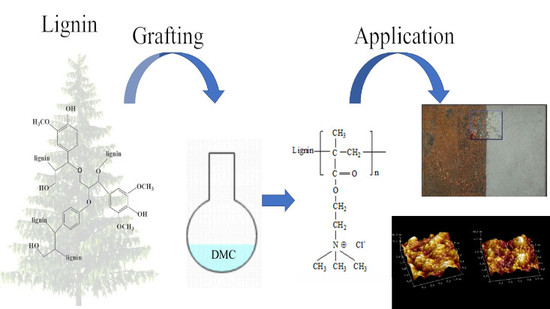
Construction of a Novel Lignin-Based Quaternary Ammonium Material with Excellent Corrosion Resistant Behavior and Its Application for Corrosion Protection
Abstract
1. Introduction
2. Materials and Experimental Method
2.1. Steel Specimen Preparation
2.2. Surface Analysis
2.3. Weight Loss Method
2.4. Electrochemical Method
3. Results and Discussion
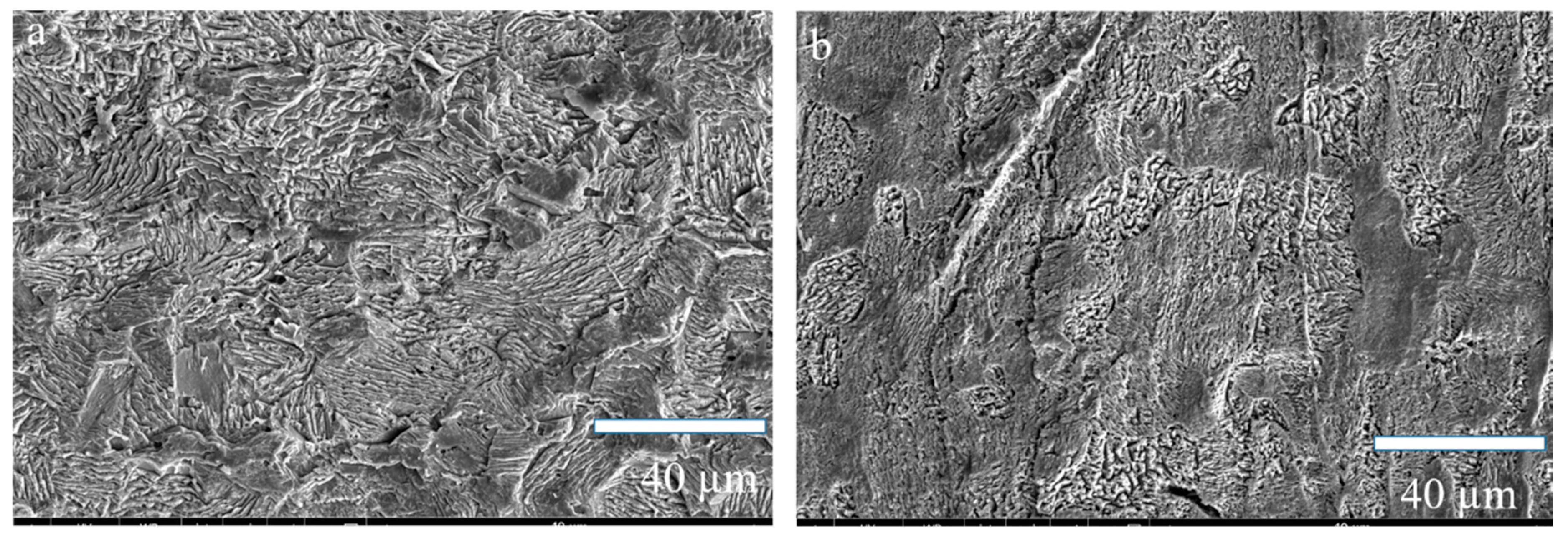
3.1. Surface Analysis
3.2. Weight Loss Experiment
3.3. Electrochemical Measurements
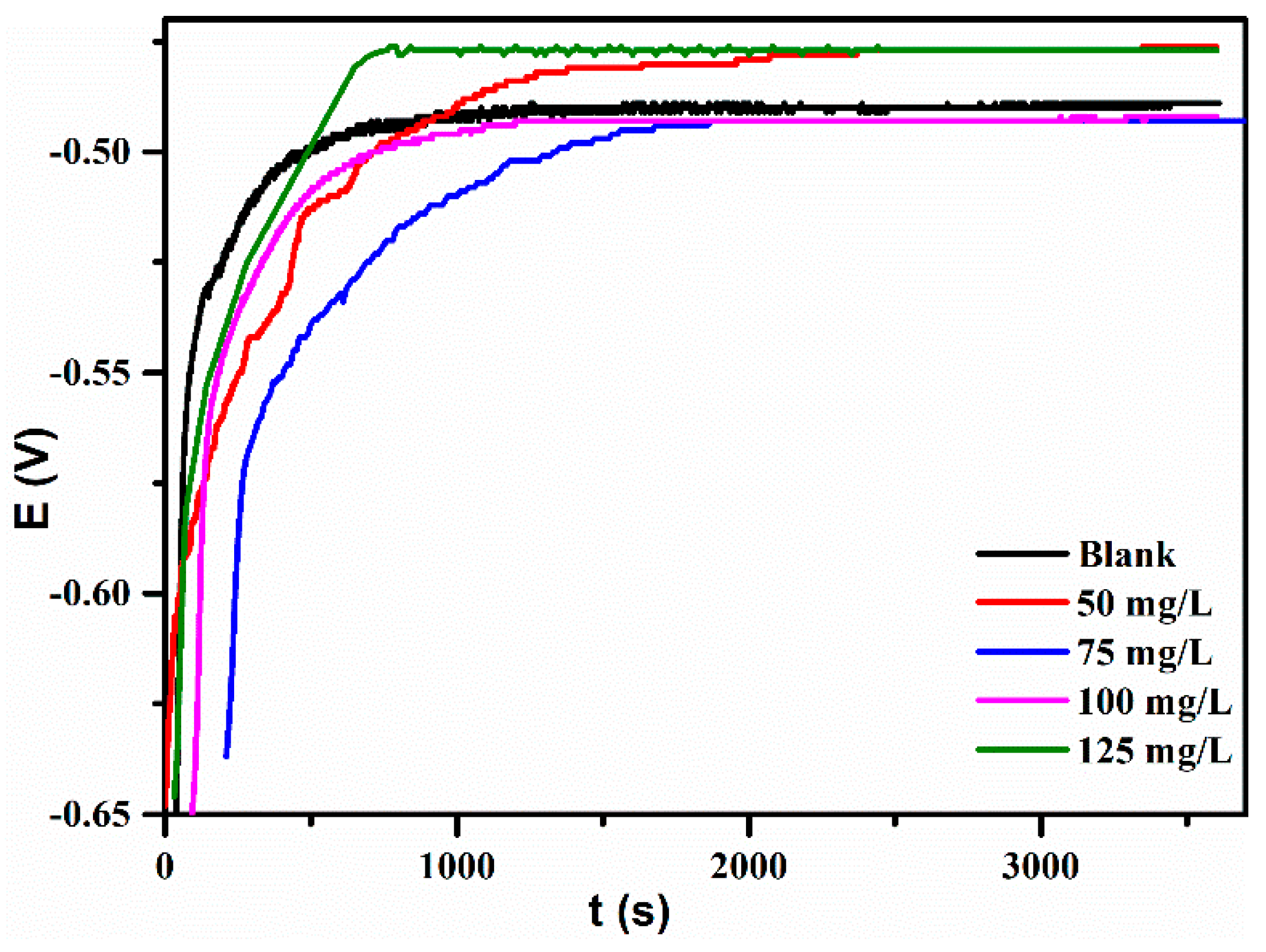
3.3.1. Open Circuit Potential
3.3.2. Polarization Studies
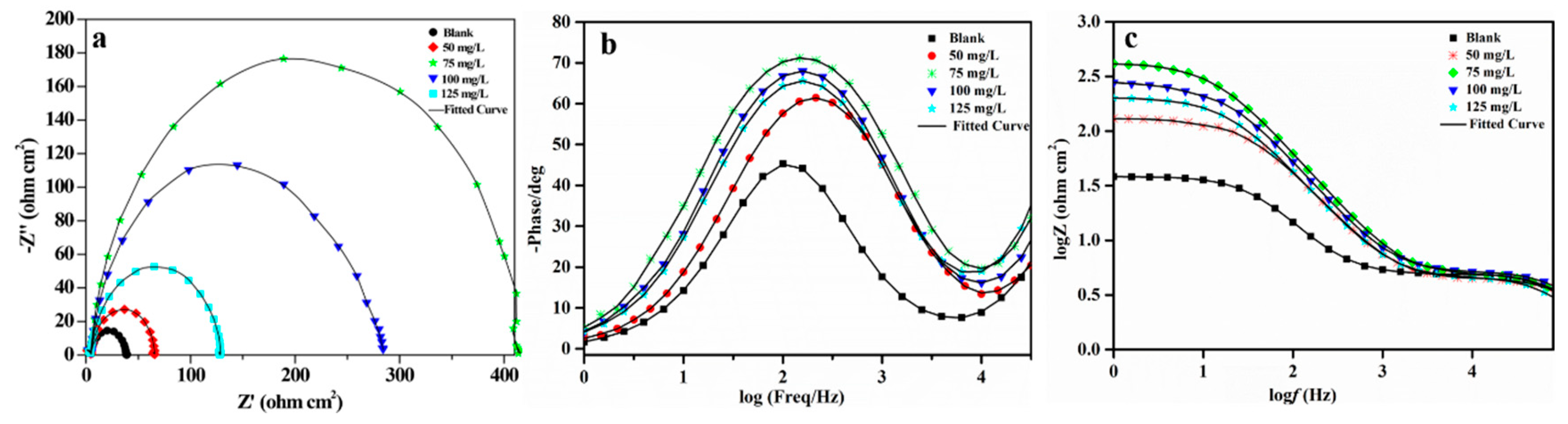
3.3.3. EIS Studies
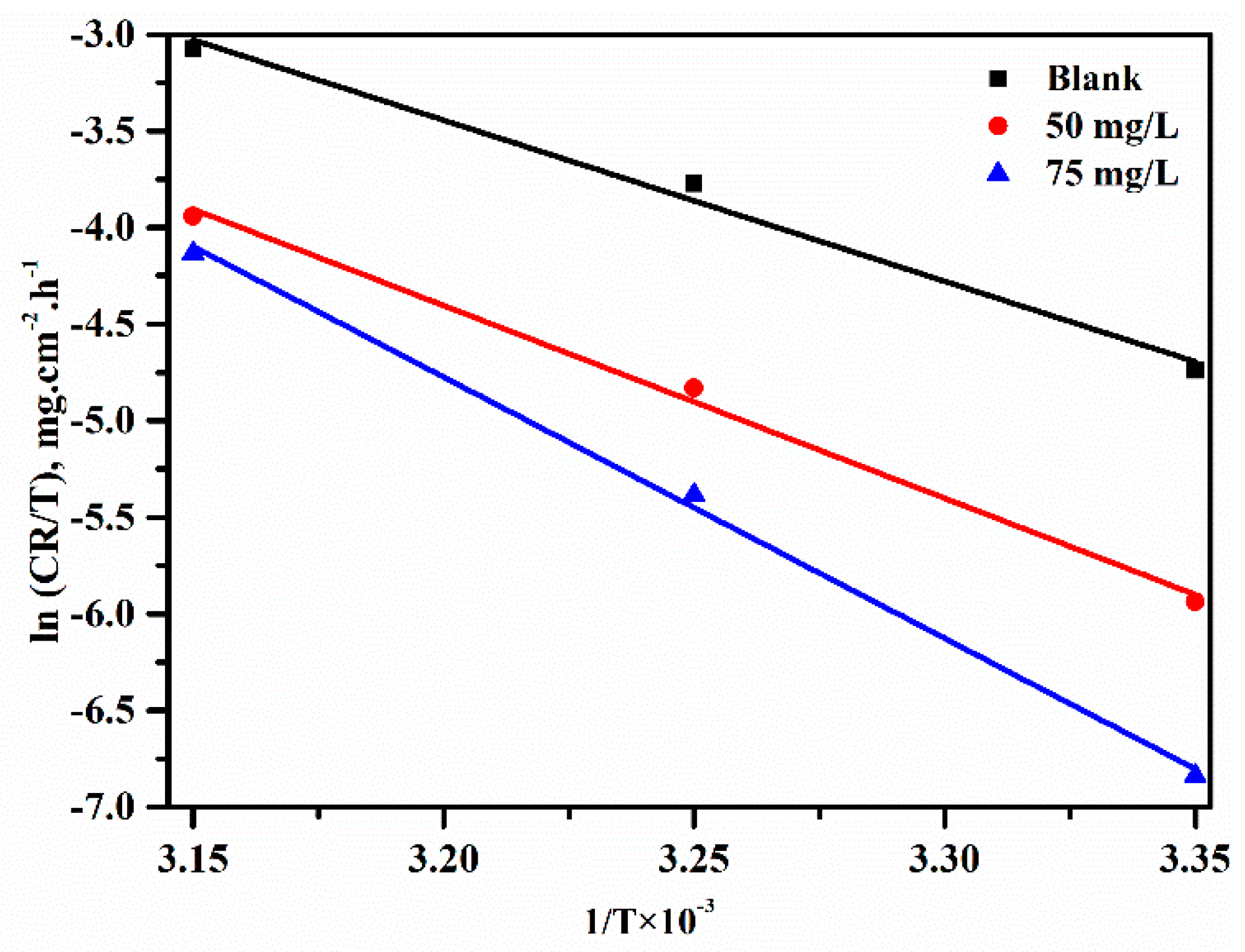
3.4. Effect of Temperature
