Investigation on the Cooling and Evaporation Behavior of Semi-Flexible Water Retaining Pavement based on Laboratory Test and Thermal-Mass Coupling Analysis
Abstract
1. Introduction
1.1. Background
1.2. Mechanism Investigation with Laboratory Test
1.3. Mechanism Investigation with Simulation Investigation
1.4. Approaches and Aims
2. Research Methods and Laboratory Test
2.1. Methodology
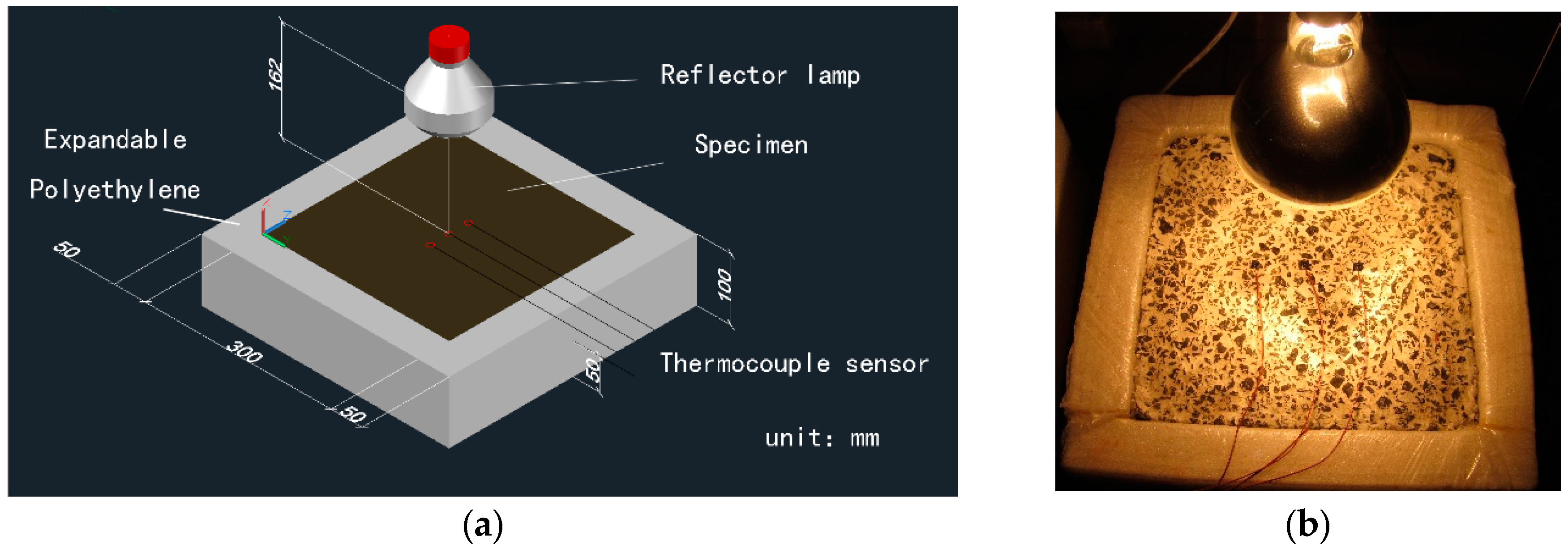
2.2. Test Devices and Preparation
2.3. Laboratory Test
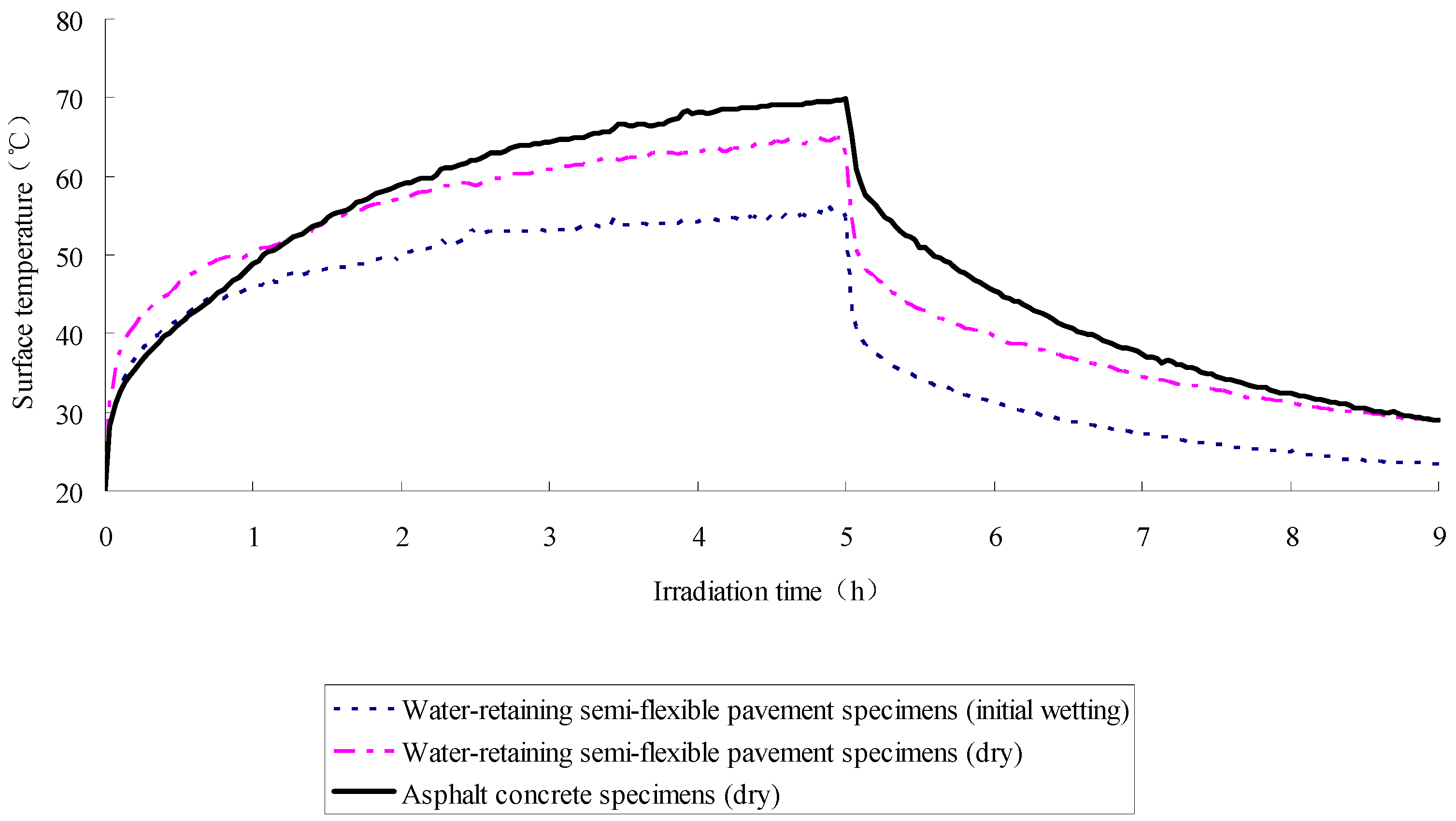
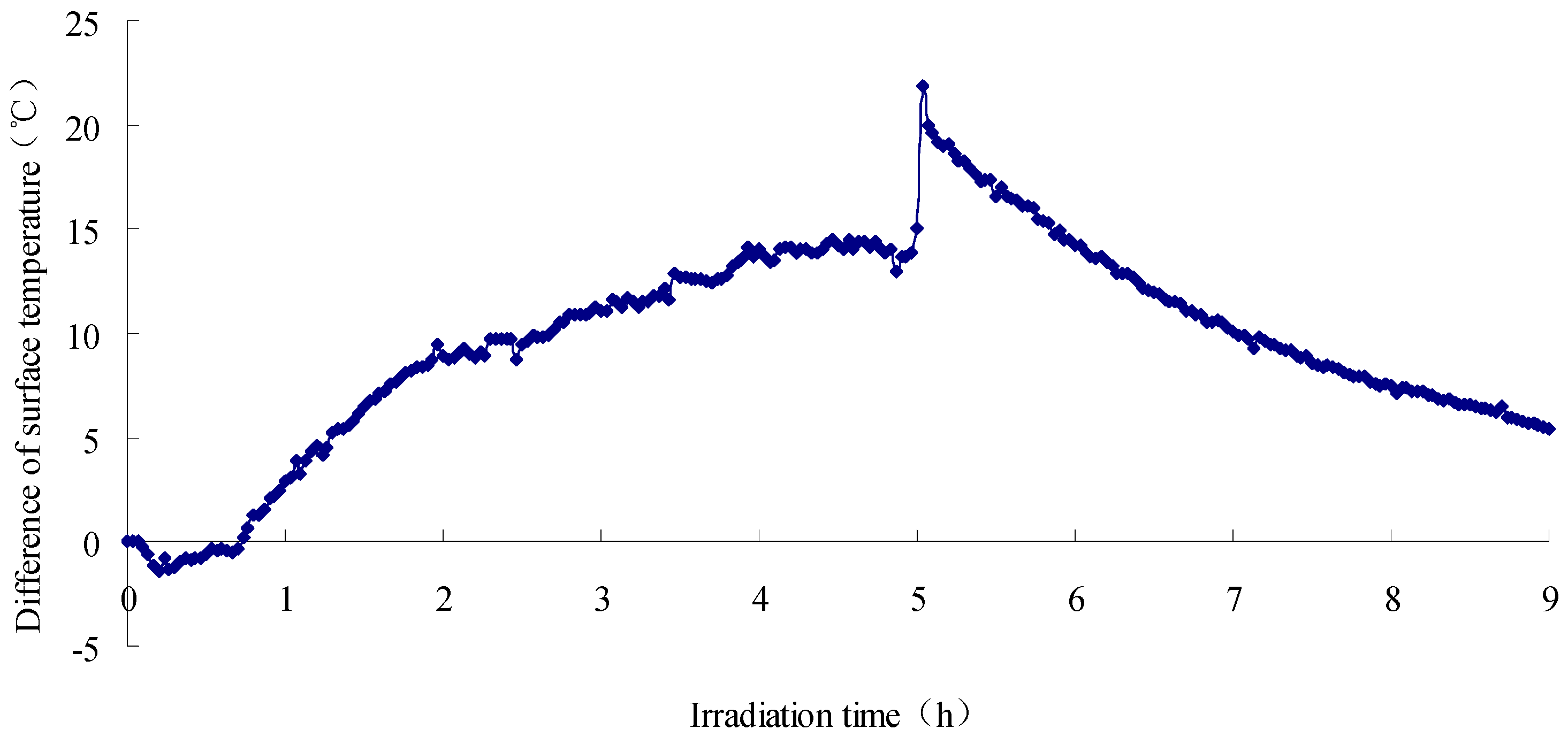
