Zinc Recovery through Electrolytic Refinement Using Insoluble Ir + Sn + Ta + PdOx/Ti Cathode to Reduce Electrical Energy Use
Abstract
1. Introduction
2. Materials and Experimental Methods
2.1. Materials
2.2. Measurement Methods
3. Results and Discussion
3.1. Analysis of the Elecments Included in Electrode Composition
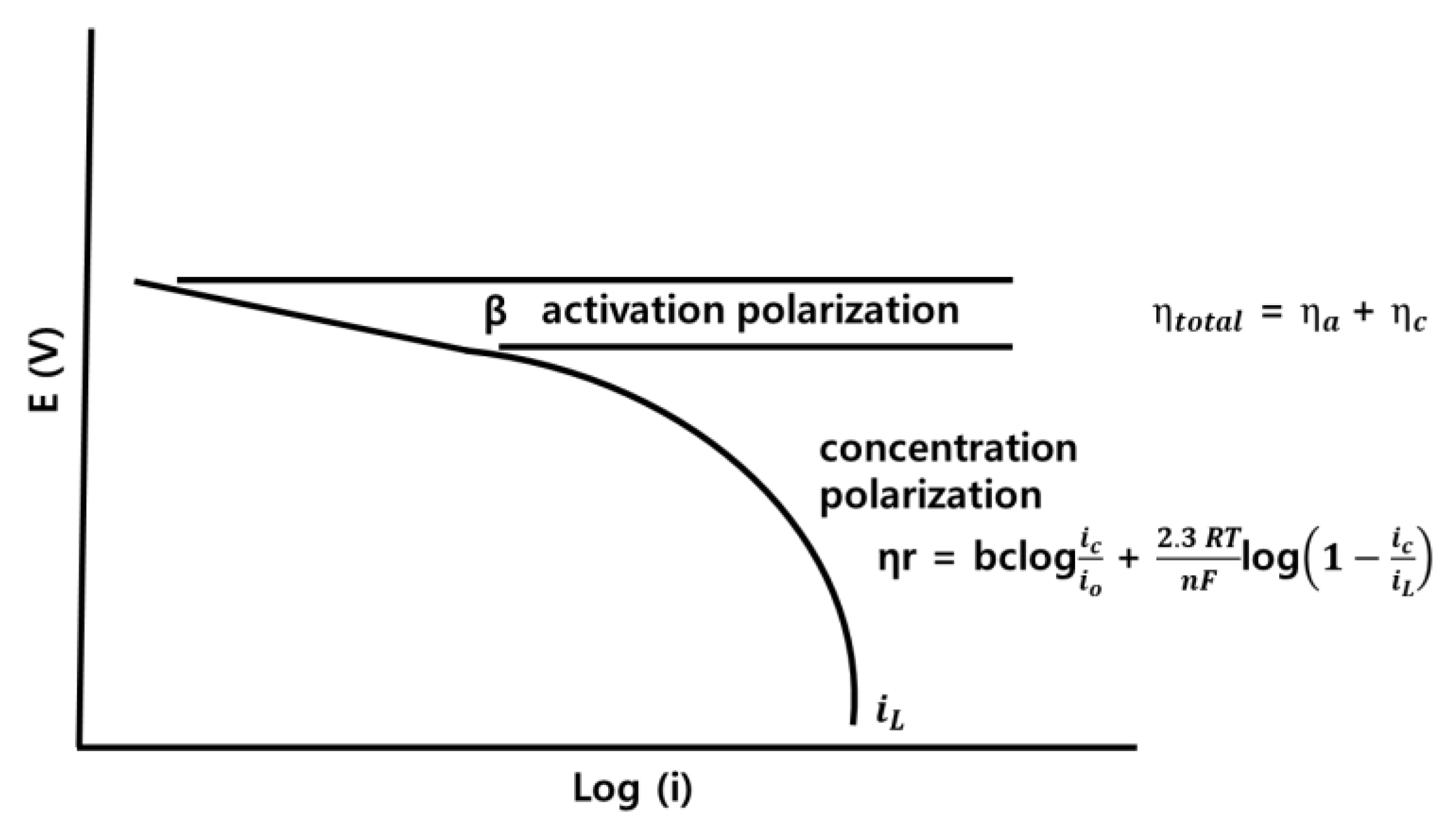
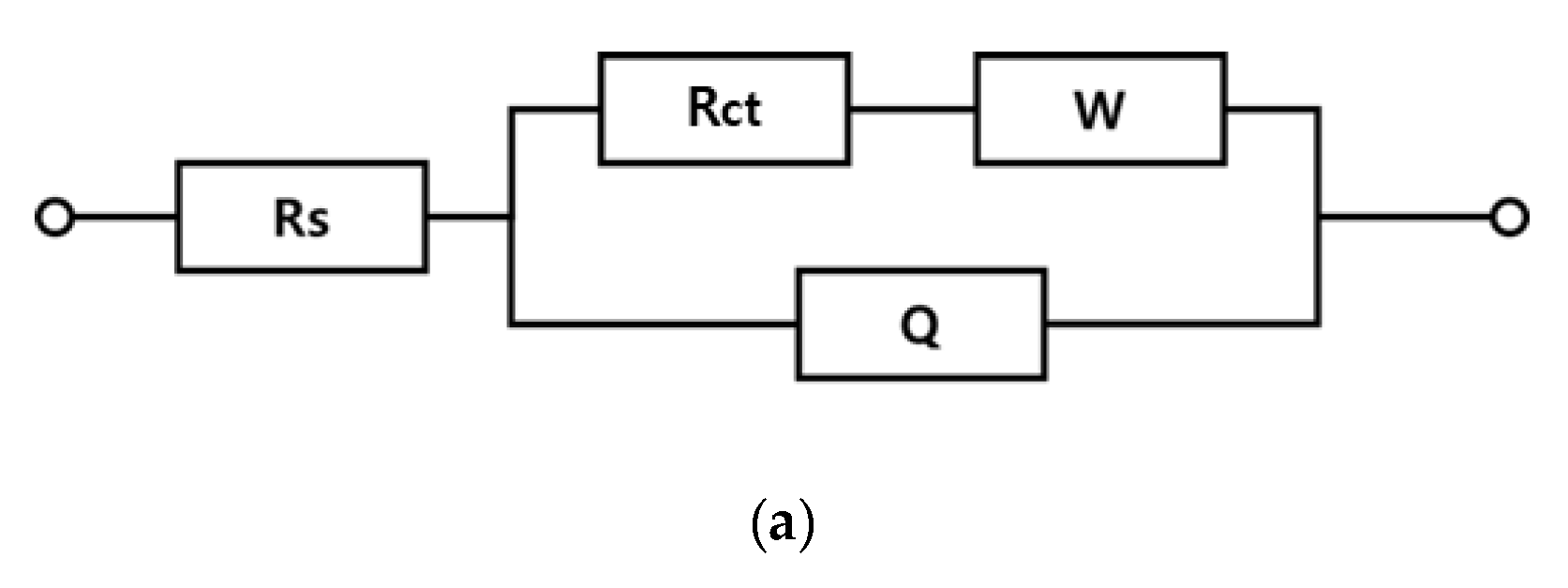
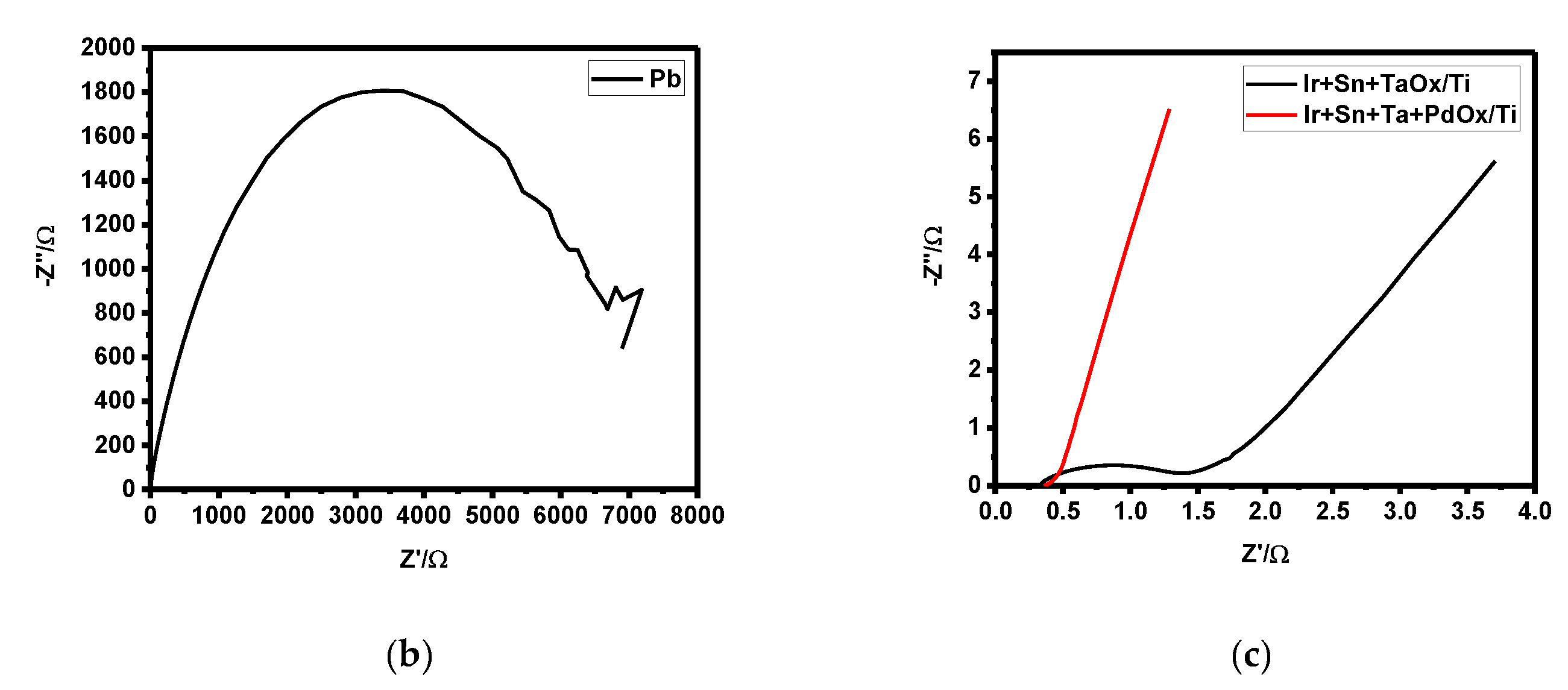
