4.1. Chemical and Mineralogical Properties
The chemical analysis of an LCD panel indicated that silicon dioxide, SiO
2, is the dominant component, constituting 24.64% of the weight (
Table 7). The second most prominent component is aluminum oxide, which corresponds to 5.10% (
w/
w); calcium oxide constituted about half of this value, i.e., 2.68% (
w/
w). Arsenic, zinc, strontium, barium, phosphorus, potassium, iron, titanium, copper and indium oxides were found in trace amounts. The presence of hydrocarbons (organic carbon bonds) was found in LCD panels, reaching 65.12% (
w/
w).
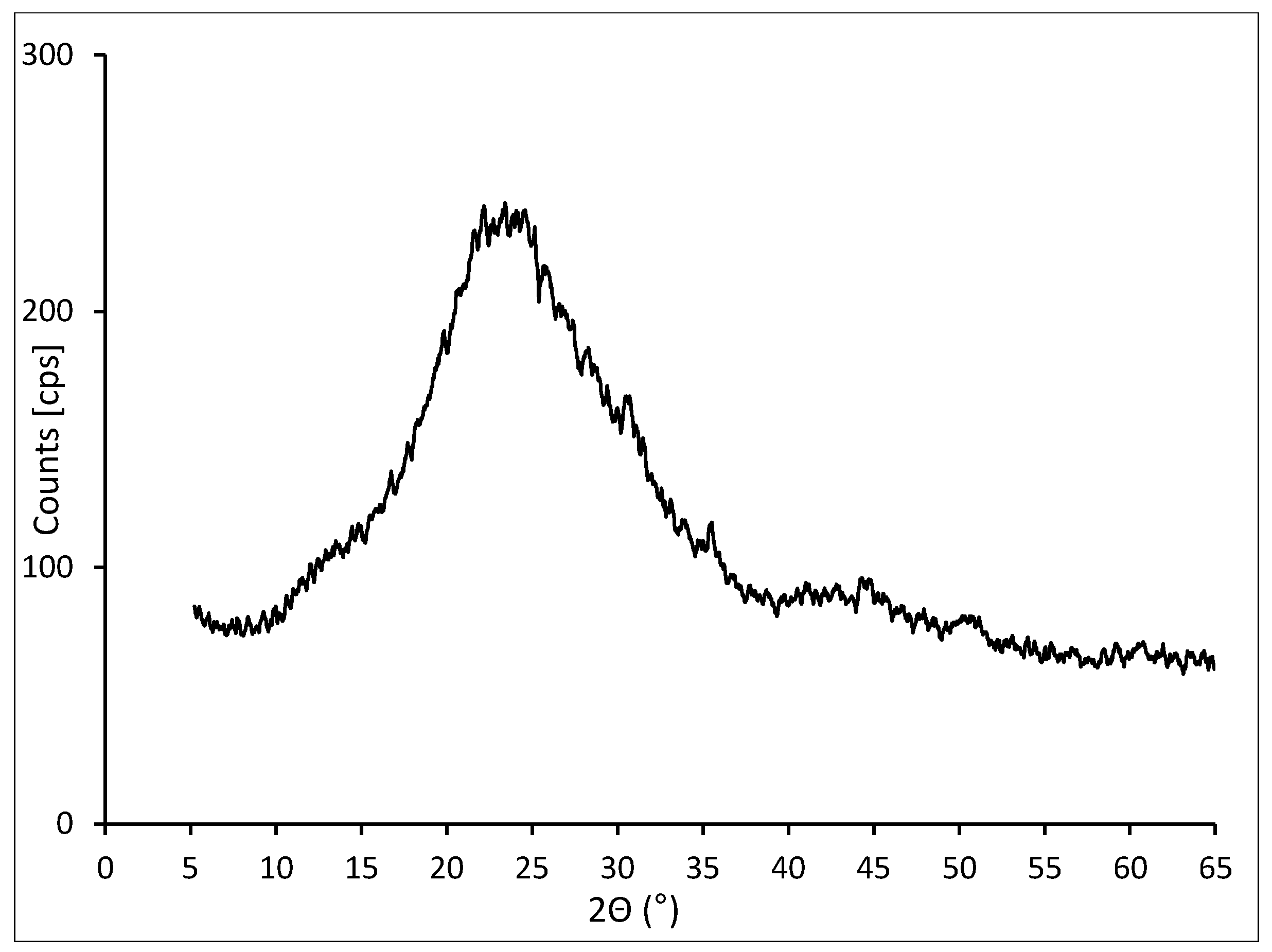
The mineral composition of the waste was analyzed with the powder method on the fraction of material smaller than 75 μm. The analysis indicated that amorphous silica SiO
2 constitutes the dominant component, which was identified by a significant background increase at 2θ 15°–35° (
Figure 1). The organic carbon bonds that did not form crystalline mineral phases remained unidentified in an investigation conducted with the XRD method.
The analysis of diffractograms confirms that in all concrete samples, the main hydration product involves phases of hydrated calcium silicates (C–S–H phase) in varying degrees of ordering. The results of reference analyses with 1% LCD addition are presented in
Figure 2. Background intensities are especially high in the case of CI.1 and CII.1 concretes with LCD addition which can be observed at 2θ approximately 20°–35°. It can be a result both by the crystallization of poorly ordered C–S–H phases as well as by the addition of amorphous LCD (
Figure 1). Additionally, intensities of peaks related to C–S–H phases in relation to peaks which can be observed on diffractograms of concretes without LCD panel addition (CI.0 and CII.0) are also higher. The main reason is probably the hydration reaction between glassy part of LCD panels and cements.
Peaks from crystalline intrusions, i.e., portlandite and quartz, were also identified in the considered cement paste samples. Crystalline phases were identified by the highest interplanar spacings
dhkl, which reached a
dhkl of 4.9 Å for portlandite and
dhkl values of1.817, 3.346 and 4.26 Å for quartz. The presence of calcium carbonate in the form of calcite was found in the pastes separated from concrete, as a product of partial carbonatization. It was identified by the basic interplanar spacings
dhkl, which amounted to 2.095, 2.285 and 3.035 Å (
Figure 2).
4.2. Physical Properties
The results of studies conducted on fresh concrete mixtures are presented in
Table 8. The obtained mean results pertaining to the physical properties of concretes are shown in
Table 9.
The air content in concrete mixtures obtained using aerating admixture exceeds 4.0% (
Table 8), which is currently required in the exposure classes from XF2 to XF4 (aggression caused by freezing/thawing with the presence of deicing agents or lack thereof), in accordance with EN 206 [
53].
The results of studies on porosity indicate an advantageous distribution, size and content of pores in all considered concretes. Total air content
A in concretes exceeds 5% (In Danish requirements, it should amount at least to 3.5%, regardless of the exposure class [
55]), pore spacing factor
L is lower than 0.2 mm (according to Danish [
55] and German requirements, this is the maximum permissible value [
56]), and the
A300 (the percentage content of micropores with the diameter up to 300 μm) micropore content exceeds 2% (the Austrian [
57] and German requirements provide the minimum value of 1.8% [
56]).
Water absorption by weight obtained in the course of studies is lower than 6%. In line with the standards related to precast concrete paving elements (cobblestone, concrete curbs [
57,
58,
59,
60]), the following requirements must be met in terms of water absorption: Class 1, construction mark A—not determined; Class 2, construction mark B—mean value ≤ 6%. Because none of the obtained results exceed 5%, they should be considered satisfactory in relation to any class.
In the water permeability tests (
Figure 3) the samples were acted upon with the pressure up to 0.8 MPa, which corresponds to W8 water tightness class, according to PN-B-06250 standard [
43]. No seepage of water through the samples under the maximum set pressure was observed; hence, the water-tightness class was determined as W8. Following the experiment, the samples were split and the depth of water infiltration into the concrete structure was determined. The depth of infiltration does not exceed 1.5 cm in any sample, which should be considered a very good and satisfactory result. It should be emphasized that the limit value of water absorption by weight amounting to 5% and minimum W6 water-tightness class are required in the case of concretes intended for bridge construction [
61], which are considered to be exposed to the harshest environmental conditions and heaviest wear.
