Robocasting of SiO2-Based Bioactive Glass Scaffolds with Porosity Gradient for Bone Regeneration and Potential Load-Bearing Applications
Abstract
1. Introduction
2. Results and Discussion
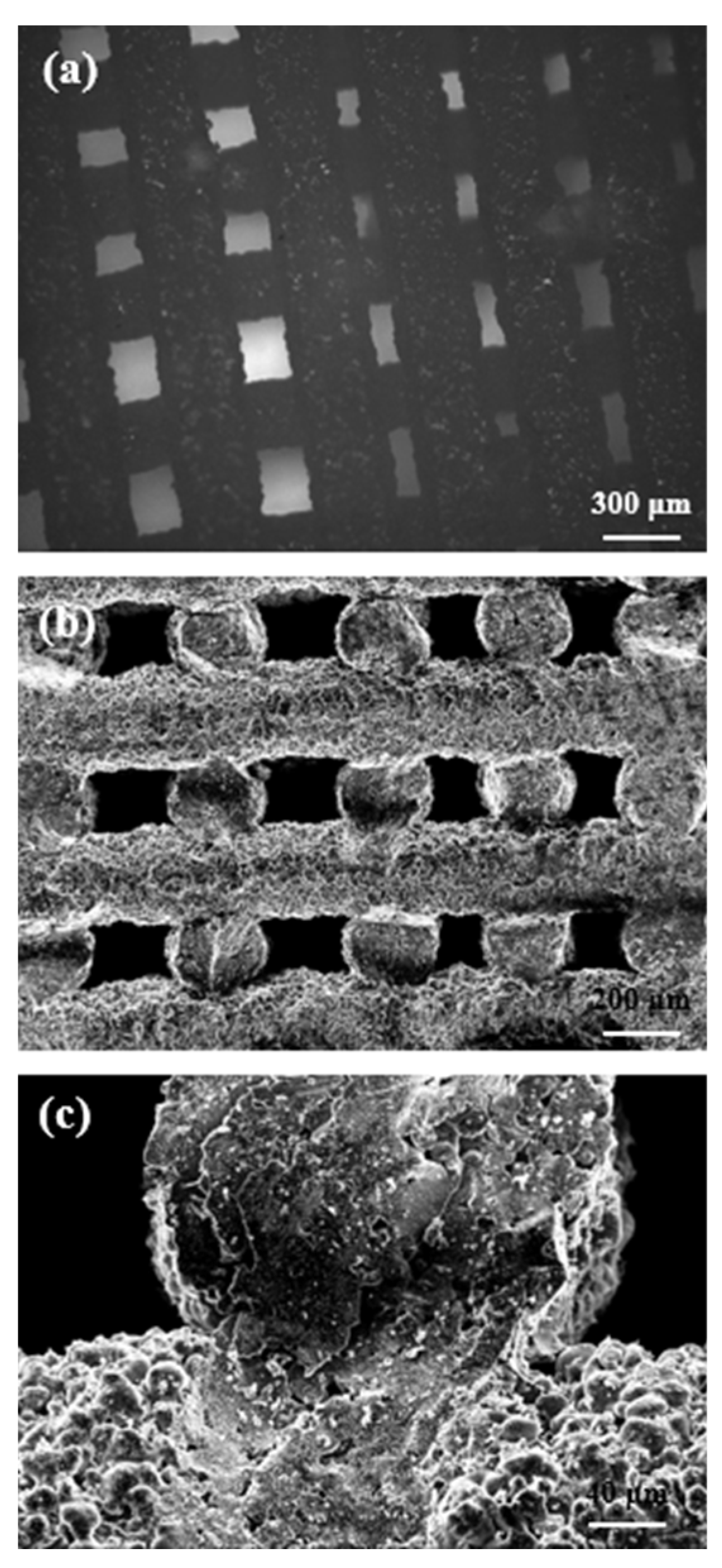
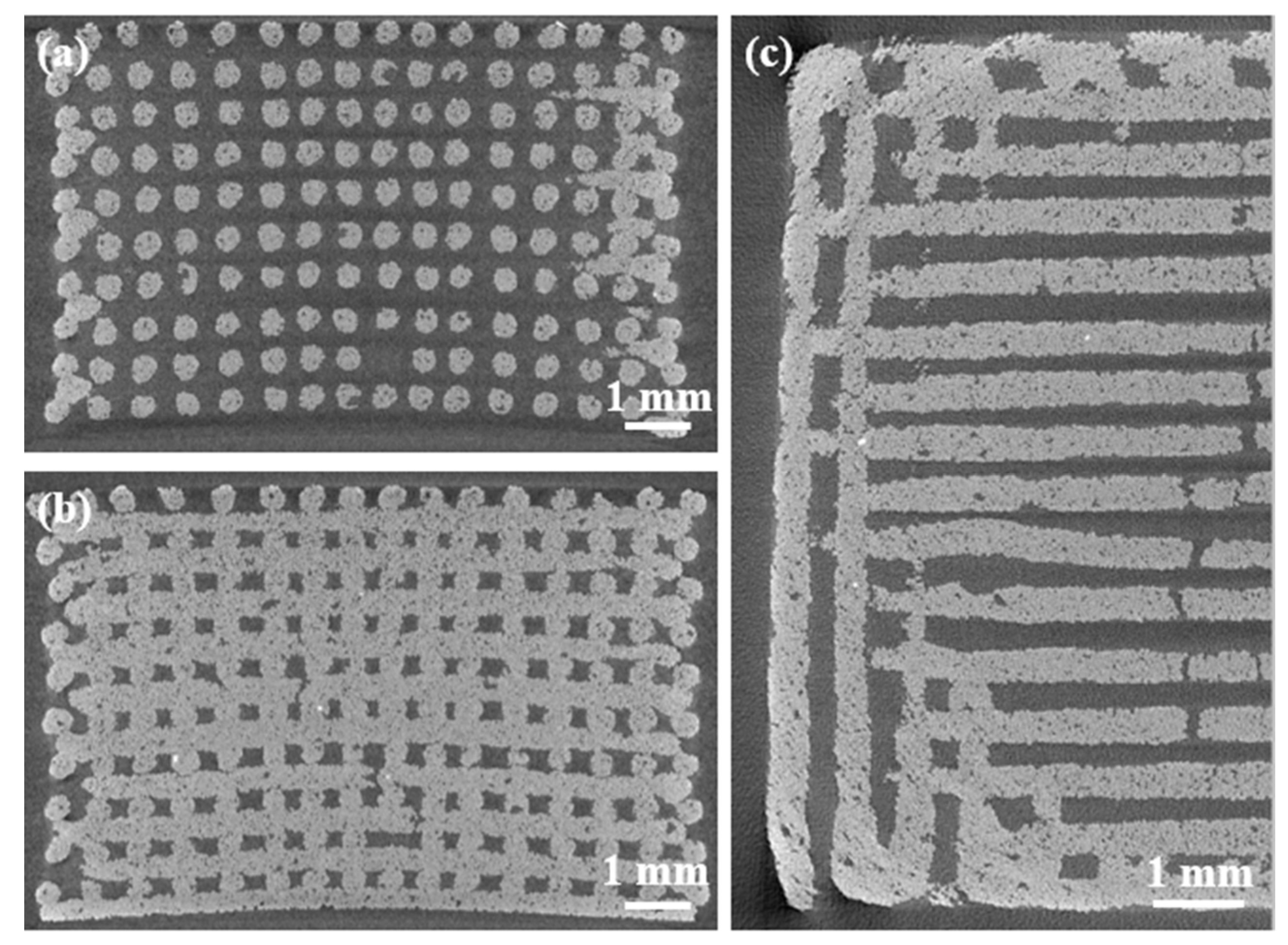
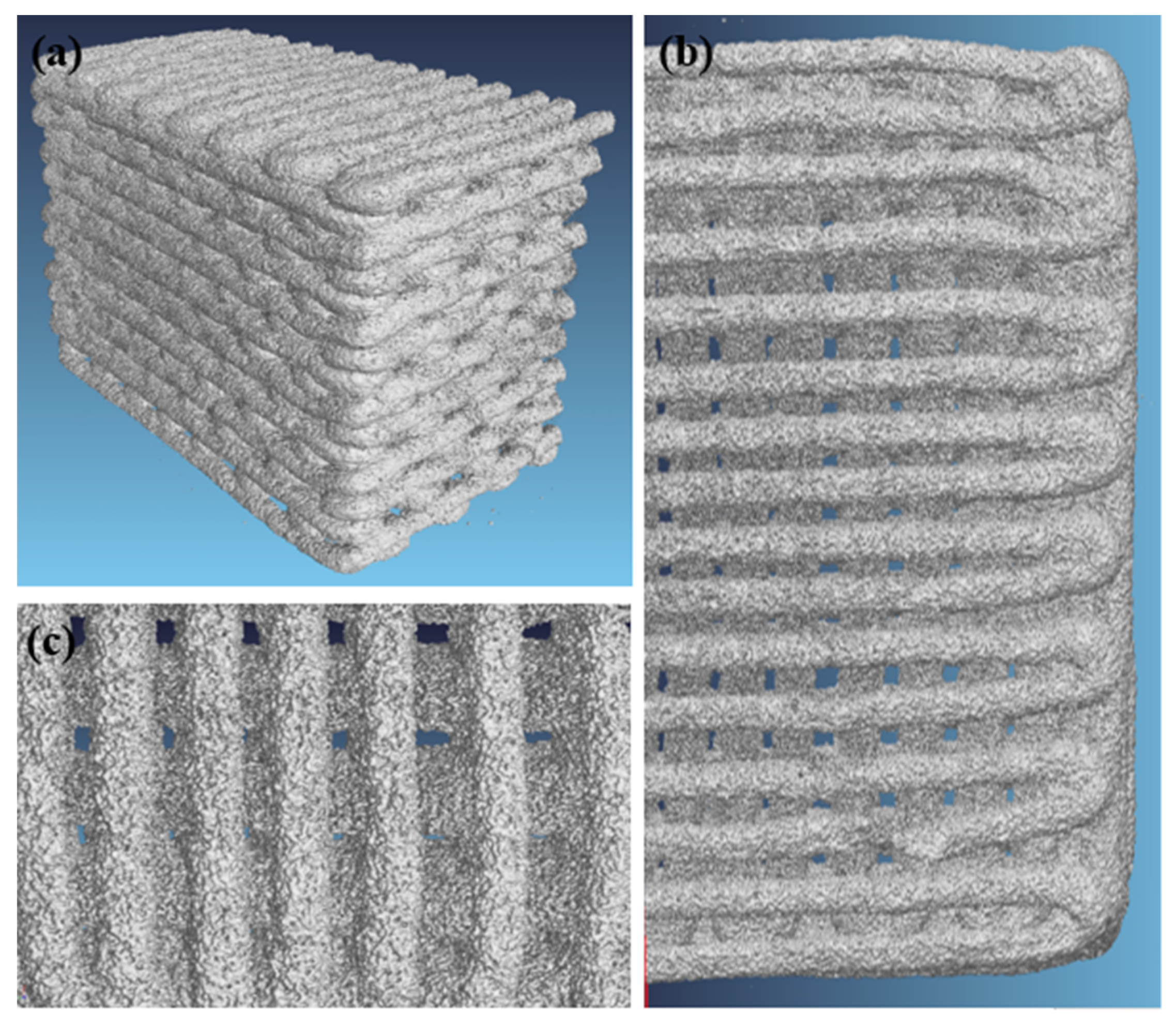
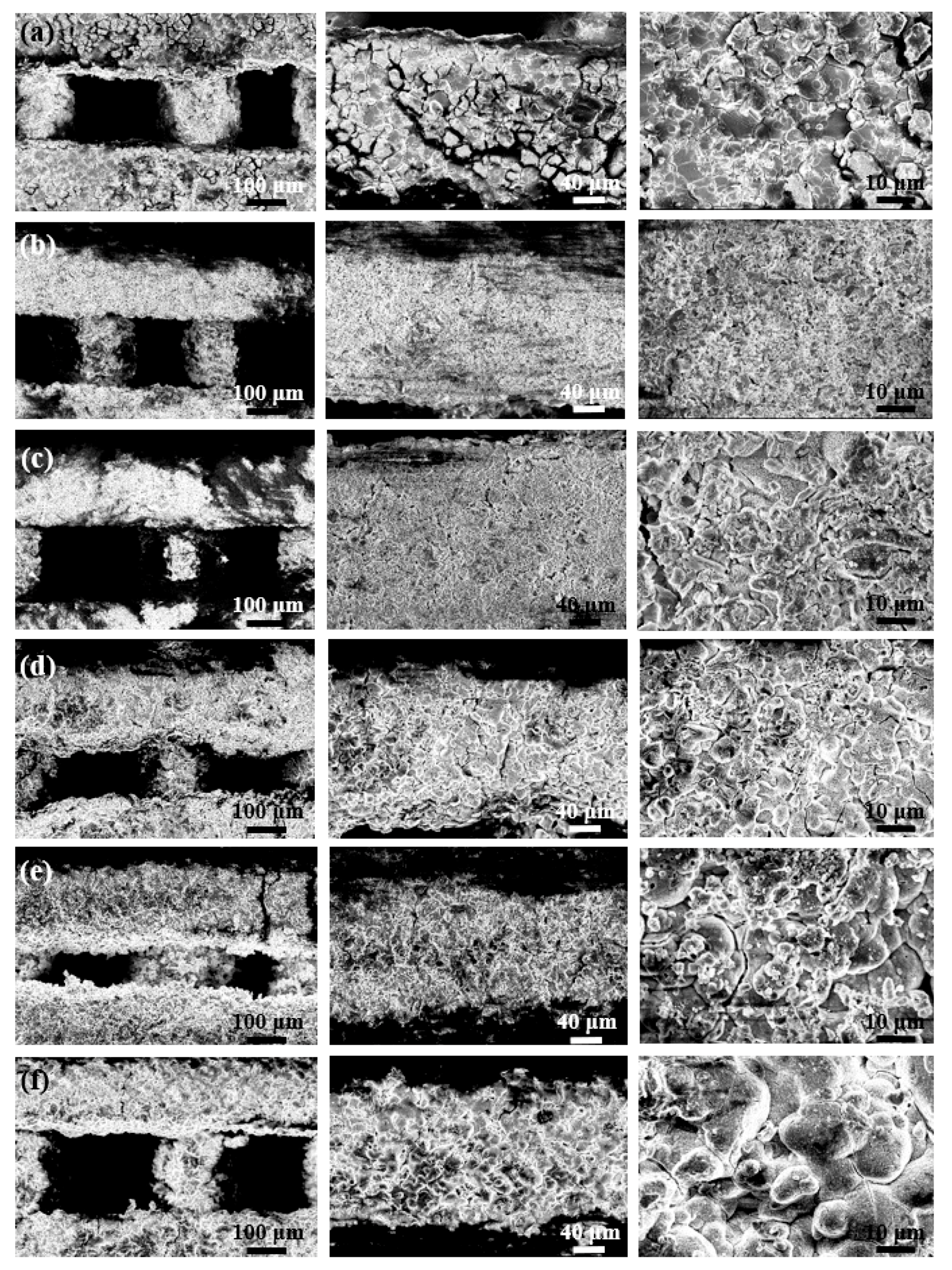
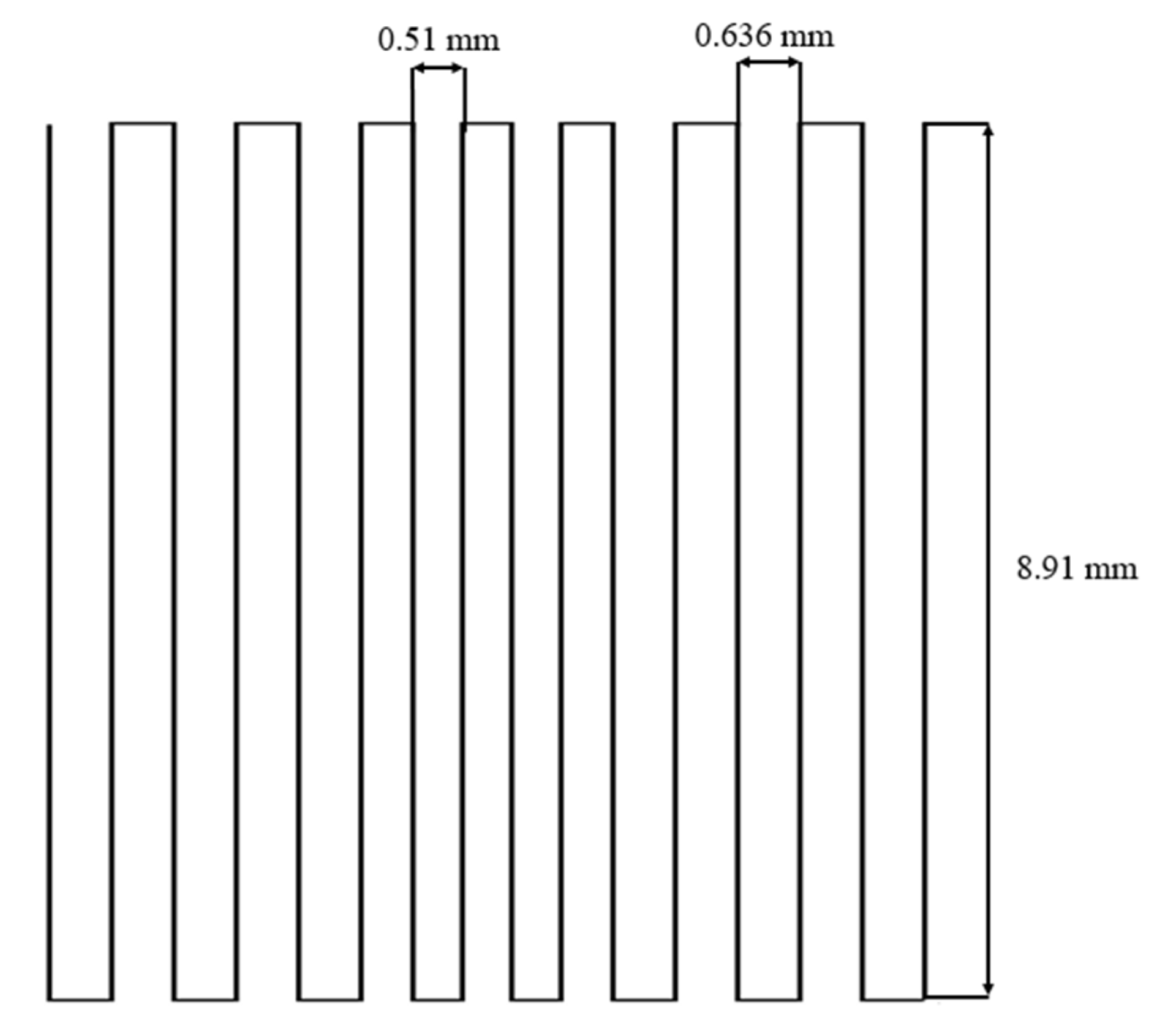
2.1. Morphology and Microstructure of Robocast Scaffolds
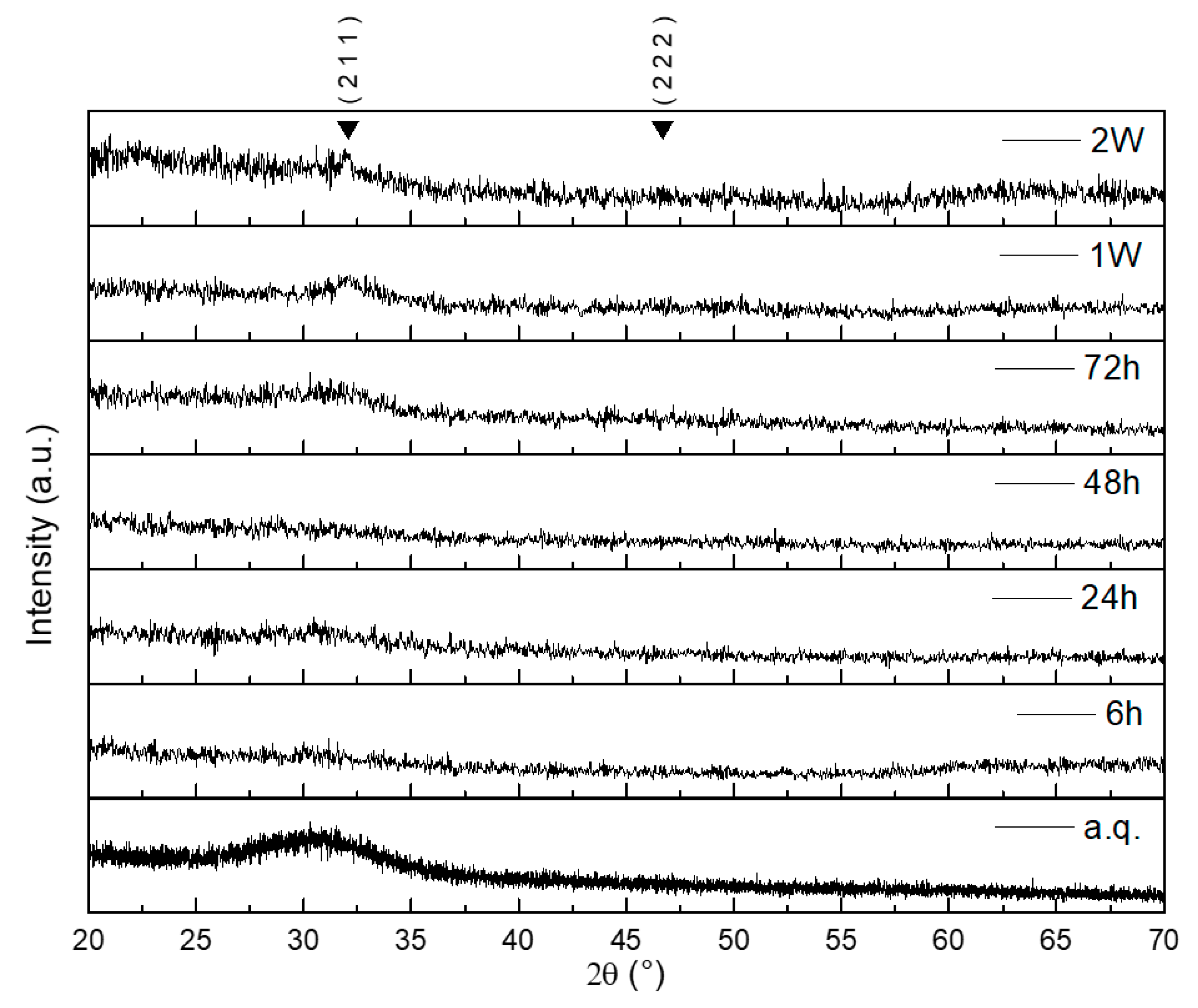
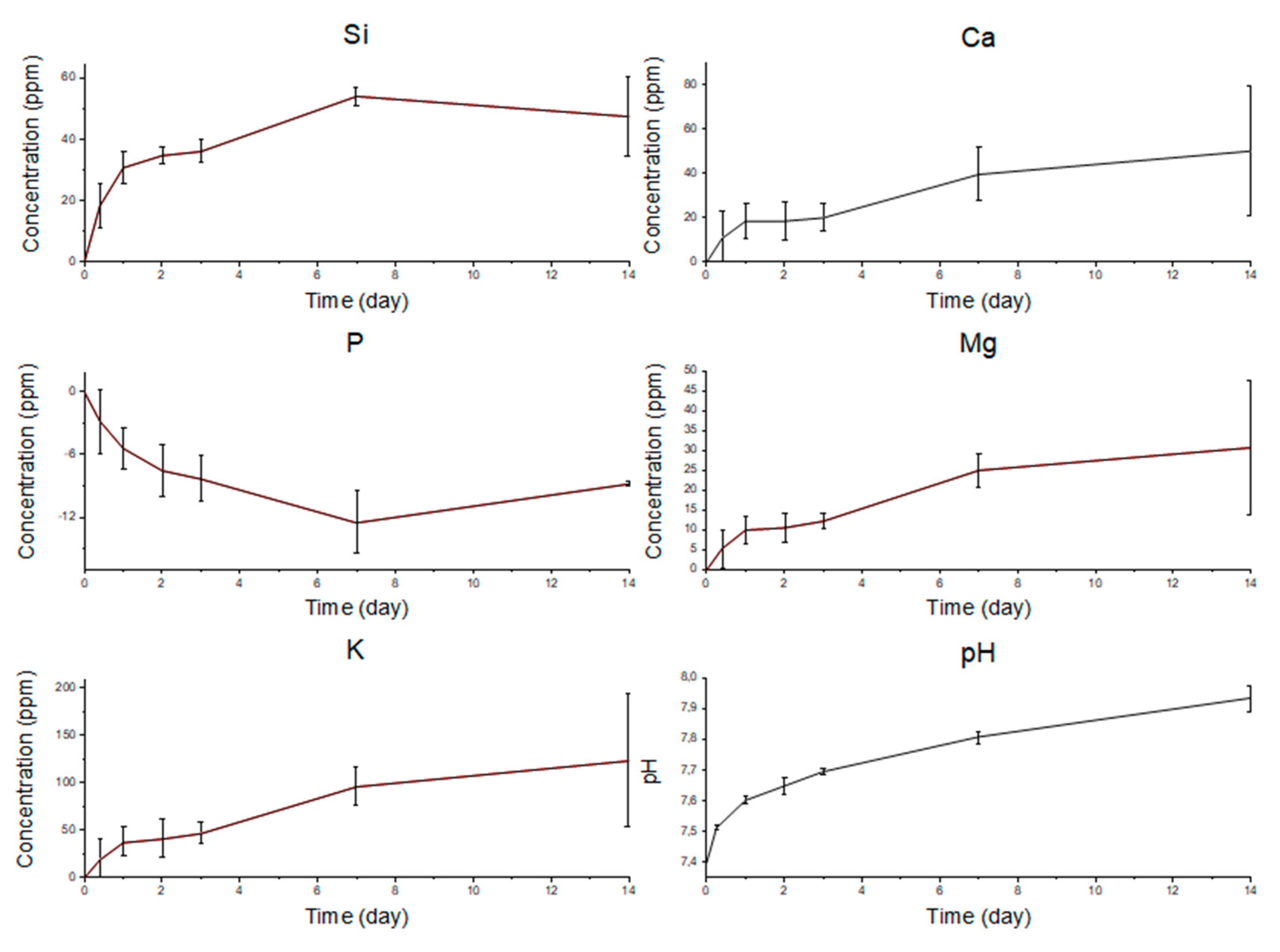
2.2. In Vitro Bioactivity
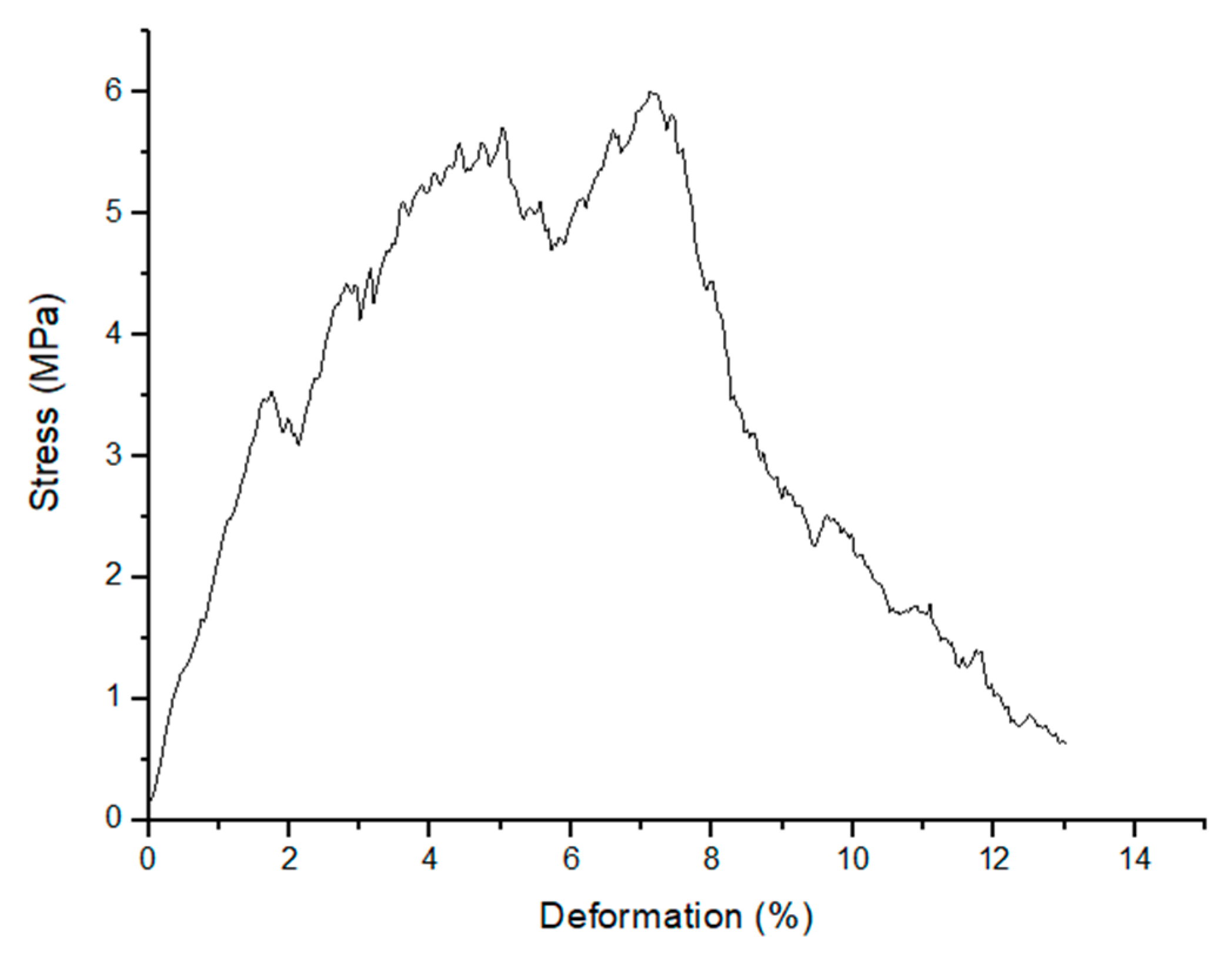
2.3. Mechanical Strength
3. Materials and Methods
3.1. Glass Production
3.2. Graded Scaffold Fabrication by Robocasting
3.3. Characterization
3.3.1. Thermal Analysis
3.3.2. X-ray Diffraction
3.3.3. In Vitro Bioactivity
3.3.4. Morphological and Structural Characterization
3.3.5. Mechanical Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fu, Q.; Saiz, E.; Rahaman, M.N.; Tomsia, A.P. Bioactive glass scaffolds for bone tissue engineering: State of the art and future perspectives. Mater. Sci. Eng. C 2011, 31, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Kinaci, A.; Neuhaus, V.; Ring, D.C. Trends in Bone Graft Use in the United States. Orthopedics 2014, 37, e783–e788. