Revisiting the Dependence of Poisson’s Ratio on Liquid Fragility and Atomic Packing Density in Oxide Glasses
Abstract
1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Characterization
3. Results and Discussion
3.1. Studied Compositions
3.2. Poisson’s Ratio vs. Packing Density
3.3. Poisson’s Ratio vs. Liquid Fragility
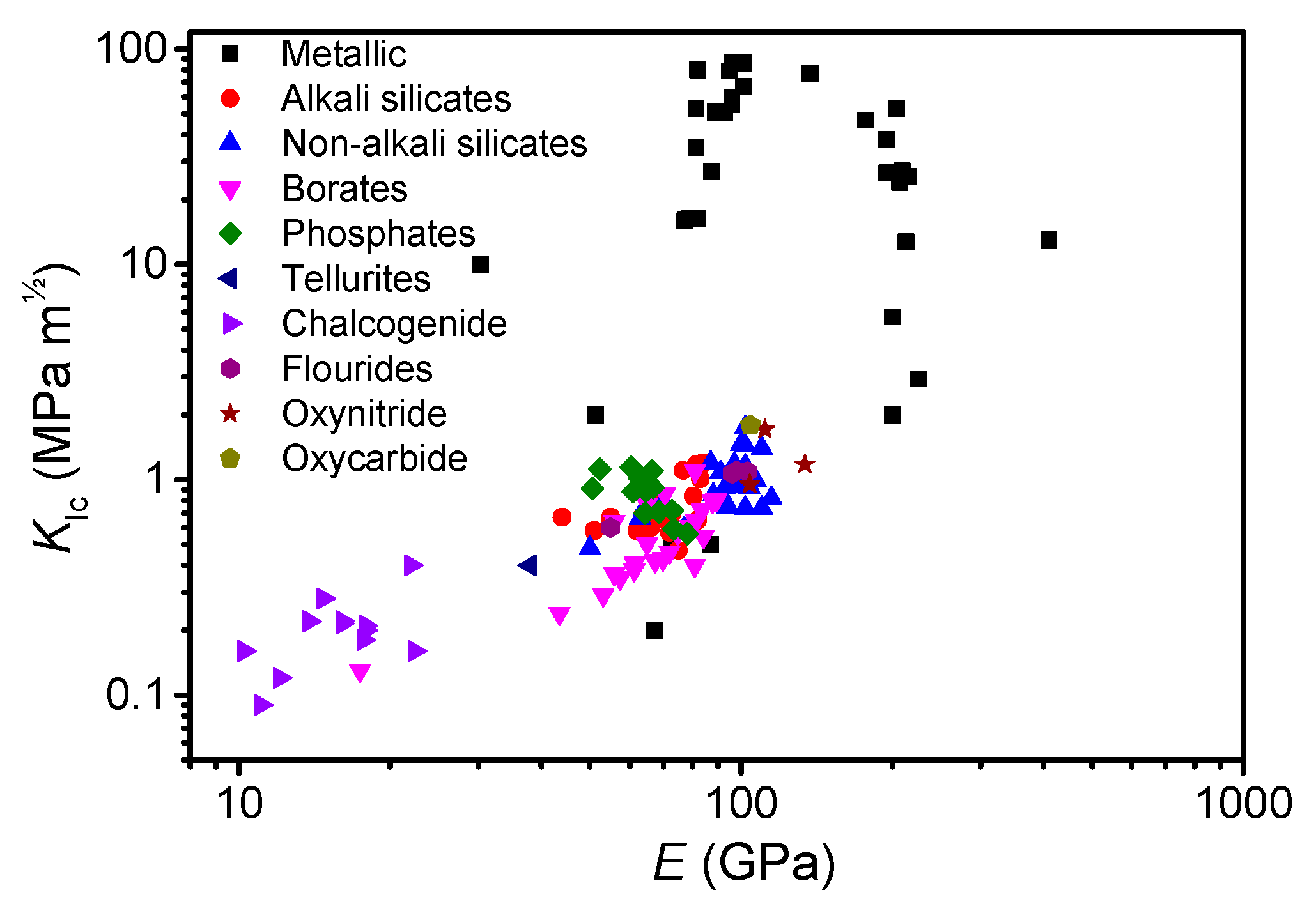
3.4. Implications for Design of Tough Oxide Glasses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wojciechowski, K.W. Remarks on “Poisson Ratio beyond the Limits of the Elasticity Theory”. J. Phys. Soc. Jpn. 2003, 72, 1819–1820. [Google Scholar] [CrossRef]
- Lakes, R.S. Foam structures with a negative Poisson’s ratio. Science 1987, 235, 1038–1040. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.E.; Nkansah, M.A.; Hutchinson, I.J.; Rogers, S.C. Molecular network design. Nature 1991, 353, 124. [Google Scholar] [CrossRef]
- Wojciechowski, K. Two-dimensional isotropic system with a negative poisson ratio. Phys. Lett. A 1989, 137, 60–64. [Google Scholar] [CrossRef]
- Bevzenko, D.; Lubchenko, V. Self-consistent elastic continuum theory of degenerate, equilibrium aperiodic solids. J. Chem. Phys. 2014, 141, 174502. [Google Scholar] [CrossRef] [PubMed]
- Greaves, G.N.; Greer, A.L.; Lakes, R.S.; Rouxel, T. Poisson’s ratio and modern materials. Nat. Mater. 2011, 10, 823–838. [Google Scholar] [CrossRef]
- Rouxel, T.; Ji, H.; Hammouda, T.; Moreac, A. Poisson’s Ratio and the Densification of Glass under High Pressure. Phys. Rev. Lett. 2008, 100, 225501. [Google Scholar] [CrossRef] [PubMed]
- Rouxel, T. Elastic Properties and Short-to Medium-Range Order in Glasses. J. Am. Ceram. Soc. 2007, 90, 3019–3039. [Google Scholar] [CrossRef]
- Wang, W.H.; Greer, A.L. Intrinsic plasticity or brittleness of metallic glasses. Philos. Mag. Lett. 2005, 85, 77–87. [Google Scholar]
- Rouxel, T.; Yoshida, S. The fracture toughness of inorganic glasses. J. Am. Ceram. Soc. 2017, 100, 4374–4396. [Google Scholar] [CrossRef]
- Karlsson, S.; Jonson, B.; Stålhandske, C. The technology of chemical glass strengthening—A review. Glass Technol. Eur. J. Glass Sci. Technol. Part A 2010, 51, 41–54. [Google Scholar]
- Wang, B.; Yu, Y.; Lee, Y.J.; Bauchy, M. Intrinsic Nano-Ductility of Glasses: The Critical Role of Composition. Front. Mater. 2015, 2, 1–9. [Google Scholar] [CrossRef]
- Yuan, F.; Huang, L. Brittle to Ductile Transition in Densified Silica Glass. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Wang, C.; Cheng, Y.-Q.; Yue, Y.; Han, X.; Zhang, Z.; Shan, Z.; Mao, S.X.; Ye, M.; Yin, Y.; et al. Electron-beam-assisted superplastic shaping of nanoscale amorphous silica. Nat. Commun. 2010, 1, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Pugh, S.F. XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Lond. Edinburgh Dublin Philos. Mag. J. Sci. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Ngai, K.L.; Wang, L.M.; Liu, R.; Wang, W.H. Microscopic dynamics perspective on the relationship between Poisson’s ratio and ductility of metallic glasses. J. Chem. Phys. 2014, 140, 044511. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.V.; Yang, B.; Yue, Y.; Bowron, D.T.; Mayers, J.; Donnan, R.S.; Dobó-Nagy, C.; Nicholson, J.W.; Fang, D.-C.; Greer, A.L.; et al. Atomic and vibrational origins of mechanical toughness in bioactive cement during setting. Nat. Commun. 2015, 6, 8631. [Google Scholar] [CrossRef] [PubMed]
- Januchta, K.; To, T.; Bødker, M.S.; Rouxel, T.; Smedskjaer, M.M. Elasticity, hardness, and fracture toughness of sodium aluminoborosilicate glasses. J. Am. Ceram. Soc. 2019, 102, 4520–4537. [Google Scholar] [CrossRef]
- To, T.; Célarié, F.; Roux-Langlois, C.; Bazin, A.; Gueguen, Y.; Orain, H.; Le Fur, M.; Burgaud, V.; Rouxel, T. Fracture toughness, fracture energy and slow crack growth of glass as investigated by the Single-Edge Precracked Beam (SEPB) and Chevron-Notched Beam (CNB) methods. Acta Mater. 2018, 146, 1–11. [Google Scholar] [CrossRef]
- Rouxel, T. Fracture surface energy and toughness of inorganic glasses. Scr. Mater. 2017, 137, 109–113. [Google Scholar] [CrossRef]
- To, T. Fracture Toughness and Fracture Energy of Inorganic and Non-Metallic Glass. Ph.D. Thesis, Université de Rennes 1, Rennes, France, 2019. [Google Scholar]
- Zhang, P.; Ma, L.; Fan, F.; Zeng, Z.; Peng, C.; Loya, P.E.; Liu, Z.; Gong, Y.; Zhang, J.; Zhang, X.; et al. Fracture toughness of graphene. Nat. Commun. 2014, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gui, G.; Li, J.; Zhong, J. Band structure engineering of graphene by strain: First-principles calculations. Phys. Rev. B 2008, 78, 1–6. [Google Scholar] [CrossRef]
- Rouse, G.B.; Kamitsos, E.I.; Risen, W.M. Brillouin spectra of mixed alkali glasses: xCs2O(1-x)Na2O5SiO2. J. Non. Cryst. Solids 1981, 45, 257–269. [Google Scholar] [CrossRef]
