Primary Stability Optimization by Using Fixtures with Different Thread Depth According To Bone Density: A Clinical Prospective Study on Early Loaded Implants
Abstract
1. Introduction
2. Materials and Methods
- i)
- Height of the residual bone crest in the programmed implant site ≥9 mm and thickness ≥7 mm;
- ii)
- Healed bone crest (almost three months elapsed after extraction or tooth loss);
- iii)
- Patient age > 18 years;
- iv)
- Patients able to examine and understand the study protocol.
- i)
- Myocardial infarction within the past six months;
- ii)
- Poorly controlled diabetes (HBA1c > 7.5%);
- iii)
- Coagulation disorders;
- iv)
- Radiotherapy to the head/neck district within the past two years;
- v)
- Present or past treatment with intravenous bisphosphonates;
- vi)
- Immunocompromised patients;
- vii)
- Psychological or psychiatric problems;
- viii)
- Alcohol or drug abuse;
- ix)
- Poor oral hygiene and motivation (full mouth plaque score > 30% and/or full mouth bleeding score > 20%);
- x)
- Uncontrolled periodontal disease.
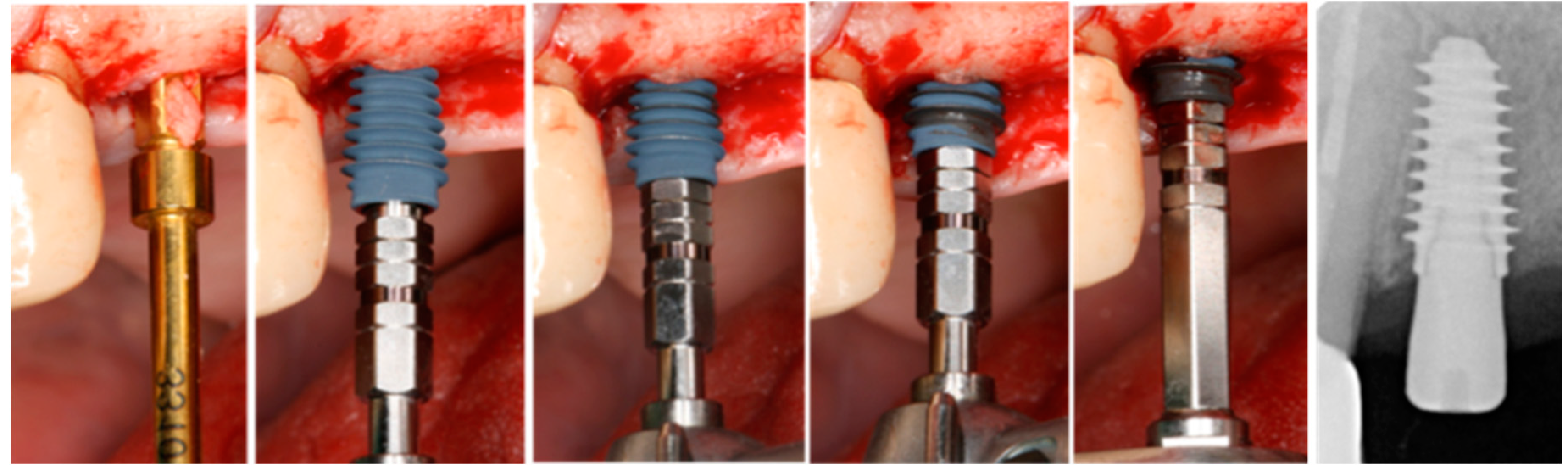
2.1. Surgical Procedure
- D1: Almost all dense cortical bone similar to oak- or maple-like in hardness;
- D2: Homogenous, dense bone similar to white pine in hardness;
- D3: Thin porous cortical and fine trabecular bone similar to balsa wood in hardness;
- D4: Little or no cortical bone, with fine trabecular bone similar to Styrofoam in hardness.
2.2. Predictor and Outcome Variables
- Implant stability (IT and ISQ) measured at implant insertion and during the first four weeks of healing.
- Marginal bone loss measured 12 months after implant insertion;
- Implant failure: Implant mobility (tested by tightening abutment screws at 35 Ncm at prosthesis delivery) or implant removal suggested by progressive marginal bone loss;
- Any complication or adverse event.
