Refitting of Zirconia Toughening into Open-Cellular Alumina Foams by Infiltration with Zirconyl Nitrate
Abstract
:1. Introduction
2. Materials and Methods
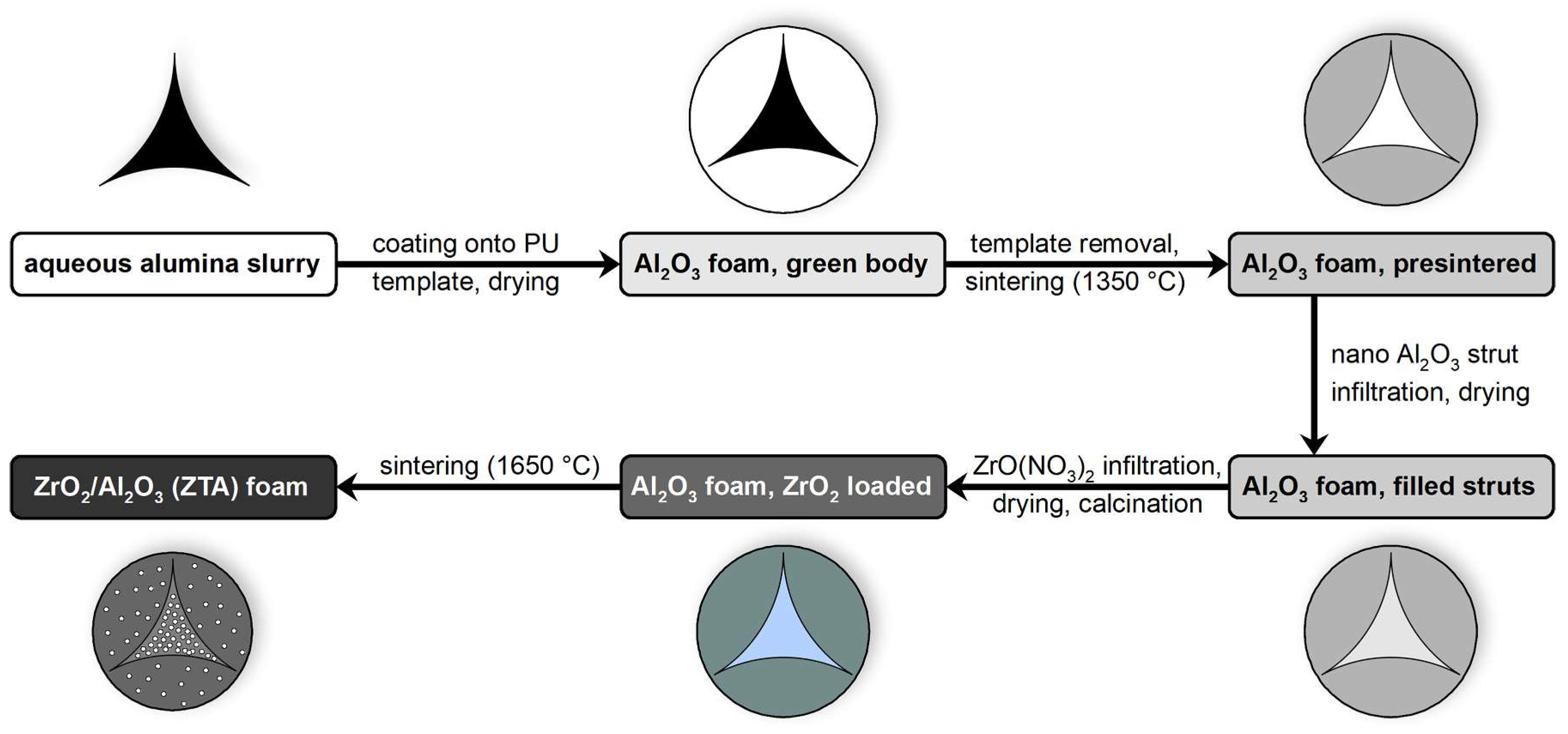
2.1. Sample Preparation
2.2. Characterization
3. Results and Discussion
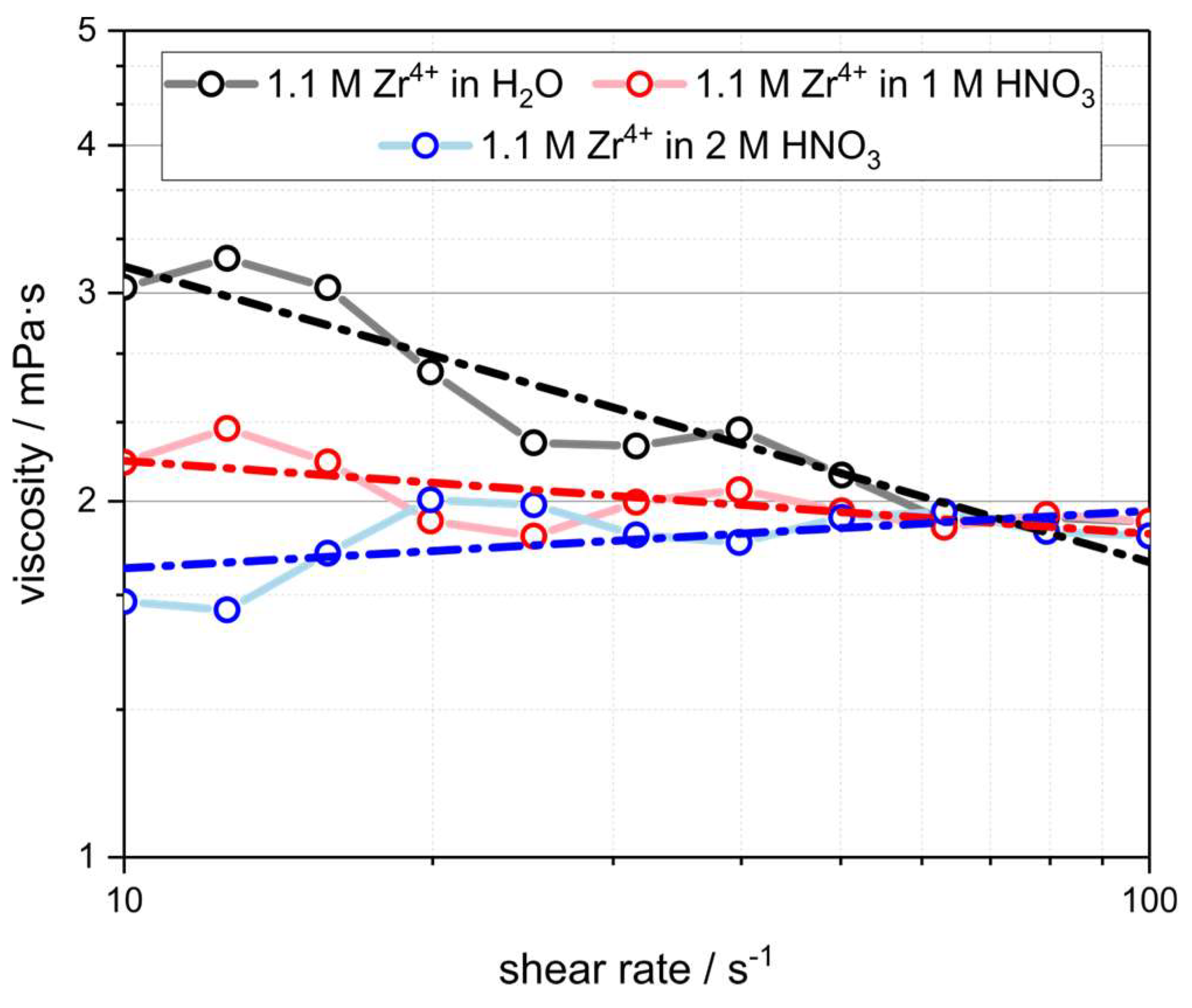
3.1. Starting Materials and Infiltration Fluids
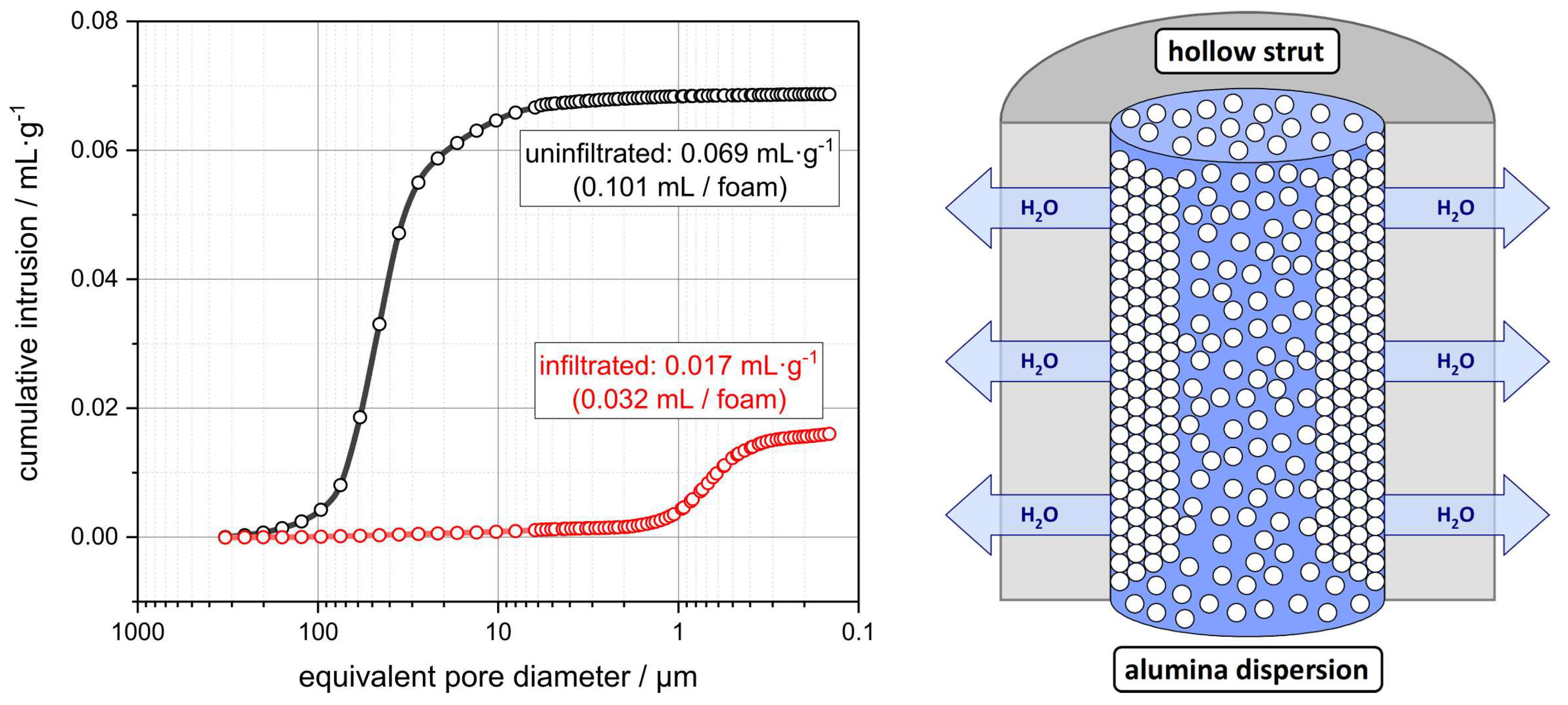
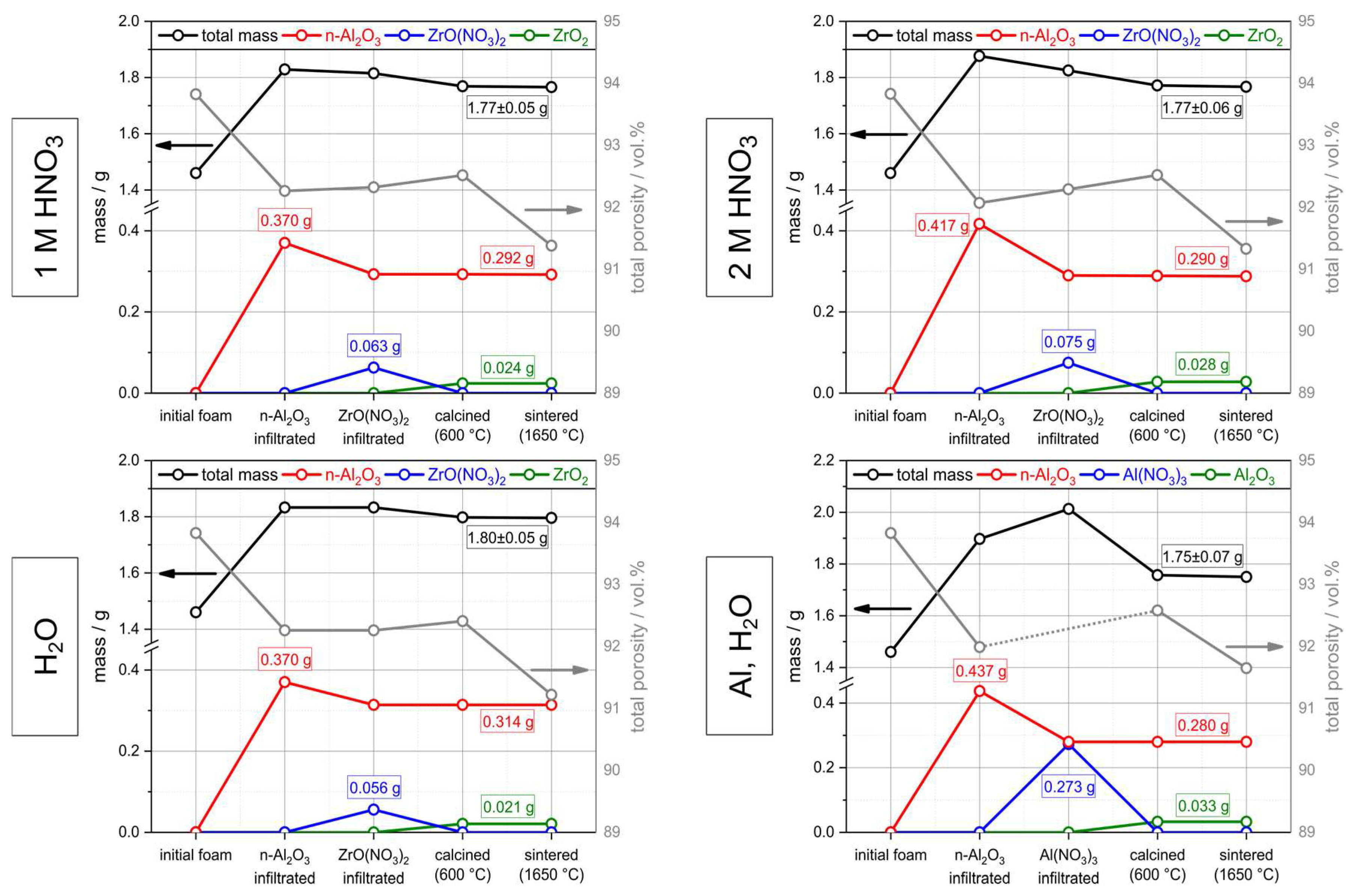
3.2. Hollow Strut Infiltration of Alumina Foams with Alumina and Zirconia
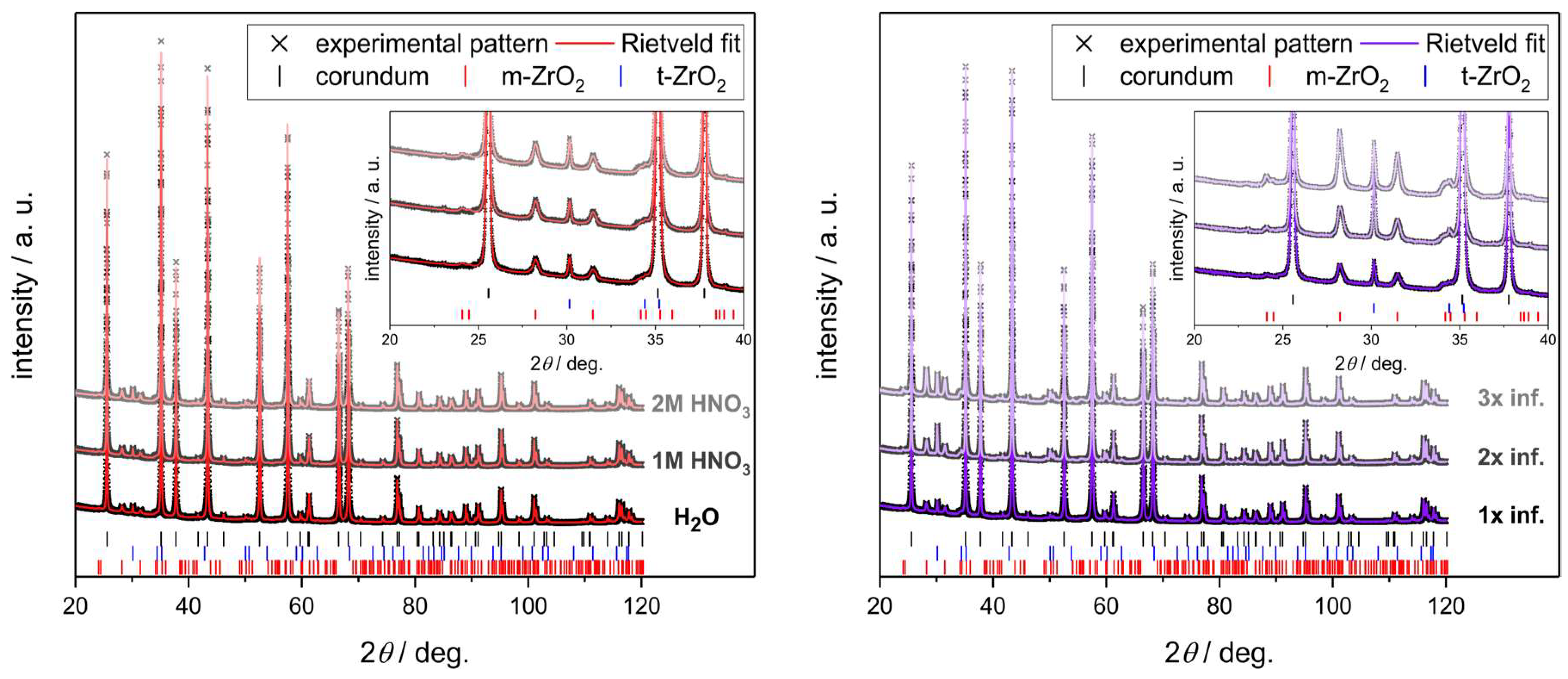
3.3. Strut Microstructure of Alumina Foams after Alumina and Zirconia Infiltration
3.4. Mechanical Behavior of Alumina Foams after Alumina and Zirconia Infiltration
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Twigg, M.V.; Richardson, J.T. Fundamentals and Applications of Structured Ceramic Foam Catalysts. Ind. Eng. Chem. Res. 2007, 46, 4166–4177. [Google Scholar] [CrossRef]
- Olson, R.A.; Martins, L.C.B. Cellular Ceramics in Metal Filtration. Adv. Eng. Mater. 2005, 7, 187–192. [Google Scholar] [CrossRef]
- Schwartzwalder, K.; Somers, H.; Somers, A.V. Method of Making Porous Ceramic Articles. U.S. Patent No. 3,090,094, 21 May 1963. [Google Scholar]
- Studart, A.R.; Gonzenbach, U.T.; Tervoort, E.; Gauckler, L.J. Processing Routes to Macroporous Ceramics: A Review. J. Am. Ceram. Soc. 2006, 89, 1771–1789. [Google Scholar] [CrossRef]
- Fey, T.; Betke, U.; Rannabauer, S.; Scheffler, M. Reticulated Replica Ceramic Foams: Processing, Functionalization, and Characterization. Adv. Eng. Mater. 2017, 19, 1700369. [Google Scholar] [CrossRef]
- Storti, E.; Farhani, M.; Aneziris, C.G.; Wöhrmeyer, C.; Parr, C. Calcium Aluminate Reactive Coatings on Carbon-Bonded Alumina Filters for Clean Steel Approaches. Steel Res. Int. 2017, 88, 1700247. [Google Scholar] [CrossRef]
- Betke, U.; Lieb, A. Micro-Macroporous Composite Materials—Preparation Techniques and Selected Applications: A Review. Adv. Eng. Mater. 2018, 20, 1800252. [Google Scholar] [CrossRef]
