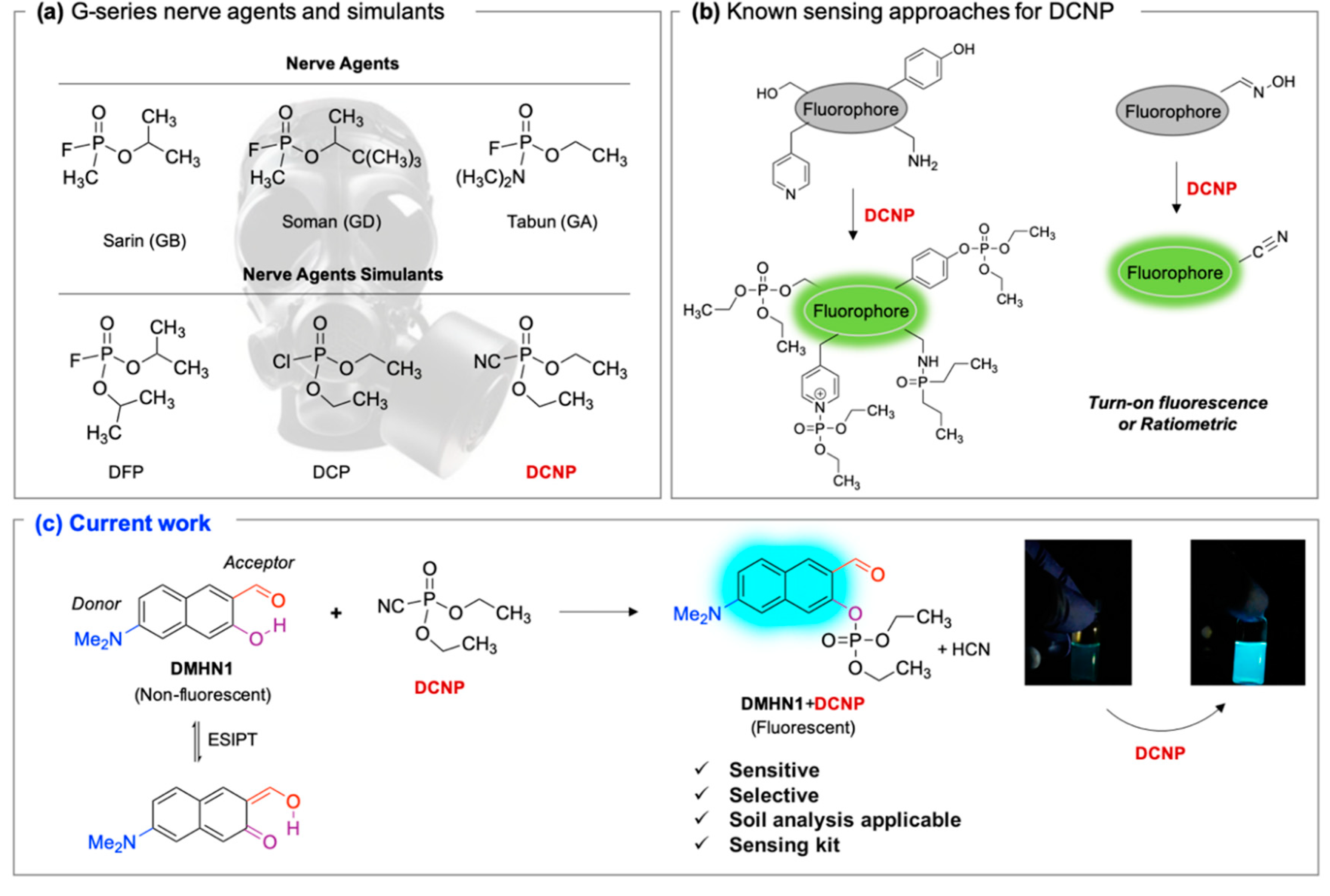
:1. Introduction
2. Materials and Methods
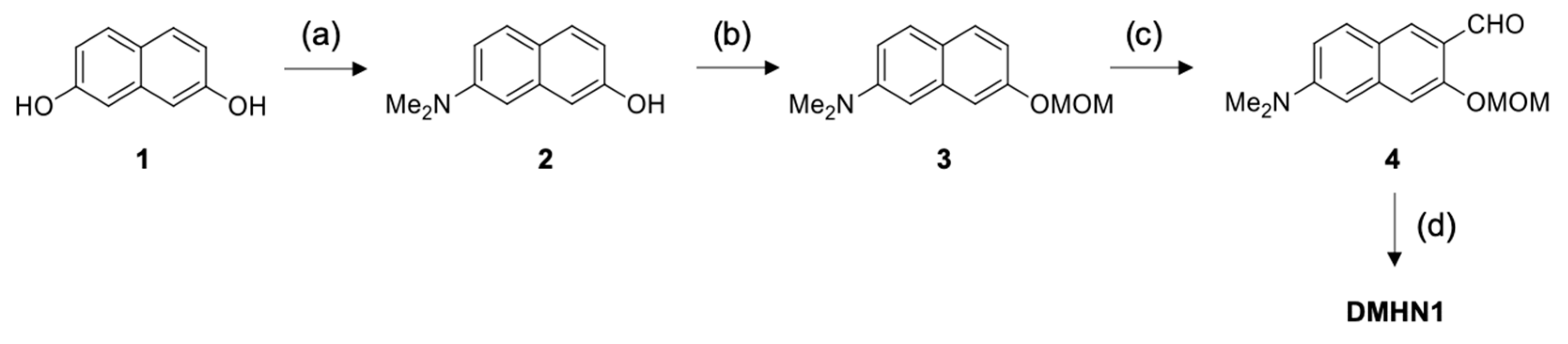
2.1. Synthesis
2.2. UV/Vis Absorption and Fluorescence Assay
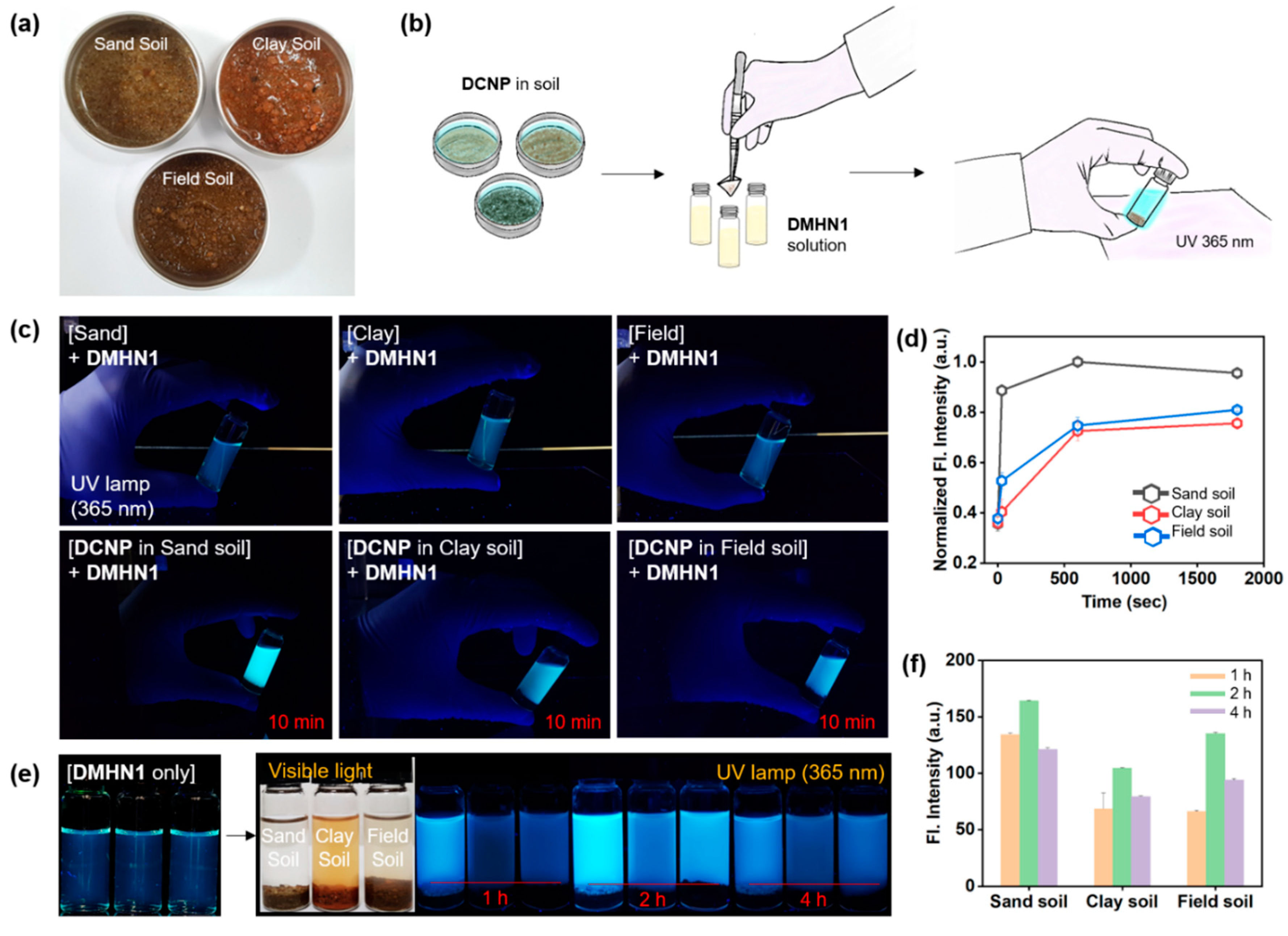
2.3. Sensing Application for DCNP-Moistened Soils
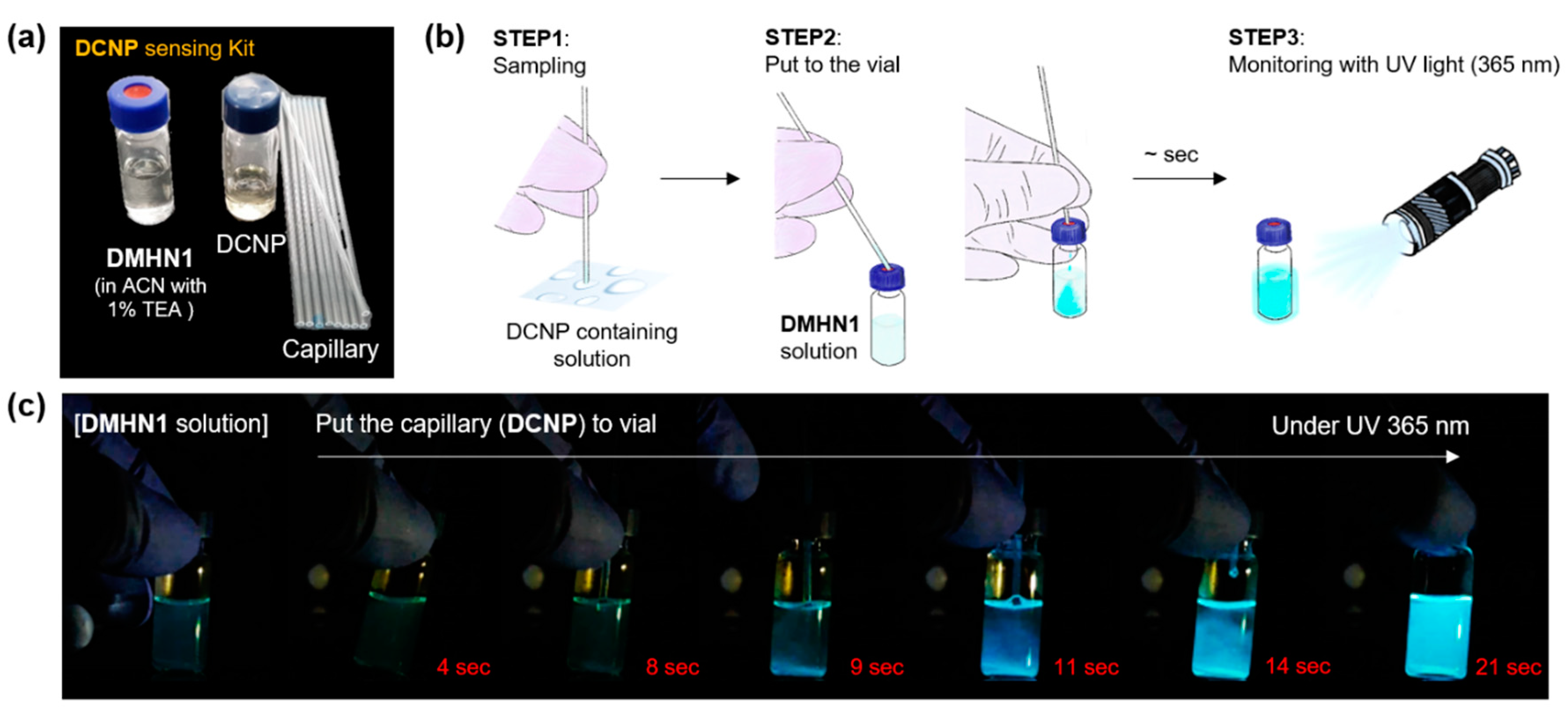
2.4. Sensing Kit Application
3. Results and Discussion
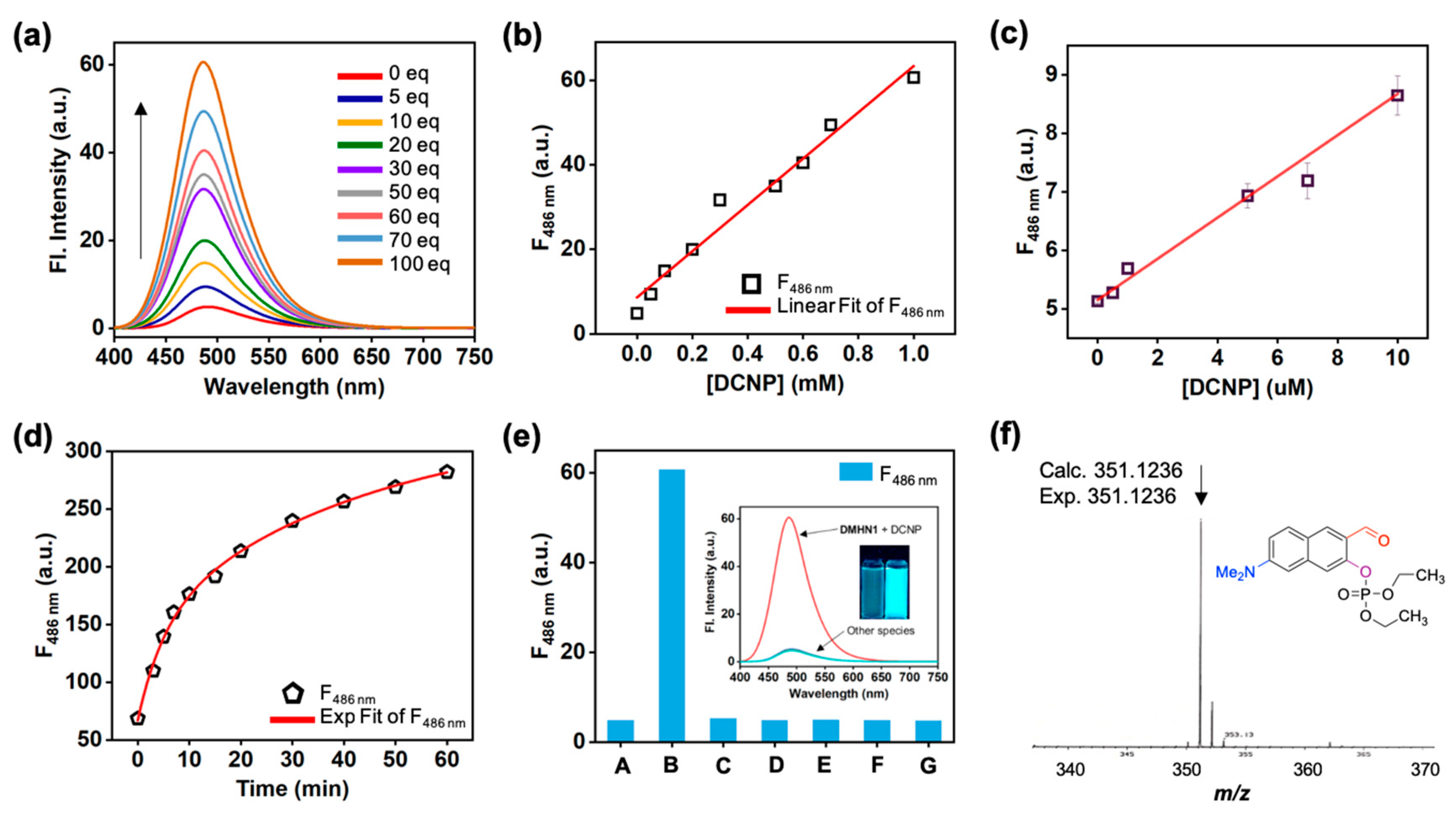
3.1. Sensing Ability of DMHN1 for DCNP
3.2. Sensing Application of DMHN1 for DCNP-Moistened Soils
3.3. Sensing Kit Test for Real-Time Detection of DCNP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DCNP | Diethylcyanophosphonate |
| DCP | Diethyl chlorophosphate |
| DMMP | Dimethyl methylphosphonate |
| TPP | Tripropyl phosphate |
| TEP | Triethyl phosphate |
| GA | Tabun |
| GB | Sarin |
| GD | Soman |
| CW | Chemical warfare |
| ESIPT | Excited state intramolecular proton transfer |
| CNS | Central nervous system |
| AChE | Acetylcholinesterase |
| ACN | Acetonitrile |
| EtOH | Ethanol |
| iPA | Isopropanol |
| DMSO | Dimethyl sulfoxide |
| DMF | N,N-dimethylformamide |
| EtOAc | Ethyl acetate |
| DI H2O | Deionized water |
| DCM | Dichloromethane |
| PTFE | Polytetrafluoroethylene |
References
- Kim, K.; Tsay, O.G.; Atwood, D.A.; Churchill, D.G. Destruction and Detection of Chemical Warfare Agents. Chem. Rev. 2011, 111, 5345–5403. [Google Scholar] [CrossRef] [PubMed]
- Eubanks, L.M.; Dickerson, T.J.; Janda, K.D. Technological advancements for the detection of and protection against biological and chemical warfare agents. Chem. Soc. Rev. 2007, 36, 458. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, M.R.; Notman, S. Supramolecular chemistry and chemical warfare agents: From fundamentals of recognition to catalysis and sensing. Chem. Soc. Rev. 2013, 42, 9251. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.M. Catalytic methods for the destruction of chemical warfare agents under ambient conditions. Chem. Soc. Rev. 2008, 37, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Bobbitt, N.S.; Mendonca, M.L.; Howarth, A.J.; Islamoglu, T.; Hupp, J.T.; Farha, O.K.; Snurr, R.Q. Metal–organic frameworks for the removal of toxic industrial chemicals and chemical warfare agents. Chem. Soc. Rev. 2017, 46, 3357–3385. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, J.; Siltanen, C.; Zhou, Q.; Revzin, A.; Simonian, A. Biosensor technology: Recent advances in threat agent detection and medicine. Chem. Soc. Rev. 2013, 42, 8733–8768. [Google Scholar] [CrossRef]
- Kientz, C. Chromatography and mass spectrometry of chemical warfare agents, toxins and related compounds: State of the art and future prospects. J. Chromatogr. A 1998, 814, 1–23. [Google Scholar] [CrossRef]
- Mäkinen, M.A.; Anttalainen, O.A.; Sillanpää, M.E.T. Ion Mobility Spectrometry and Its Applications in Detection of Chemical Warfare Agents. Anal. Chem. 2010, 82, 9594–9600. [Google Scholar] [CrossRef]
- Kittle, J.D.; Fisher, B.P.; Esparza, A.J.; Morey, A.M.; Iacono, S.T. Sensing Chemical Warfare Agent Simulants via Photonic Crystals of the Morpho didius Butterfly. ACS Omega 2017, 2, 8301–8307. [Google Scholar] [CrossRef]
- Sohn, H.; Létant, S.; Sailor, M.J.; Trogler, W.C. Detection of Fluorophosphonate Chemical Warfare Agents by Catalytic Hydrolysis with a Porous Silicon Interferometer. J. Am. Chem. Soc. 2000, 122, 5399–5400. [Google Scholar] [CrossRef]