- Srinivasarao, G.; Veeraiah, N.; Nalluri, V. Characterization and Physical Properties of PbO-As2O3 Glasses Containing Molybdenum Ions. J. Solid State Chem. 2002, 166, 104–117. [Google Scholar] [CrossRef]
- Shi, Y.; Luo, J.; Yuan, F.; Huang, L. Intrinsic ductility of glassy solids. J. Appl. Phys. 2014, 115, 043528. [Google Scholar] [CrossRef]
- Argon, A.S. Plastic deformation in metallic glasses. Acta Metall. 1979, 27, 47–58. [Google Scholar] [CrossRef]
- Yu, H.B.; Wang, W.H.; Bai, H.Y.; Wu, Y.; Chen, M.W. Relating activation of shear transformation zones to β relaxations in metallic glasses. Phys. Rev. B 2010, 81, 220201. [Google Scholar] [CrossRef]
- Mauro, J.C.; Tandia, A.; Vargheese, K.D.; Mauro, Y.Z.; Smedskjaer, M.M. Accelerating the Design of Functional Glasses through Modeling Accelerating the Design of Functional Glasses through Modeling. Chem. Mater. 2016, 28, 4267–4277. [Google Scholar] [CrossRef]
- Novikov, V.N.; Sokolov, A.P. Poisson’s ratio and the fragility of glass-forming liquids. Nature 2004, 431, 961–963. [Google Scholar] [CrossRef]
- Novikov, V.N.; Ding, Y.; Sokolov, A.P. Correlation of fragility of supercooled liquids with elastic properties of glasses. Phys. Rev. E 2005, 71, 061501. [Google Scholar] [CrossRef]
- Böhmer, R.; Angell, C.A. Correlations of the nonexponentiality and state dependence of mechanical relaxations with bond connectivity in Ge-As-Se supercooled liquids. Phys. Rev. B 1992, 45, 10091–10094. [Google Scholar] [CrossRef] [PubMed]
- Ojovan, M.I.; Lee, W.E. Fragility of oxide melts as a thermodynamic parameter. Phys. Chem. Glas. 2005, 46, 7–11. [Google Scholar]
- Gupta, P.K.; Mauro, J.C. Composition dependence of glass transition temperature and fragility. I. A topological model incorporating temperature-dependent constraints. J. Chem. Phys. 2009, 130, 94503. [Google Scholar] [CrossRef] [PubMed]
- Smedskjaer, M.M.; Mauro, J.C.; Sen, S.; Yue, Y. Quantitative Design of Glassy Materials Using Temperature-Dependent Constraint Theory. Chem. Mater. 2010, 22, 5358–5365. [Google Scholar] [CrossRef]
- Tran, T.D.; Sidebottom, D.L. Glass-Forming Dynamics of Aluminophosphate Melts Studied by Photon Correlation Spectroscopy. J. Am. Ceram. Soc. 2013, 96, 2147–2154. [Google Scholar] [CrossRef]
- Sidebottom, D.L. Fragility of network-forming glasses: A universal dependence on the topological connectivity. Phys. Rev. E 2015, 92, 1–9. [Google Scholar] [CrossRef]
- Novikov, V.N.; Sokolov, A.P. Correlation of fragility and Poisson’s ratio: Difference between metallic and nonmetallic glass formers. Phys. Rev. B 2006, 74, 064203. [Google Scholar] [CrossRef]
- Na, J.; Park, E.; Kim, Y.; Fleury, É.; Kim, W.; Kim, D. Poisson’s ratio and fragility of bulk metallic glasses. J. Mater. Res. 2008, 23, 523–528. [Google Scholar] [CrossRef]
- Wang, W.H. Correlations between elastic moduli and properties in bulk metallic glasses. J. Appl. Phys. 2006, 99, 93506. [Google Scholar] [CrossRef]
- Yannopoulos, S.N.; Johari, G.P. Glass behaviour: Poisson’s ratio and liquid’s fragility. Nature 2006, 442, E7–E8. [Google Scholar] [CrossRef]
- Duval, E.; Deschamps, T.; Saviot, L. Poisson ratio and excess low-frequency vibrational states in glasses. J. Chem. Phys. 2013, 139, 64506. [Google Scholar] [CrossRef] [PubMed]
- Makishima, A.; Mackenzie, J.D. Calculation of bulk modulus, shear modulus, and Poisson’s ratio of glass. J. Non. Cryst. Solids 1975, 17, 147–157. [Google Scholar] [CrossRef]
- Plucinski, M.; Zwanziger, J. Topological constraints and the Makishima-Mackenzie model. J. Non. Cryst. Solids 2015, 429, 20–23. [Google Scholar] [CrossRef]
- Yao, Z.Y.; Möncke, D.; Kamitsos, E.I.; Houizot, P.; Célarié, F.; Rouxel, T.; Wondraczek, L. Structure and mechanical properties of copper-lead and copper-zinc borate glasses. J. Non. Cryst. Solids 2016, 435, 55–68. [Google Scholar] [CrossRef]
- Januchta, K.; Bauchy, M.; Youngman, R.E.; Rzoska, S.J.; Bockowski, M.; Smedskjaer, M.M. Modifier field strength effects on densification behavior and mechanical properties of alkali aluminoborate glasses. Phys. Rev. Mater. 2017, 1, 063603. [Google Scholar] [CrossRef]
- Frederiksen, K.F.; Januchta, K.; Mascaraque, N.; Bauchy, M.; Rzoska, S.J.; Bockowski, M.; Smedskjaer, M.M.; E Youngman, R. Structural Compromise between High Hardness and Crack Resistance in Aluminoborate Glasses. J. Phys. Chem. B 2018, 122, 6287–6295. [Google Scholar] [CrossRef]
- Mascaraque, N.; Frederiksen, K.F.; Januchta, K.; Youngman, R.E.; Bauchy, M.; Smedskjaer, M.M. Competitive effects of modifier charge and size on mechanical and chemical resistance of aluminoborate glasses. J. Non. Cryst. Solids 2018, 499, 264–271. [Google Scholar] [CrossRef]
- Bechgaard, T.K.; Goel, A.; Youngman, R.E.; Mauro, J.C.; Rzoska, S.J.; Bockowski, M.; Jensen, L.R.; Smedskjaer, M.M. Structure and mechanical properties of compressed sodium aluminosilicate glasses: Role of non-bridging oxygens. J. Non. Cryst. Solids 2016, 441, 49–57. [Google Scholar] [CrossRef]