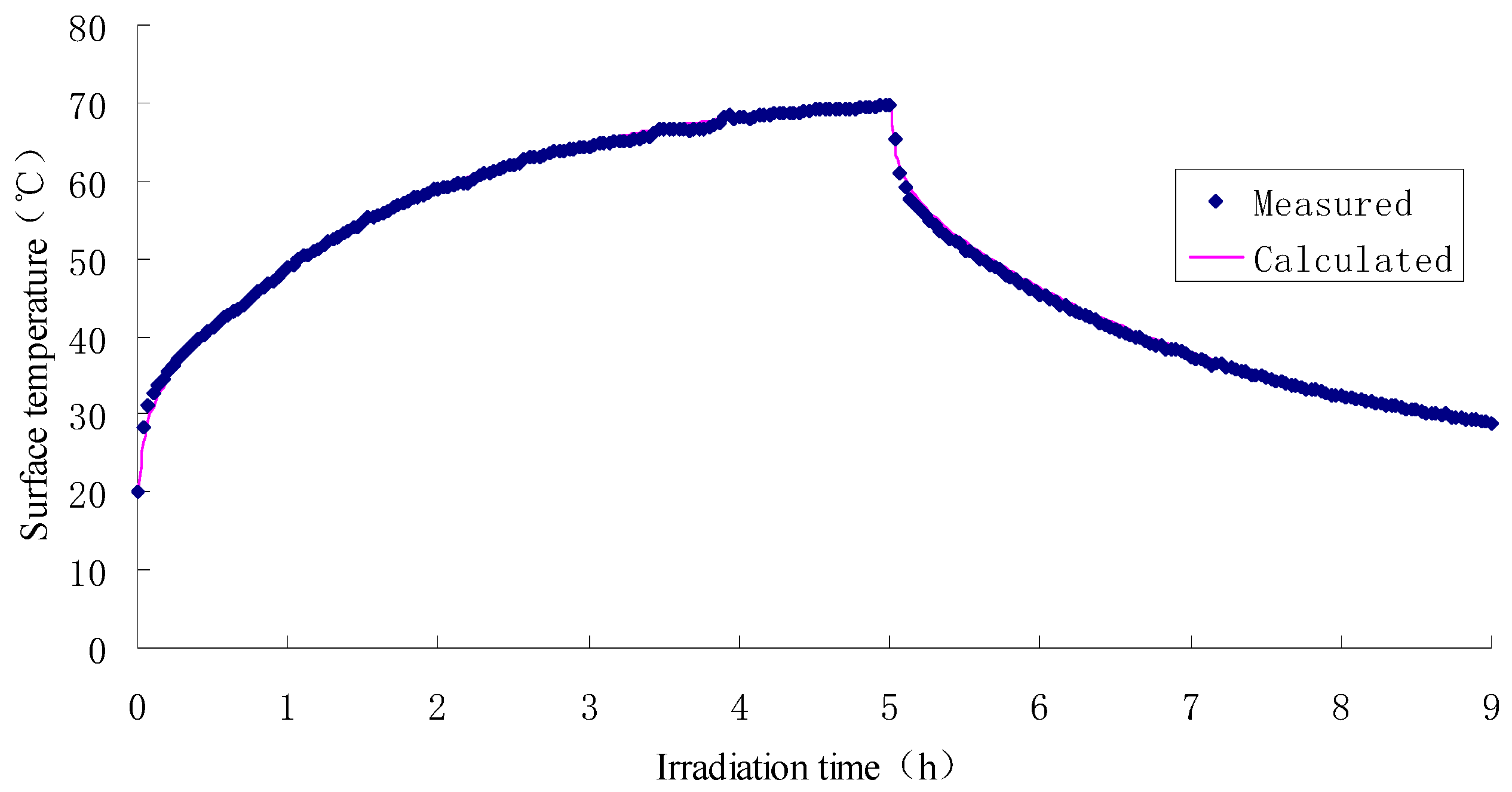
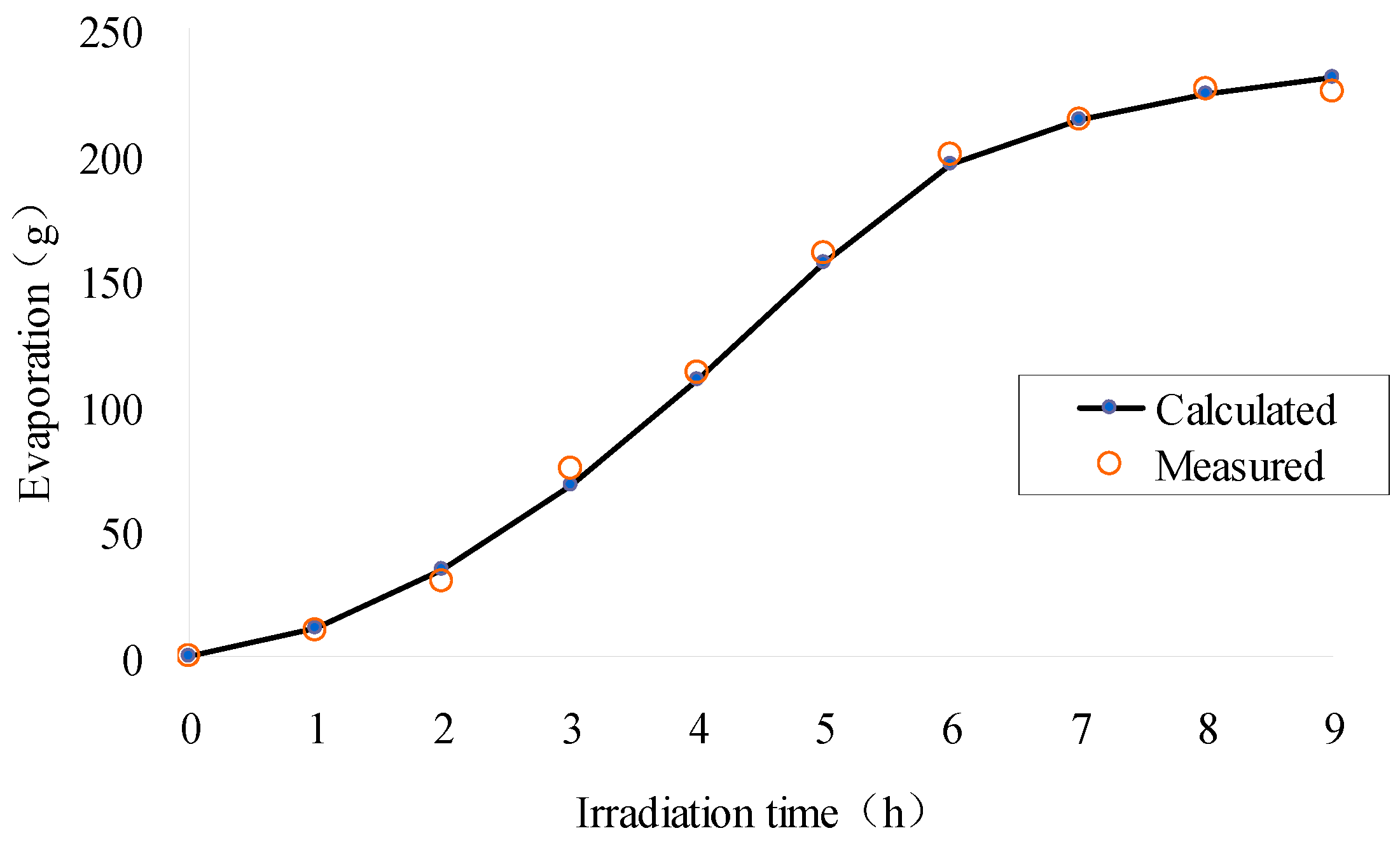
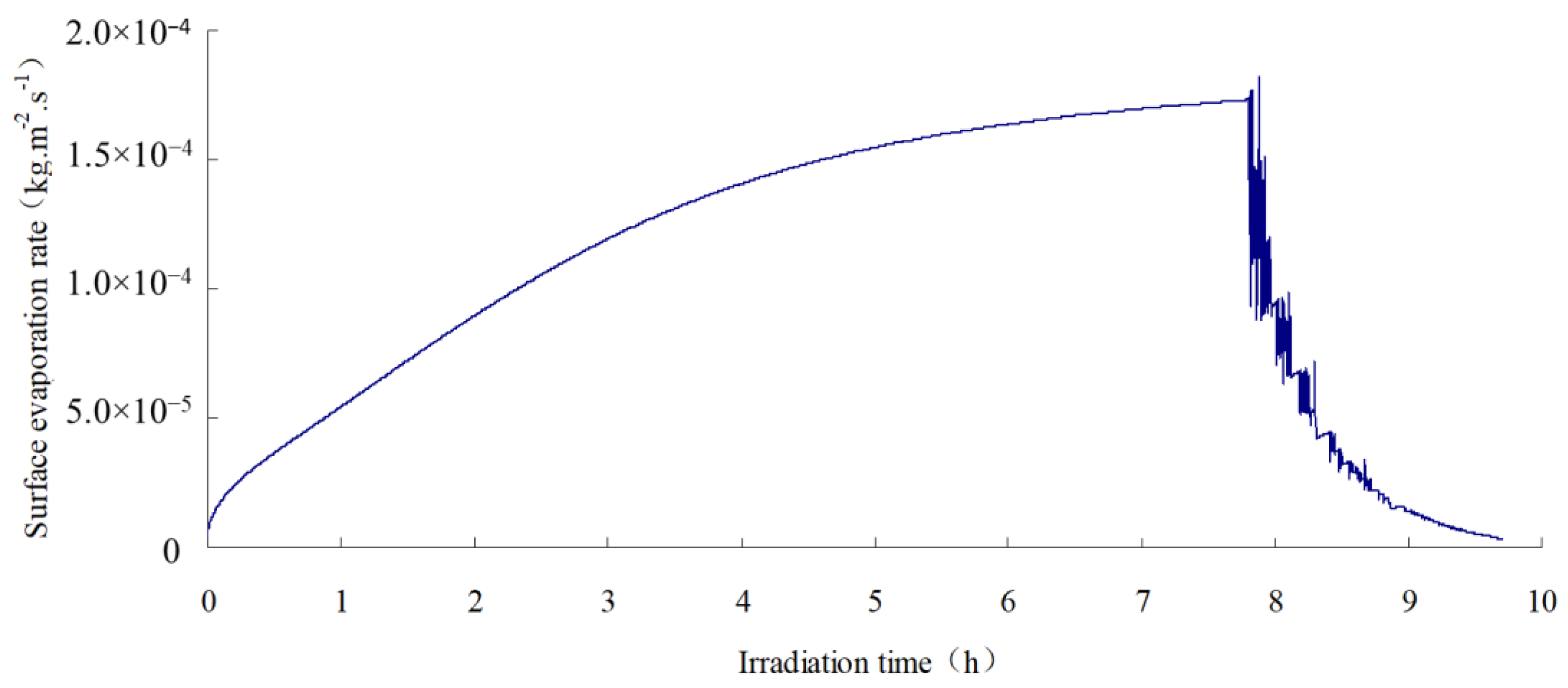
2.4. Test Results
3. Model Establishment and Governing Equations
3.1. Model Hypothesis
- (1)
- Local thermodynamic equilibrium assumptions. Namely, there is no heat transfer between the gas phase, the liquid phase and the solid phase. This assumption can guarantee the same temperature distribution of the micro-body among different phases in the sample, and the chemical potential and pressure between the phases are in equilibrium.
- (2)