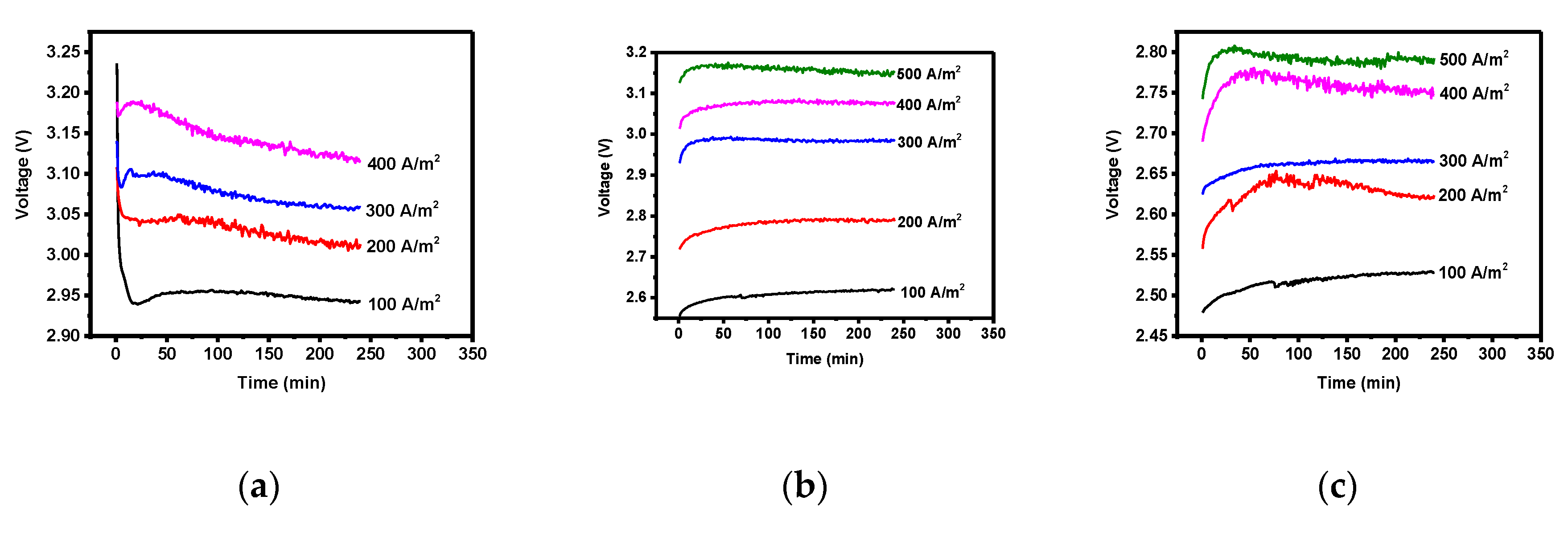
3.2. Overpotential and Electrode Resistance according to Electrode Composition
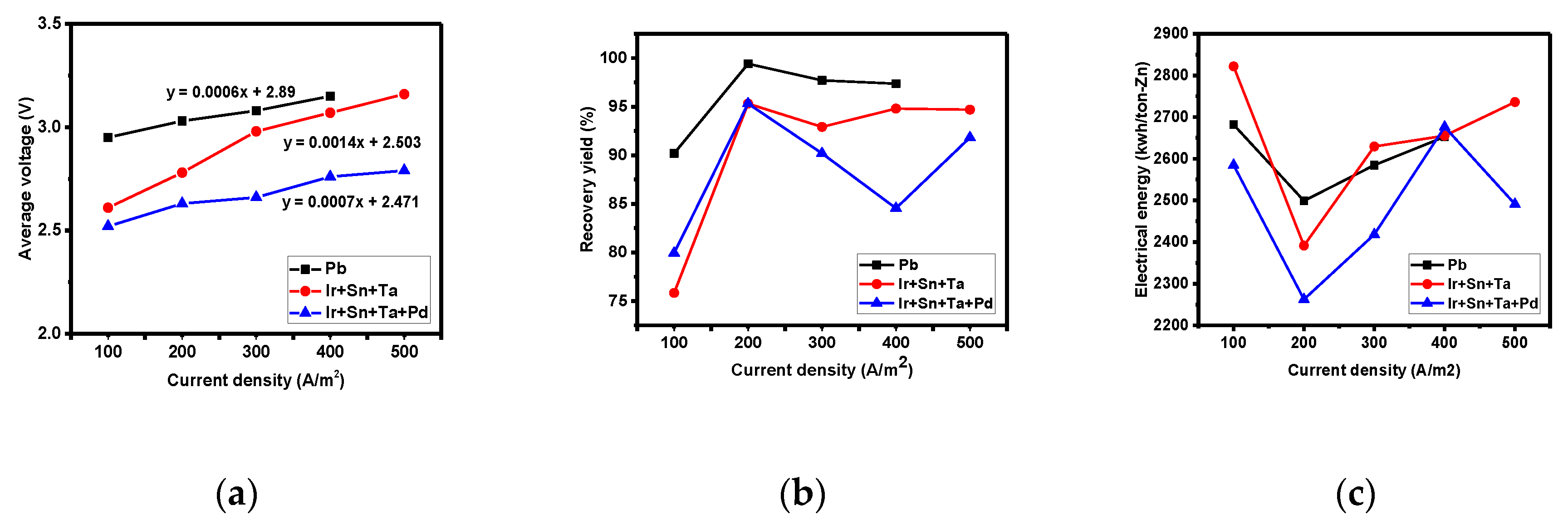