2.3. Statistical Analysis
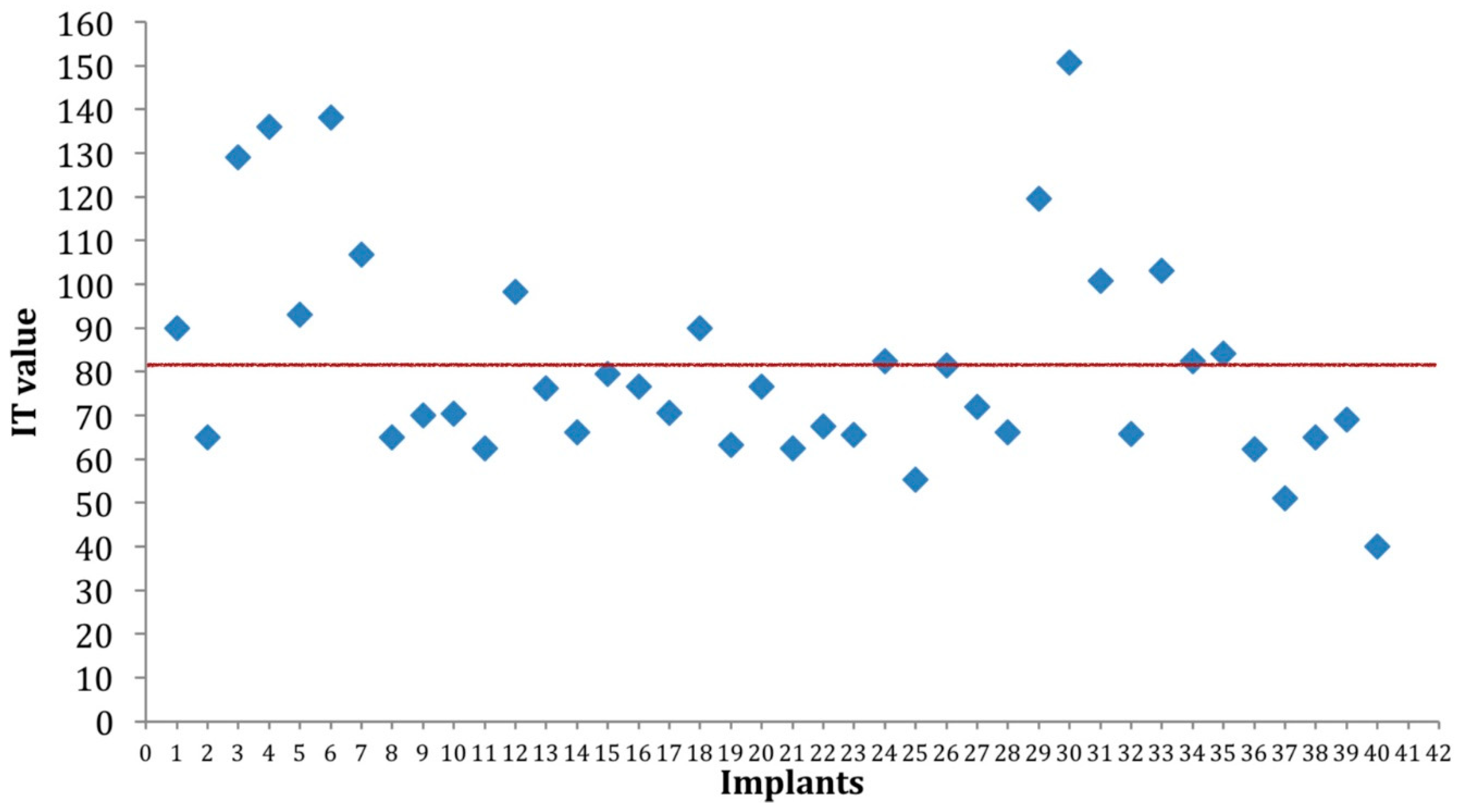
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Misch, C.E.; Perel, M.L.; Wang, H.-L.; Sammartino, G.; Galindo-Moreno, P.; Trisi, P.; Steigmann, M.; Rebaudi, A.; Palti, A.; Pikos, M.A.; et al. Implant Success, Survival, and Failure: The International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference. Implant. Dent. 2008, 17, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Brånemark, P.-I.; Hansson, H.-A.; Lindström, J. Osseointegrated Titanium Implants:Requirements for Ensuring a Long-Lasting, Direct Bone-to-Implant Anchorage in Man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Szmukler-Moncler, S.; Salama, H.; Reingewirtz, Y.; Dubruille, J.H.; Szmukler-Moncler, S. Timing of loading and effect of micromotion on bone-dental implant interface: Review of experimental literature. J. Biomed. Mater. Res. 1998, 43, 192–203. [Google Scholar] [CrossRef]
- Brunski, J.B. In vivo bone response to biomechanical loading at the bone/dental-implant interface. Adv. Dent. Res. 1999, 13, 99–119. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, S.; Wood, M.C.; Taylor, T.D. Early Wound Healing Around Endosseous Implants: A Review of the Literature. Int. J. Oral Maxillofac. Implant. 2005, 20, 425–431. [Google Scholar]
- Davies, J.E. Understanding peri-implant endosseous healing. J. Dent. Educ. 2003, 67, 932–949. [Google Scholar] [PubMed]
- Tabassum, A.; Meijer, G.J.; Wolke, J.G.C.; Jansen, J.A. Influence of surgical technique and surface roughness on the primary stability of an implant in artificial bone with different cortical thickness: A laboratory study. Clin. Oral Implants Res. 2010, 21, 213–220. [Google Scholar] [CrossRef]
- Atsumi, M.; Park, S.-H.; Wang, H.-L. Methods used to assess implant stability: Current status. Int. J. Oral Maxillofac. Implants 2007, 22, 743–754. [Google Scholar]
- Makary, C.; Rebaudi, A.; Mokbel, N.; Naaman, N. Peak insertion torque correlated to histologically and clinically evaluated bone density. Implant Dent. 2011, 20, 182–191. [Google Scholar] [CrossRef]
- Makary, C.; Rebaudi, A.; Sammartino, G.; Naaman, N. Implant primary stability determined by resonance frequency analysis: Correlation with insertion torque, histologic bone volume, and torsional stability at 6 weeks. Implant Dent. 2012, 21, 474–480. [Google Scholar] [CrossRef]
