Preparation and Properties of Wall Coatings with Calcined Shell Powder as Fillers
Abstract
1. Introduction
2. Experimental
2.1. Raw Materials
2.2. Sample Preparation
2.3. Testing and Characterization
3. Results and Discussion
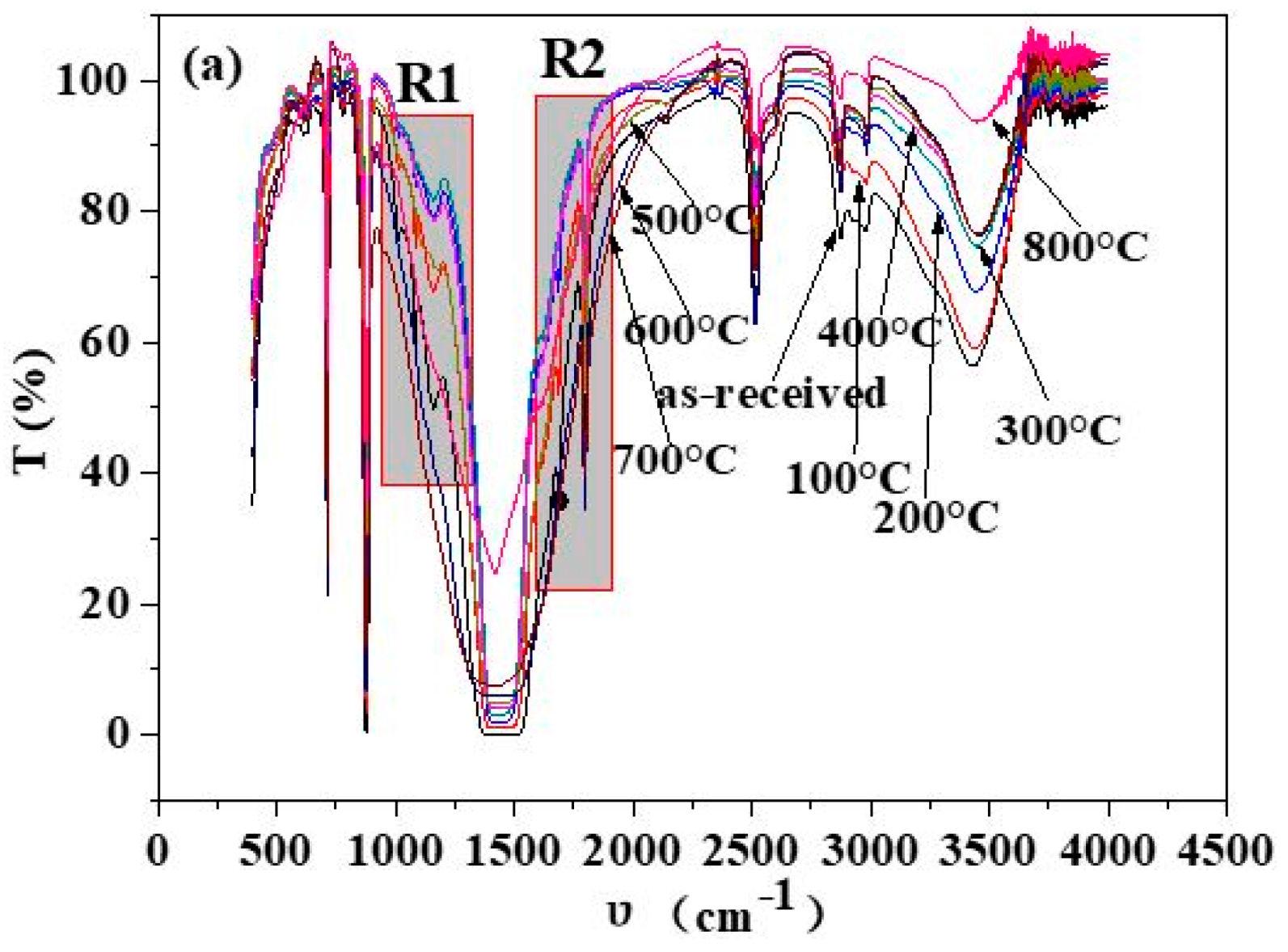
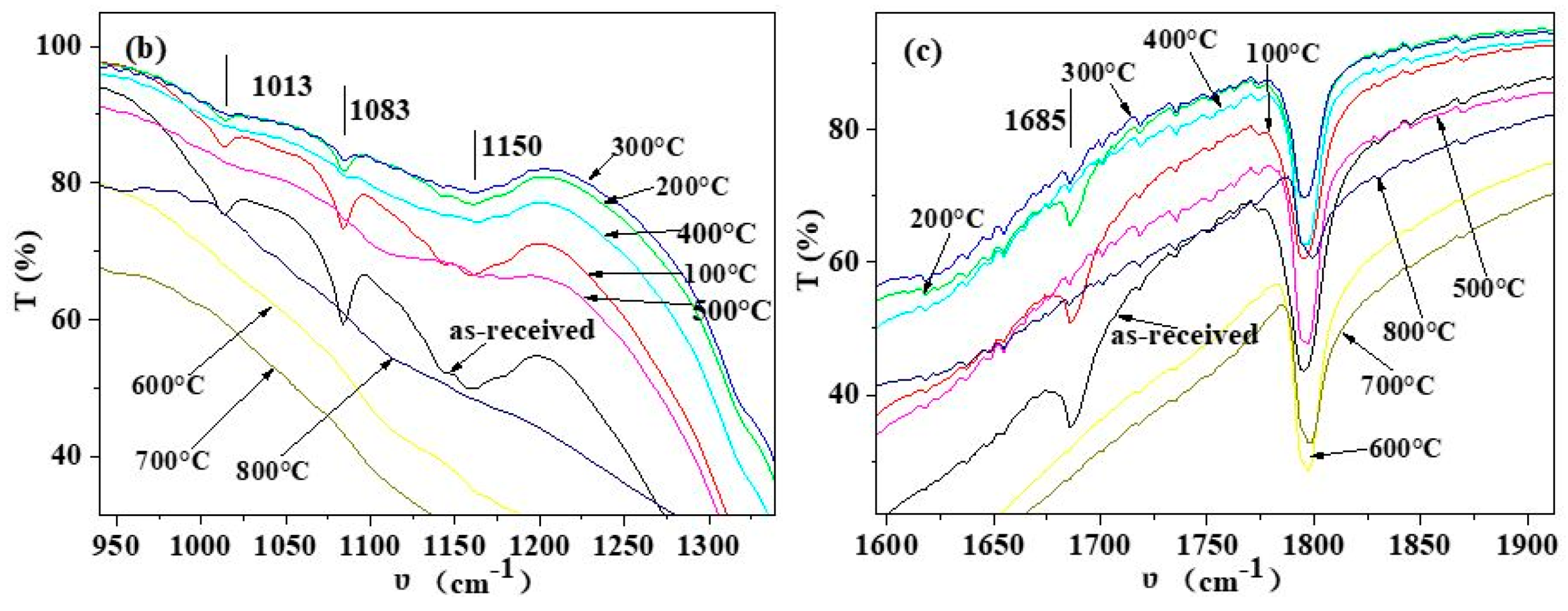
3.1. Composition of Shell Powders
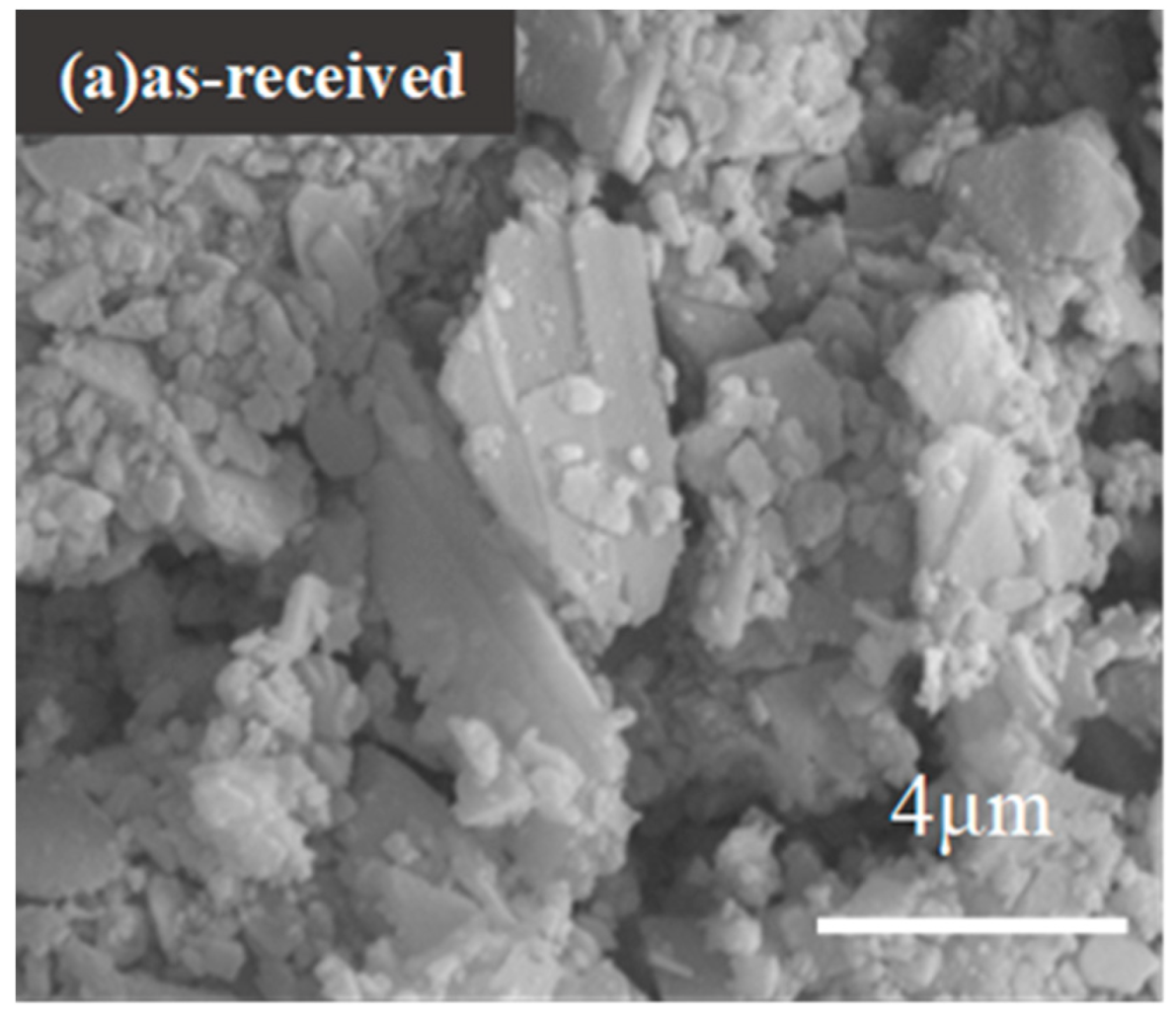
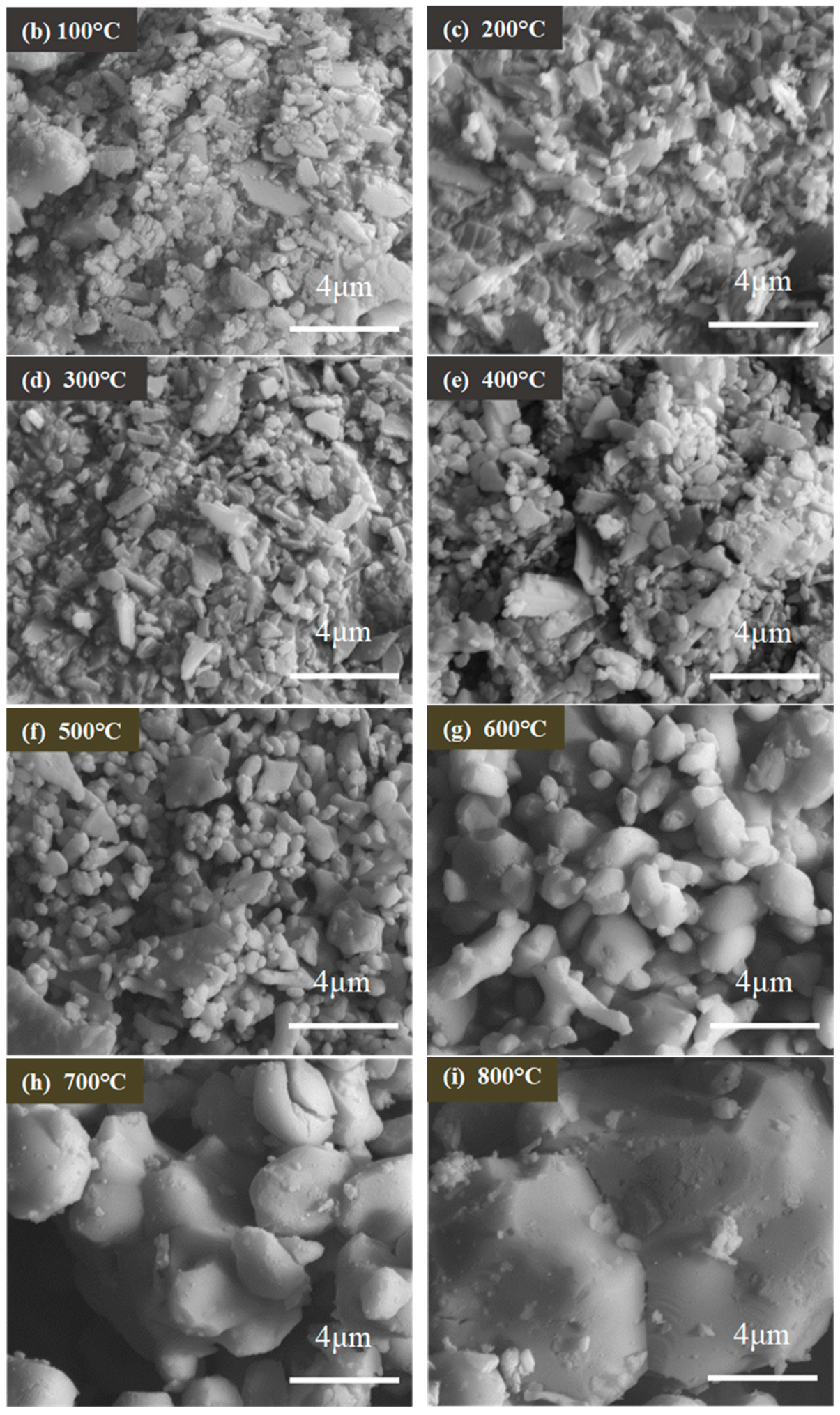
3.2. Morphologies of Shell Powders
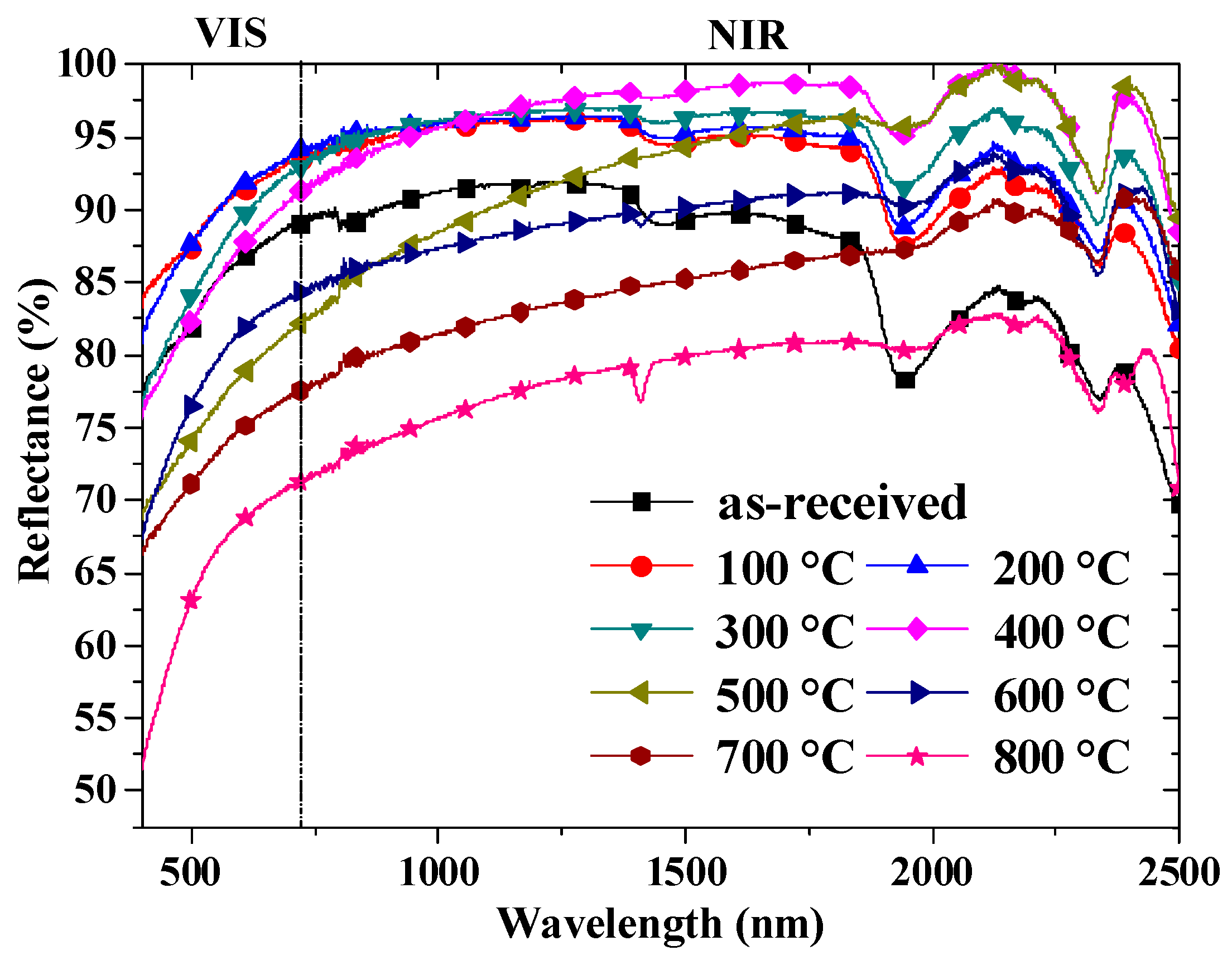
3.3. The Reflectance of the Shell Powders
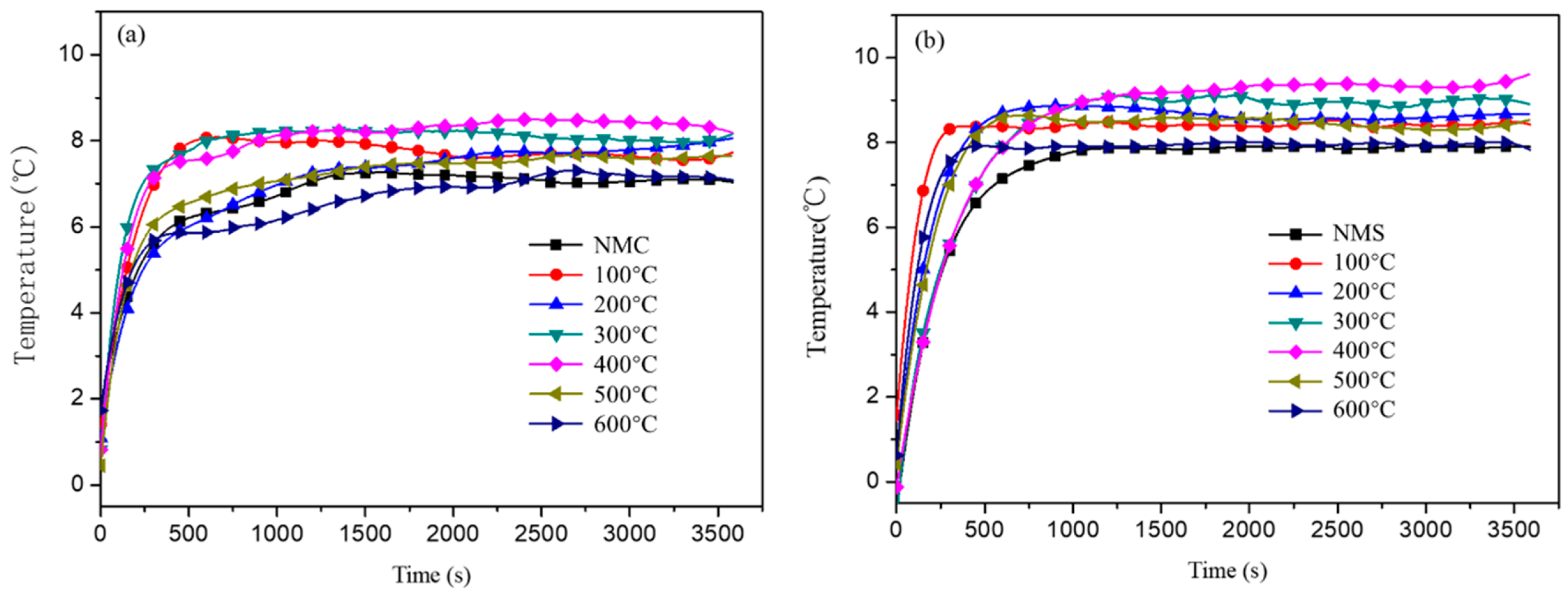
3.4. Thermal Performances of Coatings
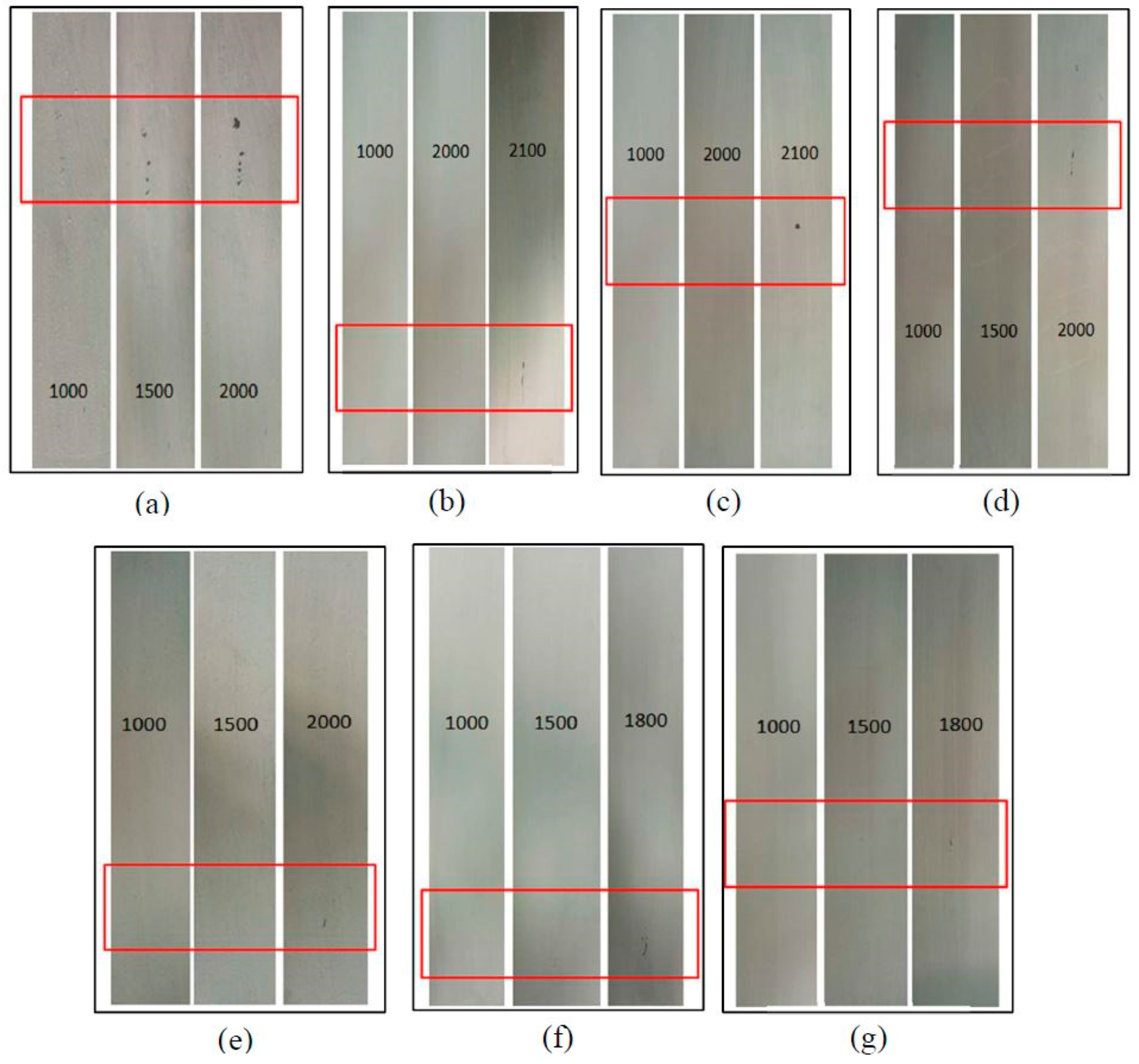
3.5. Scrub Resistance of the Coatings
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qu, J.; Song, J.R.; Qin, J.; Song, Z.N.; Zhang, W.D.; Shi, Y.X.; Zhang, T.; Zhang, H.Q.; Zhang, R.P.; He, Z.Y.; et al. Transparent thermal insulation coatings for energy efficient glass windows and curtain walls. Energy Build. 2014, 77, 1–10. [Google Scholar] [CrossRef]
- Judkoff, R. Increasing building energy efficiency through advances in materials. MRS Bull. 2008, 33, 449–454. [Google Scholar] [CrossRef]
- Shi, L.; Shuai, J.; Xu, K. Fuzzy fault tree assessment based on improved AHP for fire and explosion accidents for steel oil storage tanks. J. Hazard. Mater. 2014, 278, 529–538. [Google Scholar] [CrossRef]