- Witkiewicz, Z.; Neffe, S.; Sliwka, E.; Quagliano, J. Analysis of the Precursors, Simulants and Degradation Products of Chemical Warfare Agents. Crit. Rev. Anal. Chem. 2018, 48, 337–371. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Kim, K.; Tsay, O.G.; Atwood, D.A.; Churchill, D.G. Update 1 of: Destruction and Detection of Chemical Warfare Agents. Chem. Rev. 2015, 115, PR1–PR76. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kwon, B.; Liu, W.; Anslyn, E.V.; Wang, P.; Kim, J.S. Chromogenic/Fluorogenic Ensemble Chemosensing Systems. Chem. Rev. 2015, 115, 7893–7943. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Lee, S.; Xu, Z.; Yoon, J. Recent Progress on the Development of Chemosensors for Gases. Chem. Rev. 2015, 115, 7944–8000. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent chemosensors: The past, present and future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Ju, I.G.; Choe, Y.H.; Kim, Y.; Park, S.; Hyun, Y.-M.; Oh, M.S.; Kim, D. Hydrazine Exposé: The Next-Generation Fluorescent Probe. ACS Sens. 2019, 4, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kim, Y.; Kim, N.H.; Lee, J.; Kim, K.-H.; Jung, J.; Huh, Y.; Jang, H.-J.; Joo, J.; Park, S.; et al. A wavelength-tunable and facilely functionable D-A type naphthalene core skeleton: Synthesis, photophysical property, and bio-imaging applications for cells and tissues. Dye. Pigment. 2019, 162, 104–111. [Google Scholar] [CrossRef]
- Kim, D.; Baik, S.H.; Kang, S.; Cho, S.W.; Bae, J.; Cha, M.-Y.; Sailor, M.J.; Mook-Jung, I.; Ahn, K.H. Close Correlation of Monoamine Oxidase Activity with Progress of Alzheimer’s Disease in Mice, Observed by in Vivo Two-Photon Imaging. ACS Cent. Sci. 2016, 2, 967–975. [Google Scholar] [CrossRef]