- Makary, C.; Rebaudi, A.; Demircioglu, A.; Lahoud, P.; Naaman, N. Standard Drilling Versus Ultrasonic Implant Site Preparation: A Clinical Study at 4 Weeks After Insertion of Conical Implants. Implant Dent. 2017, 26, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Duyck, J.; Corpas, L.; Vermeiren, S.; Ogawa, T.; Quirynen, M.; Vandamme, K.; Jacobs, R.; Naert, I. Histological, histomorphometrical, and radiological evaluation of an experimental implant design with a high insertion torque. Clin. Oral Implants Res. 2010, 21, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Marconcini, S.; Giammarinaro, E.; Toti, P.; Alfonsi, F.; Covani, U.; Barone, A. Longitudinal analysis on the effect of insertion torque on delayed single implants: A 3-year randomized clinical study. Clin. Implant Dent. Relat. Res. 2018, 20, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Ottoni, J.M.P.; Oliveira, Z.F.L.; Mansini, R.; Cabral, A.M. Correlation between placement torque and survival of single-tooth implants. Int. J. Oral Maxillofac. Implants 2005, 20, 769–776. [Google Scholar] [PubMed]
- Stocchero, M.; Toia, M.; Cecchinato, D.; Becktor, J.P.; Coelho, P.G.; Jimbo, R. Biomechanical, Biologic, and Clinical Outcomes of Undersized Implant Surgical Preparation: A Systematic Review. Int. J. Oral Maxillofac. Implants 2016, 31, 1247–1263. [Google Scholar] [CrossRef]
- Degidi, M.; Daprile, G.; Piattelli, A. Influence of underpreparation on primary stability of implants inserted in poor quality bone sites: An in vitro study. J. Oral Maxillofac. Surg. 2015, 73, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Jimbo, R.; Tovar, N.; Anchieta, R.B.; Machado, L.S.; Marin, C.; Teixeira, H.S.; Coelho, P.G. The combined effects of undersized drilling and implant macrogeometry on bone healing around dental implants: An experimental study. Int. J. Oral Maxillofac. Surg. 2014, 43, 1269–1275. [Google Scholar] [CrossRef]
- O’Sullivan, D.; Sennerby, L.; Meredith, N. Measurements comparing the initial stability of five designs of dental implants: A human cadaver study. Clin. Implant Dent. Relat. Res. 2000, 2, 85–92. [Google Scholar] [CrossRef]
- Abuhussein, H.; Pagni, G.; Rebaudi, A.; Wang, H. The effect of thread pattern upon implant osseointegration. Clin. Oral Implants Res. 2010, 21, 129–136. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Kim, S.-J.; An, H.-W.; Kim, H.-S.; Ha, D.-G.; Ryo, K.-H.; Park, K.-B. The effect of the thread depth on the mechanical properties of the dental implant. J. Adv. Prosthodont. 2015, 7, 115–121. [Google Scholar] [CrossRef]