The results obtained from the frost resistance test were evaluated in accordance with PN-B-06250 [
43] (
Figure 4), which involved examining the samples and checking their mass every 25 cycles. The compressive strength tests of frozen and unfrozen samples were conducted after 150 cycles, which corresponds to the highest degree of ordinary concrete frost resistance, in line with PN-B-06250 standard [
43]. The considered samples showed no cracks, mass loss greater than 5% (the obtained values were insignificant, <0.5%); compressive strength reduction greater than 20%. High freezing–thawing resistance depends on low permeability and a low water/binder ratio. The resistance of concrete after 150 F–T cycles decreased by up to 3.8% in the case of CI and 0.7% for CII with CEM II/B-S 42.5 N. On the basis of the study results and above-mentioned criteria, the frost-resistance of considered concretes was determined as F150. Small-sized concrete elements are often exposed to aggressive impacts from the environment and thus they must have high resistance to frost corrosion, weathering or the influence of aggressive water. The studies confirmed a very high frost resistance and protection against water infiltration.
4.3. Strength Properties
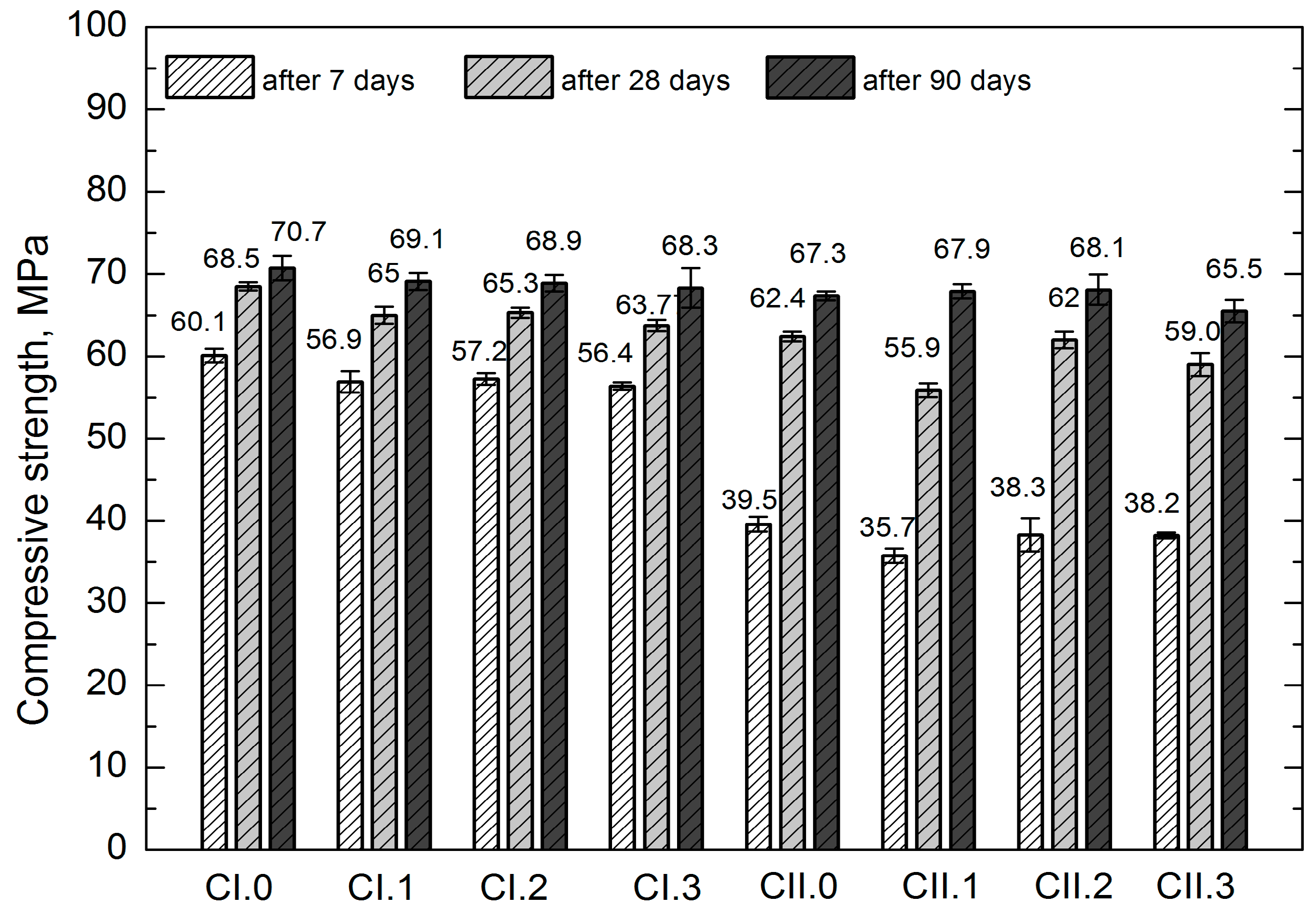
Strength tests were conducted following 7, 28 and 90 days from the sample molding. The mean values of mechanical properties of concretes are presented in
Table 10 and
Figure 5.
Prior to analyzing the results it should be emphasized that the requirements pertaining to the mechanical properties stated in the standards for precast concrete paving elements (cobblestone, concrete curbs) are related to the ready-made products. Due to their sizes that significantly differ from those of the standard samples employed in our investigations, conducting an analysis involving direct comparison of the obtained results and the standard requirements of particular products is impossible.
On the basis of the obtained results it was observed that all concretes after 28 days of maturation are characterized by the compressive strength
fc,cube#150 greater than 55 MPa, and
fc,cube#100 higher than 60 MPa. In light of the standards related to precast concrete paving elements (cobblestone, concrete curbs), the compressive strength is not accounted for in requirements and standard recommendations. It is only possible to refer to the previous versions of German standards, where—in the case of cobblestone—the compressive strength of the product should be higher than 60 MPa, and individual results should be greater than 50 MPa, in accordance with DIN 18501 [
62]. Therefore, the
fc,cube#100 results (more applicable, because the height of cobblestones usually does not exceed 100 mm) obtained from all studied concretes should be considered satisfactory. It should be noted that following 90 days of maturation, all concretes were classified as C50/60, i.e., higher than or equal to the class obtained after 28 days.
The requirements related to tensile strength in bending of cobblestone and concrete curbs are as follows: Class 1, construction mark S—characteristic strength 3.5 MPa, and minimal 2.8 MPa; Class 2, construction mark T—characteristic strength 4.0 MPa, and minimal 3.2 MPa, Class 3, construction mark U—characteristic strength 5.0 MPa, and minimal 4.0 MPa [
59,
60]. Since all the obtained results exceed 7 MPa, they should be considered satisfactory in relation to all classes of these elements.
The requirements for splitting tensile strength of cobblestone are as follows: characteristic strength of at least 3.6 MPa, individual results cannot be lower than 2.9 MPa [
58]. The results obtained in the research exceeded 4 MPa and thus they are satisfactory. Similar findings on strength of glass sand concrete were reported previously by Du and Tan [
63].
While analyzing the strength properties, slightly smaller values were observed for the concretes with LCD admixture, in relation to the reference concretes CI.0 and CII.0. The differences in the obtained values are differentiated, exhibiting no clear tendency. They are most pronounced in the tensile strength test; however, due to the high coefficient of variation (over 10%), they cannot be subjected to a comparative analysis. Generally, it can be stated that lower values were usually found in the concretes with LCD addition. Such observation can be explained both by a higher air content in the concrete mixtures with LCD admixture (the considered concretes exhibited diversified consistency with low degree of liquidity within two classes), as well as the conchoidal fracture of LCD grains. The conchoidal fracture, due to its glassy and smooth texture, deteriorates the mechanical adhesiveness of cement paste to aggregate granules, despite its low porosity found while examining the interfacial transition zone (ITZ). Nevertheless, the low share of the admixture should reduce its significance. It should also be emphasized that extending the maturation time substantially mitigates the differences in compressive strength values. Following 90 days of maturation, all concretes reached the same class. which is especially prominent while applying the CEM II/B-S 42.5 N cement that is characterized by a slower improvement of the strength parameters in relation to CEM I 42.5 R.