- Yin, H.; Li, H.; Xia, G.; Ruan, C.; Shi, Y.; Wang, H.; Jin, M.; Ding, D. A novel non-fluorescent excited state intramolecular proton transfer phenomenon induced by intramolecular hydrogen bonds: An experimental and theoretical investigation. Sci. Rep. 2016, 6, 19774. [Google Scholar] [CrossRef]
- Kim, D.; Xuan, Q.P.; Moon, H.; Jun, Y.W.; Ahn, K.H. Synthesis of Benzocoumarins and Characterization of Their Photophysical Properties. Asian J. Org. Chem. 2014, 3, 1089–1096. [Google Scholar] [CrossRef]
- Gotor, R.; Costero, A.M.; Gil, S.; Parra, M.; Martínez-Máñez, R.; Sancenón, F.; Martínez-Máñez, R. A Molecular Probe for the Highly Selective Chromogenic Detection of DFP, a Mimic of Sarin and Soman Nerve Agents. Chem. A Eur. J. 2011, 17, 11994–11997. [Google Scholar] [CrossRef]
- Royo, S.; Costero, A.M.; Parra, M.; Gil, S.; Martínez-Máñez, R.; Sancenón, F. Chromogenic, Specific Detection of the Nerve-Agent Mimic DCNP (a Tabun Mimic). Chem. A Eur. J. 2011, 17, 6931–6934. [Google Scholar] [CrossRef] [PubMed]
- Barba-Bon, A.; Costero, A.M.; Gil, S.; Martínez-Máñez, R.; Sancenón, F. Selective chromo-fluorogenic detection of DFP (a Sarin and Soman mimic) and DCNP (a Tabun mimic) with a unique probe based on a boron dipyrromethene (BODIPY) dye. Org. Biomol. Chem. 2014, 12, 8745–8751. [Google Scholar] [CrossRef] [PubMed]
- Goud, D.R.; Pardasani, D.; Tak, V.; Dubey, D.K. A highly selective visual detection of tabun mimic diethyl cyanophosphate (DCNP): Effective discrimination of DCNP from other nerve agent mimics. RSC Adv. 2014, 4, 24645. [Google Scholar] [CrossRef]
- Gotor, R.; Costero, A.M.; Gavina, P.; Gil, S. Ratiometric double channel borondipyrromethene based chemodosimeter for the selective detection of nerve agent mimics. Dye. Pigment. 2014, 108, 76–83. [Google Scholar] [CrossRef]