- The mass flux due to temperature gradient is not considered (Soret effect) [27].
- (3)
- The deformation of the porous skeleton (solid phase) is not considered, that is, the porosity of the SFWRP material is constant throughout the analysis.
- (4)
- The influence of the matrix potential in the medium is much greater than the gravitational potential, so the effect of gravity is ignored.
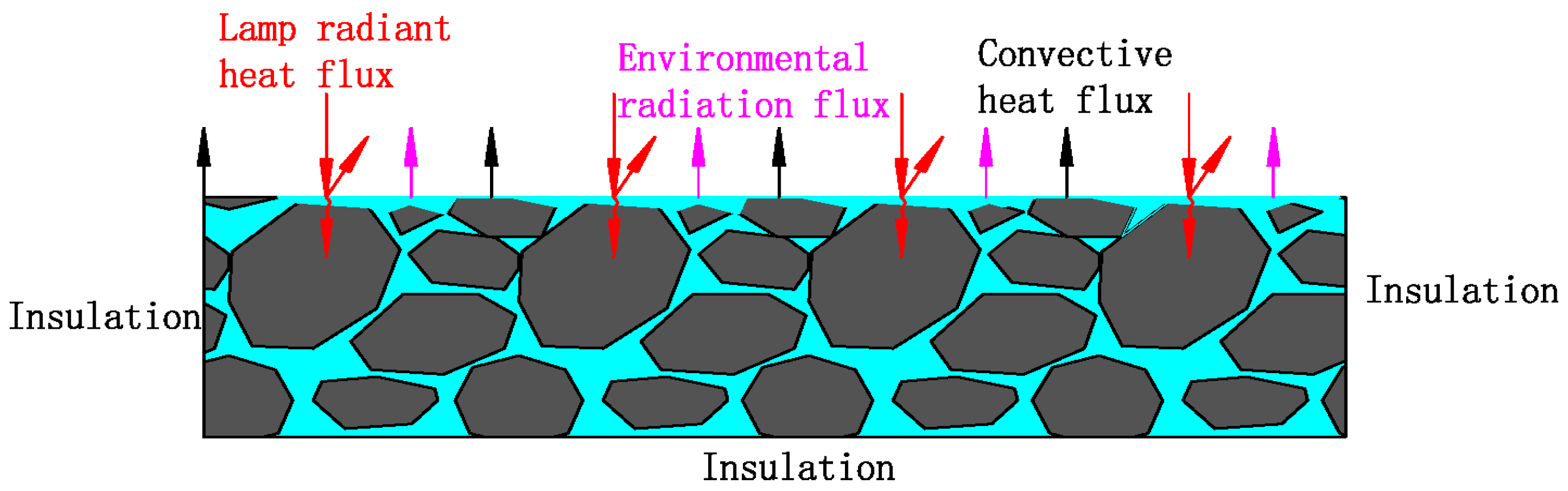
3.2. Control Equations and Model Boundary Conditions
3.3. Model Parameters
4. Simulation Model and Results for Validation
4.1. Model of Asphalt Mixture
4.2. Model of Semi-Flexible Water Retaining Pavement
4.3. Simulation of Model Template for Further Application
5. Conclusions
- The thermodynamic based prediction model of SFWRP considering humidity was developed; this model can be used to evaluate the evaporation behavior of SFWRP based on multi-phase thermal-mass theory. In addition, the exposure test in laboratory was also designed and con-ducted to validate this model. Noting that there is no need to measure the temperature of field pavement, which is time saving and economically beneficial as well.