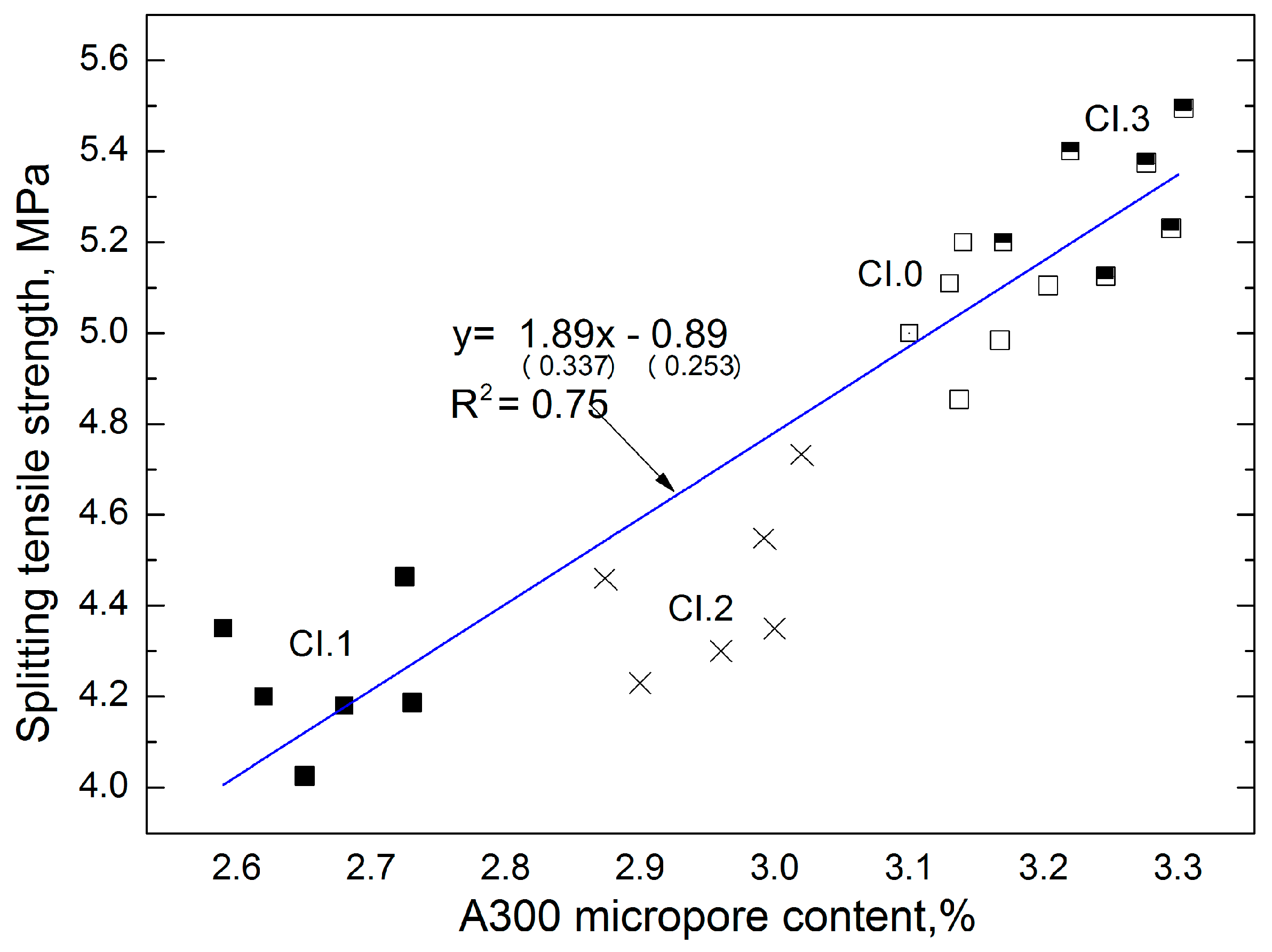
The graphs (
Figure 6) show the dependence of the different properties of CI concrete. The dependence of A300 micropore content on the splitting tensile strength for concrete is shown in
Figure 6. In this study, the determined micropore content directly corresponds to the splitting tensile strength of concrete CI. The linear trend was characterized by a coefficient of correlation
R2 = 0.75 and relatively low errors in the intercept. The error is considered to be small if the quotation estimator standard error and the estimator is less than 30%. In the first case it is 17% and in the second it is 28%.
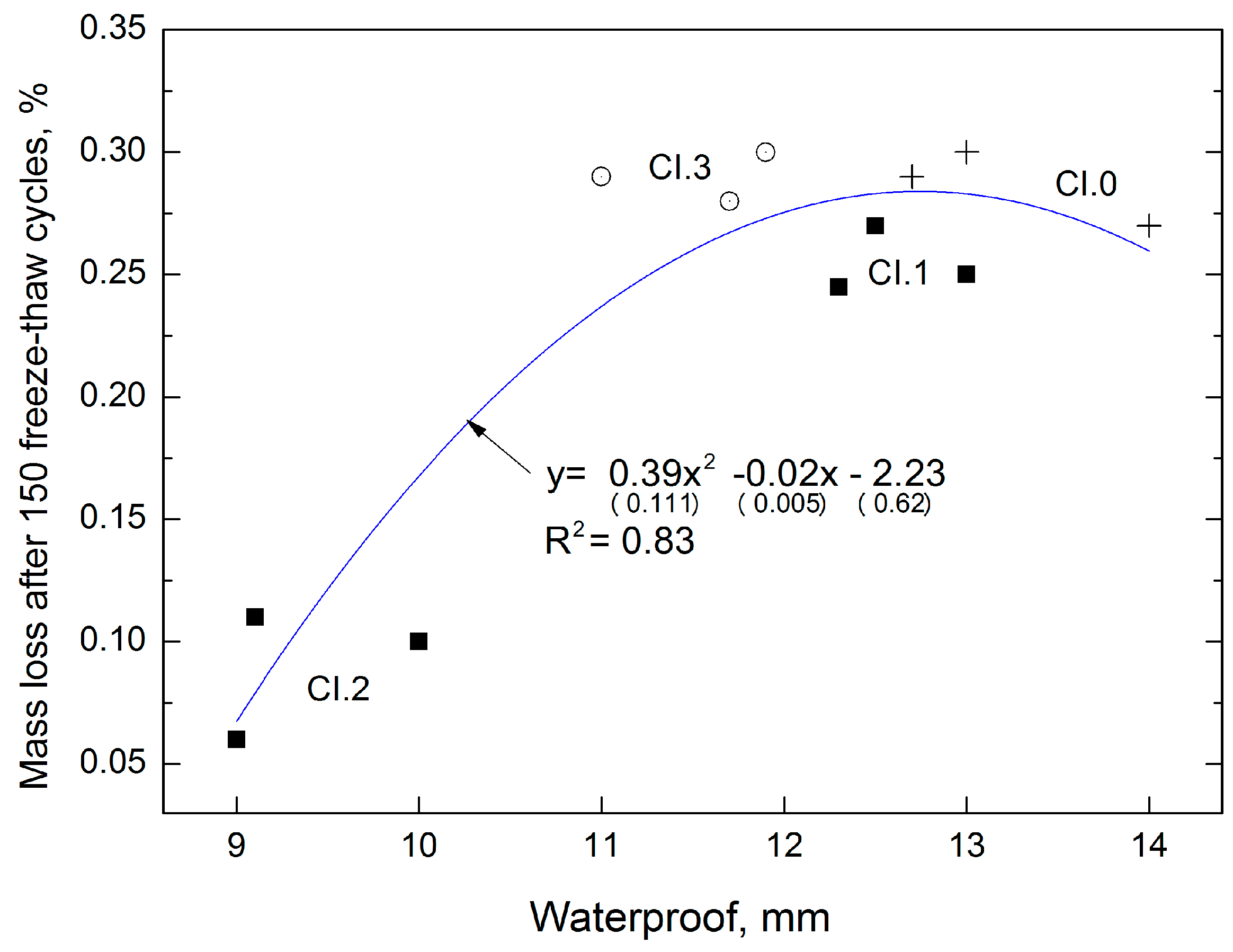
The following model (
Figure 7) presents the extent to which the characteristic of CI concrete affects the frost resistance the indirectly defines the corrosion resistance of the material.