- Barba-Bon, A.; Costero, A.M.; Gil, S.; Harriman, A.; Sancenón, F.; Barba-Bon, A. Highly Selective Detection of Nerve-Agent Simulants with BODIPY Dyes. Chem. A Eur. J. 2014, 20, 6339–6347. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Tsay, O.G.; Murale, D.P.; Jeong, J.A.; Segev, A.; Churchill, D.G. Novel and selective detection of Tabun mimics. Chem. Commun. 2014, 50, 7531–7534. [Google Scholar] [CrossRef]
- Gotor, R.; Gavina, P.; Ochando, L.E.; Chulvi, K.; Lorente, A.; Martínez-Máñez, R.; Costero, A.M. BODIPY dyes functionalized with 2-(2-dimethylaminophenyl)ethanol moieties as selective OFF–ON fluorescent chemodosimeters for the nerve agent mimics DCNP and DFP. RSC Adv. 2014, 4, 15975–15982. [Google Scholar] [CrossRef]
- Das, A.K.; Goswami, S.; Quah, C.K.; Fun, H.-K. Relay recognition of F−and a nerve-agent mimic diethyl cyano-phosphonate in mixed aqueous media: Discrimination of diethyl cyanophosphonate and diethyl chlorophosphate by cyclization induced fluorescence enhancement. RSC Adv. 2016, 6, 18711–18717. [Google Scholar] [CrossRef]
- Balamurugan, A.; Lee, H.-I. A Visible Light Responsive On–Off Polymeric Photoswitch for the Colorimetric Detection of Nerve Agent Mimics in Solution and in the Vapor Phase. Macromolecules 2016, 49, 2568–2574. [Google Scholar] [CrossRef]
- Gupta, M.; Lee, H.-I. A Pyrene Derived CO 2 -Responsive Polymeric Probe for the Turn-On Fluorescent Detection of Nerve Agent Mimics with Tunable Sensitivity. Macromolecules 2017, 50, 6888–6895. [Google Scholar] [CrossRef]
- Jang, Y.J.; Mulay, S.V.; Kim, Y.; Jorayev, P.; Churchill, D.G. Nerve agent simulant diethyl chlorophosphate detection using a cyclization reaction approach with high stokes shift system. New J. Chem. 2017, 41, 1653–1658. [Google Scholar] [CrossRef]