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, I.C.; Hutmacher, D.W.; Schuetz, M.A.; Epari, D.R.; Henkel, J.; Steck, R.; Glatt, V.; Choong, P.F.M.; Woodruff, M.A. Bone Regeneration Based on Tissue Engineering Conceptions-A 21st Century Perspective. Bone Res. 2014, 1, 216–248. [Google Scholar]
- Baino, F.; Marshall, M.; Kirk, N.; Vitale-Brovarone, C. Design, selection and characterization of novel glasses and glass-ceramics for use in prosthetic applications. Ceram. Int. 2016, 42, 1482–1491. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Chatzistavrou, X.; Newby, P.; Boccaccini, A.R. Bioactive glass and glass-ceramic scaffolds for bone tissue engineering. In Bioactive Glasses: Materials, Properties and Applications; Woodhead Publishing: Cambridge, UK, 2011; Volume 3, pp. 107–128. ISBN 9781845697686. [Google Scholar]
- Salgado, A.J.; Coutinho, O.P.; Reis, R.L. Bone tissue engineering: State of the art and future trends. Macromol. Biosci. 2004, 4, 743–765. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R. Reprint of: Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2015, 23, S53–S82. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Fiume, E.; Miola, M.; Verné, E. Bioactive sol-gel glasses: processing, properties and applications. Int. J. Appl. Ceram. Technol. 2018, 15, 841–860. [Google Scholar] [CrossRef]