- Bockowski, M.; Strąk, P.; Grzegory, I.; Porowski, S. High Pressure Solution Growth of Gallium Nitride. In Technology of Gallium Nitride Crystal Growth; Ehrentraut, D., Meissner, E., Bockowski, M., Eds.; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2010; Volume 133, pp. 207–234. [Google Scholar]
- Østergaard, M.B.; Youngman, R.E.; Svenson, M.N.; Bockowski, M.; Jensen, L.R.; Smedskjaer, M.M.; Rzoska, S.J. Temperature-dependent densification of sodium borosilicate glass. RSC Adv. 2015, 5, 78845–78851. [Google Scholar] [CrossRef]
- Januchta, K.; Youngman, R.E.; Jensen, L.R.; Smedskjaer, M.M. Mechanical property optimization of a zinc borate glass by lanthanum doping. J. Non. Cryst. Solids 2019, 520, 119461. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chaleogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Wells, A.F. Structural Inorganic Chemistry; Oxford University Press: Oxford, UK, 1945. [Google Scholar]
- Zheng, Q.; Mauro, J.C.; Yue, Y. Reconciling calorimetric and kinetic fragilities of glass-forming liquids. J. Non. Cryst. Solids 2017, 456, 95–100. [Google Scholar] [CrossRef]
- Wang, W.H.; Dong, C.; Shek, C.H. Bulk metallic glasses. Mater. Sci. Eng. R 2004, 44, 45–89. [Google Scholar] [CrossRef]
- Schroers, J.; Johnson, W.L. Ductile Bulk Metallic Glass. Phys. Rev. Lett. 2004, 93, 255506. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Bai, H.Y. Poisson’s ratio and plasticity in CuZrAl bulk metallic glasses. Mater. Sci. Eng. A 2008, 485, 1–4. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wang, G.; Wang, R.J.; Zhao, D.Q.; Pan, M.X.; Wang, W.H. Super Plastic Bulk Metallic Glasses at Room Temperature. Science 2007, 315, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Daucé, R.; Keding, R.; Sangleboeuf, J.-C. On the relations between ISE and structure in some RE(Mg)SiAlO(N) glasses. J. Mater. Sci. 2008, 43, 7239–7246. [Google Scholar] [CrossRef]
- Ecolivet, C.; Verdier, P. Proprietes elastiques et indices de refraction de verres azotes. Mater. Res. Bull. 1984, 19, 227–231. [Google Scholar] [CrossRef]
- Sellappan, P.; Rouxel, T.; Célarié, F.; Becker, E.; Houizot, P.; Conradt, R. Composition dependence of indentation deformation and indentation cracking in glass. Acta Mater. 2013, 61, 5949–5965. [Google Scholar] [CrossRef]
- Pedone, A.; Malavasi, G.; Cormack, A.N.; Segre, U.; Menziani, M.C. Insight into Elastic Properties of Binary Alkali Silicate Glasses; Prediction and Interpretation through Atomistic Simulation Techniques. Chem. Mater. 2007, 19, 3144–3154. [Google Scholar] [CrossRef]
- Kodama, M. Velocity of Sound in and Elastic Properties of Rb2O-B2O3 Glasses. Jpn. J. Appl. Phys. 1995, 34, 2570–2574. [Google Scholar] [CrossRef]
- Shaw, R.R.; Uhlmann, D.R. Effect of phase separation on the properties of simple glasses II. Elastic properties. J. Non. Cryst. Solids 1971, 5, 237–263. [Google Scholar] [CrossRef]
- El-Mallawany, R. Quantitative analysis of elastic moduli of tellurite glasses. J. Mater. Res. 1990, 5, 2218–2222. [Google Scholar] [CrossRef]
- Limbach, R.; Winterstein-Beckmann, A.; Dellith, J.; Möncke, D.; Wondraczek, L. Plasticity, crack initiation and defect resistance in alkali-borosilicate glasses: From normal to anomalous behavior. J. Non. Cryst. Solids 2015, 417, 15–27. [Google Scholar] [CrossRef]
- Svenson, M.N.; Guerette, M.; Huang, L.; Lönnroth, N.; Mauro, J.C.; Rzoska, S.J.; Bockowski, M.; Smedskjaer, M.M. Universal behavior of changes in elastic moduli of hot compressed oxide glasses. Chem. Phys. Lett. 2016, 651, 88–91. [Google Scholar] [CrossRef]
- Hwa, L.G.; Hsieh, K.J.; Liu, L.C. Elastic moduli of low-silica calcium alumino-silicate glasses. Mater. Chem. Phys. 2002, 78, 105–110. [Google Scholar] [CrossRef]
- Hwa, L.-G.; Lu, C.-L.; Liu, L.-C. Elastic moduli of calcium alumino-silicate glasses studied by Brillouin scattering. Mater. Res. Bull. 2000, 35, 1285–1292. [Google Scholar] [CrossRef]
- Winterstein-Beckmann, A.; Möncke, D.; Palles, D.; Kamitsos, E.; Wondraczek, L. A Raman-spectroscopic study of indentation-induced structural changes in technical alkali-borosilicate glasses with varying silicate network connectivity. J. Non. Cryst. Solids 2014, 405, 196–206. [Google Scholar] [CrossRef]
- Schroeder, J.; Mohr, R. Rayleigh and Brillouin Scattering in. J. Am. Ceram. Soc. 1973, 56, 510–514. [Google Scholar] [CrossRef]
- Yoshida, S.; Sanglebœuf, J.C.; Rouxel, T. Quantitative evaluation of indentation-induced densification in glass. J. Mater. Res. 2005, 20, 3404–3412. [Google Scholar] [CrossRef]
- Matusita, K.; Sakka, S.; Osaka, A.; Soga, N.; Kunugi, M. Elastic modulus of mixed alkali glass. J. Non. Cryst. Solids 1974, 16, 308–312. [Google Scholar] [CrossRef]