- Manna, A.; Jana, K.; Guchhait, N.; Goswami, S. Discrimination of tabun mimic diethyl cyanophosphonate from sarin mimic diethyl chlorophosphate via Zn(ii)-triggered photoinduced electron transfer-decoupled excited state intramolecular proton transfer processes. New J. Chem. 2017, 41, 6661–6666. [Google Scholar] [CrossRef]
- Jang, Y.J.; Mulay, S.V.; Kim, Y.; Nguyen, T.T.; Churchill, D.G. Fluorescent Sensing of a Nerve Agent Simulant with Dual Emission over Wide pH Range in Aqueous Solution. Chem. A Eur. J. 2017, 23, 7785–7790. [Google Scholar]
- Climent, E.; Biyikal, M.; Gawlitza, K.; Dropa, T.; Urban, M.; Costero, A.M.; Martínez-Máñez, R.; Rurack, K. Determination of the chemical warfare agents Sarin, Soman and Tabun in natural waters employing fluorescent hybrid silica materials. Sens. Actuators B Chem. 2017, 246, 1056–1065. [Google Scholar] [CrossRef]
- Lu, Z.; Fan, W.; Shi, X.; Black, C.A.; Fan, C.; Wang, F. A highly specific BODIPY-based fluorescent probe for the detection of nerve-agent simulants. Sens. Actuators B Chem. 2018, 255, 176–182. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Oh, H.; Wu, D.; Kim, M.H.; Yoon, J. An ESIPT fluorescent probe and a nanofiber platform for selective and sensitive detection of a nerve gas mimic. Chem. Commun. 2018, 54, 2276–2279. [Google Scholar] [CrossRef]
- Dey, N.; Jha, S.; Bhattacharya, S. Visual detection of a nerve agent simulant using chemically modified paper strips and dye-assembled inorganic nanocomposite. Analyst 2018, 143, 528–535. [Google Scholar] [CrossRef]
- Zeng, L.; Zeng, H.; Jiang, L.; Wang, S.; Hou, J.-T.; Yoon, J. A Single Fluorescent Chemosensor for Simultaneous Discriminative Detection of Gaseous Phosgene and a Nerve Agent Mimic. Anal. Chem. 2019. [Google Scholar] [CrossRef]