- Gmeiner, R.; Deisinger, U.; Schönherr, J.; Lechner, B.; Detsch, R.; Boccaccini, A.R.; Stampfl, J. Additive manufacturing of bioactive glasses and silicate bioceramics. J. Ceram. Sci. Technol. 2015, 6, 75–86. [Google Scholar]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Baino, F.; Fiume, E.; Barberi, J.; Kargozar, S.; Marchi, J.; Massera, J.; Verné, E. Processing methods for making porous bioactive glass-based scaffolds-A state-of-the-art review. Int. J. Appl. Ceram. Technol. 2019, 16, 1762–1796. [Google Scholar] [CrossRef]
- Mehrali, M.; Metselaar, H.S.C.; Yarmand, H.; Osman, N.A.A.; Adib Kadri, N.; Gharehkhani, S.; Shirazi, S.F.S. A review on powder-based additive manufacturing for tissue engineering: selective laser sintering and inkjet 3D printing. Sci. Technol. Adv. Mater. 2015, 16, 033502. [Google Scholar]
- Cesarano, J. Robocasting of Ceramics and Composites Using Fine Particle Suspensions. In Proceedings of the Second Annual Technology and Business Conference, Orlando, FL, USA, 17–19 October 1999. [Google Scholar]
- Franco, J.; Hunger, P.; Launey, M.E.; Tomsia, A.P.; Saiz, E. Direct write assembly of calcium phosphate scaffolds using a water-based hydrogel. Acta Biomater. 2010, 6, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Saiz, E.; Tomsia, A.P. Direct ink writing of highly porous and strong glass scaffolds for load-bearing bone defects repair and regeneration. Acta Biomater. 2011, 7, 3547–3554. [Google Scholar] [CrossRef] [PubMed]
- Nommeots-Nomm, A.; Lee, P.D.; Jones, J.R. Direct ink writing of highly bioactive glasses. J. Eur. Ceram. Soc. 2018, 38, 837–844. [Google Scholar] [CrossRef]
- Liu, X.; Rahaman, M.N.; Hilmas, G.E.; Bal, B.S. Mechanical properties of bioactive glass (13–93) scaffolds fabricated by robotic deposition for structural bone repair. Acta Biomater. 2013, 9, 7025–7034. [Google Scholar] [CrossRef]
- Eqtesadi, S.; Motealleh, A.; Miranda, P.; Pajares, A.; Lemos, A.; Ferreira, J.M.F. Robocasting of 45S5 bioactive glass scaffolds for bone tissue engineering. J. Eur. Ceram. Soc. 2014, 34, 107–118. [Google Scholar] [CrossRef]
- Eqtesadi, S.; Motealleh, A.; Pajares, A.; Guiberteau, F.; Miranda, P. Improving mechanical properties of 13–93 bioactive glass robocast scaffold by poly (lactic acid) and poly (ε-caprolactone) melt infiltration. J. Non. Cryst. Solids 2016, 432, 111–119. [Google Scholar] [CrossRef]