Figure 7 shows the model as well as frost resistance (mass loss of samples after 150 freeze–thaw cycles) features of concrete depending on the water resistance (water penetration).
The obtained correlations can be described by the equation , which is characterized by a coefficient of determination R2 = 0.83 and relatively low errors in the intercept (respectively 28%, 25%, 28%). The higher the water-tightness, the higher the frost resistance and the lower the mass loss. The reference concrete CI.0 is characterized by the lowest water-tightness (deepest water penetration), which corresponds to the highest mass loss during the water tightness test. An increase in LCD admixture to 2% resulted in 3-fold decrease in the mass loss of the samples.
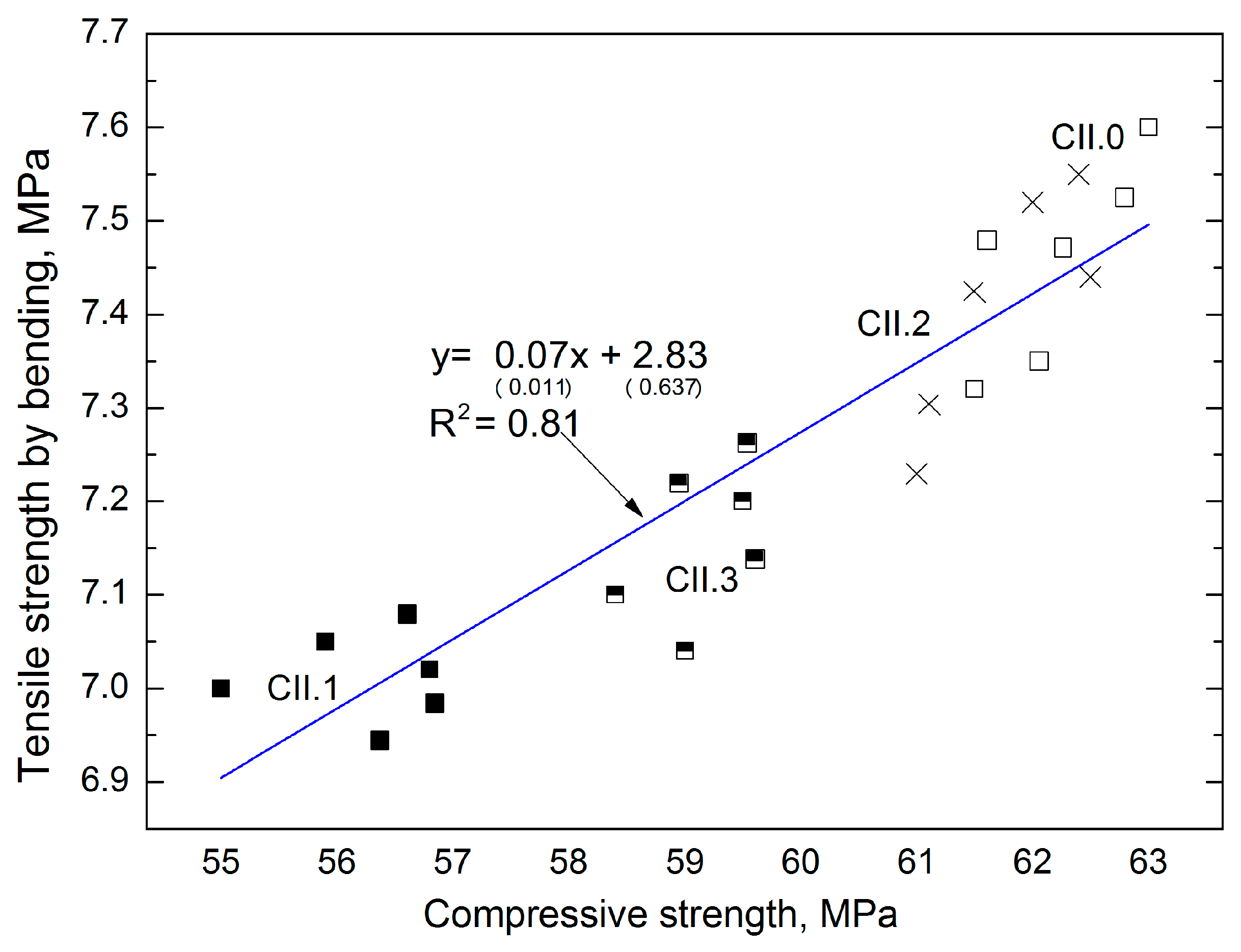
The above-mentioned dependences were not observed in the concretes with CII cement. In turn, good correlation was obtained between the compressive strength and tensile strength in bending (
Figure 8). The linear function allows a good coefficient of determination, equal to 0.81 and low errors in the intercept (respectively 14.5%, 22.5%).
Based on the research of Batayneh et al. [
64] concerning the impact of various waste materials on the properties of concretes and hardened concrete, it was found that the use of aggregate made of recycled concrete, due to the shape and texture of the grain surface, significantly reduces the workability of the concretes. Similar observations were made when using crushed plastics. In our own research, the impact of LCD additive with significantly smaller amounts and completely different mineralogical and chemical characteristics did not significantly affect the consistency of the mixtures.
In turn, the use of crushed glass as a fine aggregate increases the compressive strength of concrete, but due to the long-term increased impact of alkali on cement paste, the durability of concrete should be taken into account. This is a very important aspect of the concrete durability, however, due to extensive chemical analyzes carried out in relation to the tested concretes, according to the authors, it is irrelevant [
64].
The use of 20% recycled plastics or concrete aggregate according to Batayneh et al. [
64] reduces the compressive strength compared to reference concrete with natural aggregate. Despite the small amounts of LCD addition used in our studies, similar relationships in compressive strength values were observed in the early maturing periods (see
Figure 5). However, after a longer maturing period (90 days), the same compressive strength classes of the tested concretes were obtained.
As a result of research of Mahesh and co-workers [
65] regarding the impact of waste from polyethylene plastics, it was found that despite the reduction of early compressive strength of tested concretes (5–10% of the used waste), their compressive strengths after 28 days were comparable to the reference concrete [
65]. Similar correlations were observed in own research after the use of LCD as an additive (
Table 10).
In water permeability studies of concretes substituting a portion of fine aggregate (4–12%) with glass waste, an increase in the water permeability of concrete was observed along with the increase of the additive added [
66]. Similarly, in the present research this increase also occurred, but did not exceed 0.3%.