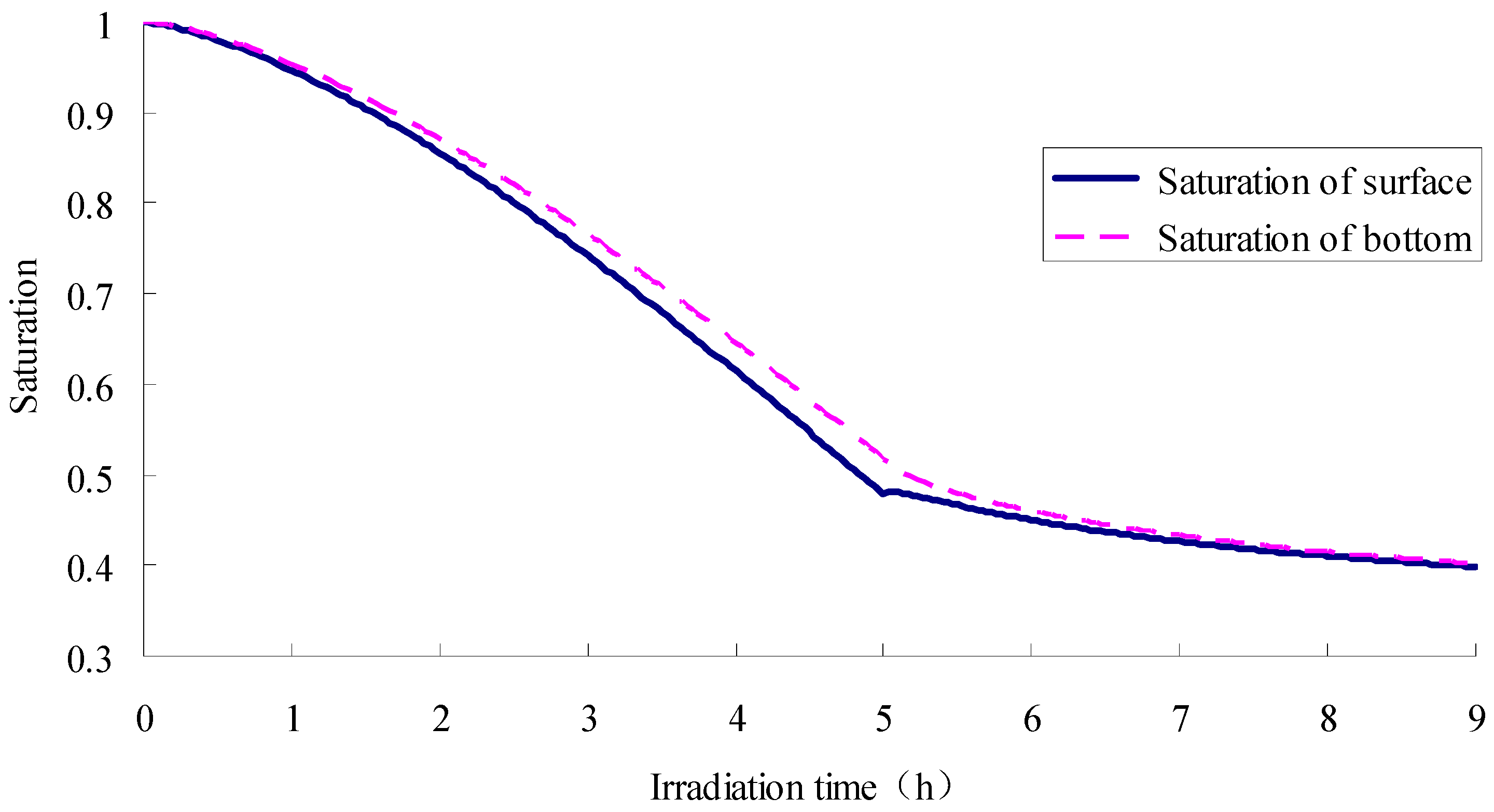
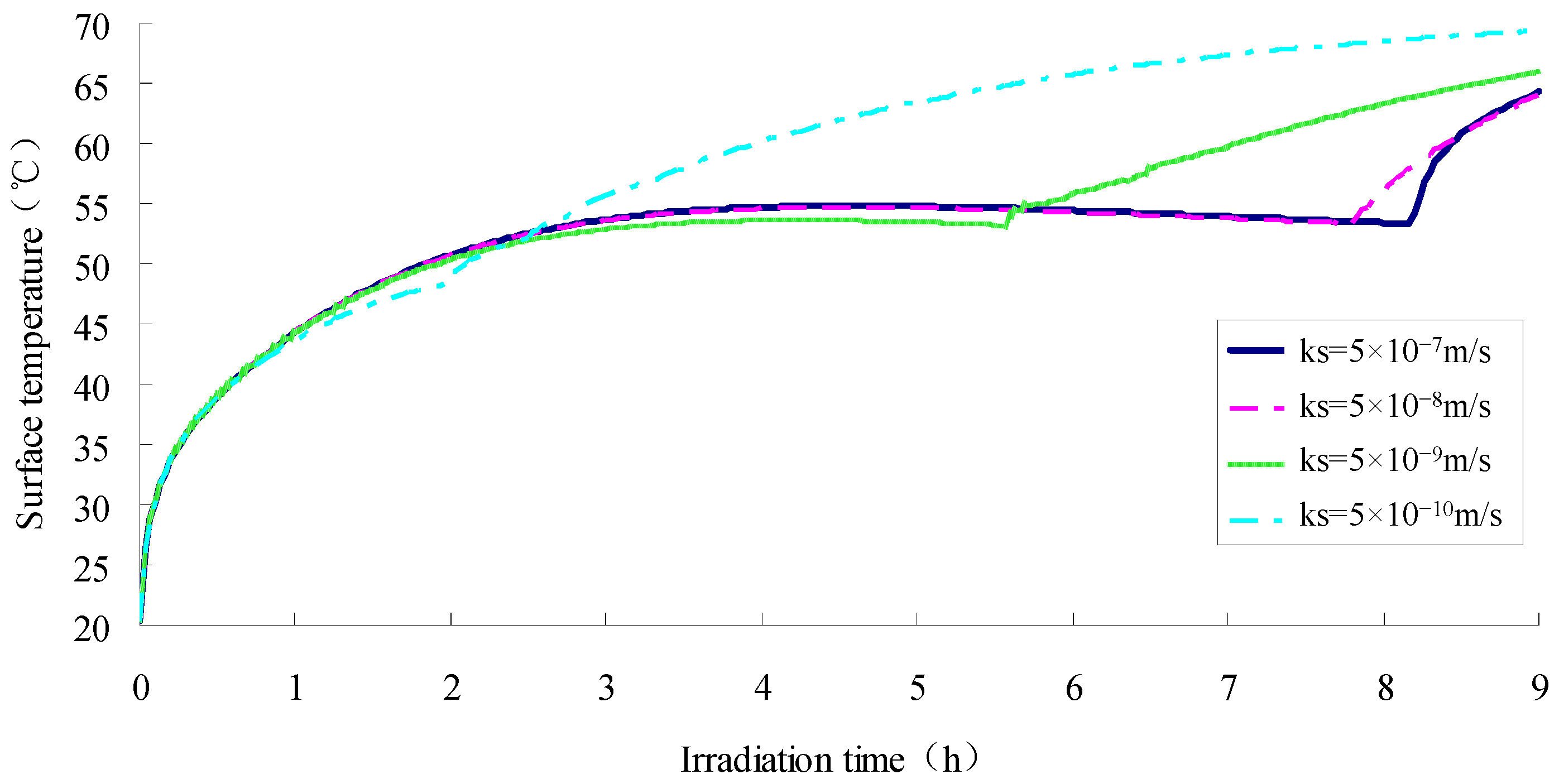
- The SFWRP material under humid conditions has better cooling capability when compared with asphalt pavement, since SFWRP material has lower thermal conductivity, and the water remained inside the sample also leads to moisture evaporation. Besides, the evaluation of the cooling capability of SFWRP material is not efficient until the surface temperature of the sample is higher than 40 °C, this condition can be satisfied in field test at noon or later during the day time (for area where the sun exposure temperature is higher than 40 °C during day time).