- Shi, Y.X.; Song, Z.N.; Zhang, W.D.; Song, J.R.; Qu, J.; Wang, Z.D.; Li, Y.W.; Xu, L.J.; Lin, J. Physicochemical properties of dirt-resistant cool white coatings for building energy efficiency. Sol. Energy Mater. Sol. Cells 2013, 110, 133–139. [Google Scholar] [CrossRef]
- Brito, J.P.; Santos, T.V.O. Thermal analysis of roofs with thermal insulation layer and reflective coatings in subtropical and equatorial climate regions in Brazil. Energy Build. 2014, 84, 466–474. [Google Scholar] [CrossRef]
- Alexis, J.; Gaussens, C.; Etcheverry, B.; Bonino, J.-P. Development of nickel–phosphorus coatings containing micro particles of talc phyllosilicates. Mater. Chem. Phys. 2013, 137, 723–733. [Google Scholar] [CrossRef][Green Version]
- Katiyar, J.K.; Sinha, S.K.; Kumar, A. Friction and wear durability study of epoxy-based polymer (SU-8) composite coatings with talc and graphite as fillers. Wear 2016, 362–363, 199–208. [Google Scholar] [CrossRef]
- Liang, J.Z.; Li, F.H. Measurement of thermal conductivity of hollow glass-bead-filled polypropylene composites. Polym. Test. 2006, 25, 527–531. [Google Scholar] [CrossRef]
- Russa, M.F.L.; Rovella, N.; Buergo, M.A.D.; Belfiore, C.M.; Pezzino, A.; Crisci, G.M.; Ruffolo, S.A. Nano-TiO2 coatings for cultural heritage protection: The role of the binder on hydrophobic and self-cleaning efficacy. Prog. Org. Coat. 2016, 91, 1–8. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Tri, P.N.; Nguyen, T.D.; El Aidani, R.; Trinh, V.T.; Decker, C. Accelerated degradation of water borne acrylic nanocomposites used in outdoor protective coatings. Polym. Degrad. Stabil. 2016, 128, 65–76. [Google Scholar] [CrossRef]
- Taheran, M.; Navarchian, A.H.; Razavi, R.S. Optimization of wear resistance of PU/TiO coatings on aluminum surfaces. Prog. Org. Coat. 2011, 72, 486–491. [Google Scholar] [CrossRef]
- Dhoke, S.K.; Bhandari, R.; Khanna, A.S. Effect of nano-ZnO addition on the silicone-modified alkyd-based waterborne coatings on its mechanical and heat-resistance properties. Prog. Org. Coat. 2009, 64, 39–46. [Google Scholar] [CrossRef]
- Bozsaky, D. Thermodynamic tests with nano-ceramic thermal insulation coatings. Pollack Period. 2017, 12, 135–145. [Google Scholar] [CrossRef]
- Čekon, M. Spectral emissivity properties of reflective coatings. Slovak J. Civ. Eng. 2012, 20, 1–7. [Google Scholar] [CrossRef][Green Version]
- Cekon, M.; Kalousek, M.; Hraska, J.; Ingeli, R. Spectral optical properties and thermodynamic performance of reflective coatings in a mild climate zone. Energy Build. 2014, 77, 343–354. [Google Scholar] [CrossRef]
- Bozsaky, D. Recent studies on thermodynamic processes in nano-ceramic thermal insulation coatings. Pollack Period. 2019, 14, 107–116. [Google Scholar] [CrossRef]