4.4. Scanning Electron Microscopy with Energy Dispersive Spectroscopy
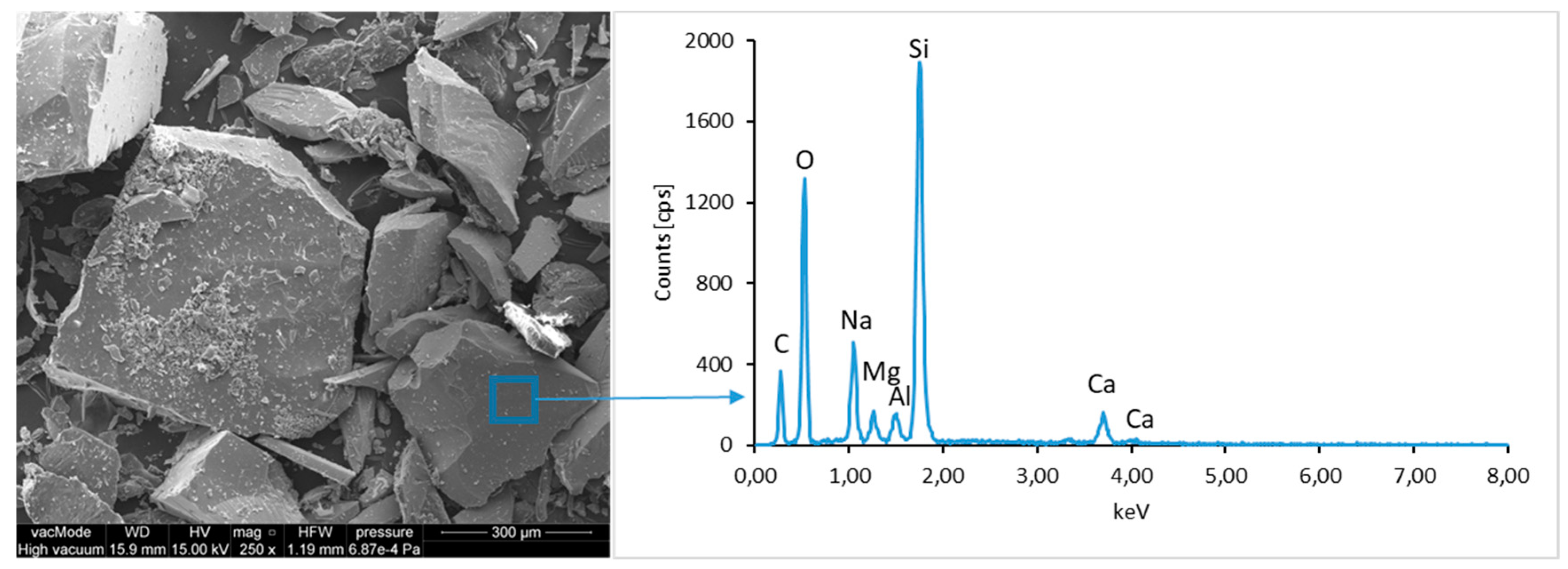
The ground LCD panels are characterized by a sharp-edged, irregular shape showing characteristic conchoidal fracture surfaces typical for crystalline phases. The panel elements indicate highly diversified grain size distribution, including grains up to 2 mm, 300–500 µm, and 20–100 µm. The chemical composition includes silicon, sodium and calcium oxides, the presence of which is connected with the glassy surface of an LCD panel (
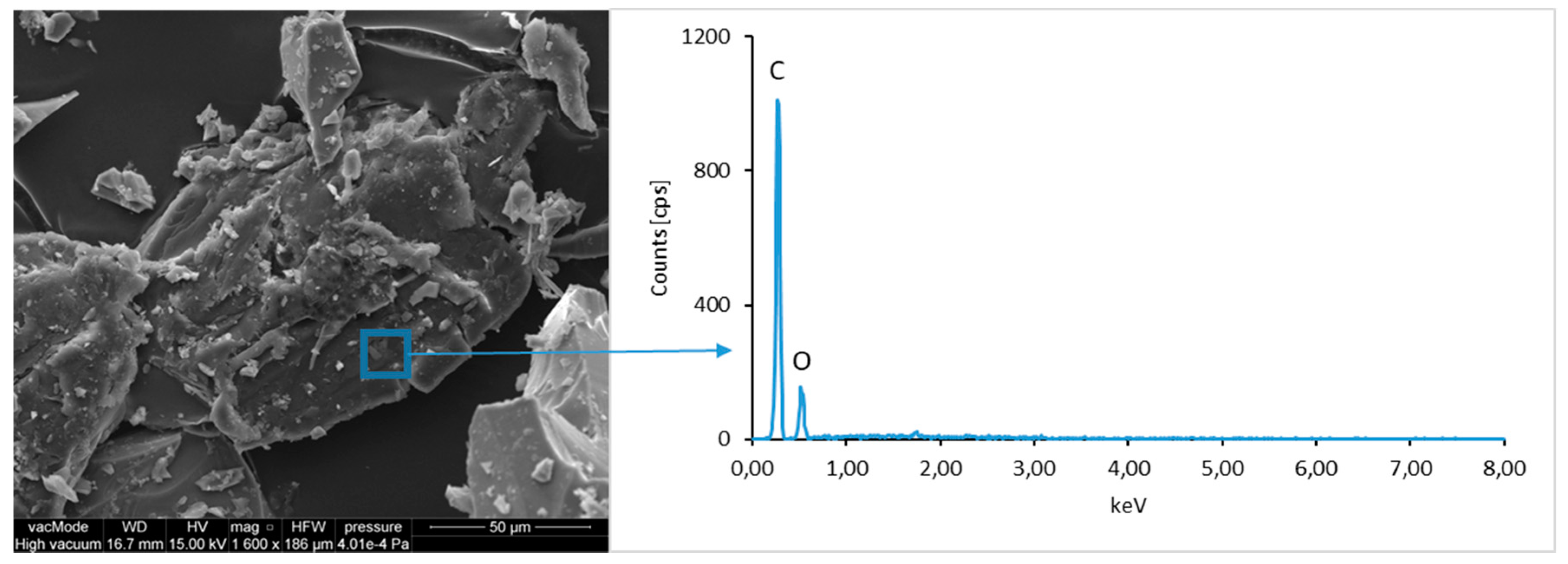
Figure 9). Additionally, organic carbon bonds are found in the waste material, originating from the plastic elements of LCD panels (
Figure 10).
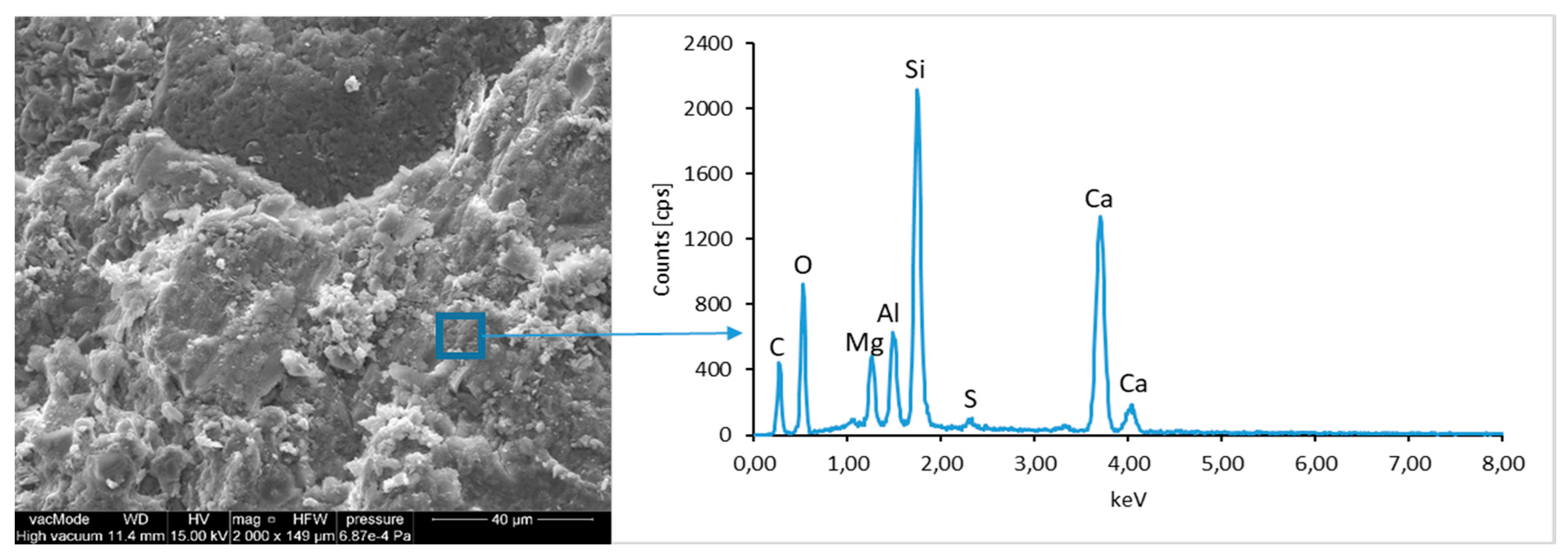
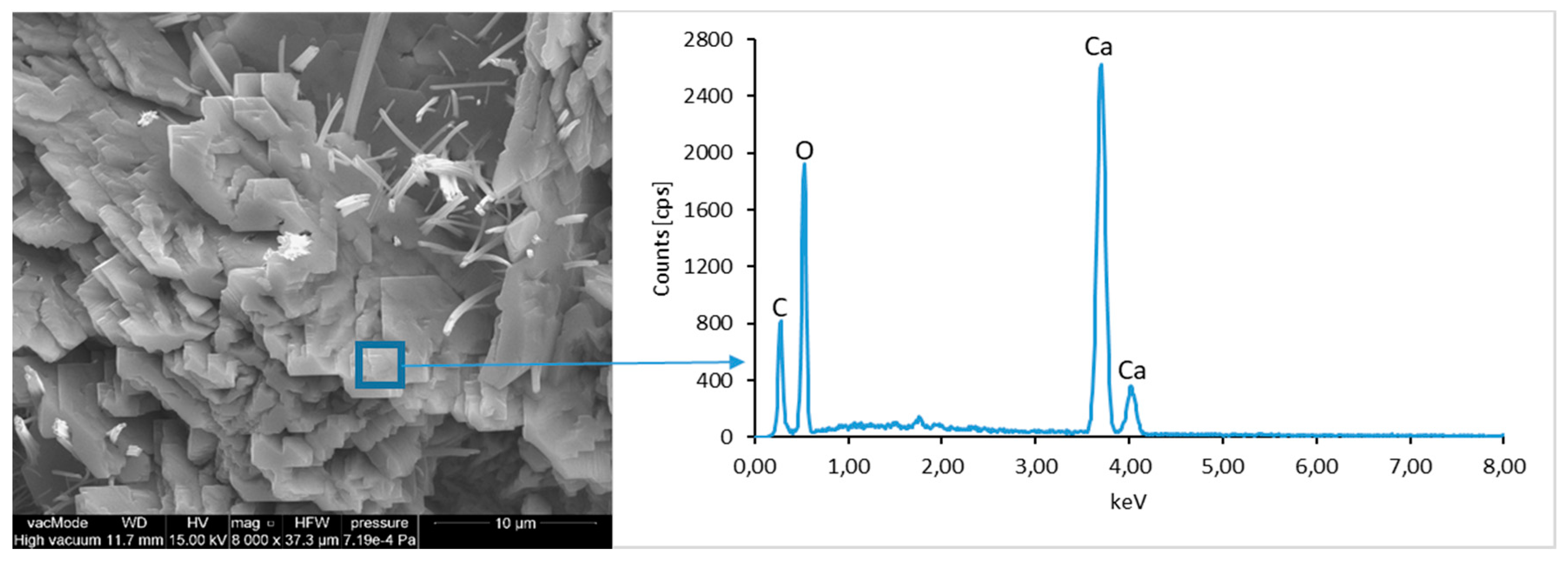
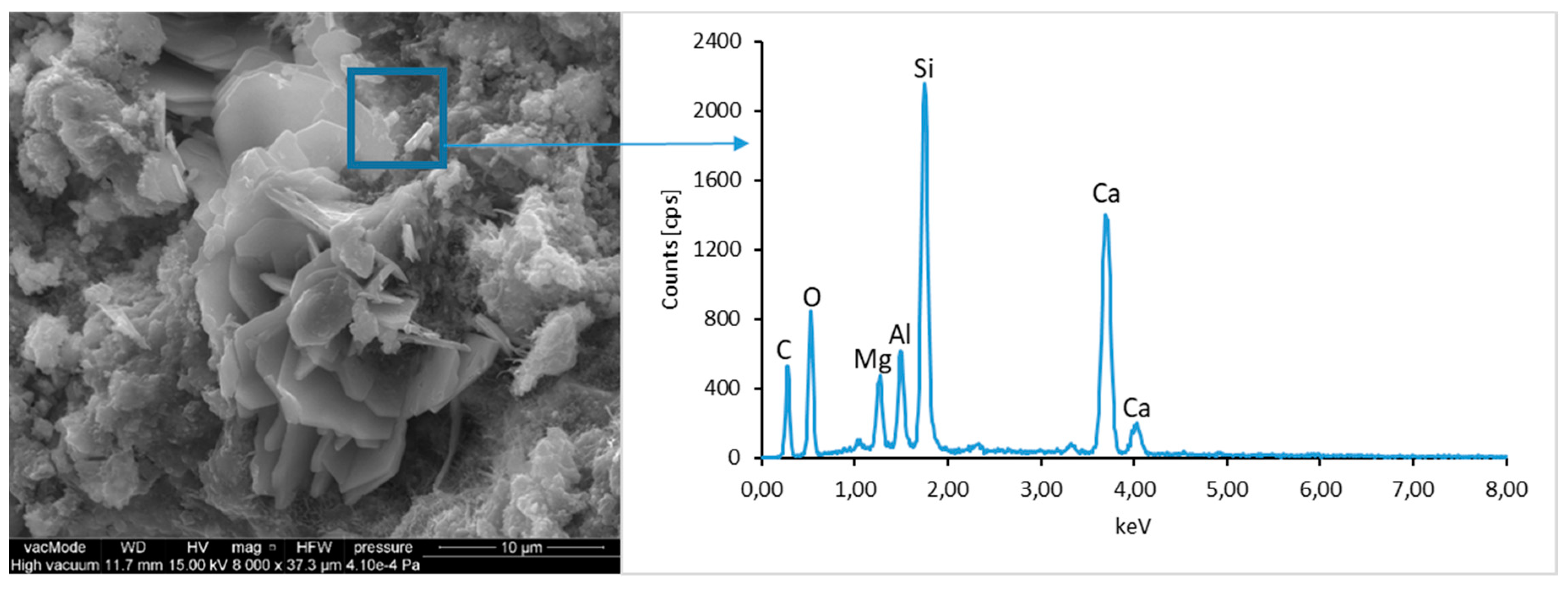
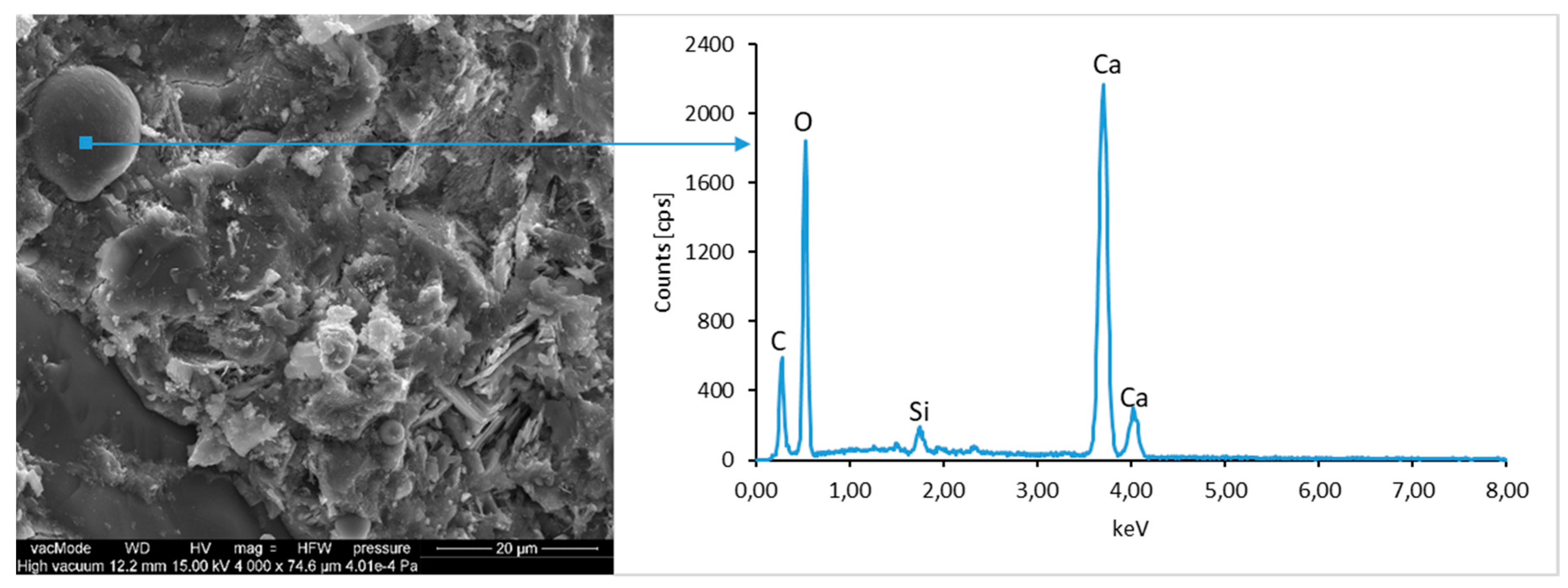
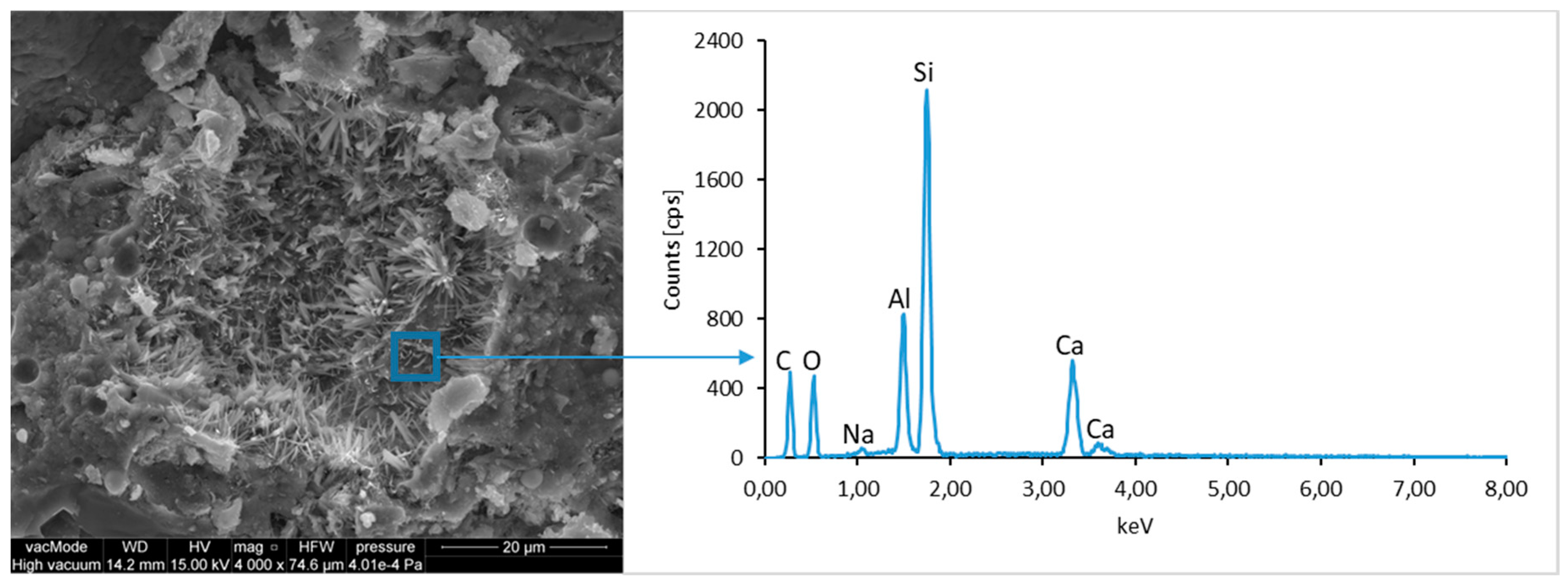
The morphological analysis of pastes and their elemental compositions indicates that the main components of concretes involve calcium, aluminum and silicon oxides. The C–S–H phase, found in the cement paste hydration products, is an important component due to its dominant share. The observation of fractures of considered concretes using a scanning electron microscope with EDS analysis show different forms of C–S–H phase. The conducted studies indicate that in CII.0 concrete, the hydration products mainly include irregular and isometric or flattened particles forming relatively compact aggregations that correspond to III and IV morphological types of C–S–H phases, according to the Diamond’s classification (