3.3. Comparative Evaluation of Recovery Yield according to Electrode Components
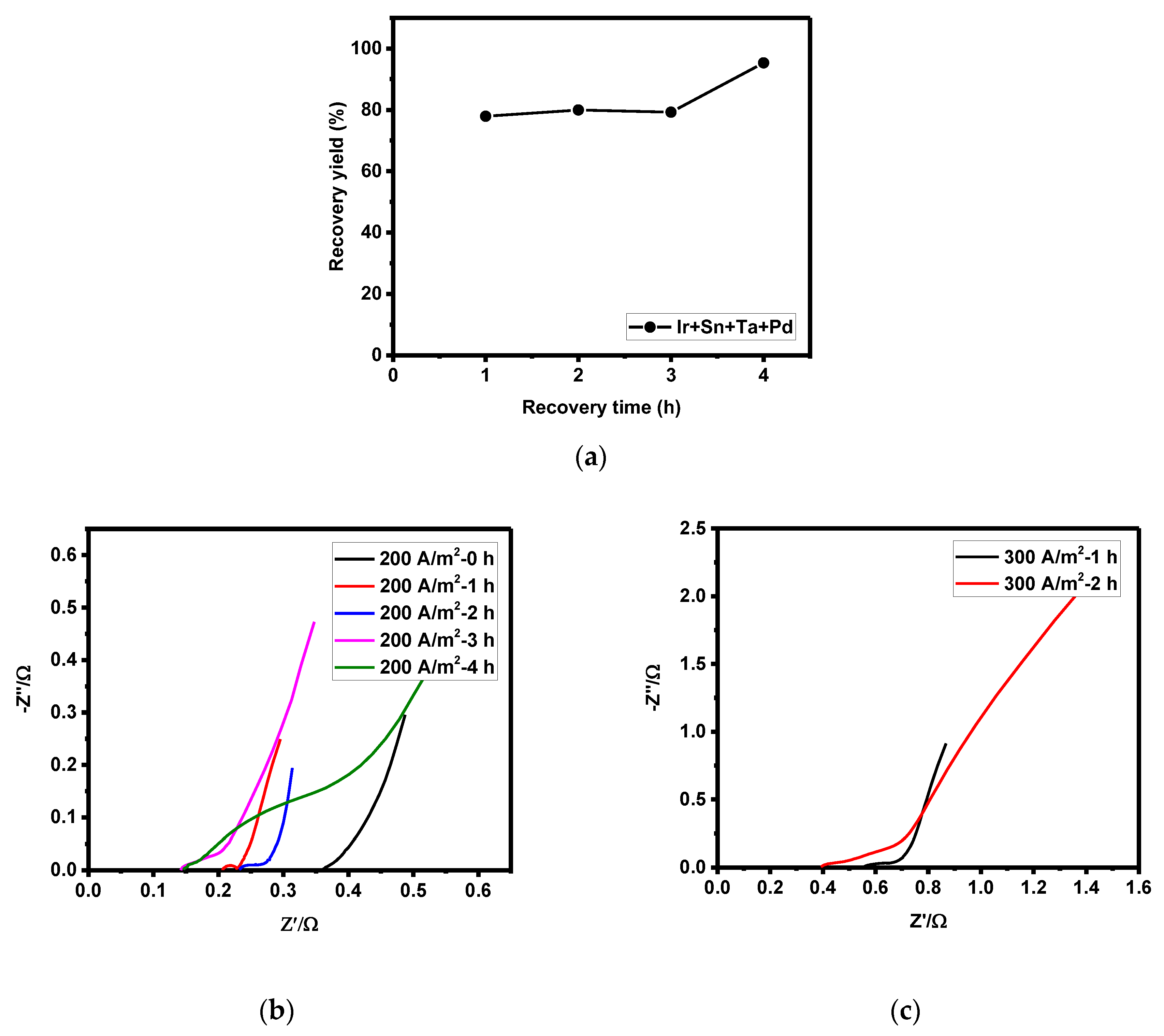
3.4. Optimization of Zinc Recovery Condition according to Recovery Time and Current Density
4. Conclusions
- This study used an alumina (Al) anode, a lead electrode, and electrodes using an insoluble catalyst, which can increase the electrode stability and conductivity, as the cathode in order to increase the zinc recovery yield.