- Atta, A.M.; Al-Lohedan, H.A.; Ezzat, A.O.; Al-Hussain, S.A. Characterization of superhydrophobic epoxy coatings embedded by modified calcium carbonate nanoparticles. Prog. Org. Coat. 2016, 101, 577–586. [Google Scholar] [CrossRef]
- He, H.W.; Li, K.X.; Wang, J.; Sun, G.H.; Li, Y.Q.; Wang, J.L. Study on thermal and mechanical properties of nano-calcium carbonate/epoxy composites. Mater. Des. 2011, 32, 4521–4527. [Google Scholar] [CrossRef]
- El-Sherbiny, S.; El-Sheikh, S.M.; Barhoum, A. Preparation and modification of nano calcium carbonate filler from waste marble dust and commercial limestone for papermaking wet end application. Powder Technol. 2015, 279, 290–300. [Google Scholar] [CrossRef]
- SHENTU, B.; Jipeng, L.; Zhixue, W. Effect of Oleic Acid-modified Nano-CaCO3 on the Crystallization Behavior and Mechanical Properties of Polypropylene. Chin. J. Chem. Eng. 2006, 14, 814–818. [Google Scholar] [CrossRef]
- Zhao, L.N.; Feng, J.D.; Wang, Z.C. In situ synthesis and modification of calcium carbonate nanoparticles via a bobbling method. Sci. China Ser. B 2009, 52, 924–929. [Google Scholar] [CrossRef]
- Tang, Z.F.; Cheng, G.J.; Chen, Y.S.; Yu, X.H.; Wang, H.L. Characteristics evaluation of calcium carbonate particles modified by surface functionalization. Adv. Powder Technol. 2014, 25, 1618–1623. [Google Scholar] [CrossRef]