- Fu, Q.; Saiz, E.; Tomsia, A.P. Bioinspired strong and highly porous glass scaffolds. Adv. Funct. Mater. 2011, 21, 1058–1063. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Motealleh, A.; Eqtesadi, S.; Civantos, A.; Pajares, A.; Miranda, P. Robocast 45S5 bioglass scaffolds: in vitro behavior. J. Mater. Sci. 2017, 52, 9179–9191. [Google Scholar] [CrossRef]
- Baino, F.; Barberi, J.; Fiume, E.; Orlygsson, G.; Massera, J.; Verné, E. Robocasting of Bioactive SiO2-P2O5-CaO-MgO-Na2O-K2O Glass Scaffolds. J. Healthc. Eng. 2019, 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Verné, E.; Bretcanu, O.; Balagna, C.; Bianchi, C.L.; Cannas, M.; Gatti, S.; Vitale-Brovarone, C. Early stage reactivity and in vitro behavior of silica-based bioactive glasses and glass-ceramics. J. Mater. Sci. Mater. Med. 2009, 20, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Fiume, E.; Verné, E.; Baino, F. Crystallization behavior of SiO2-P2O5-CaO-MgO-Na2O-K2O bioactive glass. Biomed. Glas. 2019, 5, 46–52. [Google Scholar] [CrossRef]
- Filho, O.P.; Latorre, G.P.; Hench, L.L. Effect of crystallization on apatite-layer formation of bioactive glass 45S5. J. Biomed. Mater. Res. 1996, 30, 509–514. [Google Scholar] [CrossRef]