- Rugele, K.; Lehmhus, D.; Hussainova, I.; Peculevica, J.; Lisnanskis, M.; Shishkin, A. Effect of Fly-Ash Cenospheres on Properties of Clay-Ceramic Syntactic Foams. Materials 2017, 10, 828. [Google Scholar] [CrossRef]
- Bianchi, G.; Gianella, S.; Ortona, A. Design and Additive Manufacturing of Periodic Ceramic Architectures. J. Ceram. Sci. Technol. 2017, 8, 59–66. [Google Scholar] [CrossRef]
- Maurath, J.; Willenbacher, N. 3D printing of open-porous cellular ceramics with high specific strength. J. Eur. Ceram. Soc. 2017, 37, 4833–4842. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Li, J.; Liu, C.; Lao, C.; Fu, Y.; Liu, C.; Li, Y.; Wang, P.; He, Y. 3D printing of ceramics: A review. J. Eur. Ceram. Soc. 2019, 39, 661–687. [Google Scholar] [CrossRef]
- Lehmhus, D.; Vesenjak, M.; Schampheleire, S.; Fiedler, T. From Stochastic Foam to Designed Structure: Balancing Cost and Performance of Cellular Metals. Materials 2017, 10, 922. [Google Scholar] [CrossRef] [PubMed]
- Bhate, D. Four Questions in Cellular Material Design. Materials 2019, 12, 1060. [Google Scholar] [CrossRef] [PubMed]
- Rannabauer, S.; Söffker, G.-M.; Scheunemann, M.; Betke, U.; Scheffler, M. Increased Mechanical Stability and Thermal Conductivity of Alumina Reticulated Porous Ceramics (RPC) by Nanoparticle Infiltration Processing. Adv. Eng. Mater. 2017, 19, 201700211. [Google Scholar] [CrossRef]
- Betke, U.; Sharma, K.S.K.; Rodak, A.; Rannabauer, S.; Lieb, A.; Scheffler, F.; Scheffler, M. Manufacturing of an electrically conducting cellular Cu-SiC material by metal salt infiltration and chemical reduction (MESCAL). Mater. Lett. 2016, 185, 201–203. [Google Scholar] [CrossRef]
- Protective Coatings; Wen, M.; Dušek, K. (Eds.) Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Colombo, P. Conventional and novel processing methods for cellular ceramics. Philos. Trans. R. Soc. A 2006, 364, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Brezny, R.; Green, D.J. Factors Controlling the Fracture Resistance of Brittle Cellular Materials. J. Am. Ceram. Soc. 1991, 74, 1061–1065. [Google Scholar] [CrossRef]
- Brown, D.D.; Green, D.J. Investigation of Strut Crack Formation in Open Cell Alumina Ceramics. J. Am. Ceram. Soc. 1994, 77, 1467–1472. [Google Scholar] [CrossRef]