- Berglundh, T.; Abrahamsson, I.; Lang, N.P.; Lindhe, J. De novo alveolar bone formation adjacent to endosseous implants. Clin. Oral Implants Res. 2003, 14, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Adell, R.; Lekholm, U.; Rockler, B.; Brånemark, P.I. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int. J. Oral Surg. 1981, 10, 387–416. [Google Scholar] [CrossRef]
- Khang, W.; Feldman, S.; Hawley, C.E.; Gunsolley, J. A multi-center study comparing dual acid-etched and machined-surfaced implants in various bone qualities. J. Periodontol. 2001, 72, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implants Res. 2009, 20, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, G.; Mendonça, D.B.S.; Aragão, F.J.L.; Cooper, L.F. Advancing dental implant surface technology—From micron-to nanotopography. Biomaterials 2008, 29, 3822–3835. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Yang, D.-J.; Yeo, S.; An, H.-W.; Ryoo, K.H.; Park, K.-B. The cytocompatibility and osseointegration of the Ti implants with XPEED(R) surfaces. Clin. Oral Implants Res. 2012, 23, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Baltayan, S.; Pi-Anfruns, J.; Aghaloo, T.; Moy, P.K. The Predictive Value of Resonance Frequency Analysis Measurements in the Surgical Placement and Loading of Endosseous Implants. J. Oral Maxillofac. Surg. 2016, 74, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Ostman, P.-O.; Hellman, M.; Sennerby, L. Direct implant loading in the edentulous maxilla using a bone density-adapted surgical protocol and primary implant stability criteria for inclusion. Clin. Implant Dent. Relat. Res. 2005, 7, S60–S69. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, M.M.; Hart, C.N.; Halbritter, S.A.; Morton, D.; Buser, D. Early loading of nonsubmerged titanium implants with a chemically modified sand-blasted and acid-etched surface: 6-month results of a prospective case series study in the posterior mandible focusing on peri-implant crestal bone changes and implant stability quotient (ISQ) values. Clin. Implant Dent. Relat. Res. 2009, 11, 338–347. [Google Scholar] [PubMed]