- Nommeots-Nomm, A.; Ligorio, C.; Bodey, A.J.; Cai, B.; Jones, J.R.; Lee, P.D.; Poologasundarampillai, G. Four-dimensional imaging and quantification of viscous flow sintering within a 3D printed bioactive glass scaffold using synchrotron X-ray tomography. Mater. Today Adv. 2019, 2, 100011. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Bohner, M.; Lemaitre, J. Can bioactivity be tested in vitro with SBF solution? Biomaterials 2009, 30, 2175–2179. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Synthesis of mechanically stiff and bioactive hybrid hydrogels for bone tissue engineering applications. Chem. Eng. J. 2017, 317, 119–131. [Google Scholar] [CrossRef]
- Nommeots-Nomm, A.; Labbaf, S.; Devlin, A.; Todd, N.; Geng, H.; Solanki, A.K.; Tang, H.M.; Perdika, P.; Pinna, A.; Ejeian, F.; et al. Highly degradable porous melt-derived bioactive glass foam scaffolds for bone regeneration. Acta Biomater. 2017, 57, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Izquierdo-Barba, I.; Terasaki, O.; Arcos, D.; Sakamoto, Y.; López-Noriega, A. Ordered Mesoporous Bioactive Glasses for Bone Tissue Regeneration. Chem. Mater. 2006, 18, 3137–3144. [Google Scholar]