- Voigt, C.; Jäckel, E.; Aneziris, C.G.; Hubálková, J. Investigations of reticulated porous alumina foam ceramics based on different coating techniques with the aid of μCT and statistical characteristics. Ceram. Int. 2013, 39, 2415–2422. [Google Scholar] [CrossRef]
- Pu, X.; Liu, X.; Qiu, F.; Huang, L. Novel Method to Optimize the Structure of Reticulated Porous Ceramics. J. Am. Ceram. Soc. 2004, 87, 1392–1394. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, D.; Tan, S.; Zhang, Z. Improvement in the Strut Thickness of Reticulated Porous Ceramics. J. Am. Ceram. Soc. 2001, 84, 1654–1656. [Google Scholar] [CrossRef]
- Yao, X.; Tan, S.; Huang, Z.; Jiang, D. Effect of recoating slurry viscosity on the properties of reticulated porous silicon carbide ceramics. Ceram. Int. 2006, 32, 137–142. [Google Scholar] [CrossRef]
- Yao, X.; Yang, Y.; Liu, X.; Huang, Z. Effect of recoating slurry compositions on the microstructure and properties of SiC reticulated porous ceramics. J. Eur. Ceram. Soc. 2013, 33, 2909–2914. [Google Scholar] [CrossRef]
- Jun, I.-K.; Kong, Y.-M.; Lee, S.-H.; Kim, H.-E.; Kim, H.-W.; Goretta, K.C. Reinforcement of a Reticulated Porous Ceramic by a Novel Infiltration Technique. J. Am. Ceram. Soc. 2006, 89, 2317–2319. [Google Scholar] [CrossRef]
- Vogt, U.F.; Gorbar, M.; Dimopoulos-Eggenschwiler, P.; Broenstrup, A.; Wagner, G.; Colombo, P. Improving the properties of ceramic foams by a vacuum infiltration process. J. Eur. Ceram. Soc. 2010, 30, 3005–3011. [Google Scholar] [CrossRef]
- Liang, X.; Li, Y.; Liu, J.; Sang, S.; Chen, Y.; Li, B.; Aneziris, C.G. Fabrication of SiC reticulated porous ceramics with multi-layered struts for porous media combustion. Ceram. Int. 2016, 42, 13091–13097. [Google Scholar] [CrossRef]
- Chen, X.; Betke, U.; Rannabauer, S.; Peters, P.C.; Söffker, G.M.; Scheffler, M. Improving the Strength of ZTA Foams with Different Strategies: Immersion Infiltration and Recoating. Materials 2017, 10, 735. [Google Scholar] [CrossRef]