- The overpotential of the lead, three-component, and four-component electrodes were 0.49, 1.20, and 1.13 η, respectively, while the concentration polarizations were 0.40, 1.16, and 1.10 ηc,. The overpotential was highly affected by the concentration polarization. A higher concentration polarization was found to generate a higher reduction in reactants, and thus the three- and four-component electrodes more actively recovered zinc compared to the lead electrode. Furthermore, the order of electrode resistance was the lead, the three-component, and the four-component electrodes.
- Using this sequence, the four-component electrode was identified as that with the greatest potential to lower the amount of required electrical energy due to its high reactivity for zinc recovery and low electrode resistance.
- At up to 3 h of zinc recovery time, the recovery yield was about 77% at the current density of 200 A/m2, which was lower than that at a current density of 300 A/m2 (recovery yield: about 80%). After 3 h of recovery time, however, the electrode resistance (reaction surface resistance due to zinc recovery) and the increase in overpotential with time were lower at a current density of 200 A/m2, significantly enhancing the zinc recovery yield to over 95%.
- In conclusion, an increase in recovery yield is accompanied by a lower overpotential. As zinc recovery occurs on the electrode surface, the electrode with a smaller increase in activation polarization and resistance was determined to be more effective for zinc recovery.
Author Contributions
Funding
Conflicts of Interest
References
- Lee, S.H.; Jo, Y.M. Review of national policies on the utilization of waste metal resources. KIC News 2010, 13, 2–9. [Google Scholar]
- Frioui, S.; Oumeddour, R.; Lacour, S. Highly selective extraction of metal ions from dilute solutions by hybrid electrodialysis technology. Sep. Purif. Technol. 2017, 174, 264–274. [Google Scholar] [CrossRef]
- Virolainen, S.; Ibana, D.; Raatero, E. Recovery of indium from indium tin oxide by solvent extraction. Hydrometallurgy 2011, 107, 56–61. [Google Scholar] [CrossRef]
- Babilas, D.; Dydo, P. Selective zinc recovery from electroplating wastewaters by electrodialysis enhanced with complex formation. Sep. Purif. Technol. 2018, 192, 419–428. [Google Scholar] [CrossRef]