Figure 11). In the remaining concretes, i.e., CII.1, CI.0 and CI.1, the morphology of C–S–H phase samples indicates type I in the Diamond’s classification that corresponds to well-crystallized fibrous structure (
Figure 12,
Figure 13 and
Figure 18). The needle-like forms taper at their ends and intertwine, filling the space between them. The length of C–S–H fibers amounts to 0.5–10 µm and their diameter amounts to about 2 µm.
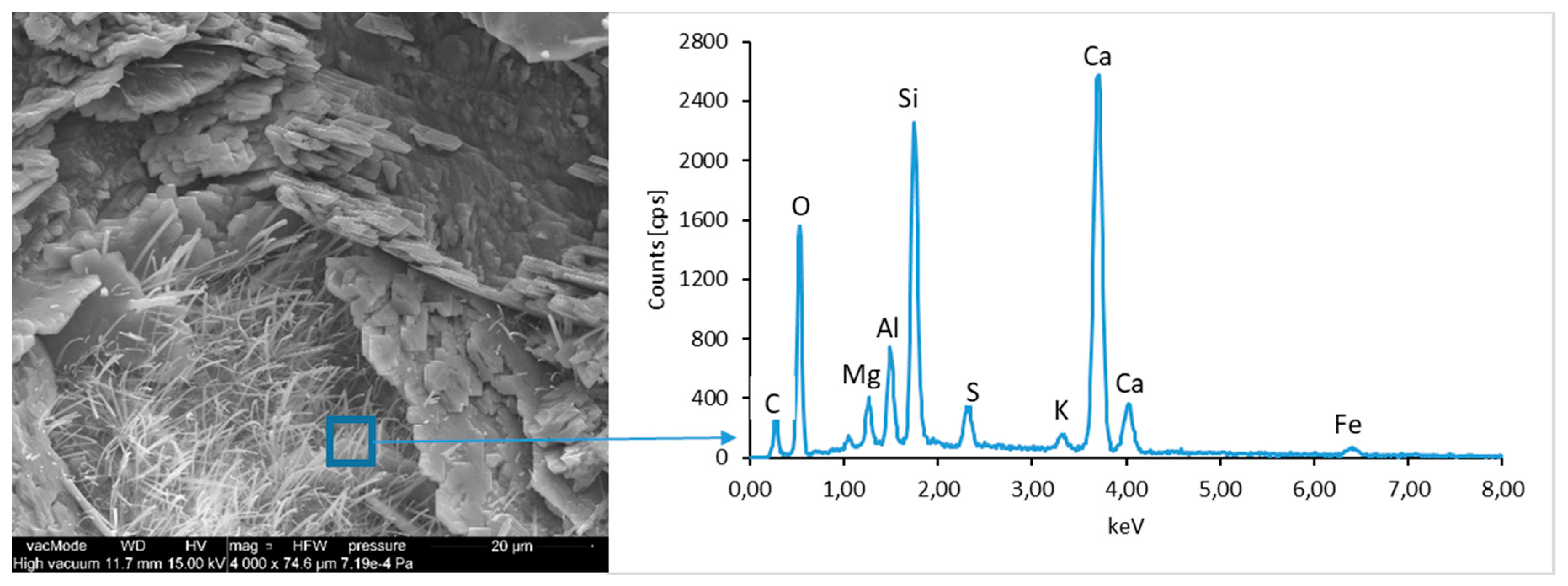
The calcium hydroxide Ca(OH)
2 (portlandite) found in cement pastes forms hexagonal lamella with the thickness of about 0.5 µm. Lamellar, rosette-forming aggregations of portlandite are embedded into the C–S–H phase (
Figure 13 and
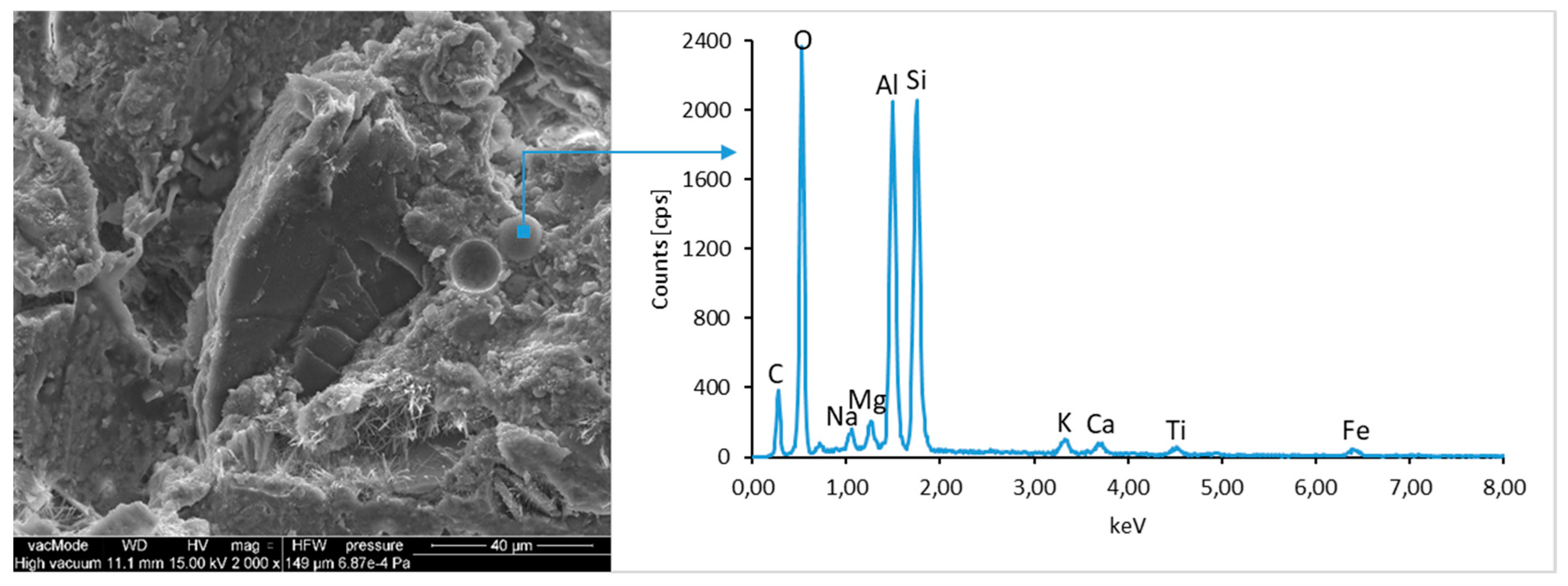
Figure 15). The micro-stricture of the paste corresponding to the concretes with fly ash addition is more compact, which results from the increased content of C–S–H phase being a product of a reaction between aluminosilicate enamel and calcium hydroxide from hydrolysis of silicate phases. It is characterized by the presence of characteristic spherical fly ash forms, which are well-preserved particles with the diameter of approximately 20 µm (
Figure 14 and
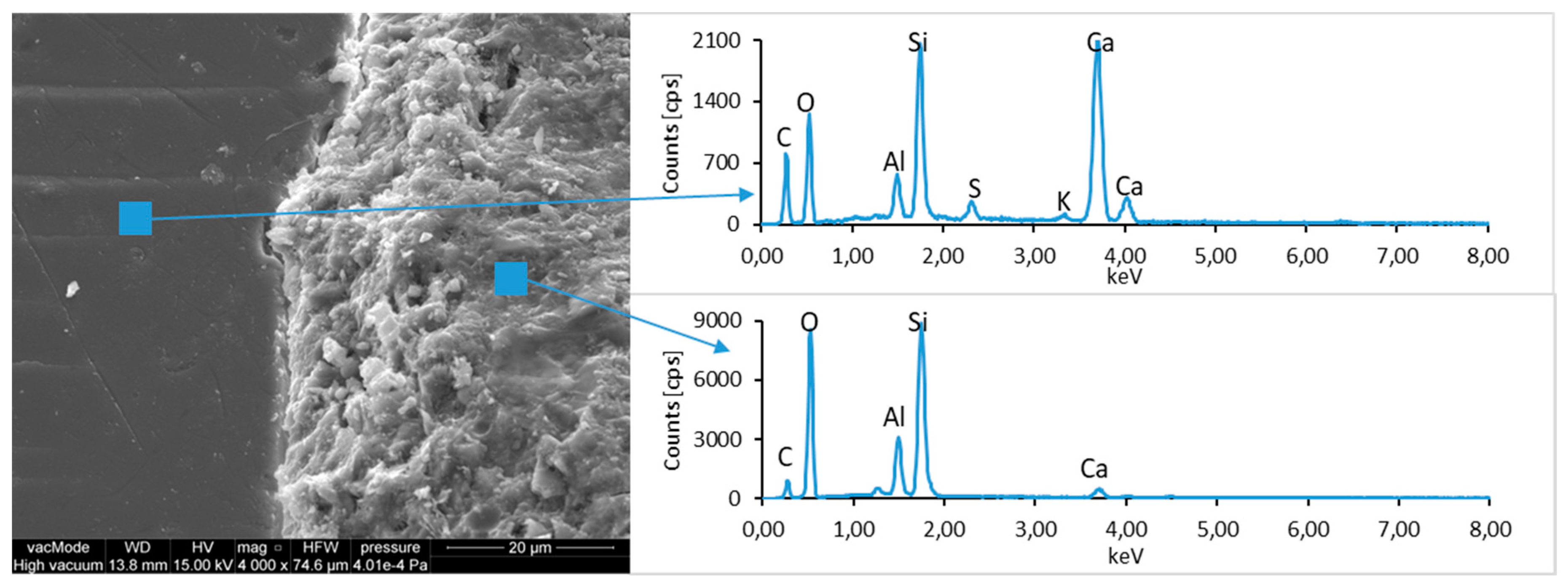
Figure 16). The observation of paste–LCD panel interface showed that the area in its vicinity is tightly filled (
Figure 17). This layer does not exhibit increased porosity and no scratches or cracks are visible; thus, the paste is characterized by good adhesion to the LCD panel.