- Gallucci, G.O.; Benic, G.I.; Eckert, S.E.; Papaspyridakos, P.; Schimmel, M.; Schrott, A.; Weber, H.P. Consensus statements and clinical reco mmendations for implant loading protocols. Int. J. Oral Maxillofac. Implants 2014, 29, 287–290. [Google Scholar] [CrossRef]
- Hicklin, S.P.; Schneebeli, E.; Chappuis, V.; Janner, S.F.M.; Buser, D.; Brägger, U. Early loading of titanium dental implants with an intra-operatively conditioned hydrophilic implant surface after 21 days of healing. Clin. Oral Implants Res. 2016, 27, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.E. Bone classification, training keys to implant success. Dent. Today 1989, 8, 39. [Google Scholar] [PubMed]
- Galindo-Moreno, P.; León-Cano, A.; Ortega-Oller, I.; Monje, A.; O’Valle, F.; Catena, A. Marginal bone loss as success criterion in implant dentistry: Beyond 2 mm. Clin. Oral Implants Res. 2015, 26, e28–e34. [Google Scholar] [CrossRef] [PubMed]
- Wentaschek, S.; Scheller, H.; Schmidtmann, I.; Hartmann, S.; Weyhrauch, M.; Weibrich, G.; Lehmann, K.M. Sensitivity and Specificity of Stability Criteria for I mmediately Loaded Splinted Maxillary Implants. Clin. Implant Dent. Relat. Res. 2015, 17, e542–e549. [Google Scholar] [CrossRef] [PubMed]
- Trisi, P.; Perfetti, G.; Baldoni, E.; Berardi, D.; Colagiovanni, M.; Scogna, G. Implant micromotion is related to peak insertion torque and bone density. Clin. Oral Implants Res. 2009, 20, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Lioubavina-Hack, N.; Lang, N.P.; Karring, T. Significance of primary stability for osseointegration of dental implants. Clin. Oral Implants Res. 2006, 17, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Maiorana, C.; Farronato, D.; Pieroni, S.; Cicciu, M.; Andreoni, D.; Santoro, F. A Four-Year Survival Rate Multicenter Prospective Clinical Study on 377 Implants: Correlations Between Implant Insertion Torque, Diameter, and Bone Quality. J. Oral Implantol. 2015, 41, e60–e65. [Google Scholar] [CrossRef]
- Cannizzaro, G.; Leone, M.; Ferri, V.; Viola, P.; Gelpi, F.; Esposito, M. I mmediate loading of single implants inserted flapless with medium or high insertion torque: A 6-month follow-up of a split-mouth randomised controlled trial. Eur. J. Oral Implantol. 2012, 5, 333–342. [Google Scholar] [PubMed]
- Greenstein, G.; Cavallaro, J. Implant Insertion Torque: Its Role in Achieving Primary Stability of Restorable Dental Implants. Compend. Contin. Educ. Dent. 2017, 38, 88–95. [Google Scholar]