- Lim, K.H.; Shon, B.H. Study on recovery of heavy metals from red mud by using the ultrasonic waves. J. Korea Acad. Industr. Coop. Soc. 2015, 16, 906–913. [Google Scholar] [CrossRef]
- Kurama, H.; Goktepe, F. Recovery of zinc from waste material using hydrometallurgical processes. Environ. Prog. 2003, 22, 161–166. [Google Scholar] [CrossRef]
- Modin, O.; Fuad, N.; Rauch, S. Microbial electrochemical recovery of zinc. Electrochim. Acta 2017, 248, 58–63. [Google Scholar] [CrossRef]
- Cui, W.; Chen, Z.; Yu, Q.; Zhu, W.; Li, H.; Wang, H. Preparation of Ti/PbO2-ZrO2 composite anode for Zn electrowining. Int. J. Electrochem. Sci. 2018, 13, 1400–1412. [Google Scholar] [CrossRef]
- Chen, G.; Chen, X.; Yue, P.L. Electrochemical behavior of novel Ti/IrOx-Sb2O5-SnO2 anodes. J. Phys. Chem. B 2002, 106, 4364–4369. [Google Scholar] [CrossRef]
- Kakooei, S.; Ismail, M.C.; Wahjoedi, B.A. Electrchemical study of Iridium oxide coating on stainless steel substrate. Int. J. Electrochem. Sci. 2013, 8, 3290–3301. [Google Scholar]
- Wang, Y.; Xiao, S.; Cai, X.; Bao, W.; Reutt-Robey, J.; Fuhrer, M.S. Electronic transport properties of Ir-decorated graphene. Sci. Rep. 2015, 5, 15764–15770. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Huang, S.M.; Lin, Y.H.; Lee, T.C.; Liu, H.; Zhang, X.X.; Chen, R.S.; Huang, Y.S. Low temperature electrical transport properties of RuO2 and IrO2 single crystals. J. Phys. Condens. Matter 2004, 16, 8035–8041. [Google Scholar] [CrossRef]
- Ryabtsev, S.V.; Levlev, V.M.; Samoylov, A.M.; Kuschev, S.B.; Soldatenko, S.A. Microsturcture and electrical properties of palladium oxide thin films for oxidizing gases detection. Thin Solid Films 2017, 636, 751–759. [Google Scholar] [CrossRef]
- Park, J.E.; Yang, S.K.; Kim, J.H.; Park, M.J.; Lee, E.S. Electrocatalytic activity of Pd/Ir/Sn/Ta/TiO2 composite electrodes. Energies 2018, 11, 3356. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumar, V.; Singh, R.J. Review of hydrometallurgical recovery of zinc from industrial wastes. Resour. Conserv. Recy. 2001, 33, 1–22. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801–13822. [Google Scholar] [CrossRef] [PubMed]
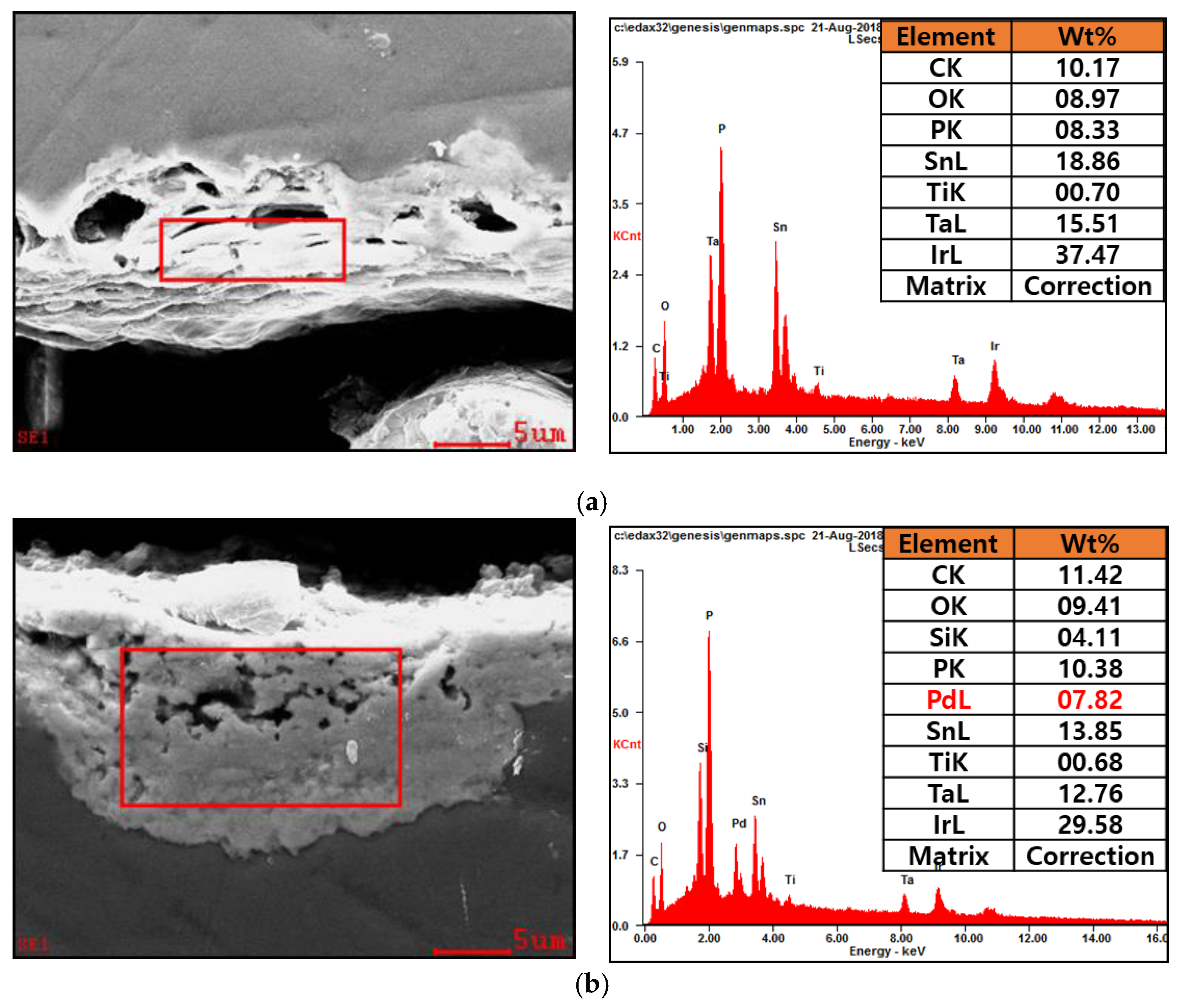

| Sample | Solution Condition | Activation Polarization (ηa) | Concentration Polarization (ηc) | Activation + Concentration Polarization (η) | Electrode Resistance (Rct, Ω) | Solution Conductivity at 25 °C (mS/cm) |