- Xing, R.; Qin, Y.; Guan, X.; Song, L.; Yu, H.; Li, P. Comparison of antifungal activities of scallop shell, oyster shell and their pyrolyzed products. Egypt. J. Aquat. Res. 2013, 39, 83–90. [Google Scholar] [CrossRef]
- Luo, H.B.; Huang, G.; Fu, X.Y.; Liu, X.L.; Zheng, D.C.; Peng, J.; Zhang, K.; Huang, B.; Fan, L.Q.; Chen, F.H.; et al. Waste oyster shell as a kind of active filler to treat the combined wastewater at an estuary. J. Environ. Sci.-China 2013, 25, 2047–2055. [Google Scholar] [CrossRef]
- Moustafa, H.; Youssef, A.M.; Duquesne, S.; Darwish, N.A. Characterization of bio-filler derived from seashell wastes and its effect on the mechanical, thermal, and flame retardant properties of ABS composites. Polym. Compos. 2017, 38, 2788–2797. [Google Scholar] [CrossRef]
- Suteu, D.; Bilba, D.; Aflori, M.; Doroftei, F.; Lisa, G.; Badeanu, M.; Malutan, T. The Seashell Wastes as Biosorbent for Reactive Dye Removal from Textile Effluents. Clean-Soil Air Water 2012, 40, 198–205. [Google Scholar] [CrossRef]
- Rujitanapanich, S.; Kumpapan, P.; Wanjanoi, P. Synthesis of Hydroxyapatite from Oyster Shell via Precipitation. Energy Procedia 2014, 56, 112–117. [Google Scholar] [CrossRef]
- Santhosh, S.; Prabu, S.B. Thermal stability of nano hydroxyapatite synthesized from sea shells through wet chemical synthesis. Mater. Lett. 2013, 97, 121–124. [Google Scholar] [CrossRef]