- Hench, L.L. Bioceramics: from concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- “nScrypt”. Available online: https://www.nscrypt.com/wpcontent/ uploads/2018/11/2018-3Dn-Brochure.pdf (accessed on December 2017).
- Macon, A.; Kim, T.; Valliant, E.; Goetschius, K.; Brow, R.; Day, D.; Hoppe, A.; Boccaccini, A.; Kim, I.; Ohtsuki, C.; et al. A unified in vitro evaluation for apatite-forming ability of bioactive glasses and their variants. J. Mater. Sci. Mater. Med. 2015, 26, 115. [Google Scholar] [CrossRef] [PubMed]
- Radon, J. Uber die bestimmung von funktionen durch ihre integralwerte langs geweisser mannigfaltigkeiten (on the determination of functions from their integrals along certain manifolds). Berichte Saechsische Akad. der Wissenschaften. 1917, 29, 262–277. [Google Scholar]
- Beyerer, J.; Puente León, F. Die Radontransformation in der digitalen Bildverarbeitung (The Radon Transform in Digital Image Processing). Autom 2002, 50, 472. [Google Scholar] [CrossRef]
- Doube, M.; Klosowski, M.M.; Arganda-Carreras, I.; Cordelières, F.P.; Dougherty, R.P.; Jackson, J.S.; Schmid, B.; Hutchinson, J.R.; Shefelbine, S.J. BoneJ: Free and extensible bone image analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ; U. S. National Institutes of Health: Bethesda, MD, USA, 1997–2018.