| Probes | λex/λem (nm) Sensing Media) | Detection Limit | Selectivity | Reaction Condition (time) | Application | Reference |
|---|---|---|---|---|---|---|
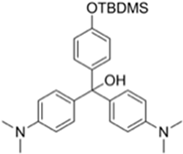 | 266/n.r. (CH3CN-H2O) | 30 ppm | ○ (Multi-sensing: DFP) | 25 °C (30 s) | Polyurethane membrane vapor test | [21] |
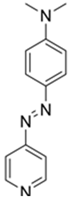 | 475/n.r. (CH3CN-H₂O) | 0.9 mM | ○ (Multi-sensing: DCP) | 25 °C (10 min) | Polyurethane film vapor test | [22] |
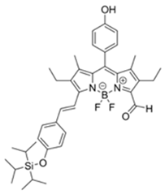 | 555/625 (CH3CN) | 0.91 ppm (visible), 0.36 ppm (Fl) | ○ (Multi-sensing: DFP) | 25 °C (n.r.) | Silica gel plate and polyethylene oxide membrane vapor test | [23] |
 | 410/n.r. (DMSO-TEA) | 3 mM | ○ (Multi-sensing: DCP) | 25 °C (60 min) | Chemogenic response test | [24] |
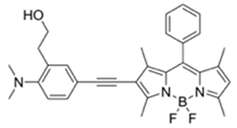 | 530/555 (CH3CN-H₂O) | 2.7 ppm (in CH3CN) | ○ (Multi-sensing: DFP | 25 °C (n.r.) | Hydrogel coated polyethylene strip vapor test, silica strip test | [25] |
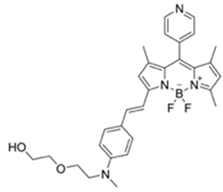 | 530/575 (CH3CN) | 4.52 ppm (visible), 4.01 ppm(Fl) | ○ (Multi-sensing: DFP) | 25 °C (n.r.) | Silica gel plate test, polyethylene oxide membrane vapor test | [26] |
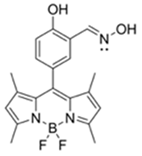 | 499/508 (HEPES buffer) | 92.2 μM | ○ (Multi-sensing: DCP, DEMP) | n.r. | n.r. | [27] |
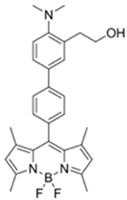 | 470/507 (CH3CN) | 7 ppm (CH3CN), 4 ppm (H₂O-CH3CN) | ○ | 25 °C (~ s) | Hydrogel coated polyethylene strip vapor test | [28] |
 | 320, 410/486 (CH3CN-H₂O) | 3.09 μM | ○ (Relay sensing with F- ion) | 25 °C (~ s) | TLC plate emerging test | [29] |
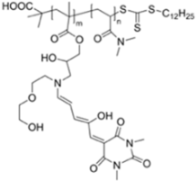 | 550/n.a. (Dioxane) | 1 mM | ○ | n.r. | Vapor test | [30] |
 | 345/375-395 (THF-H₂O) | 0.1 mM | ○ (Multi-sensing) | n.r. | Quartz plate vapor test | [31] |