- Trisi, P.; Rao, W. Bone classification: Clinical-histomorphometric comparison. Clin. Oral Implants Res. 1999, 10, 1–7. [Google Scholar]
- Rebaudi, A. The ray setting procedure: A new method for implant planning and i mmediate prosthesis delivery. Int. J. Periodontics Restor. Dent. 2007, 27, 267–275. [Google Scholar]
- Li, H.; Liang, Y.; Zheng, Q. Meta-Analysis of Correlations Between Marginal Bone Resorption and High Insertion Torque of Dental Implants. Int. J. Oral Maxillofac. Implants 2015, 30, 767–772. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khayat, P.G.; Arnal, H.M.; Tourbah, B.I.; Sennerby, L. Clinical outcome of dental implants placed with high insertion torques (up to 176 Ncm). Clin. Implant Dent. Relat. Res. 2013, 15, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Berardini, M.; Trisi, P.; Sinjari, B.; Rutjes, A.W.S.; Caputi, S. The Effects of High Insertion Torque Versus Low Insertion Torque on Marginal Bone Resorption and Implant Failure Rates: A Systematic Review with Meta-Analyses. Implant Dent. 2016, 25, 532. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.B.V.; Shimano, A.C.; Macedo, A.P.; Valente, M.L.C.; Reis, A.C.D. Influence of torsional strength on different types of dental implant platforms. Implant Dent. 2015, 24, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Aldahlawi, S.; Demeter, A.; Irinakis, T. The effect of implant placement torque on crestal bone remodeling after 1 year of loading. Clin. Cosmet. Investig. Dent. 2018, 10, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Alfonsi, F.; Derchi, G.; Tonelli, P.; Toti, P.; Marchionni, S.; Covani, U. The effect of insertion torque on the clinical outcome of single implants: A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2016, 18, 588–600. [Google Scholar] [CrossRef]
- Grandi, T.; Guazzi, P.; Samarani, R.; Grandi, G. Clinical outcome and bone healing of implants placed with high insertion torque: 12-month results from a multicenter controlled cohort study. Int. J. Oral Maxillofac. Surg. 2013, 42, 516–520. [Google Scholar] [CrossRef]
- Palacios-Garzón, N.; Mauri-Obradors, E.; Labrés, X.R.; Estrugo-Devesa, A.; Jané-Salas, E.; López-López, J. Comparison of Marginal Bone Loss Between Implants with Internal and External Connections: A Systematic Review. Int. J. Oral Maxillofac. Implants. 2018, 33, 580–589. [Google Scholar] [CrossRef]