- Salvini, V.R.; Luchini, B.; Aneziris, C.G.; Pandolfelli, V.C. Innovation in ceramic foam filters manufacturing process. Int. J. Appl. Ceram. Technol. 2019, 16, 378–388. [Google Scholar] [CrossRef]
- Adler, J.; Teichgräber, M.; Standke, G. Offenzellige Schaumkeramik mit hoher Festigkeit und Verfahren zu deren Herstellung. DE 1996, 196, 638–639. [Google Scholar]
- Ortona, A.; Molinari, M.; Romelli, L. Open Cell Foam Material. European Patent 1382590, 30 June 2004. [Google Scholar]
- Gianella, S.; Gaia, D.; Ortona, A. High Temperature Applications of Si-SiC Cellular Ceramics. Adv. Eng. Mater. 2012, 14, 1074–1081. [Google Scholar] [CrossRef]
- Wang, J.; Stevens, R. Zirconia-toughened alumina (ZTA) ceramics. J. Mater. Sci. 1989, 24, 3421–3440. [Google Scholar] [CrossRef]
- Lange, F.F. Transformation toughening: Part 1 Size effects associated with the thermodynamics of constrained transformations. J. Mater. Sci. 1982, 17, 225–234. [Google Scholar] [CrossRef]
- Lange, F.F. Transformation toughening: Part 2 Contribution to fracture toughness. J. Mater. Sci. 1982, 17, 235–239. [Google Scholar] [CrossRef]
- Lange, F.F. Transformation toughening: Part 4 Fabrication, fracture toughness and strength of Al2O3-ZrO2 composites. J. Mater. Sci. 1982, 17, 247–254. [Google Scholar] [CrossRef]
- Ahmad, R.; Ha, J.-H.; Song, I.-H. Particle-stabilized ultra-low density zirconia toughened alumina foams. J. Eur. Ceram. Soc. 2013, 33, 2559–2564. [Google Scholar] [CrossRef]
- He, X.; Zhang, Y.Z.; Mansell, J.P.; Su, B. Zirconia toughened alumina ceramic foams for potential bone graft applications: Fabrication, bioactivation, and cellular responses. J. Mater. Sci. Mater. Med. 2008, 19, 2743–2749. [Google Scholar] [CrossRef] [PubMed]
- Betke, U.; Proemmel, S.; Rannabauer, S.; Lieb, A.; Scheffler, M.; Scheffler, F. Silane functionalized open-celled ceramic foams as support structure in metal organic framework composite materials. Microporous Mesoporous Mater. 2017, 239, 209–220. [Google Scholar] [CrossRef]
- Betke, U.; Dalicho, S.; Rannabauer, S.; Lieb, A.; Scheffler, F.; Scheffler, M. Impact of Slurry Composition on Properties of Cellular Alumina: A Computed Tomographic Study. Adv. Eng. Mater. 2017, 19, 1700138. [Google Scholar] [CrossRef]