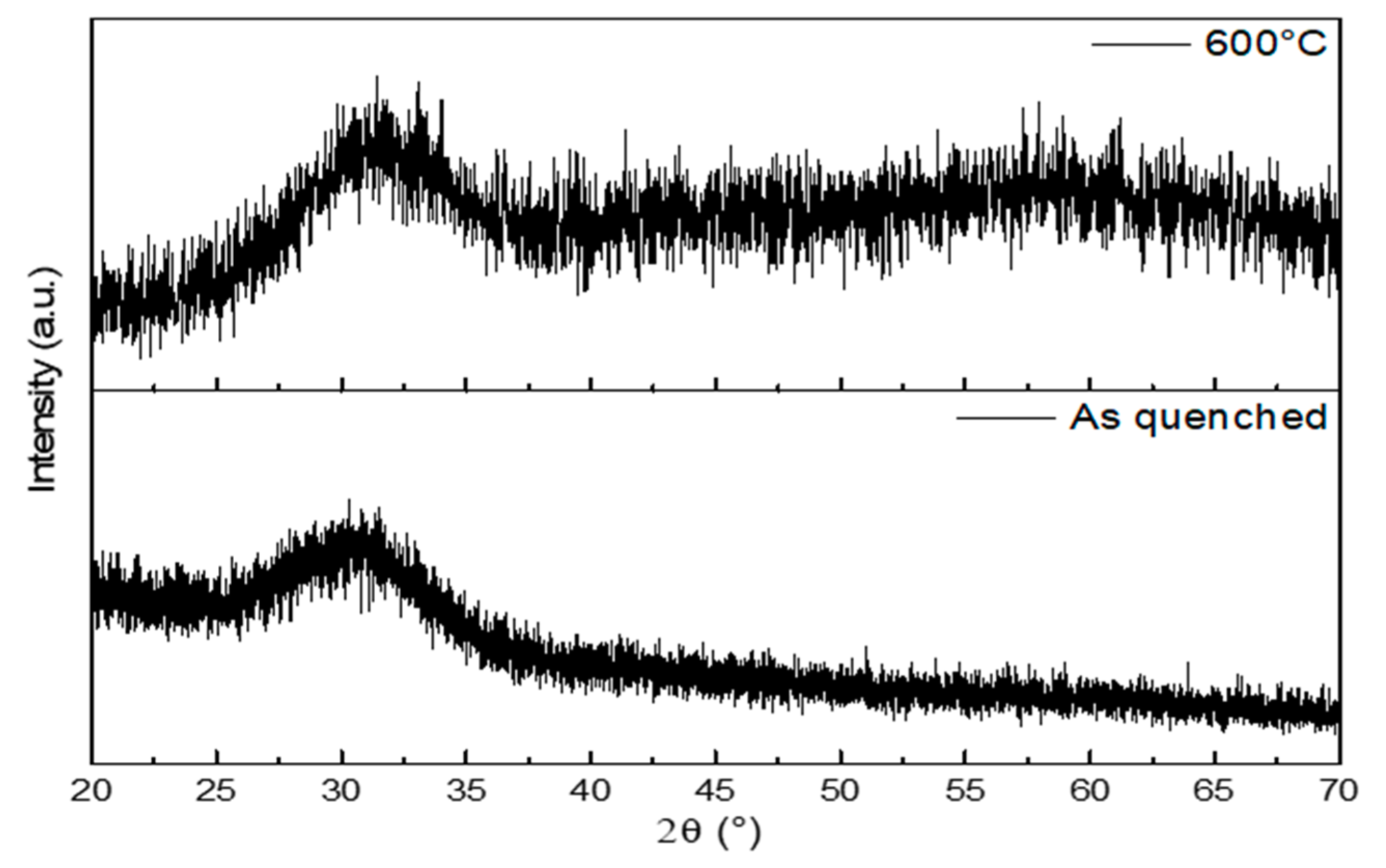

| Thermal Analysis Used | Characteristic Temperature | Temperature (°C) |
|---|---|---|
| DSC (thermal properties) | Tg | 547 |
| Tx | 760 | |
| Tc | 806 | |
| Tm | 1004 | |
| HSM (viscous behavior) | TFS | 585 |
| TB | 800 | |
| TFP | 1050 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barberi, J.; Baino, F.; Fiume, E.; Orlygsson, G.; Nommeots-Nomm, A.; Massera, J.; Verné, E. Robocasting of SiO2-Based Bioactive Glass Scaffolds with Porosity Gradient for Bone Regeneration and Potential Load-Bearing Applications. Materials 2019, 12, 2691. https://doi.org/10.3390/ma12172691
Barberi J, Baino F, Fiume E, Orlygsson G, Nommeots-Nomm A, Massera J, Verné E. Robocasting of SiO2-Based Bioactive Glass Scaffolds with Porosity Gradient for Bone Regeneration and Potential Load-Bearing Applications. Materials. 2019; 12(17):2691. https://doi.org/10.3390/ma12172691
Chicago/Turabian StyleBarberi, Jacopo, Francesco Baino, Elisa Fiume, Gissur Orlygsson, Amy Nommeots-Nomm, Jonathan Massera, and Enrica Verné. 2019. "Robocasting of SiO2-Based Bioactive Glass Scaffolds with Porosity Gradient for Bone Regeneration and Potential Load-Bearing Applications" Materials 12, no. 17: 2691. https://doi.org/10.3390/ma12172691
APA StyleBarberi, J., Baino, F., Fiume, E., Orlygsson, G., Nommeots-Nomm, A., Massera, J., & Verné, E. (2019). Robocasting of SiO2-Based Bioactive Glass Scaffolds with Porosity Gradient for Bone Regeneration and Potential Load-Bearing Applications. Materials, 12(17), 2691. https://doi.org/10.3390/ma12172691