- Rajendran, V.; Khaliafa, F.A.; El-Batal, H.A. Investigation of acoustical parameters in binary X LiO2 (100 X) SiO2 glasses. Indian J. Phys. 1995, 69A, 237–242. [Google Scholar]
- Gaafar, M.; El-Aal, N.A.; Gerges, O.; El-Amir, G. Elastic properties and structural studies on some zinc-borate glasses derived from ultrasonic, FT-IR and X-ray techniques. J. Alloys Compd. 2009, 475, 535–542. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A.P.; Bhatti, S.S. Elastic moduli of some mixed alkali borate glasses. J. Mater. Sci. 1989, 24, 1539–1542. [Google Scholar] [CrossRef]
- Carini, G.; Carini, G.; D’Angelo, G.; Tripodo, G.; Bartolotta, A.; Salvato, G. Ultrasonic relaxations, anharmonicity, and fragility in lithium borate glasses. Phys. Rev. B 2005, 72, 1–10. [Google Scholar] [CrossRef]
- Soga, N.; Yamanaka, H.; Hisamoto, C.; Kunugi, M. Elastic properties and structure of alkaline-earth silicate glasses. J. Non. Cryst. Solids 1976, 22, 67–76. [Google Scholar] [CrossRef]
- Striepe, S.; Smedskjaer, M.M.; Deubener, J.; Bauer, U.; Behrens, H.; Potuzak, M.; Youngman, R.E.; Mauro, J.C.; Yue, Y. Elastic and micromechanical properties of isostatically compressed soda-lime-borate glasses. J. Non. Cryst. Solids 2013, 364, 44–52. [Google Scholar] [CrossRef]
- Scannell, G.; Laille, D.; Célarié, F.; Huang, L.; Rouxel, T. Interaction between Deformation and Crack Initiation under Vickers Indentation in Na2O-TiO2-SiO2 Glasses. Front. Mater. 2017, 4, 175. [Google Scholar] [CrossRef]
- Rajendran, V.; Begum, A.N.; Azooz, M.A.; Batal, F.H. El Microstructural dependence on relevant physical-mechanical properties on SiO2-Na2O-CaO-P2O5 biological glasses. Biomaterials 2002, 23, 4263–4275. [Google Scholar] [CrossRef]
- Hermansen, C.; Matsuoka, J.; Yoshida, S.; Yamazaki, H.; Kato, Y.; Yue, Y. Densification and plastic deformation under microindentation in silicate glasses and the relation to hardness and crack resistance. J. Non. Cryst. Solids 2013, 364, 40–43. [Google Scholar] [CrossRef]
- El-Moneim, A.A. Acoustical and structural properties of 65SiO2-15PbO-5CaO-(15-x)K2O-xNa2O glass system. Mater. Chem. Phys. 1998, 52, 36–40. [Google Scholar] [CrossRef]
- Burkhard, D.J. Elastic properties of alkali silicate glasses with iron oxide: Relation to glass structure. Solid State Commun. 1997, 101, 903–907. [Google Scholar] [CrossRef]
- Osaka, A.; Ariyoshi, K.; Takahashi, K. Network structure of alkali germanosilicate glasses. J. Non. Cryst. Solids 1986, 83, 335–343. [Google Scholar] [CrossRef]
- Januchta, K.; Sun, R.; Huang, L.; Bockowski, M.; Rzoska, S.J.; Jensen, L.R.; Smedskjaer, M.M. Deformation and cracking behavior of La2O3-doped oxide glasses with high Poisson’s ratio. J. Non. Cryst. Solids 2018, 494, 86–93. [Google Scholar] [CrossRef]
- Kjeldsen, J.; Smedskjaer, M.M.; Mauro, J.C.; Yue, Y. On the origin of the mixed alkali effect on indentation in silicate glasses. J. Non. Cryst. Solids 2014, 406, 22–26. [Google Scholar] [CrossRef]
- Pönitzsch, A.; Nofz, M.; Wondraczek, L.; Deubener, J. Bulk elastic properties, hardness and fatigue of calcium aluminosilicate glasses in the intermediate-silica range. J. Non. Cryst. Solids 2016, 434, 1–12. [Google Scholar] [CrossRef]
- Makishima, A.; Tamura, Y.; Sakaino, T. Elastic Moduli and Refractive Indices of Aluminosilicate Glasses Containing Y2O3, La2O3, and TiO2. J. Am. Ceram. Soc. 1978, 61, 247–249. [Google Scholar] [CrossRef]
- She, J.; Sawamura, S.; Wondraczek, L. Scratch hardness of rare-earth substituted calcium aluminosilicate glasses. J. Non. Cryst. Solids X 2019, 1, 100010. [Google Scholar] [CrossRef]
- Tanabe, S.; Hirao, K.; Soga, N. Elastic Properties and Molar Volume of Rare-Earth Aluminosilicate Glasses. J. Am. Ceram. Soc. 1992, 75, 503–506. [Google Scholar] [CrossRef]
- Yamane, M.; Okuyama, M. Coordination number of aluminum ions in alkali-free alumino-silicate glasses. J. Non. Cryst. Solids 1982, 52, 217–226. [Google Scholar] [CrossRef]
- Ashizuka, M.; Masuda, T.; Ishida, E. Elastic Modulus, Hardness and Fracture Toughness of Ca3(PO4)2-Al2O3-SiO2 Glasses. J. Ceram. Assoc. Jpn. 1985, 93, 433–441. [Google Scholar] [CrossRef][Green Version]
- Aakermann, K.G.; Januchta, K.; Pedersen, J.A.; Svenson, M.N.; Rzoska, S.J.; Bockowski, M.; Mauro, J.C.; Guerette, M.; Huang, L.; Smedskjaer, M.M. Indentation deformation mechanism of isostatically compressed mixed alkali aluminosilicate glasses. J. Non. Cryst. Solids 2015, 426, 175–183. [Google Scholar] [CrossRef]
- Kannappan, A.; Thirumaran, S.; Palani, R. Elastic and mechanical properties of glass specimen by ultrasonic method. J. Eng. Appl. Sci. 2009, 4, 27–31. [Google Scholar]
- Möncke, D.; Kamitsos, E.I.; Palles, D.; Limbach, R.; Winterstein-Beckmann, A.; Honma, T.; Yao, Z.; Rouxel, T.; Wondraczek, L. Transition and post-transition metal ions in borate glasses: Borate ligand speciation, cluster formation, and their effect on glass transition and mechanical properties. J. Chem. Phys. 2016, 145, 124501. [Google Scholar] [CrossRef] [PubMed]