- Franco, A., Jr.; Roberts, S.G. Surface mechanical analyses by Hertzian indentation. Cerâmica 2004, 50, 94–106. [Google Scholar] [CrossRef]
- DIN EN 623-2:1993-11. Hochleistungskeramik; Monolithische Keramik; Allgemeine und strukturelle Eigenschaften; Teil 2: Bestimmung von Dichte und Porosität; Beuth Verlag: Berlin, Germany, 1993. [Google Scholar]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Coelho, A.A. Topas Academic V5; Coelho Software: Brisbane, Australia, 2012. [Google Scholar]
- Weibull, W. A Statistical Distribution Function of Wide Applicability. J. Appl. Mech. 1951, 20, 293–297. [Google Scholar]
- Ronninger, C. Visual-XSel 14; CRGRAPH: Starnberg, Germany, 2018. [Google Scholar]
- Betke, U.; Klaus, M.; Eggebrecht, J.G.; Scheffler, M.; Lieb, A. MOFs meet macropores: Dynamic direct crystallization of the microporous aluminum isophthalate CAU-10 on reticulated open-cellular alumina foams. Microporous Mesoporous Mater. 2018, 265, 43–56. [Google Scholar] [CrossRef]
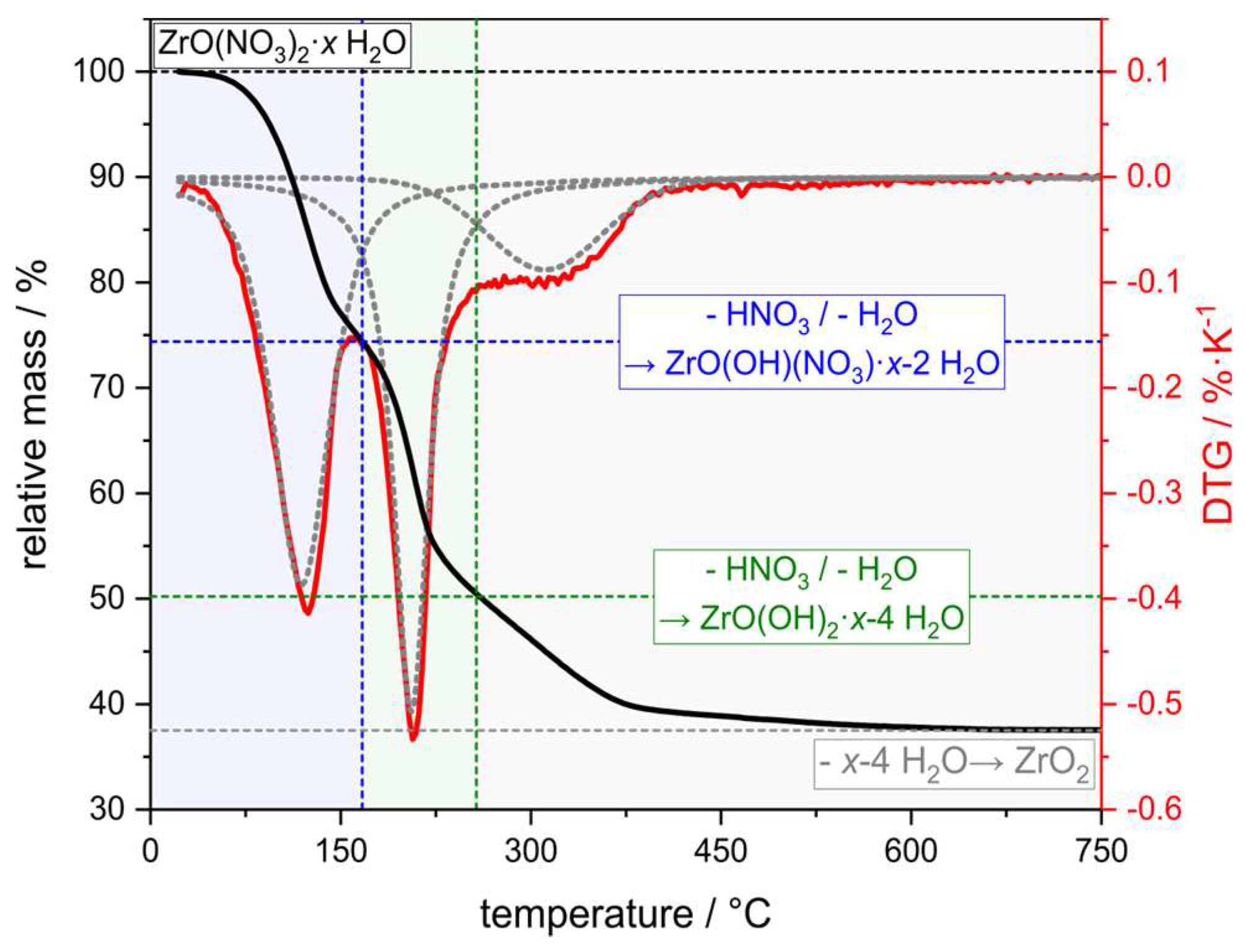
- Campbell, P.F.; Ortner, M.H.; Anderson, C.J. Differential Thermal Analysis and Thermogravimetric Analysis of Fission Product Oxides and Nitrates to 1500 °C. Anal. Chem. 1961, 33, 58–61. [Google Scholar] [CrossRef]
- Feichtenschlager, B.; Lomoschitz, C.J.; Kickelbick, G. Tuning the self-assembled monolayer formation on nanoparticle surfaces with different curvatures: Investigations on spherical silica particles and plane-crystal-shaped zirconia particles. J. Colloid Interface Sci. 2011, 360, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
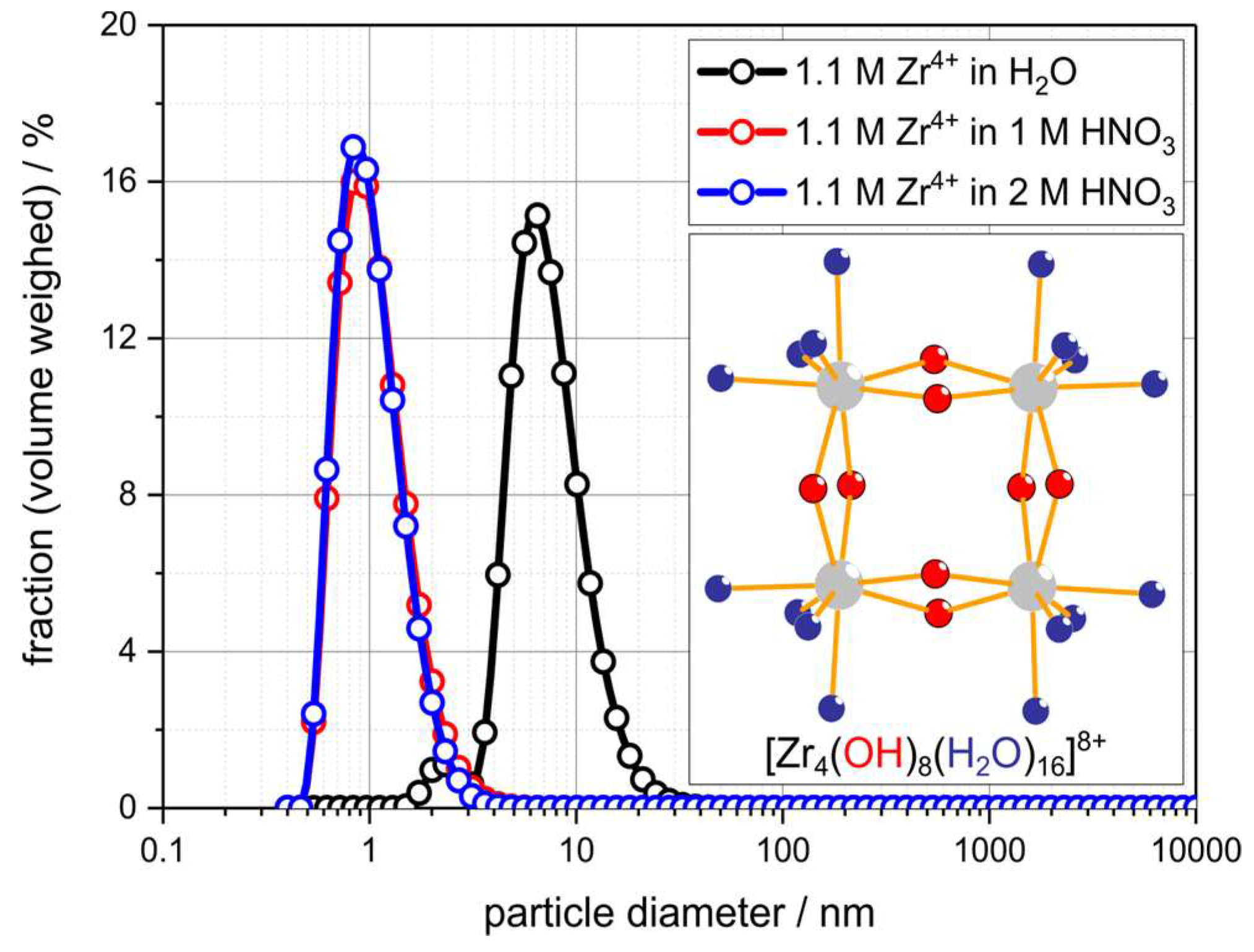
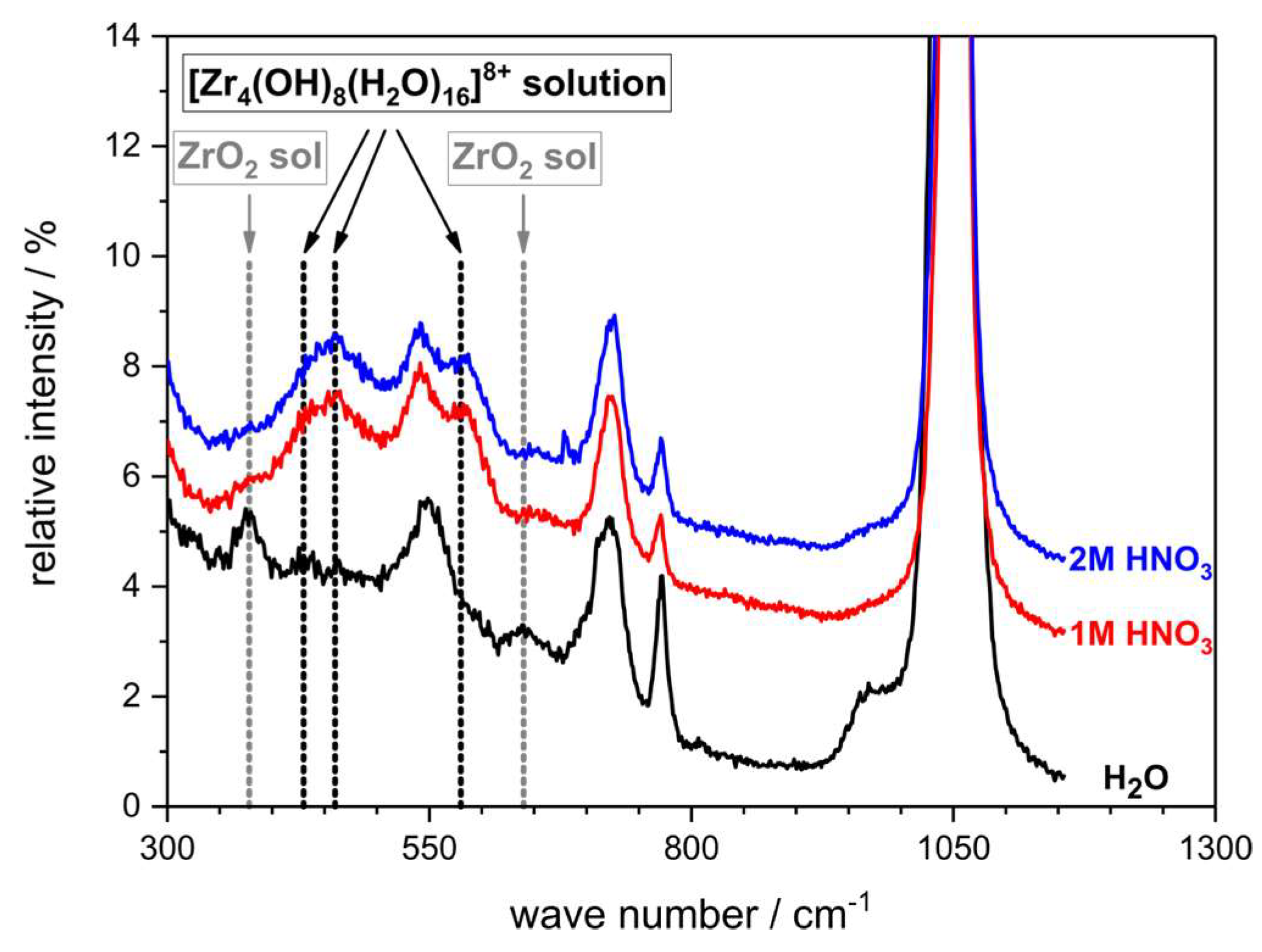
- Singhal, A.; Toth, L.M.; Lin, J.S.; Affholter, K. Zirconium(IV) Tetramer/Octamer Hydrolysis Equilibrium in Aqueous Hydrochloric Acid Solution. J. Am. Chem. Soc. 1996, 118, 11529–11534. [Google Scholar] [CrossRef]
- Southon, P.D.; Bartlett, J.R.; Woolfrey, J.L.; Ben-Nissan, B. Formation and Characterization of an Aqueous Zirconium Hydroxide Colloid. Chem. Mater. 2002, 14, 4313–4319. [Google Scholar] [CrossRef]