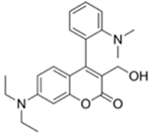 | 388/460 (CHCl3) | 0.044 nM (DCP) | × (selective for DCP) | 25 °C (5 min) | n.r. | [32] |
 | 365/430, 559 (DMSO-TEA) | 1.6 μM (DCP) | × (selective for DCP) | 25 °C (n.r.) | Vapor test | [33] |
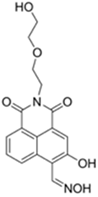 | 458/570 (PBS buffer) | 21.9 nM | ○ (Multi-sensing: DCP) | 25 °C (10 min) | Silica plate vapor test | [34] |
 | 550/635 (MES buffer) | 90.8 pM | × | 25 °C (10 min) | Water test | [35] |
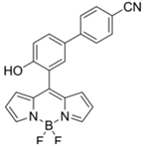 | 480/520 (DMF-TEA/ DMAP) | 20.7 ppb (DCP) | × (selective for DCP) | 25 °C (10 min) | Paper strip test | [36] |
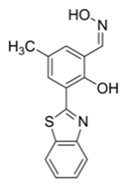 | 410/480 (DMF) | 1.3 nM | ○ | 25 °C (4 min) | Vapor test | [37] |
 | 465/550 (H₂O) | 10.8 μM | ○ | 25 °C (30 min) | Paper test vapor test | [38] |
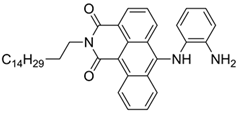 | 520/588 (CHCl3-TEA) | 88 nM (DCP) 72 μM (Phosgene) | ○ | 25 °C (2 min) | Polystyrene membrane vapor test | [39] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, Y.; Kim, D. A Selective Fluorescence Turn-On Probe for the Detection of DCNP (Nerve Agent Tabun Simulant). Materials 2019, 12, 2943. https://doi.org/10.3390/ma12182943
Jung Y, Kim D. A Selective Fluorescence Turn-On Probe for the Detection of DCNP (Nerve Agent Tabun Simulant). Materials. 2019; 12(18):2943. https://doi.org/10.3390/ma12182943
Chicago/Turabian StyleJung, Yuna, and Dokyoung Kim. 2019. "A Selective Fluorescence Turn-On Probe for the Detection of DCNP (Nerve Agent Tabun Simulant)" Materials 12, no. 18: 2943. https://doi.org/10.3390/ma12182943
APA StyleJung, Y., & Kim, D. (2019). A Selective Fluorescence Turn-On Probe for the Detection of DCNP (Nerve Agent Tabun Simulant). Materials, 12(18), 2943. https://doi.org/10.3390/ma12182943