- Saddeek, Y.B. Structural and acoustical studies of lead sodium borate glasses. J. Alloys Compd. 2009, 467, 14–21. [Google Scholar] [CrossRef]
- El-moneim, A.A. Interpretation of elastic properties and structure of TiO2–CaO–Al2O3–B2O3 glasses. Phys. Chem. Glas. 2004, 45, 15–20. [Google Scholar]
- Hwa, L.; Chao, W. Velocity of sound and elastic properties of lanthanum gallo-germanate glasses. Mater. Chem. Phys. 2005, 94, 37–41. [Google Scholar] [CrossRef]
- Zheng, Q.; Potuzak, M.; Mauro, J.C.; Smedskjaer, M.M.; Youngman, R.E.; Yue, Y. Composition-structure-property relationships in boroaluminosilicate glasses. J. Non. Cryst. Solids 2012, 358, 993–1002. [Google Scholar] [CrossRef]
- Saddeek, Y.B. Ultrasonic study and physical properties of some borate glasses. Mater. Chem. Phys. 2004, 83, 222–228. [Google Scholar] [CrossRef]
- Saddeek, Y.B.; El Latif, L.A. Effect of TeO2 on the elastic moduli of sodium borate glasses. Phys. B Condens. Matter 2004, 348, 475–484. [Google Scholar] [CrossRef]
- Saddeek, Y.B.; Abousehly, A.M.; Hussien, S. Synthesis and several features of the Na2O-B2O3-Bi2O3-MoO3 glasses. J. Phys. D Appl. Phys. 2007, 40, 4674–4681. [Google Scholar] [CrossRef]
- Saddeek, Y.B.; Gaafar, M. Physical and structural properties of some bismuth borate glasses. Mater. Chem. Phys. 2009, 115, 280–286. [Google Scholar] [CrossRef]
- Limbach, R.; Rodrigues, B.; Möncke, D.; Wondraczek, L. Elasticity, deformation and fracture of mixed fluoride-phosphate glasses. J. Non. Cryst. Solids 2015, 430, 99–107. [Google Scholar] [CrossRef]
- Ota, R.; Soga, N. Elastic properties of fluoride glasses under pressure and temperature. J. Non. Cryst. Solids 1983, 56, 105–110. [Google Scholar] [CrossRef]
- Brassington, M.; Hailing, T.; Miller, A.; Saunders, G. Elastic constants of a fluorozirconate glass. Mater. Res. Bull. 1981, 16, 613–621. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Hwa, L. Temperature dependence of elastic properties of ZBLAN glasses. Mater. Chem. Phys. 2000, 65, 306–309. [Google Scholar] [CrossRef]
- Saddeek, Y.B.; Shaaban, E.R.; Aly, K.A.; Sayed, I. Characterization of some lead vanadate glasses. J. Alloys Compd. 2009, 478, 447–452. [Google Scholar] [CrossRef]
- Rajendran, V.; Palanivelu, N.; Modak, D.; Chaudhuri, B. Ultrasonic Investigation on Ferroelectric BaTiO3 Doped 80V2O5-20PbO Oxide Glasses. Phys. Status Solidi 2000, 180, 467–477. [Google Scholar] [CrossRef]
- Rajendran, V.; Palanivelu, N.; Chaudhuri, B.; Goswami, K. Characterisation of semiconducting V2O5-Bi2O3-TeO2 glasses through ultrasonic measurements. J. Non. Cryst. Solids 2003, 320, 195–209. [Google Scholar] [CrossRef]
- Halimah, M.; Eevon, C. Comprehensive study on the effect of Gd2O3 NPs on elastic properties of zinc borotellurite glass system using non-destructive ultrasonic technique. J. Non. Cryst. Solids 2019, 511, 10–18. [Google Scholar] [CrossRef]
- Zainal, A.S.; Mansor, H.; Sidek, H.A.; Daud, W.M.; Zainul, H.; Zaidan, A.W.; Halimah, M.K.; Talib, Z.A. Ultrasonic Study and Physical Properties of Borotellurite Glasses. Am. J. Appl. Sci. 2005, 2, 1541–1546. [Google Scholar]
- Nazrin, S.; Halimah, M.; Muhammad, F.; Yip, J.; Hasnimulyati, L.; Faznny, M.; Hazlin, M.; Zaitizila, I. The effect of erbium oxide in physical and structural properties of zinc tellurite glass system. J. Non. Cryst. Solids 2018, 490, 35–43. [Google Scholar] [CrossRef]
- Halimah, M.K.; Daud, W.M.; Sidek, H.A.A. Elastic properties of TeO2-B2O3-Ag2O glasses. Ionics 2010, 16, 807–813. [Google Scholar] [CrossRef]
- Umair, M.; Yahya, A. Elastic and structural changes of xNa2O-(35-x)V2O5-65TeO2 glass system with increasing sodium. Mater. Chem. Phys. 2013, 142, 549–555. [Google Scholar] [CrossRef]
- Souri, D.; Salehizadeh, S.A. Glass transition, fragility, and structural features of amorphous nickel-tellurate-vanadate samples. J. Therm. Anal. Calorim. 2013, 112, 689–695. [Google Scholar] [CrossRef]
- El-Mallawany, R.; Elkhoshkhany, N.; Afifi, H. Ultrasonic studies of (TeO2)50-(V2O5)50-x(TiO2)x glasses. Mater. Chem. Phys. 2006, 95, 321–327. [Google Scholar] [CrossRef]
- Souri, D. Fragility, DSC and elastic moduli studies on tellurite–vanadate glasses containing molybdenum. Measurement 2011, 44, 1904–1908. [Google Scholar] [CrossRef]
- Souri, D. Crystallization kinetic of Sb-V2O5-TeO2 glasses investigated by DSC and their elastic moduli and Poisson’s ratio. Phys. B Condens. Matter 2015, 456, 185–190. [Google Scholar] [CrossRef]
- Elkhoshkhany, N.; El-Mallawany, R.; Syala, E. Mechanical and thermal properties of TeO2-Bi2O3-V2O5-Na2O-TiO2 glass system. Ceram. Int. 2016, 42, 19218–19224. [Google Scholar] [CrossRef]
- Sidkey, M.; Gaafar, M. Ultrasonic studies on network structure of ternary TeO2-WO3-K2O glass system. Phys. B: Condens. Matter 2004, 348, 46–55. [Google Scholar] [CrossRef]