- Ostwald, W. Über die Geschwindigkeitsfunktion der Viskosität disperser Systeme. I. Kolloid-Z. 1925, 36, 99–117. [Google Scholar] [CrossRef]
- Giesche, H. Mercury Porosimetry: A General (Practical) Overview. Part. Part. Syst. Charact. 2006, 23, 9–19. [Google Scholar] [CrossRef]
- Betke, U.; Lieb, A.; Scheffler, F.; Scheffler, M. Manufacturing of Reticulated Open-Cellular Aluminum Nitride Ceramic Foams from Aqueous AlN Suspensions. Adv. Eng. Mater. 2017, 19, 1600660. [Google Scholar] [CrossRef]
- Ostrowski, T.; Rödel, J. Evolution of Mechanical Properties of Porous Alumina during Free Sintering and Hot Pressing. J. Am. Ceram. Soc. 1999, 82, 3080–3086. [Google Scholar] [CrossRef]
- Maizza, G.; Grasso, S.; Sakka, Y. Systematic study on densification of alumina fine powder during milliwave sintering at 28 GHz. J. Asian Ceram. Soc. 2014, 2, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Ripperger, S.; Gösele, W.; Alt, C.; Loewe, T. Filtration, 1. Fundamentals. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 1–38. [Google Scholar]
- Salmang, H.; Scholze, H.; Telle, R. Keramik, 7th ed.; Springer: Berlin, Germany; New York, NY, USA, 2007. [Google Scholar]
- Ashby, M.F.; Mehl-Medalist, R.F. The mechanical properties of cellular solids. Metall. Trans. A 1983, 14, 1755–1769. [Google Scholar] [CrossRef]
- Oliveira, F.A.C.; Dias, S.; Vaz, M.F.; Fernandes, J.C. Behaviour of open-cell cordierite foams under compression. J. Eur. Ceram. Soc. 2006, 26, 179–186. [Google Scholar] [CrossRef]
- De Sousa, E.; Rambo, C.R.; Hotza, D.; Novaes de Oliveira, A.P.; Fey, T.; Greil, P. Microstructure and properties of LZSA glass-ceramic foams. Mater. Sci. Eng. A 2008, 476, 89–97. [Google Scholar] [CrossRef]
- Colombo, P.; Hellmann, J.R.; Shelleman, D.L. Mechanical Properties of Silicon Oxycarbide Ceramic Foams. J. Am. Ceram. Soc. 2001, 84, 2245–2251. [Google Scholar] [CrossRef]
- Chaim, R. Pressureless sintered ATZ and ZTA ceramic composites. J. Mater. Sci. 1992, 27, 5597–5602. [Google Scholar] [CrossRef]