- Rostami-Vartooni, A.; Nasrollahzadeh, M.; Alizadeh, M. Green synthesis of seashell supported silver nanoparticles using Bunium persicum seeds extract: Application of the particles for catalytic reduction of organic dyes. J. Colloid Interface Sci. 2016, 470, 268–275. [Google Scholar] [CrossRef]
- Zhang, P.G.; Tang, J.W.; Tang, Q.; Zhang, M.Z.; Shen, L.W.; Tian, W.B.; Zhang, Y.M.; Sun, Z.M. Shell powder as a novel bio-filler for thermal insulation coatings. Chin. J. Chem. Eng. 2019, 27, 452–458. [Google Scholar] [CrossRef]
- Tang, Q.; Zhang, Y.M.; Zhang, P.G.; Shi, J.J.; Tian, W.B.; Sun, Z.M. Preparation and properties of thermal insulation coatings with a sodium stearate-modified shell powder as a filler. Int. J. Miner. Metall. Mater. 2017, 24, 1192–1199. [Google Scholar] [CrossRef]
- Shen, L.W.; Zhang, Y.M.; Zhang, P.G.; Shi, J.J.; Sun, Z.M. Effect of TiO2 pigment gradation on the properties of thermal insulation coatings. Int. J. Miner. Metall. Mater. 2016, 23, 1466–1474. [Google Scholar] [CrossRef]
- Sophia, M.; Sakthieswaran, N. Waste shell powders as valuable bio-filler in gypsum plaster—Efficient waste management technique by effective utilization. J. Clean. Prod. 2019, 220, 74–86. [Google Scholar] [CrossRef]