- Tallarico, M.; Fiorellini, J.; Nakajima, Y.; Omori, Y.; Takahisa, I.; Canullo, L. Mechanical Outcomes, Microleakage, and Marginal Accuracy at the Implant-Abutment Interface of Original versus Nonoriginal Implant Abutments: A Systematic Review of In Vitro Studies. Biomed. Res. Int. 2018, 2018, 2958982. [Google Scholar] [CrossRef]
- Baldi, D.; Lombardi, T.; Colombo, J.; Cervino, G.; Perinetti, G.; Di Lenarda, R.; Stacchi, C. Correlation between Insertion Torque and Implant Stability Quotient in Tapered Implants with Knife-Edge Thread Design. Biomed. Res. Int. 2018, 2018, 7201093. [Google Scholar] [CrossRef] [PubMed]
- Lages, F.S.; Oliveira, D.W.D.; Costa, F.O. Relationship between implant stability measurements obtained by insertion torque and resonance frequency analysis: A systematic review. Clin. Implant Dent. Relat. Res. 2018, 20, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Oates, T.W.; Valderrama, P.; Bischof, M.; Nedir, R.; Jones, A.; Simpson, J.; Toutenburg, H.; Cochran, D.L. Enhanced implant stability with a chemically modified SLA surface: A randomized pilot study. Int. J. Oral Maxillofac. Implant. 2007, 22, 755–760. [Google Scholar]
- McCullough, J.J.; Klokkevold, P.R. The effect of implant macro-thread design on implant stability in the early post-operative period: A randomized, controlled pilot study. Clin. Oral Implant. Res. 2017, 28, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Cicciù, M.; Fiorillo, L.; Herford, A.S.; Crimi, S.; Bianchi, A.; D’Amico, C.; Laino, L.; Cervino, G. Bioactive Titanium Surfaces: Interactions of Eukaryotic and Prokaryotic Cells of Nano Devices Applied to Dental Practice. Biomedicines 2019, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Cicciù, M. Nanobiomaterials in dentistry: What’s the consequent level. Eur. J. Dent. 2018, 12, 161–162. [Google Scholar] [CrossRef] [PubMed]
- Mangano, C.; Shibli, J.A.; Pires, J.T.; Luongo, G.; Piattelli, A.; Iezzi, G. Early Bone Formation around I mmediately Loaded Transitional Implants Inserted in the Human Posterior Maxilla: The Effects of Fixture Design and Surface. Biomed. Res. Int. 2017, 2017, 4152506. [Google Scholar] [CrossRef] [PubMed]

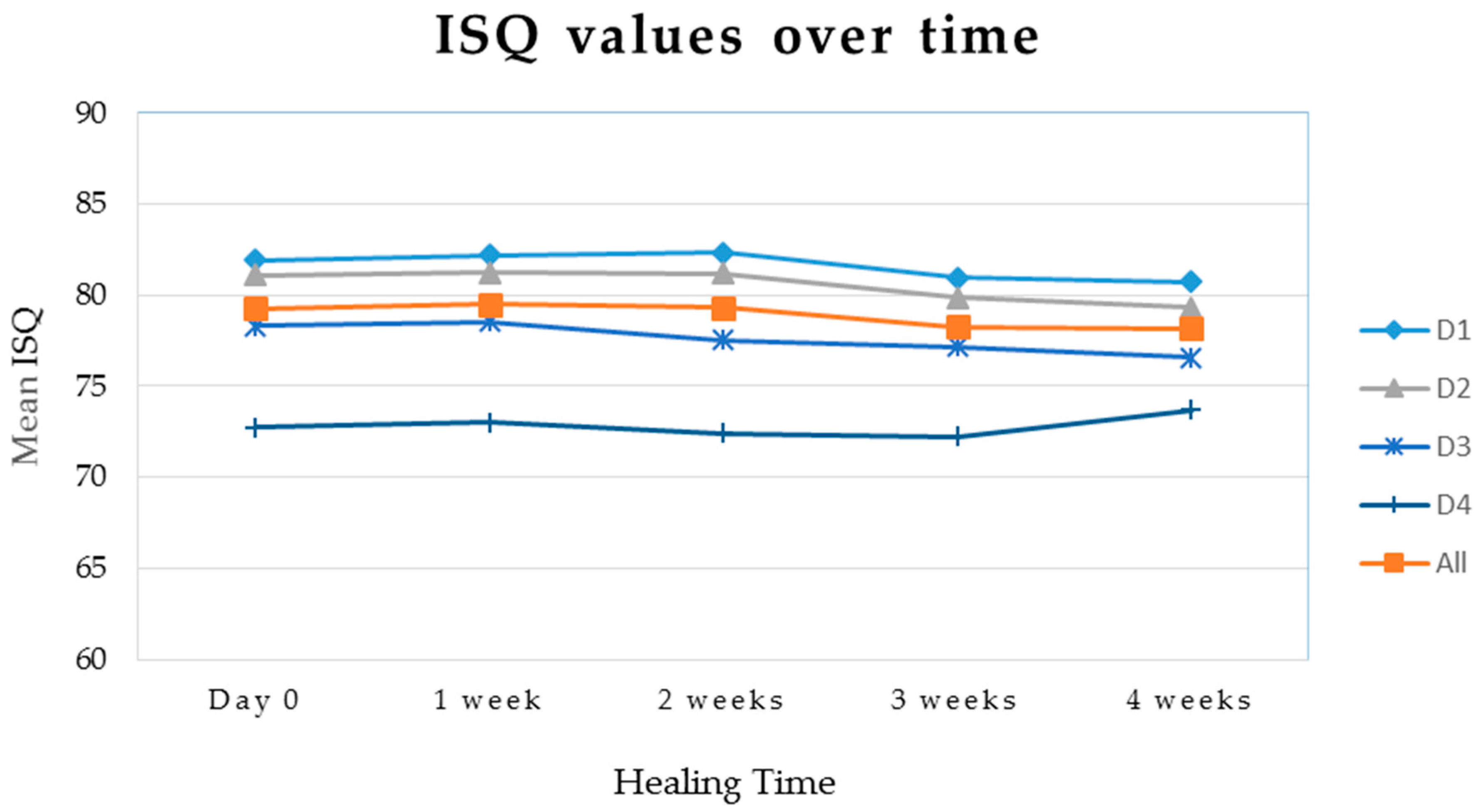
| Measurement | Bone Type | Mean Value | Standard Deviation | N | p-value |
|---|---|---|---|---|---|
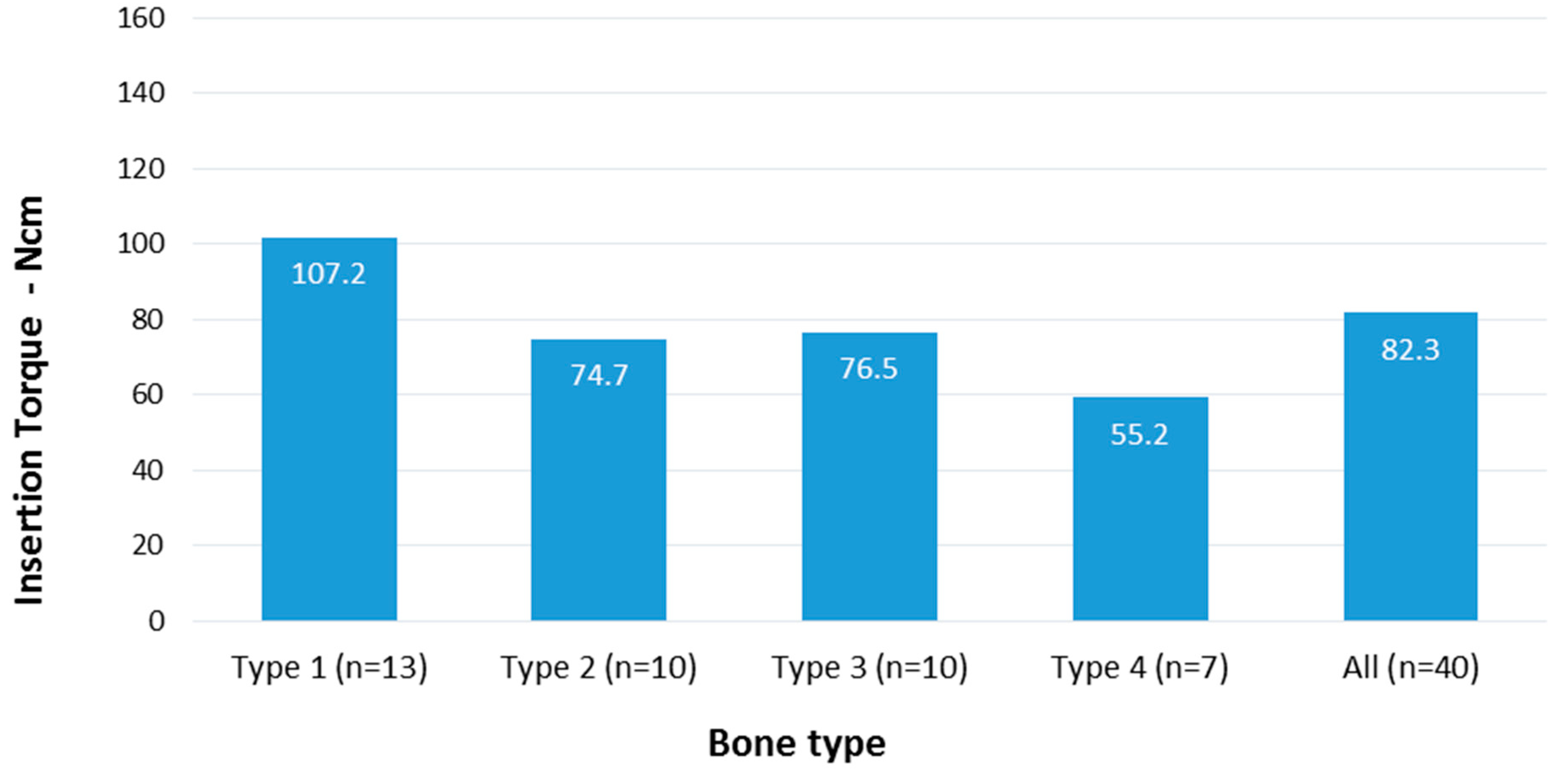
| IT | All | 82.3 | 33.2 | 40 | 0.003 |
| 1 | 107.2 | 35.6 | 13 | ||
| 2 | 74.7 | 14.0 | 10 | ||