- Abbas, S.; Maleksaeedi, S.; Kolos, E.; Ruys, A.J. Processing and Properties of Zirconia-Toughened Alumina Prepared by Gelcasting. Materials 2015, 8, 4344–4362. [Google Scholar] [CrossRef] [Green Version]
- Lanin, A.G.; Muravin, E.L.; Popov, V.P.; Turchin, V.N. Thermal shock resistance and thermal-mechanical processing of sapphire. J. Eur. Ceram. Soc. 2003, 23, 455–468. [Google Scholar] [CrossRef]
- Curtis, C.E.; Doney, L.M.; Johnson, J.R. Some Properties of Hafnium Oxide, Hafnium Silicate, Calcium Hafnate, and Hafnium Carbide. J. Am. Ceram. Soc. 1954, 37, 458–465. [Google Scholar] [CrossRef]
- Zierath, B.; Greil, P.; Stumpf, M.; Fey, T. Enforcing of Mechanical Properties of Alumina Foams. In Ceramic Engineering and Science Proceedings Vol. 37, Issue 4: Advances in Ceramic Armor, Bioceramics, and Porous Materials; LaSalvia, J.C., Narayan, R., Colombo, P., Fukushima, M., Gyekenyesi, A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 149–162. [Google Scholar]
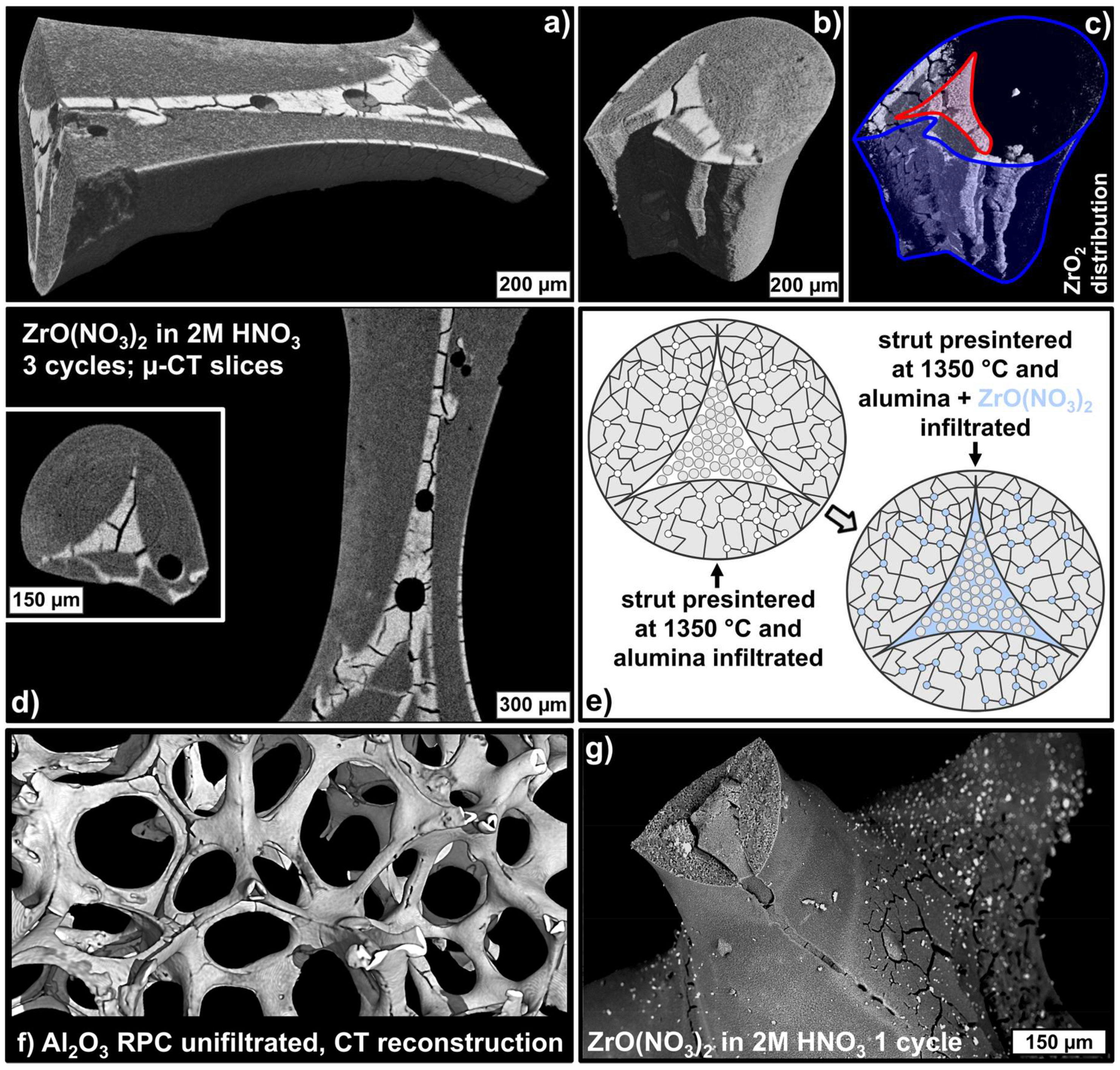
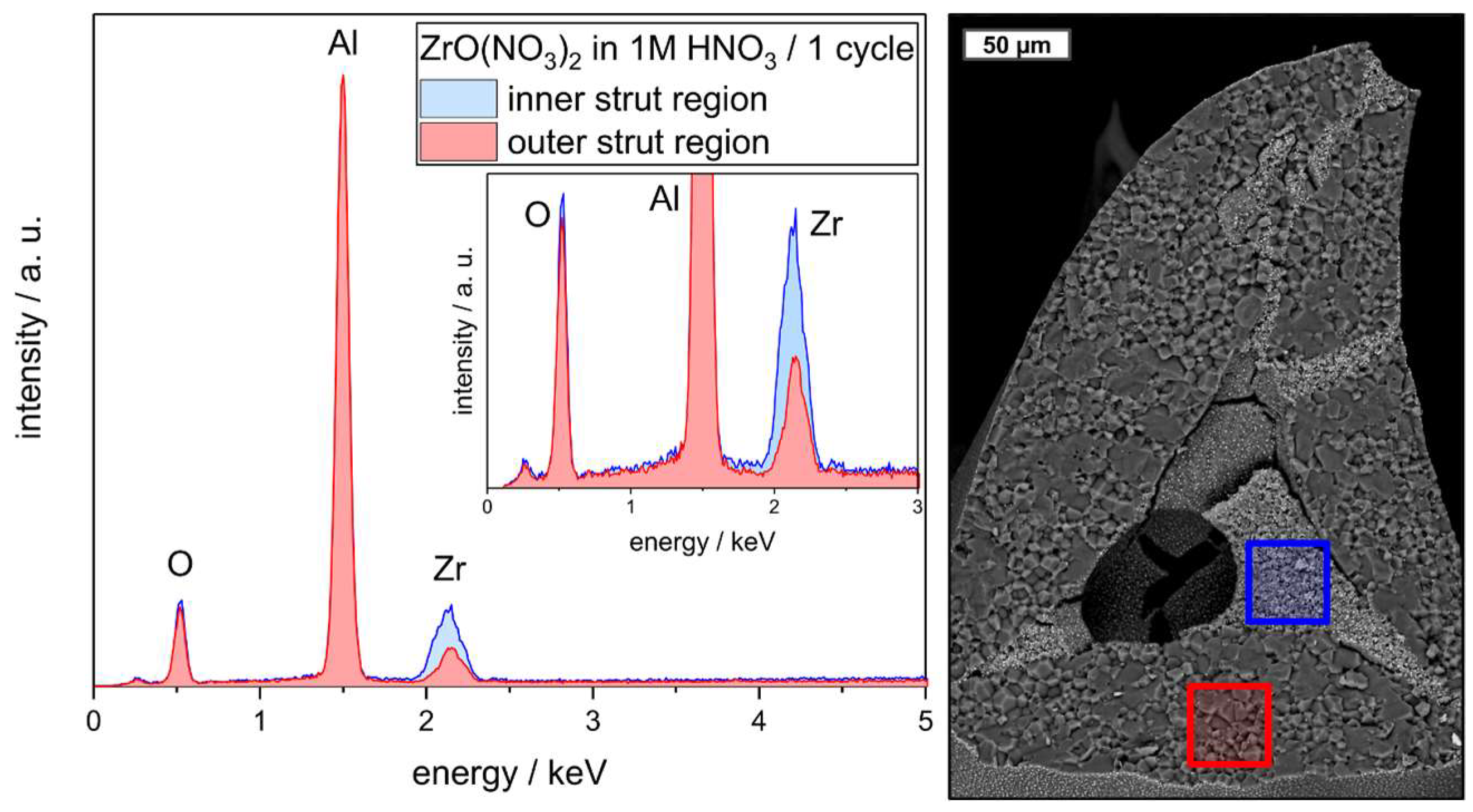
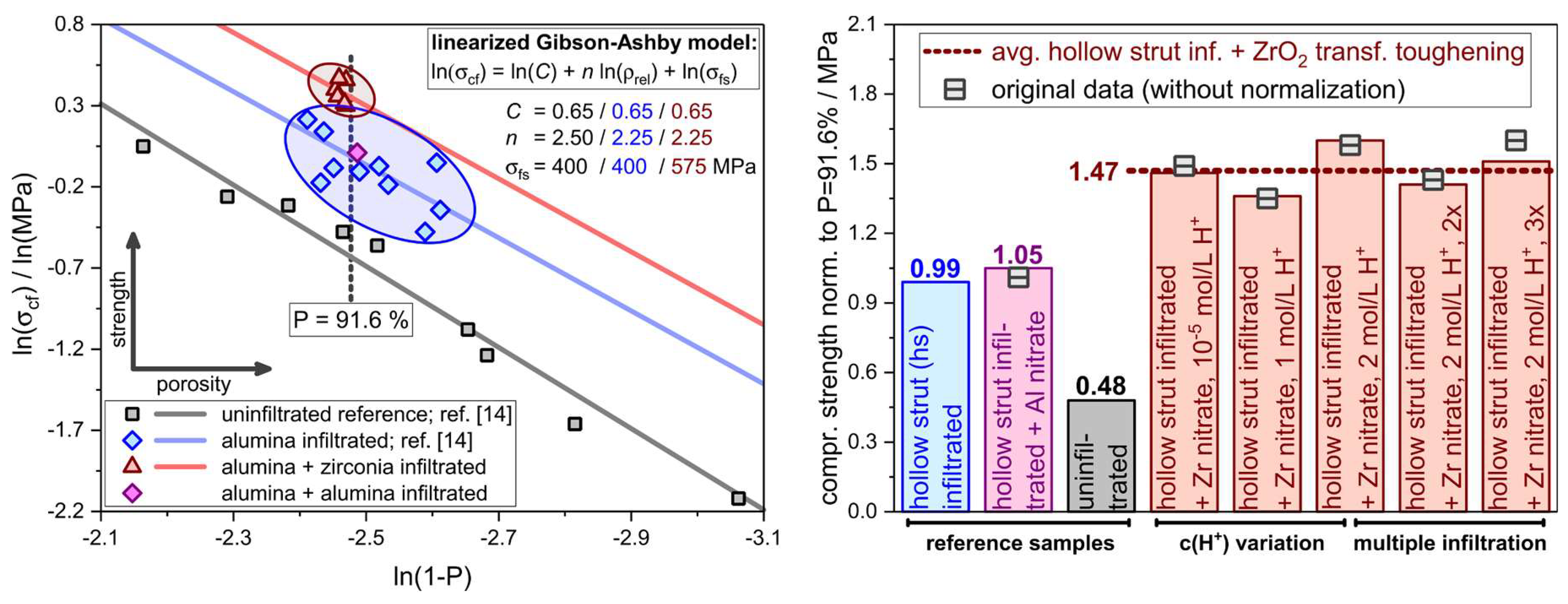
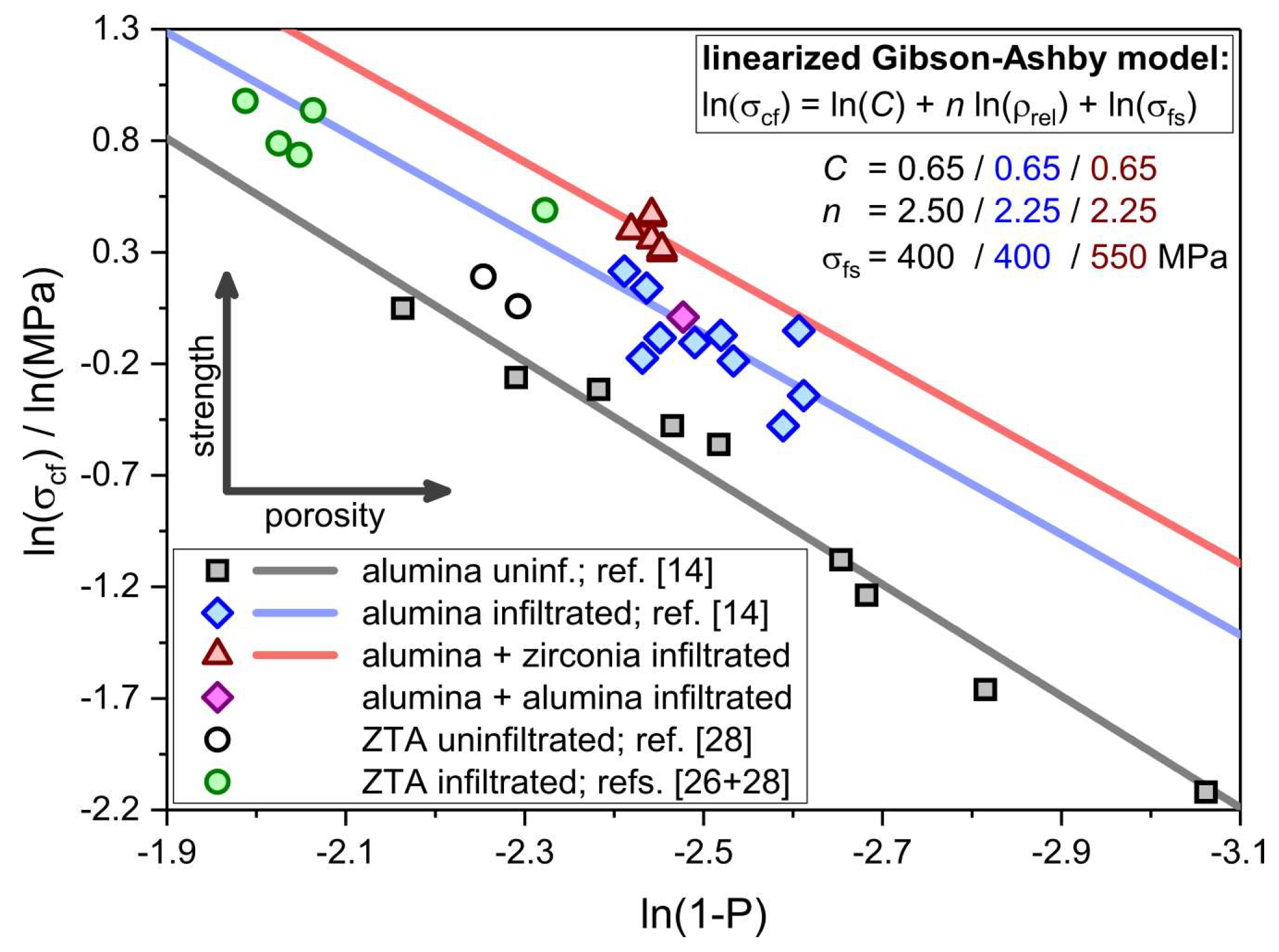
| Component | Material | Dispersion Foam Manufacturing | Dispersion Strut Infiltration |
|---|---|---|---|
| Alumina powder | CT 3000 SG, d50 = 0.5 µm a | 78.3 wt.% | -/- |
| TM-DAR, d50 = 0.1 µm a | -/- | 63.8 wt.% | |
| Dispersant | demineralized water | 19.6 wt.% | 35.5 wt.% |
| Deflocculant | ethanolammonium citrate (Dolapix CE64) | 0.8 wt.% | 0.6 wt.% |
| Binder | polyvinylalcohol (Optapix PA 4G) | 1.2 wt.% | -/- |
| Defoamer | polyalkylene glycolether (Contraspum K1012) | 0.1 wt.% | 0.1 wt.% |
| Fluid | Solvent/wt.% | Metal Salt/wt.% | Density/g∙cm−3 | c(Mn+)/mol∙L−1 | OxideContent /g∙L−1 | d50,3 a /nm | K b /mPa∙s |
|---|---|---|---|---|---|---|---|
| TM-DAR H2O | 36 | 64 | -/- | -/- | 638 | 236 ± 88 | 14.0 ± 1.0 |
| ZrO(NO3)2 H2O | 70 | 30 | 1.19 ± 0.02 | 1.09 ± 0.02 | 134.0 ± 1.6 | 7.8 ± 3.6 | 5.6 ± 0.5 |
| ZrO(NO3)2 1M HNO3 | 70 | 30 | 1.23 ± 0.02 | 1.13 ± 0.02 | 138.8 ± 1.8 | 1.1 ± 0.5 | 2.5 ± 0.2 |
| ZrO(NO3)2 2M HNO3 | 70 | 30 | 1.26 ± 0.02 | 1.15 ± 0.02 | 141.2 ± 1.7 | 1.1 ± 0.4 | 1.6 ± 0.1 |