|---|---|---|---|---|---|---|
| Pb | Sulfuric acid-3 M, Zn-100 g/L | 0.09 | 0.40 | 0.49 | 5902.00 | 405.20 |
| Ir + Sn + Ta | 0.04 | 1.16 | 1.20 | 0.98 | ||
| Ir + Sn + Ta + Pd | 0.03 | 1.10 | 1.13 | 0.02 |
| Sample | Current Density (A/m2) | Recovery Time (h) | Activation Polarization (ηa) | Concentration Polarization (ηc) | Activation + Concentration Polarization (η) | Electrode Resistance (Rct, Ω) | Solution Conductivity at 25 °C (mS/cm) |
|---|---|---|---|---|---|---|---|
| Ir + Sn + Ta + Pd | 200 | 0 | 0.03 | 1.10 | 1.13 | 0.02 | 446.30 |
| 1 | 0.10 | 1.30 | 1.40 | 0.03 | 422.70 | ||
| 2 | 0.08 | 1.29 | 1.37 | 0.06 | 394.10 | ||
| 3 | 0.10 | 1.30 | 1.40 | 0.24 | 411.70 | ||
| 4 | 0.11 | 1.29 | 1.40 | 0.25 | 409.20 | ||
| 300 | 1 | 0.11 | 1.28 | 1.39 | 0.11 | 416.40 | |
| 2 | 0.10 | 1.29 | 1.39 | 0.13 | 373.30 |
| Sample | Current Density (A/m2) | Activation Polarization (ηa) | Concentration Polarization (ηc) | Activation + Concentration Polarization (η) | Electrode Resistance (Rct, Ω) | Solution Conductivity at 25 °C (mS/cm) |
|---|---|---|---|---|---|---|
| Ir + Sn + Ta + Pd | 0 | 0.03 | 1.10 | 1.13 | 0.02 | 446.30 |
| 100 | 0.04 | 1.29 | 1.33 | 0.10 | 436.50 | |
| 200 | 0.10 | 1.30 | 1.40 | 0.03 | 422.70 | |
| 300 | 0.11 | 1.28 | 1.39 | 0.11 | 416.40 | |
| 400 | 0.06 | 1.30 | 1.36 | 0.13 | 415.20 | |
| 500 | 0.12 | 1.28 | 1.40 | 0.27 | 402.70 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Park, J.E.; Lee, E.S. Zinc Recovery through Electrolytic Refinement Using Insoluble Ir + Sn + Ta + PdOx/Ti Cathode to Reduce Electrical Energy Use. Materials 2019, 12, 2779. https://doi.org/10.3390/ma12172779
Kim J-H, Park JE, Lee ES. Zinc Recovery through Electrolytic Refinement Using Insoluble Ir + Sn + Ta + PdOx/Ti Cathode to Reduce Electrical Energy Use. Materials. 2019; 12(17):2779. https://doi.org/10.3390/ma12172779
Chicago/Turabian StyleKim, Ji-Hyun, Jung Eun Park, and Eun Sil Lee. 2019. "Zinc Recovery through Electrolytic Refinement Using Insoluble Ir + Sn + Ta + PdOx/Ti Cathode to Reduce Electrical Energy Use" Materials 12, no. 17: 2779. https://doi.org/10.3390/ma12172779
APA StyleKim, J.-H., Park, J. E., & Lee, E. S. (2019). Zinc Recovery through Electrolytic Refinement Using Insoluble Ir + Sn + Ta + PdOx/Ti Cathode to Reduce Electrical Energy Use. Materials, 12(17), 2779. https://doi.org/10.3390/ma12172779