4.5. Leaching Test
The analysis of the obtained results pertaining to heavy metal leaching from integral/monolithic forms of concretes with LCD waste (3%) is presented in
Table 11. The obtained leaching test results were compared with the permissible leaching values that constituted a criterion for the Regulation of Minister of Environment [
67,
68] and landfilling waste other than inert and hazardous [
69].
Conducting the leaching test on all concrete samples created a strict environmental regime enabling their release and indicating a very low mobility. All considered elements in the eluates obtained using the EN 12457-4 [
49] and ML methods indicated the concentration which was below the limit of detection. The chemical investigations showed that the total content of particular elements in the analyzed concrete, i.e., arsenic, zinc, strontium, barium, phosphorus, potassium, iron, titanium, and copper, did not exceed the permissible concentration for discharging wastewater to water or soil (including Group A soil, i.e., protected areas); the limit values pertaining to hazardous substances were not exceeded either [
67,
68]. The trace amounts of arsenic, zinc, strontium, barium, phosphorus, potassium, iron, titanium, and copper are 10
4–10
6 fold lower than the permissible values.
The concentrations of the elements mentioned above in the CI.3 and CII.3 concretes are at a comparable and similar level. The effect of LCD addition on concrete on leaching is practically imperceptible. Only elements and substances such as Zn, As, CH
2, Sn, Ba, Cu were observed at the detection limit level. It was expected considering XRF results of LCD panel (
Table 7) in which low concentrations of elements such as Sr, Sn, Zn, As, Ti, Ba, Sb were noted. In addition, adding LCD up to a maximum of 3% to the weight of concrete can only slightly affect the chemical composition of concrete without lowering its class.
Concrete matrix that immobilizes trace amounts of the elements found in LCD prevents their release into the environment and mitigates the risk of hazardous substances infiltrating into soil. This is confirmed by the test of water permeability through concrete, in which no water seepage through the samples occurred under the maximum set pressure.
The physicochemical properties of concrete, connected with the specific properties of cement hydration products (main component of concrete) and the concrete production technology make it one of the best environments for an efficient management of industrial wastes. Hydrated calcium silicates, especially C–S–H phase, are responsible for the immobilizing properties of cement pastes (including the resulting mortars and concretes) [
70,
71,
72,
73]. The wastes managed in this way are much more geochemically stable than traditionally landfilled wastes. This solution contributes to reducing the area required for waste storage, which is directly connected with decreasing the environmental fees incurred by the enterprises landfilling LCD wastes.