- Seo, J.H.; Park, S.M.; Yang, B.J.; Jang, J.G. Calcined Oyster Shell Powder as an Expansive Additive in Cement Mortar. Materials 2019, 12, 1322. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.I.; Kim, M.Y.; Park, H.G.; Yi, S.T. Effect of partial replacement of sand with dry oyster shell on the long-term performance of concrete. Constr. Build. Mater. 2010, 24, 758–765. [Google Scholar] [CrossRef]
- Lertwattanaruk, P.; Makul, N.; Siripattarapravat, C. Utilization of ground waste seashells in cement mortars for masonry and plastering. J. Environ. Manag. 2012, 111, 133–141. [Google Scholar] [CrossRef]
- Dauphin, Y.; Guzman, N.; Denis, A.; Cuif, J.P.; Ortlieb, L. Microstructure, nanostructure and composition of the shell of Concholepas concholepas (Gastropoda, Muricidae). Aquat. Living Resour. 2003, 16, 95–103. [Google Scholar] [CrossRef]




| Components | Mass/g | Mass Ratio/% |
|---|---|---|
| Emulsion | 50.0 | 37.51 |
| Water | 30.0 | 22.51 |
| Calcined shell powder | 15.0 | 11.25 |
| Titanium dioxide | 20.0 | 15.00 |
| Hollow microspheres | 5.0 | 3.75 |
| Antifoaming agent | 4.8 | 3.60 |
| Film-forming agent | 2.5 | 1.88 |
| Dispersant | 6.0 | 4.50 |
| Temperature (°C) | Total Reflectance | Reflectance in VIS | Reflectance in NIR |
|---|---|---|---|
| Room temperature | 0.848 | 0.812 | 0.859 |
| 100 °C | 0.902 | 0.889 | 0.912 |
| 200 °C | 0.910 | 0.889 | 0.921 |
| 300 °C | 0.934 | 0.856 | 0.942 |
| 400 °C | 0.956 | 0.852 | 0.978 |
| 500 °C | 0.903 | 0.749 | 0.912 |