- The saturated permeability coefficient of the SFWRP material should not be less than 5 × 10−9 m·s−1 according to the simulation.
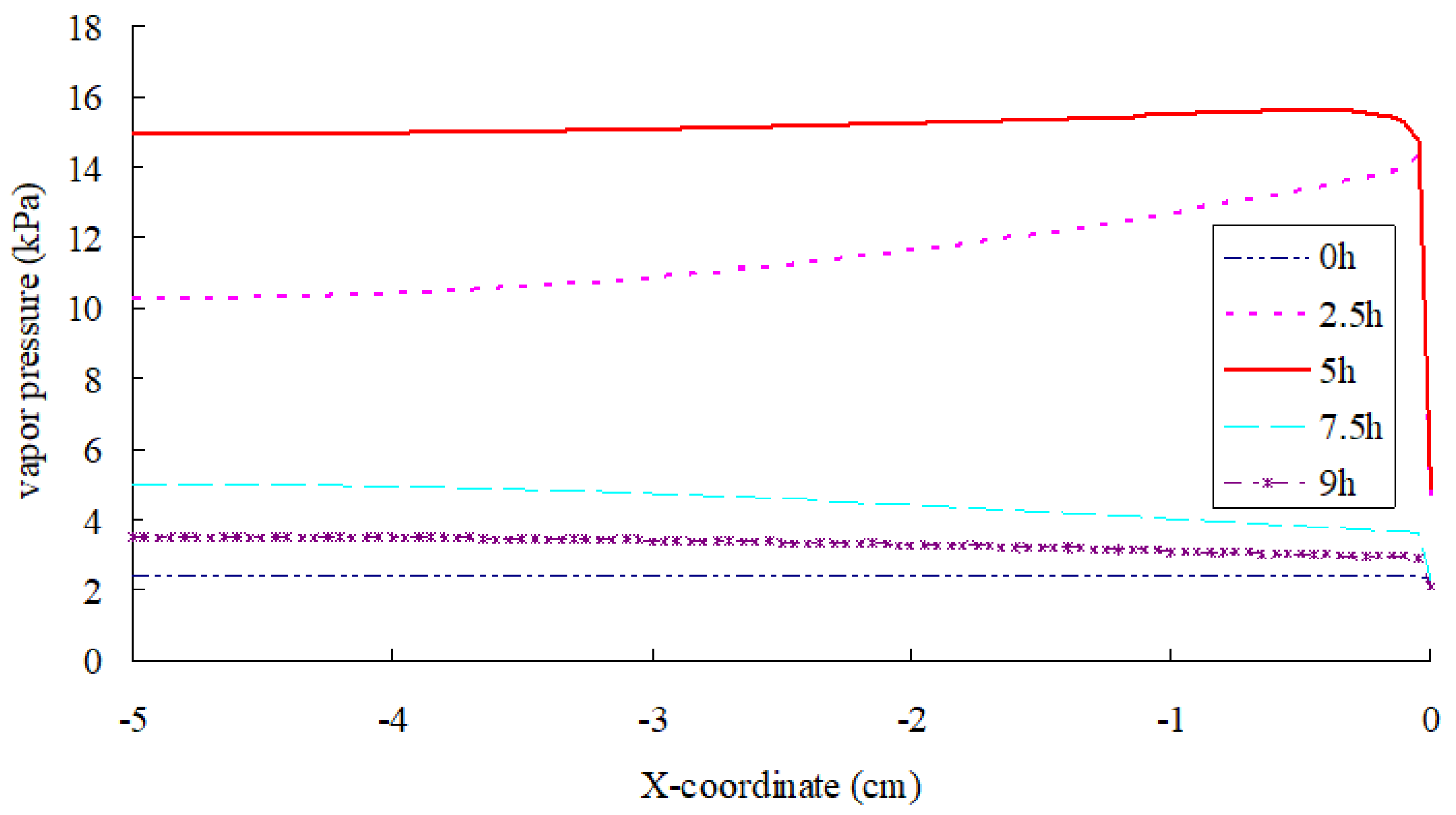
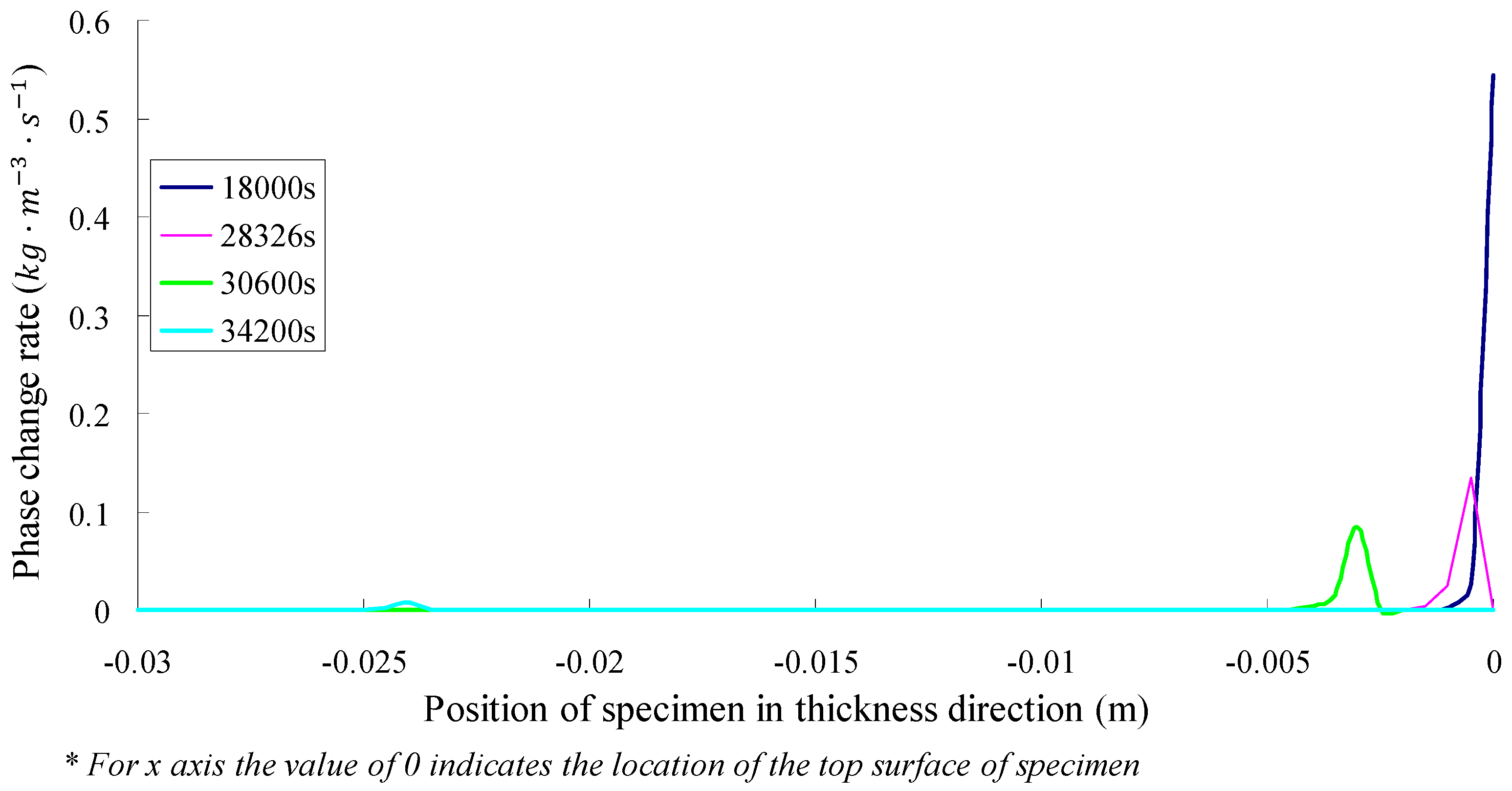
- According to the simulation, before the top surface of SFWRP is dried, the phase change of inside material occurs in a region of 3.5 mm below the top surface, which is less than the influence depth in the soil evaporation model. On the other hand, the phase transition occurs in a small range near the drier end.
- The SFWRP should adopt the mortar considering the water seepage and water retention, and the base asphalt mixture can provide sufficient strength both in cohesive bond and interlocking; the SFWRP material should be placed on the top layer in pavement structure, which can effectively motivate the migration of water vapor to the surface.
Author Contributions
Funding
Conflicts of Interest
References
- Lemonsu, A.; Viguié, V.; Daniel, M.; Masson, V. Vulnerability to heat waves: Impact of urban expansion scenarios on urban heat island and heat stress in Paris (France). Urban Clim. 2015, 14, 586–605. [Google Scholar] [CrossRef]
- Gao, X.; Fan, Z.; Zhang, J.; Liu, S. Micromechanical model for asphalt mixture coupling inter-particle effect and imperfect interface. Constr. Build. Mater. 2017, 148, 696–703. [Google Scholar] [CrossRef]
- Leng, Z.; Zhang, Z.; Zhang, Y.; Wang, Y.; Yu, H.; Ling, T. Laboratory evaluation of electromagnetic density gauges for hot-mix asphalt mixture density measurement. Constr. Build. Mater. 2018, 158, 1055–1064. [Google Scholar] [CrossRef]
- Qu, X.; Liu, Q.; Guo, M.; Wang, D.; Oeser, M. Study on the effect of aging on physical properties of asphalt binder from a microscale perspective. Constr. Build. Mater. 2018, 187, 718–729. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; You, Z.; Yang, X.; Huang, Z. Characterising the asphalt concrete fracture performance from X-ray CT Imaging and finite element modelling. Int. J. Pavement Eng. 2018, 19, 307–318. [Google Scholar] [CrossRef]
- Diefenderfer, B.K.; Al-Qadi, I.L.; Diefenderfer, S.D. Model to Predict Pavement Temperature Profile: Development and Validation. J. Transp. Eng. 2006, 132, 162–167. [Google Scholar] [CrossRef]
- Qin, Y. A review on the development of cool pavements to mitigate urban heat island effect. Renew. Sustain. Energy Rev. 2015, 52, 445–459. [Google Scholar] [CrossRef]
- Sha, A. Material and structure of eco-friendly pavements. Int. J. Pavement Res. Technol. 2012, 5, 4. [Google Scholar]
- He, J.; Hoyano, A. Experimental study of cooling effects of a passive evaporative cooling wall constructed of porous ceramics with high water soaking-up ability. Build. Environ. 2010, 45, 461–472. [Google Scholar] [CrossRef]
- Li, H.; Harvey, J.; Ge, Z. Experimental investigation on evaporation rate for enhancing evaporative cooling effect of permeable pavement materials. Constr. Build. Mater. 2014, 65, 367–375. [Google Scholar] [CrossRef]
- Mrawira, D.M.; Luca, J. Thermal Properties and Transient Temperature Response of Full-Depth Asphalt Pavements. Transp. Res. Rec. J. Transp. Res. Board 2007, 1809, 160–171. [Google Scholar] [CrossRef]