- El-Moneim, A.A. DTA and IR absorption spectra of vanadium tellurite glasses. Mater. Chem. Phys. 2002, 73, 318–322. [Google Scholar] [CrossRef]
- Damodaran, K.V.; Rao, K.J. Elastic properties of phosphotungstate glasses. J. Mater. Sci. 1989, 24, 2380–2386. [Google Scholar] [CrossRef]
- Miura, T.; Watanabe, T.; Benino, Y.; Komatsu, T. Unusual Elastic and Mechanical Behaviors of Copper Phosphate Glasses with Different Copper Valence States. J. Am. Ceram. Soc. 2001, 84, 2401–2408. [Google Scholar] [CrossRef]
- Mierzejewski, A.; Saunders, G.; Sidek, H.; Bridge, B. Vibrational properties of samarium phosphate glasses. J. Non. Cryst. Solids 1988, 104, 323–332. [Google Scholar] [CrossRef]
- Higazy, A.; Bridge, B. Elastic constants and structure of the vitreous system Co3O4P2O5. J. Non. Cryst. Solids 1985, 72, 81–108. [Google Scholar] [CrossRef]
- Rajendran, V.; Devi, A.G.; Azooz, M.; El-Batal, F. Physicochemical studies of phosphate based P2O5-Na2O-CaO-TiO2 glasses for biomedical applications. J. Non. Cryst. Solids 2007, 353, 77–84. [Google Scholar] [CrossRef]
- Muthupari, S.; Raghavan, S.L.; Rao, K. Elastic properties of binary AO3-P2O5 and ternary Na2O-AO3-P2O5 (A-Mo or W) glasses. Mater. Sci. Eng. B 1996, 38, 237–244. [Google Scholar] [CrossRef]
- Damodaran, K.V.; Rao, K.J. Elastic Properties of Alkali Phosphomolybdate Glasses. J. Am. Ceram. Soc. 1989, 72, 533–539. [Google Scholar] [CrossRef]
- Zhong, J.; Bray, P. Change in boron coordination in alkali borate glasses, and mixed alkali effects, as elucidated by NMR. J. Non. Cryst. Solids 1989, 111, 67–76. [Google Scholar] [CrossRef]
- Ehrt, D. Zinc and manganese borate glasses-phase separation, crystallization, photoluminescence and structure. Phys. Chem. Glas. Eur. J. Glas. Sci. Technol. B 2013, 54, 65–75. [Google Scholar]
- Kajinami, A.; Harada, Y.; Inoue, S.; Deki, S.; Umesaki, N. The Structural Analysis of Zinc Borate Glass by Laboratory EXAFS and X-ray Diffraction Measurements. Jpn. J. Appl. Phys. 1999, 38, 132. [Google Scholar] [CrossRef]
- Ponader, C.W.; Brown, G.E. Rare earth elements in silicate glass/melt systems: I. Effects of composition on the coordination environments of La, Gd, and Yb. Geochim. Cosmochim. Acta 1989, 53, 2893–2903. [Google Scholar] [CrossRef]
- Takaishi, T.; Jin, J.; Uchino, T.; Yoko, T. Structural Study of PbO–B2O3 Glasses by X-ray Diffraction and 11B MAS NMR Techniques. J. Am. Ceram. Soc. 2000, 48, 2543–2548. [Google Scholar] [CrossRef]
- Miyaji, F.; Sakka, S. Structure of PbO-Bi2O3-Ga2O3 glasses. J. Non. Cryst. Solids 1991, 134, 77–85. [Google Scholar] [CrossRef]
- Piguet, J.L.; Shelby, J.E. Transformation-Range Behavior of Li2O-(Al, Ga)2O3-SiO2 Glasses. J. Am. Ceram. Soc. 1985, 68, 232–233. [Google Scholar] [CrossRef]
- Farrow, L.; Vogel, E. Raman spectra of phosphate and silicate glasses doped with the cations Ti, Nb and Bi. J. Non. Cryst. Solids 1992, 143, 59–64. [Google Scholar] [CrossRef]
- Farges, F. Ab initio and experimental pre-edge investigations of the Mn K-edge XANES in oxide-type materials. Phys. Rev. B Condens. Matter Mater. Phys. 2005, 71, 1–14. [Google Scholar] [CrossRef]
- Schneider, M.; Richter, W.; Keding, R.; Rüssel, C. XPS investigations on coordination and valency of Ti in fresnoite glasses and glass ceramics. J. Non. Cryst. Solids 1998, 226, 273–280. [Google Scholar] [CrossRef]
- Maekawa, T.; Yokokawa, T.; Niwa, K. Optical Spectra of Transition Metals in Na2O-P2O5 Glasses. Bull. Chem. Soc. Jpn. 1969, 42, 2102–2106. [Google Scholar] [CrossRef]
- Boudlich, D.; Bih, L.; Archidi, M.E.H.; Haddad, M.; Yacoubi, A.; Nadiri, A.; Elouadi, B. Infrared, Raman, and Electron Spin Resonance Studies of Vitreous Alkaline Tungsten Phosphates and Related Glasses. J. Am. Ceram. Soc. 2010, 85, 623–630. [Google Scholar] [CrossRef]
- Poirier, G.; Ottoboni, F.S.; Cassanjes, F.C.; Remonte, A.; Messaddeq, Y.; Ribeiro, S.J.L. Redox Behavior of Molybdenum and Tungsten in Phosphate Glasses. J. Phys. Chem. B 2008, 112, 4481–4487. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Komatsu, T.; Matusita, K. Unique physical properties and fragility of 50CuOx-50P2O5 glasses. J. Non. Cryst. Solids 1996, 201, 222–230. [Google Scholar] [CrossRef]
- Kamiya, K.; Okasaka, K.; Wada, M.; Nasu, H.; Yoko, T. Extended X-ray Absorption Fine Structure (EXAFS) Study on the local Environment around Copper in Low Thermal Expansion Copper Aluminosilicate Glasses. J. Am. Ceram. Soc. 1992, 75, 477–478. [Google Scholar] [CrossRef]
- Dimitrov, V.; Dimitriev, Y.; Montenero, A. IR spectra and structure of V2O5-GeO2-Bi2O3 glasses. J. Non. Cryst. Solids 1994, 180, 51–57. [Google Scholar] [CrossRef]
- Dimitriev, Y.; Dimitrov, V.; Arnaudov, M.; Topalov, D. IR-spectral study of vanadate vitreous systems. J. Non. Cryst. Solids 1983, 57, 147–156. [Google Scholar] [CrossRef]