| 600 °C | 0.851 | 0.756 | 0.862 |
| 700 °C | 0.788 | 0.721 | 0.801 |
| 800 °C | 0.742 | 0.645 | 0.752 |
| Temperature (°C) | Total Reflectance | Reflectance in VIS | Reflectance in NIR |
|---|---|---|---|
| RT | 0.721 | 0.741 | 0.688 |
| 100 °C | 0.735 | 0.785 | 0.701 |
| 200 °C | 0.756 | 0.801 | 0.721 |
| 300 °C | 0.781 | 0.839 | 0.756 |
| 400 °C | 0.796 | 0.846 | 0.765 |
| 500 °C | 0.665 | 0.773 | 0.623 |
| 600 °C | 0.649 | 0.739 | 0.601 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Liu, Y.; Tang, Q.; Zhang, P.; Zhang, Y.; Sun, Z. Preparation and Properties of Wall Coatings with Calcined Shell Powder as Fillers. Materials 2019, 12, 2213. https://doi.org/10.3390/ma12142213
Chen C, Liu Y, Tang Q, Zhang P, Zhang Y, Sun Z. Preparation and Properties of Wall Coatings with Calcined Shell Powder as Fillers. Materials. 2019; 12(14):2213. https://doi.org/10.3390/ma12142213
Chicago/Turabian StyleChen, Chun, Yongchao Liu, Qiang Tang, Peigen Zhang, Yamei Zhang, and Zhengming Sun. 2019. "Preparation and Properties of Wall Coatings with Calcined Shell Powder as Fillers" Materials 12, no. 14: 2213. https://doi.org/10.3390/ma12142213
APA StyleChen, C., Liu, Y., Tang, Q., Zhang, P., Zhang, Y., & Sun, Z. (2019). Preparation and Properties of Wall Coatings with Calcined Shell Powder as Fillers. Materials, 12(14), 2213. https://doi.org/10.3390/ma12142213