- Wang, D. Prediction of Asphalt Pavement Temperature Profile During FWD Testing: Simplified Analytical Solution with Model Validation Based on LTPP Data. J. Transp. Eng. 2013, 139, 109–113. [Google Scholar] [CrossRef]
- Thomas, H.R.; He, Y. A coupled heat-moisture transfer theory for deformable unsaturated soil and its algorithmic implementation. Int. J. Numer. Methods Eng. 1997, 40, 3421–3441. [Google Scholar] [CrossRef]
- Daly, E.; Porporato, A. A Review of Soil Moisture Dynamics: From Rainfall Infiltration to Ecosystem Response. Environ. Eng. Sci. 2005, 22, 9–24. [Google Scholar] [CrossRef]
- Feddes, R.; Kabat, P.; Van Bakel, P.; Bronswijk, J.; Halbertsma, J. Modelling soil water dynamics in the unsaturated zone—State of the art. J. Hydrol. 1988, 100, 69–111. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Ge, H.; Fazio, P.; Chen, G. Numerical investigation for thermal performance of exterior walls of residential buildings with moisture transfer in hot summer and cold winter zone of China. Energy Build. 2015, 93, 259–268. [Google Scholar] [CrossRef]
- Abahri, K.; Belarbi, R.; Trabelsi, A. Contribution to analytical and numerical study of combined heat and moisture transfers in porous building materials. Build. Environ. 2011, 46, 1354–1360. [Google Scholar] [CrossRef]
- Litavcova, E.; Korjenic, A.; Korjenic, S.; Pavluš, M.; Sarhadov, I.; Seman, J.; Bednar, T. Diffusion of moisture into building materials: A model for moisture transport. Energy Build. 2014, 68, 558–561. [Google Scholar] [CrossRef]
- Philip, J.R.; De Vries, D.A. Moisture movement in porous materials under temperature gradients. Trans. Am. Geophys. Union 1957, 38, 222. [Google Scholar] [CrossRef]
- Zhang, J.; Datta, A.K. Some Considerations in Modeling of Moisture Transport in Heating of Hygroscopic Materials. Dry. Technol. 2004, 22, 1983–2008. [Google Scholar] [CrossRef]
- Qu, X.; Liu, Q.; Wang, C.; Wang, D.; Oeser, M. Effect of Co-Production of Renewable Biomaterials on the Performance of Asphalt Binder in Macro and Micro Perspectives. Materials 2018, 11, 244. [Google Scholar] [CrossRef]
- Ling, T.; Zhao, Z.; Li, C.; Dong, Q.; Zheng, Z.; Zhang, Y.; Liu, Y.; Zheng, X. Technology Guide for Application of Semi-Flexible Pavement; Chongqing Jiaotong University; Reseach Institute of Highway Ministry of Transport: Chongqing, China, 2009. [Google Scholar]
- Scholz, M.; Grabowiecki, P. Review of permeable pavement systems. Build. Environ. 2007, 42, 3830–3836. [Google Scholar] [CrossRef]
- Al-Rousan, A.A. Reduction of fuel consumption in gasoline engines by introducing HHO gas into intake manifold. Int. J. Hydrogen Energy 2010, 35, 12930–12935. [Google Scholar] [CrossRef]
- Bazzaz, M.; Darabi, M.K.; Little, D.N.; Garg, N. A Straightforward Procedure to Characterize Nonlinear Viscoelastic Response of Asphalt Concrete at High Temperatures. Transp. Res. Rec. J. Transp. Res. Board 2018, 2672, 481–492. [Google Scholar] [CrossRef]
- Darabi, M.K.; Huang, C.-W.; Bazzaz, M.; Masad, E.A.; Little, D.N. Characterization and validation of the nonlinear viscoelastic-viscoplastic with hardening-relaxation constitutive relationship for asphalt mixtures. Constr. Build. Mater. 2019, 216, 648–660. [Google Scholar] [CrossRef]