- Smedskjaer, M.M.; Youngman, R.E.; Striepe, S.; Potuzak, M.; Bauer, U.; Deubener, J.; Behrens, H.; Mauro, J.C.; Yue, Y. Irreversibility of Pressure Induced Boron Speciation Change in Glass. Sci. Rep. 2014, 4, 3770. [Google Scholar] [CrossRef]
- Svenson, M.N.; Bechgaard, T.K.; Fuglsang, S.D.; Pedersen, R.H.; Tjell, A.Ø.; Østergaard, M.B.; Youngman, R.E.; Mauro, J.C.; Rzoska, S.J.; Bockowski, M.; et al. Composition-Structure-Property Relations of Compressed Borosilicate Glasses. Phys. Rev. Appl. 2014, 2, 1–9. [Google Scholar] [CrossRef]
- Hassan, A.K.; Börjesson, L.; Torell, L.M. Relaxations in Complex Systems The boson peak in glass formers of increasing fragility. J. Non. Cryst. Solids 1994, 172, 154–160. [Google Scholar] [CrossRef]
- Sanditov, D.S.; Mashanov, A.A.; Sanditov, B.D.; Mantatov, V.V. Fragility and anharmonicity of lattice vibrations of glass-forming systems. Glas. Phys. Chem. 2008, 34, 389–393. [Google Scholar] [CrossRef]
- Nascimento, M.L.F.; Aparicio, C. Viscosity of strong and fragile glass-forming liquids investigated by means of principal component analysis. J. Phys. Chem. Solids 2007, 68, 104–110. [Google Scholar] [CrossRef]
- Bechgaard, T.K.; Mauro, J.C.; Bauchy, M.; Yue, Y.; Lamberson, L.A.; Jensen, L.R.; Smedskjaer, M.M. Fragility and configurational heat capacity of calcium aluminosilicate glass-forming liquids. J. Non. Cryst. Solids 2017, 461, 24–34. [Google Scholar] [CrossRef]
- Ersundu, A.; Çelikbilek, M.; Aydin, S. Characterization of B2O3 and/or WO3 containing tellurite glasses. J. Non. Cryst. Solids 2012, 358, 641–647. [Google Scholar] [CrossRef]
- Battezzati, L. Is There a Link between Melt Fragility and Elastic Properties of Metallic Glasses? Mater. Trans. 2006, 46, 2915–2919. [Google Scholar] [CrossRef][Green Version]
- Stepniewska, M.; Januchta, K.; Zhou, C.; Qiao, A.; Smedskjaer, M.M.; Yue, Y. Anomalous Cracking in a Metal-Organic Framework Glass. ChemRxiv 2019, 1–18. [Google Scholar]
- Irwin, G.R. Analysis of stresses and strains near the end of a crack traversing a plate. Spie Milest Ser MS 1997, 137, 16. [Google Scholar]
- Lewandowski, J.J.; Gu, X.J.; Nouri, A.S.; Poon, S.J.; Shiflet, G.J. Tough Fe-based bulk metallic glasses. Appl. Phys. Lett. 2008, 92, 91918. [Google Scholar] [CrossRef]
- Conner, R.; Rosakis, A.; Johnson, W.; Owen, D. Fracture toughness determination for a beryllium-bearing bulk metallic glass. Scr. Mater. 1997, 37, 1373–1378. [Google Scholar] [CrossRef]
- Yuan, C.C.; Xi, X.K. On the correlation of Young’s modulus and the fracture strength of metallic glasses. J. Appl. Phys. 2011, 109, 33515. [Google Scholar] [CrossRef]
- Keryvin, V.; Hoang, V.H.; Shen, J. Intermetallics Hardness, toughness, brittleness and cracking systems in an iron-based bulk metallic glass by indentation. Intermetallics 2009, 17, 211–217. [Google Scholar] [CrossRef]
- Conner, R.; Dandliker, R.; Johnson, W. Mechanical properties of tungsten and steel fiber reinforced Zr41.25Ti13.75Cu12.5Ni10Be22.5 metallic glass matrix composites. Acta Mater. 1998, 46, 6089–6102. [Google Scholar] [CrossRef]
- Tiegel, M.; Hosseinabadi, R.; Kühn, S.; Herrmann, A.; Rüssel, C.; Hosseinabhadi, R. Young’s modulus, Vickers hardness and indentation fracture toughness of alumino silicate glasses. Ceram. Int. 2015, 41, 7267–7275. [Google Scholar] [CrossRef]
- Matzke, H.; Toscano, E.; Routbort, J.; Reimann, K. Temperature Dependence and Fracture Toughness and Elastic Moduli of a Waste Glass. J. Am. Ceram. Soc. 1986, 69, 138–139. [Google Scholar] [CrossRef]
- Sampaio, J.A.; Baesso, M.; Gama, S.; Coelho, A.; Eiras, J.; Santos, I.; Santos, I. Rare earth doping effect on the elastic moduli of low silica calcium aluminosilicate glasses. J. Non. Cryst. Solids 2002, 304, 293–298. [Google Scholar] [CrossRef]
- Shinkai, N.; Bradt, R.C.; Rindone, G.E. Elastic Modulus and Fracture Toughness of Ternary PbO-ZnO-B2O3 Glasses. J. Am. Ceram. Soc. 1982, 65, 123–126. [Google Scholar] [CrossRef]
- Le Bourhis, E.; Gadaud, P.; Guin, J.-P.; Tournerie, N.; Zhang, X.-H.; Lucas, J.; Rouxel, T. Temperature dependence of the mechanical behaviour of a GeAsSe glass. Scr. Mater. 2001, 45, 317–323. [Google Scholar] [CrossRef]
- Guin, J.; Rouxel, T.; Sanglebœuf, J.; Rennes, D.; De Beaulieu, C.; Melscoe, I.; Lucas, J. Hardness, Toughness, and Scratchability of Germanium–Selenium Chalcogenide Glasses. J. Am. Ceram. Soc. 2002, 85, 1545–1552. [Google Scholar] [CrossRef]
- Lowhaphandu, P.; Lewandowski, J. Fracture toughness and notched toughness of bulk amorphous alloy: Zr-Ti-Ni-Cu-Be. Scr. Mater. 1998, 38, 1811–1817. [Google Scholar] [CrossRef]
- Lewandowski, J.J. Effects of Annealing and Changes in Stress State on Fracture Toughness of Bulk Metallic Glass. Mater. Trans. 2001, 42, 633–637. [Google Scholar] [CrossRef]
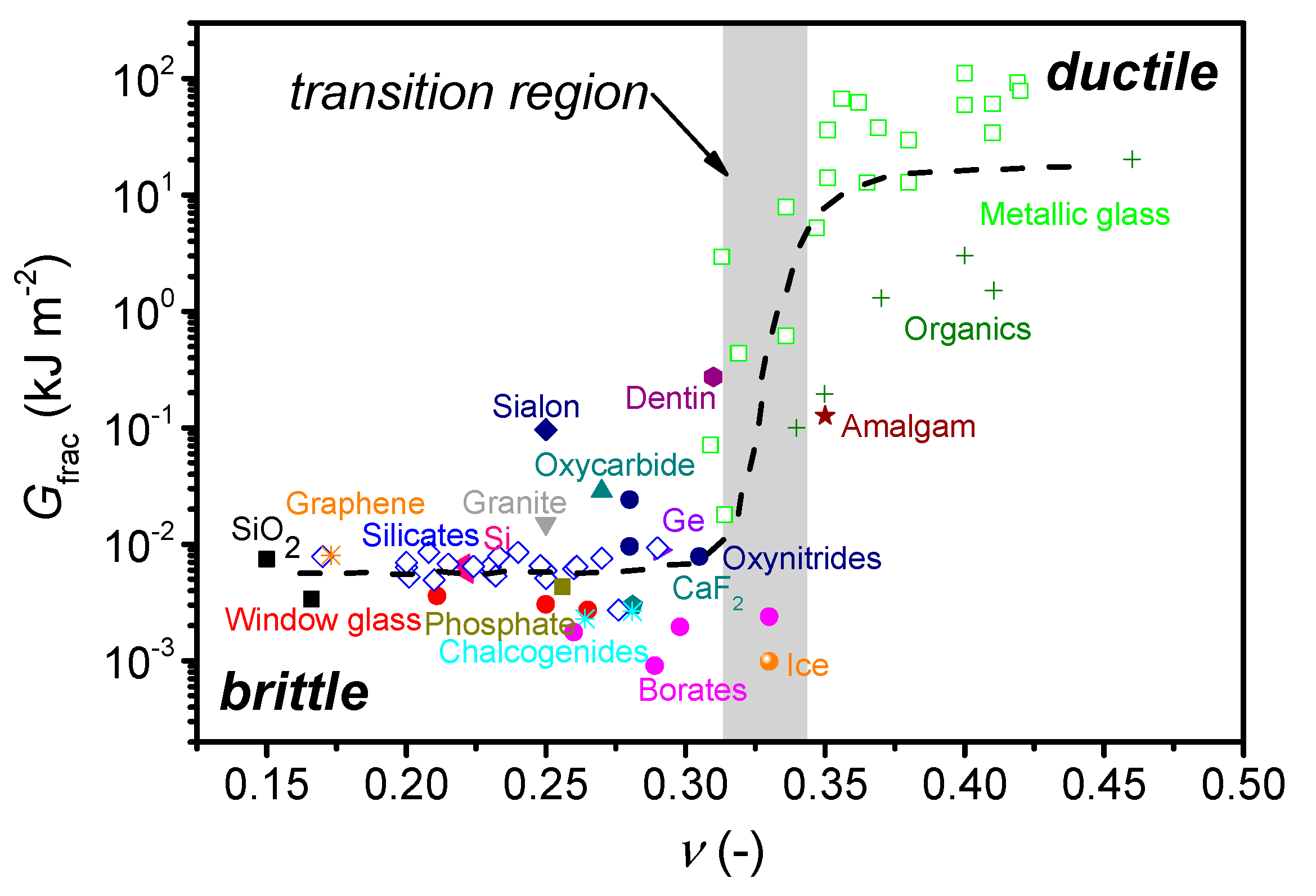
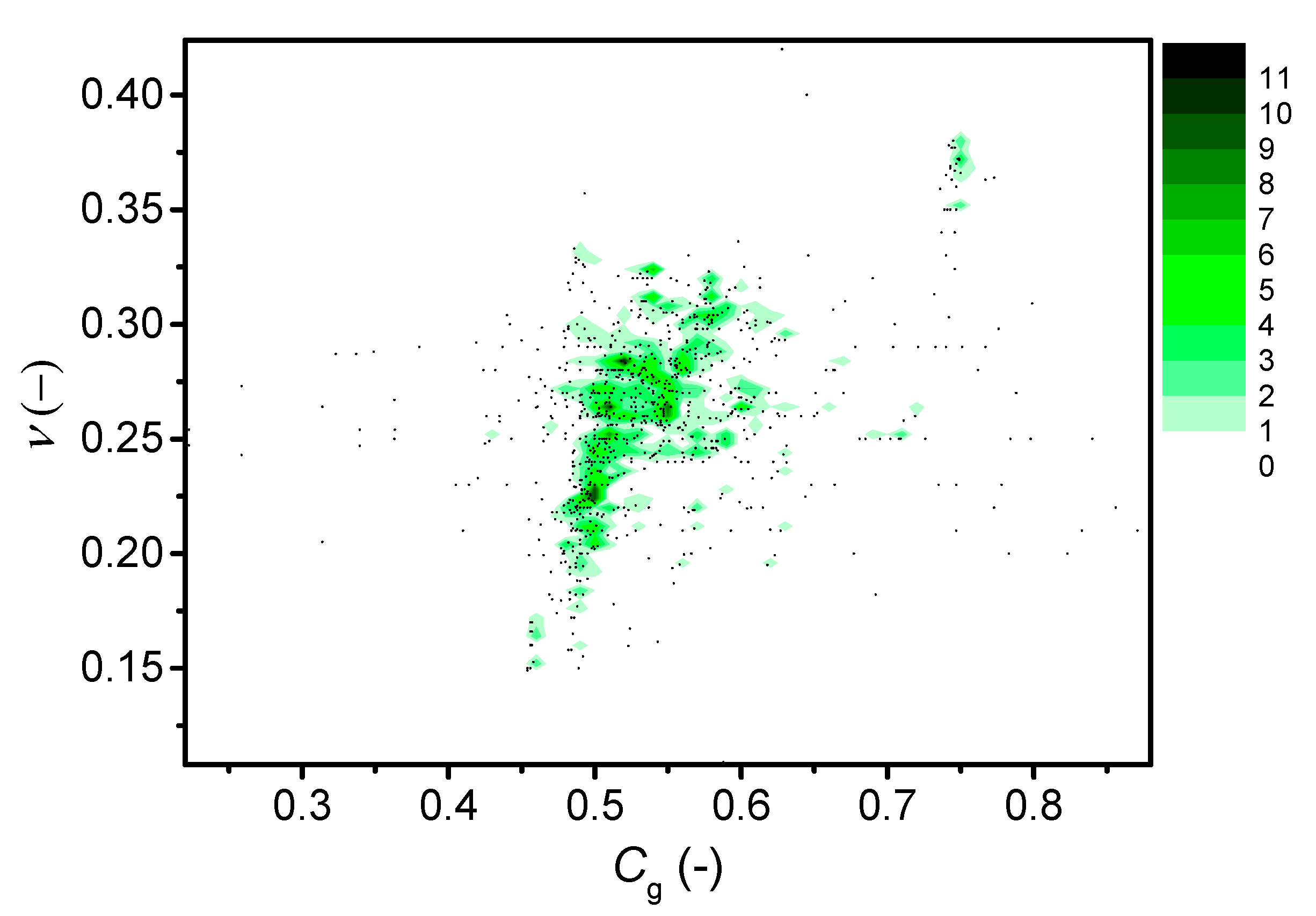
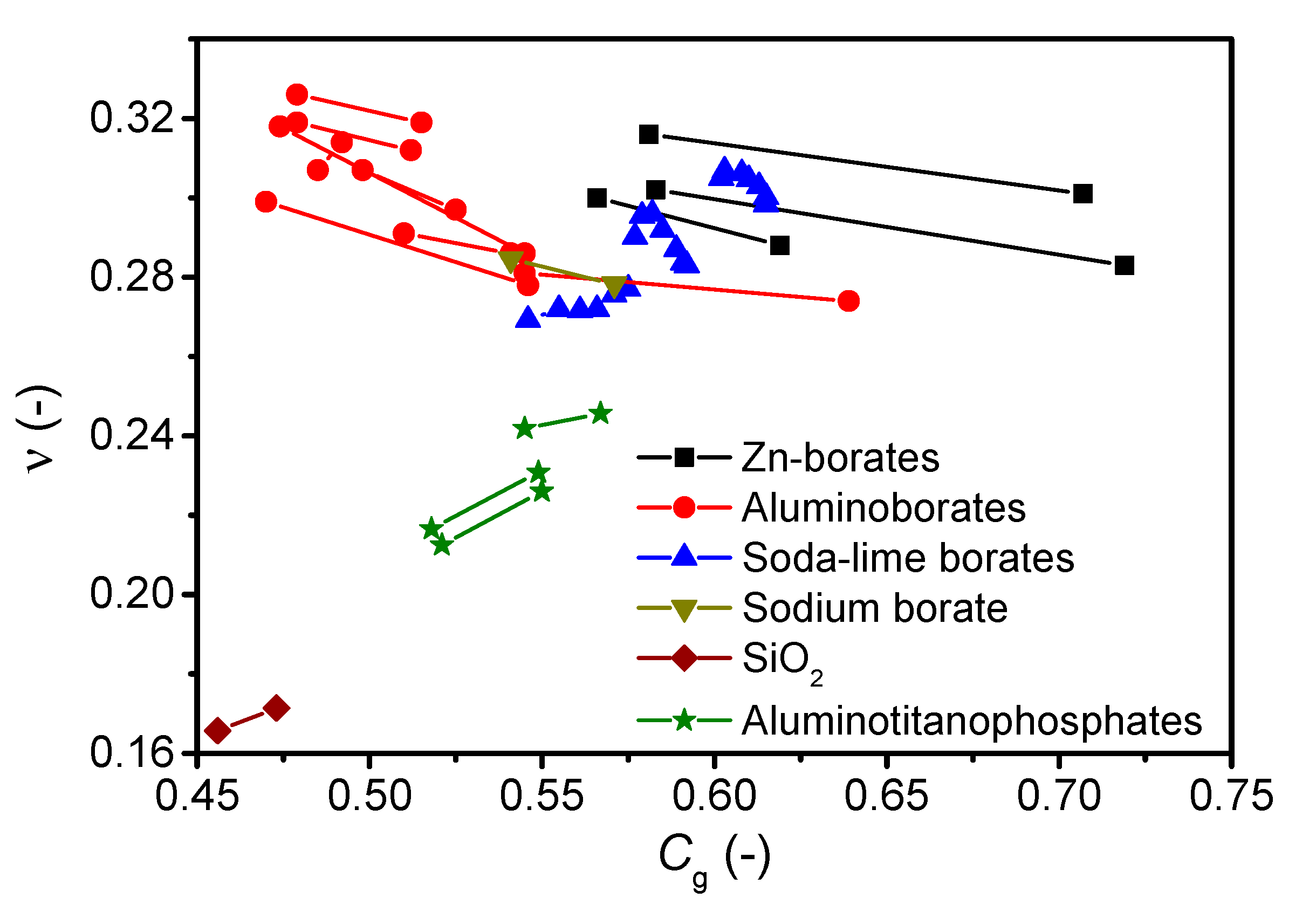