| Al(NO3)3 H2O | 25 | 75 | 1.43 ± 0.02 | 1.91 ± 0.03 | 97.2 ± 2.4 | 1.1 ± 0.3 | 18.2 ± 0.2 |
| Processing Level | Geometric Porosity a/% | Strut Thickness (CT) b/mm | Cell Size (CT) b/mm |
|---|---|---|---|
| initial foam (1350 °C) | 93.8 ± 0.2 | 0.43 ± 0.17 | 2.6 ± 0.2 |
| alumina-infiltrated | 92.1 ± 0.2 | 0.51 ± 0.13 | 2.6 ± 0.2 |
| alumina and zirconia infiltrated | 92.5 ± 0.2 | 0.45 ± 0.14 | 2.5 ± 0.2 |
| sintered foam (1650 °C) | 91.5 ± 0.2 | 0.42 ± 0.14 | 2.4 ± 0.2 |
| Sample | Infiltration | Initial Porosity a/% | Final Porosity b/% | ZrO2/wt.% (m:t) | σcf/MPa | σcf,min/MPa |
|---|---|---|---|---|---|---|
| Zr_H2O | Al2O3/ZrO(NO3)2 in demin. H2O | 93.8 ± 0.2 | 91.4 ± 0.3 | 1.2 (79:21) | 1.49 ± 0.36 | 0.79 |
| Zr_1M_HNO3 | Al2O3/ZrO(NO3)2 in 1M HNO3 | 93.8 ± 0.2 | 91.5 ± 0.4 | 1.4 (80:20) | 1.35 ± 0.21 | 0.97 |
| Zr_2M_HNO3 | Al2O3/ZrO(NO3)2 in 2M HNO3 | 93.8 ± 0.2 | 91.5 ± 0.4 | 1.6 (79:21) | 1.58 ± 0.24 | 1.14 |
| Zr_2M_HNO3_2x | Al2O3/ZrO(NO3)2 in 2M HNO3 (2x) | 93.8 ± 0.2 | 91.4 ± 0.4 | 3.3 (71:29) | 1.43 ± 0.29 | 0.96 |
| Zr_2M_HNO3_3x | Al2O3/ZrO(NO3)2 in 2M HNO3 (3x) | 94.0 ± 0.1 | 91.5 ± 0.3 | 4.9 (84:16) | 1.60 ± 0.07 | 1.45 |
| Zr_Y_2M_HNO3 | Al2O3/ZrO(NO3)2 + Y in 2M HNO3 | 93.7 ± 0.1 | 91.5 ± 0.4 | 1.4 (13:87) | 1.37 ± 0.26 | 0.87 |
| Al_H2O_ref. | Al2O3/Al(NO3)3 in H2O | 93.8 ± 0.2 | 91.7 ± 0.5 | -/- | 1.01 ± 0.28 | 0.43 |
| uninf._ref. c | -/- | -/- | 91.8 ± 0.3 | -/- | 0.56 ± 0.21 | 0.24 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betke, U.; Scheunemann, M.; Scheffler, M. Refitting of Zirconia Toughening into Open-Cellular Alumina Foams by Infiltration with Zirconyl Nitrate. Materials 2019, 12, 1886. https://doi.org/10.3390/ma12121886
Betke U, Scheunemann M, Scheffler M. Refitting of Zirconia Toughening into Open-Cellular Alumina Foams by Infiltration with Zirconyl Nitrate. Materials. 2019; 12(12):1886. https://doi.org/10.3390/ma12121886
Chicago/Turabian StyleBetke, Ulf, Marcel Scheunemann, and Michael Scheffler. 2019. "Refitting of Zirconia Toughening into Open-Cellular Alumina Foams by Infiltration with Zirconyl Nitrate" Materials 12, no. 12: 1886. https://doi.org/10.3390/ma12121886