- Datta, A.K. Porous media approaches to studying simultaneous heat and mass transfer in food processes. I: Problem formulations. J. Food Eng. 2007, 80, 80–89. [Google Scholar] [CrossRef]
- Mößner, M.; Radespiel, R. Flow simulations over porous media – Comparisons with experiments. Comput. Fluids 2017, 154, 358–370. [Google Scholar] [CrossRef]
- Datta, A.K. Porous media approaches to studying simultaneous heat and mass transfer in food processes. II: Property data and representative results. J. Food Eng. 2007, 80, 96–110. [Google Scholar] [CrossRef]
- Zhang, Z.; Kong, N. Nonequilibrium Thermal Dynamic Modeling of Porous Medium Vacuum Drying Process. Math. Probl. Eng. 2012, 2012, 1–22. [Google Scholar] [CrossRef]
- Javaherdeh, K.; Nejad, M.M.; Moslemi, M. Natural convection heat and mass transfer in MHD fluid flow past a moving vertical plate with variable surface temperature and concentration in a porous medium. Eng. Sci. Technol. Int. J. 2015, 18, 423–431. [Google Scholar] [CrossRef]
- Liao, C.-M.; Chiu, K.-H. Wind tunnel modeling the system performance of alternative evaporative cooling pads in Taiwan region. Build. Environ. 2002, 37, 177–187. [Google Scholar] [CrossRef]



| Index | Test Result | Results from RTFO Test | ||
|---|---|---|---|---|
| Penetration (100 g 5 s) | 15 °C | 25.1 | - | - |
| 25 °C | 64.1 | Mass Loss (%) | 0.116 | |
| 30 °C | 115.3 | - | - | |
| 135 °C Viscosity (Pa·s) | 0.709 | 5 °C Ductility (cm) | 2.8 | |
| 5 °C Ductility (cm) | 7.9 | - | - | |
| Softening Point TR and B (°C) | 48.7 | Penetration Ratio (%) | 74.6 | |
| Parameter | SFWRP (Dry) | Water | Water Vapor | Dry Air | Asphalt Concrete |
|---|---|---|---|---|---|
| Density ρ/Kg·m−3 | 2120 | 992.2 | - | 1.128 | 2356 |
| Thermal Conductivity λ/w·(m·K)−1 | 1.010 | 0.635 | 0.0206 | 0.0276 | 1.112 |
| Specific heat capacity cp/J·(kg·K)−1 | 734 | 4174 | 1885.3 | 1005 | 823 |
| Absorption rate αs | 0.85 | - | - | - | 0.93 |
| Emissivity ε | 0.85 | - | - | - | 0.93 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Q.; Wang, C.; Xiong, C.; Li, X.; Wang, H.; Ling, T. Investigation on the Cooling and Evaporation Behavior of Semi-Flexible Water Retaining Pavement based on Laboratory Test and Thermal-Mass Coupling Analysis. Materials 2019, 12, 2546. https://doi.org/10.3390/ma12162546
Dong Q, Wang C, Xiong C, Li X, Wang H, Ling T. Investigation on the Cooling and Evaporation Behavior of Semi-Flexible Water Retaining Pavement based on Laboratory Test and Thermal-Mass Coupling Analysis. Materials. 2019; 12(16):2546. https://doi.org/10.3390/ma12162546
Chicago/Turabian StyleDong, Qiang, Chonghui Wang, Chuhua Xiong, Xiulei Li, Hainian Wang, and Tianqing Ling. 2019. "Investigation on the Cooling and Evaporation Behavior of Semi-Flexible Water Retaining Pavement based on Laboratory Test and Thermal-Mass Coupling Analysis" Materials 12, no. 16: 2546. https://doi.org/10.3390/ma12162546
APA StyleDong, Q., Wang, C., Xiong, C., Li, X., Wang, H., & Ling, T. (2019). Investigation on the Cooling and Evaporation Behavior of Semi-Flexible Water Retaining Pavement based on Laboratory Test and Thermal-Mass Coupling Analysis. Materials, 12(16), 2546. https://doi.org/10.3390/ma12162546