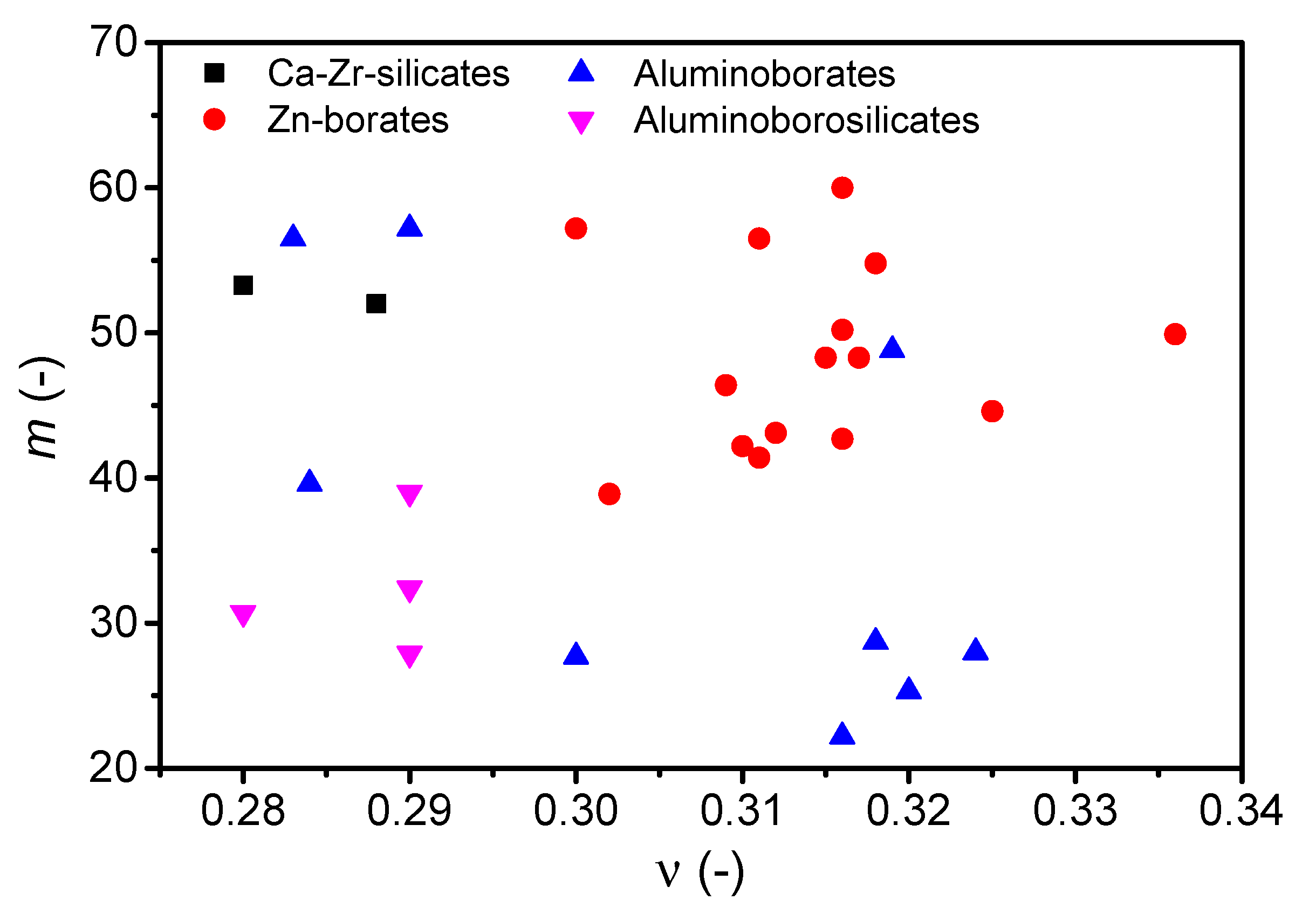

| Composition (mol%) | Cg (-) | Tg (°C) | m (-) | ν (-) | |
|---|---|---|---|---|---|
| Ca-Zr-Silicates | 45CaO-5ZrO2-50SiO2 | 0.523 | 789.2 | 53.3 | 0.280 |
| 50CaO-5ZrO2-45SiO2 | 0.524 | 806.2 | 52.0 | 0.288 | |
| Zn-Borates | 55ZnO-45B2O3 (a) | 0.566 (a) | 556.5 | 57.2 | 0.300 (a) |
| 2La2O3-53ZnO-45B2O3 (a) | 0.565 (a) | 557.4 | 56.5 | 0.311 (a) | |
| 5La2O3-50ZnO-45B2O3 (a) | 0.572 (a) | 565.3 | 60.0 | 0.316 (a) | |
| 10La2O3-45ZnO-45B2O3 (a) | 0.580 (a) | 552.4 | 54.8 | 0.318 (a) | |
| 5La2O3-10GeO2-50ZnO-35B2O3 | 0.554 | 576.3 | 41.4 | 0.311 | |
| 2Ta2O5-53ZnO-45B2O3 | 0.581 | 559.6 | 42.7 | 0.316 | |
| 5Ta2O5-50ZnO-45B2O3 | 0.577 | 563.7 | 48.3 | 0.315 | |
| 2Ta2O5-55ZnO-43B2O3 | 0.583 | 547.6 | 49.9 | 0.336 | |
| 5Ta2O5-55ZnO-40B2O3 | 0.550 | 563.7 | 48.3 | 0.317 | |
| 10Sb2O3-55ZnO-35B2O3 | 0.498 | 502.4 | 39.9 | 0.278 | |
| 2La2O3-55ZnO-43B2O3 | 0.583 | 533.0 | 38.9 | 0.302 | |
| 5La2O3-55ZnO-40B2O3 | 0.551 | 557.1 | 46.4 | 0.309 | |
| 10La2O3-55ZnO-35B2O3 | 0.539 | 542.2 | 42.2 | 0.310 | |
| 2La2O3-2Ta2O5-53ZnO-43B2O3 | 0.617 | 538.7 | 50.2 | 0.316 | |
| 5La2O3-2Ta2O5-50ZnO-43B2O3 | 0.580 | 539.5 | 44.6 | 0.325 | |
| 5La2O3-5Ta2O5-50ZnO-40B2O3 | 0.569 | 547.2 | 43.1 | 0.312 | |
| Aluminoborates | 25MgO-20Al2O3-55B2O3 (b) | 0.565 (b) | 636 (b) | 56.5 | 0.283 |
| 25CaO-20Al2O3-55B2O3 (b) | 0.551 (b) | 615 (b) | 54.8 | 0.220 | |
| 25SrO-20Al2O3-55B2O3 (b) | 0.537 (b) | 590 (b) | 60.0 | 0.266 | |
| 25BaO-20Al2O3-55B2O3 (b) | 0.545 (b) | 554 (b) | 57.2 | 0.290 | |
| 18.75Li2O-6.25BaO-20Al2O3-55B2O3 | 0.531 | 484.4 | 39.6 | 0.284 | |
| 20Li2O-5MgO-20Al2O3-55B2O3(c) | 0.553 (c) | 482 (c) | 40.6 | 0.247 | |
| 25Cs2O-20Al2O3-55B2O3 (d) | 0.479 (d) | 416 (d) | 48.8 | 0.319 (d) | |
| 25Cs2O-5Ga2O3-15Al2O3-55B2O3 | 0.480 | 421.2 | 28.0 | 0.324 | |
| 25Cs2O-10Ga2O3-10Al2O3-55B2O3 | 0.474 | 418.7 | 25.3 | 0.320 | |
| 25Cs2O-2Ta2O3-18Al2O3-55B2O3 | 0.474 | 432.1 | 28.7 | 0.318 | |
| 23Cs2O-2Ta2O3-20Al2O3-55B2O3 | 0.470 | 433.6 | 22.2 | 0.316 | |
| 21Cs2O-4Ta2O3-20Al2O3-55B2O3 | 0.475 | 449.7 | 27.7 | 0.300 | |
| Aluminoborosilicates | 25Na2O-75SiO2 (e) | 0.49 (e) | 475 (e) | 33.3 | 0.25 (e) |
| 25Na2O-12.5B2O3-62.5SiO2 (e) | 0.52 (e) | 539 (e) | 43.8 | 0.22 (e) | |
| 25Na2O-25B2O3-50SiO2 (e) | 0.55 (e) | 544 (e) | 48.7 | 0.22 (e) | |
| 25Na2O-37.5B2O3-37.5SiO2 (e) | 0.56 (e) | 525 (e) | 48.8 | 0.24 (e) | |
| 25Na2O-50B2O3-25SiO2 (e) | 0.56 (e) | 511 (e) | 50.6 | 0.25 (e) | |
| 25Na2O-62.5B2O3-12.5SiO2 (e) | 0.56 (e) | 495 (e) | 50.2 | 0.25 (e) | |
| 25Na2O-75B2O3 (e) | 0.56 (e) | 473 (e) | 51.4 | 0.27 (e) | |
| 25Na2O-12.5Al2O3-62.5SiO2 (e) | 0.49 (e) | 567 (e) | 32.8 | 0.23 (e) | |
| 25Na2O-12.5Al2O3-12.5B2O3-50SiO2 (e) | 0.51 (e) | 545 (e) | 50.4 | 0.24 (e) | |
| 25Na2O-12.5Al2O3-25B2O3-37.5SiO2 (e) | 0.52 (e) | 514 (e) | 43.3 | 0.25 (e) | |
| 25Na2O-12.5Al2O3-37.5B2O3-25SiO2 (e) | 0.52 (e) | 493 (e) | 46.4 | 0.26 (e) | |
| 25Na2O-12.5Al2O3-50B2O3-12.5SiO2 (e) | 0.52 (e) | 480 (e) | 48.9 | 0.27 (e) | |
| 25Na2O-12.5Al2O3-62.5B2O3 (e) | 0.52 (e) | 465 (e) | 39.0 | 0.29 (e) | |
| 25Na2O-25Al2O3-50SiO2 f) | 0.49 (e) | 792 (e) | 38.5 | 0.21 (e) | |
| 25Na2O-25Al2O3-12.5B2O3-37.5SiO2 (e) | 0.49 (e) | 611 (e) | 30.0 | 0.25 (e) | |
| 25Na2O-25Al2O3-25B2O3-25SiO2 (e) | 0.49 (e) | 511 (e) | 28.4 | 0.26 (e) | |
| 25Na2O-25Al2O3-37.5B2O3-12.5SiO2 (e) | 0.50 (e) | 468 (e) | 30.7 | 0.28 (e) | |
| 25Na2O-25Al2O3-50B2O3 (e) | 0.50 (e) | 459 (e) | 32.4 | 0.29 (e) | |
| 25Na2O-30Al2O3-45B2O3 (e) | 0.50 (e) | 528 (e) | 31,9 | 0.27 (e) | |
| 25Na2O-30Al2O3-32.5B2O3-12.5SiO2 (e) | 0.50 (e) | 469 (e) | 27.9 | 0.29 (e) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Østergaard, M.B.; Hansen, S.R.; Januchta, K.; To, T.; Rzoska, S.J.; Bockowski, M.; Bauchy, M.; Smedskjaer, M.M. Revisiting the Dependence of Poisson’s Ratio on Liquid Fragility and Atomic Packing Density in Oxide Glasses. Materials 2019, 12, 2439. https://doi.org/10.3390/ma12152439
Østergaard MB, Hansen SR, Januchta K, To T, Rzoska SJ, Bockowski M, Bauchy M, Smedskjaer MM. Revisiting the Dependence of Poisson’s Ratio on Liquid Fragility and Atomic Packing Density in Oxide Glasses. Materials. 2019; 12(15):2439. https://doi.org/10.3390/ma12152439
Chicago/Turabian StyleØstergaard, Martin B., Søren R. Hansen, Kacper Januchta, Theany To, Sylwester J. Rzoska, Michal Bockowski, Mathieu Bauchy, and Morten M. Smedskjaer. 2019. "Revisiting the Dependence of Poisson’s Ratio on Liquid Fragility and Atomic Packing Density in Oxide Glasses" Materials 12, no. 15: 2439. https://doi.org/10.3390/ma12152439
APA StyleØstergaard, M. B., Hansen, S. R., Januchta, K., To, T., Rzoska, S. J., Bockowski, M., Bauchy, M., & Smedskjaer, M. M. (2019). Revisiting the Dependence of Poisson’s Ratio on Liquid Fragility and Atomic Packing Density in Oxide Glasses. Materials, 12(15), 2439. https://doi.org/10.3390/ma12152439