| 3 | 76.5 | 31.1 | 10 | ||
| 4 | 55.2 | 22.6 | 7 | ||
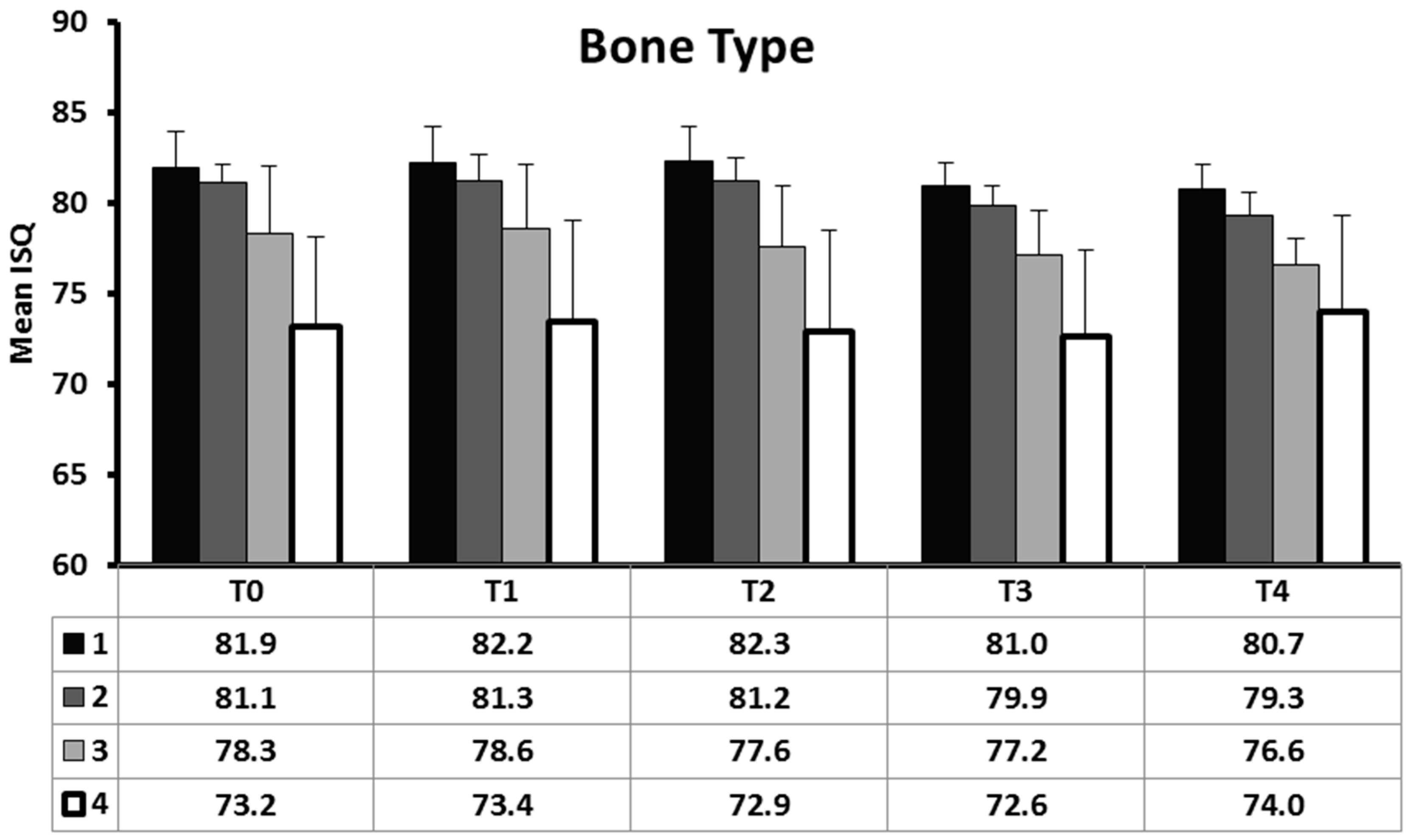
| ISQ T0 | All | 79.3 | 4.3 | 40 | <0.001 |
| 1 | 81.9 | 2.0 | 13 | ||
| 2 | 81.1 | 1.0 | 10 | ||
| 3 | 78.3 | 3.7 | 10 | ||
| 4 | 73.2 | 4.9 | 7 | ||
| ISQ T1 | All | 79.5 | 4.4 | 40 | <0.001 |
| 1 | 82.2 | 2.0 | 13 | ||
| 2 | 81.3 | 1.4 | 10 | ||
| 3 | 78.6 | 3.6 | 10 | ||
| 4 | 73.4 | 5.6 | 7 | ||
| ISQ T2 | All | 79.3 | 4.6 | 40 | <0.001 |
| 1 | 82.3 | 1.9 | 13 | ||
| 2 | 81.2 | 1.3 | 10 | ||
| 3 | 77.6 | 3.4 | 10 | ||
| 4 | 72.9 | 5.6 | 7 | ||
| ISQ T3 | All | 78.3 | 3.8 | 40 | <0.001 |
| 1 | 81.0 | 1.3 | 13 | ||
| 2 | 79.9 | 1.1 | 10 | ||
| 3 | 77.2 | 2.4 | 10 | ||
| 4 | 72.6 | 4.8 | 7 | ||
| ISQ T4 | All | 78.1 | 3.5 | 40 | <0.001 |
| 1 | 80.7 | 1.4 | 13 | ||
| 2 | 79.3 | 1.2 | 10 | ||
| 3 | 76.6 | 1.5 | 10 | ||
| 4 | 74.0 | 5.3 | 7 |
| Group | Rho Coefficient | Significance |
|---|---|---|
| D1 | −0.32 | 0.28 NS |
| D2 | 0.35 | 0.31 NS |
| D3 | 0.47 | 0.16 NS |
| D4 | 0.95 | 0.0008 S |
| Overall | 0.55 | 0.0002 S |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makary, C.; Menhall, A.; Zammarie, C.; Lombardi, T.; Lee, S.Y.; Stacchi, C.; Park, K.B. Primary Stability Optimization by Using Fixtures with Different Thread Depth According To Bone Density: A Clinical Prospective Study on Early Loaded Implants. Materials 2019, 12, 2398. https://doi.org/10.3390/ma12152398
Makary C, Menhall A, Zammarie C, Lombardi T, Lee SY, Stacchi C, Park KB. Primary Stability Optimization by Using Fixtures with Different Thread Depth According To Bone Density: A Clinical Prospective Study on Early Loaded Implants. Materials. 2019; 12(15):2398. https://doi.org/10.3390/ma12152398
Chicago/Turabian StyleMakary, Christian, Abdallah Menhall, Carole Zammarie, Teresa Lombardi, Seung Yeup Lee, Claudio Stacchi, and Kwang Bum Park. 2019. "Primary Stability Optimization by Using Fixtures with Different Thread Depth According To Bone Density: A Clinical Prospective Study on Early Loaded Implants" Materials 12, no. 15: 2398. https://doi.org/10.3390/ma12152398
APA StyleMakary, C., Menhall, A., Zammarie, C., Lombardi, T., Lee, S. Y., Stacchi, C., & Park, K. B. (2019). Primary Stability Optimization by Using Fixtures with Different Thread Depth According To Bone Density: A Clinical Prospective Study on Early Loaded Implants. Materials, 12(15), 2398. https://doi.org/10.3390/ma12152398