3.5. Mechanism of the Corrosion Inhibitor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khadiri, A.; Saddik, R.; Bekkouche, K.; Aouniti, A.; Hammouti, B.; Benchat, N.; Bouachrine, M.; Solmaz, R. Gravimetric, electrochemical and quantum chemical studies of some pyridazine derivatives as corrosion inhibitors for mild steel in 1 M HCl solution. J. Taiwan. Inst. Chem. Eng. 2016, 58, 552–564. [Google Scholar] [CrossRef]
- Kowsari, E.; Arman, S.Y.; Shahini, M.H.; Zandi, H.; Ehsani, A.; Naderi, R.; PourghasemiHanza, A.; Mehdipour, M. In situ synthesis, electrochemical and quantum chemical analysis of an amino acid-derived ionic liquid inhibitor for corrosion protection of mild steel in 1 M HCl solution. Corros. Sci. 2016, 112, 73–85. [Google Scholar] [CrossRef]
- Baux, J.; Caussé, N.; Esvan, J.; Delaunay, S.; Tireau, J.; Roy, M.; You, D.; Pébère, N. Impedance analysis of film-forming amines for the corrosion protection of a carbon steel. Electrochim. Acta 2018, 283, 699–707. [Google Scholar] [CrossRef]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On coating techniques for surface protection: A review. J. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef]
- Ulaeto, S.B.; Pancrecious, J.K.; Rajan, T.P.D.; Pai, B.C. Noble Metal-Metal Oxide Hybrid Nanoparticles; Woodhead Publishing: Cambridge, UK, 2019; pp. 341–372. [Google Scholar]
- Abu-Dalo, M.A.; Al-Rawashdeh, N.A.; Mutlaq, A.A. Green approachto corrosion inhibition of mild steel by ligninsulfonatein acidic media. J. Iron. Steel. Res. Int. 2016, 23, 722–732. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Zheng, Y.; Zhang, N.; Zhang, C. Multi-functionalization of magnetic graphene by surface-initiated icar atrp mediated by polydopamine chemistry for adsorption and speciation of arsenic. Appl. Surf. Sci. 2019, 478, 15–25. [Google Scholar] [CrossRef]
- Maharana, H.S.; Katiyar, P.K.; Mondal, K. Structure dependent super-hydrophobic and corrosion resistant behavior of electrodeposited ni-mose2-mwcnt coating. Appl. Surf. Sci. 2019, 478, 26–37. [Google Scholar] [CrossRef]
- Feizi Mohazzab, B.; Jaleh, B.; Kakuee, O.; Fattah-alhosseini, A. Formation of titanium carbide on the titanium surface using laser ablation in N-heptane and investigating its corrosion resistance. Appl. Surf. Sci. 2019, 478, 623–635. [Google Scholar] [CrossRef]
- Noor, E.A. The inhibition of mild steel corrosion in phosphoric acid solutions by some N-heterocyclic compounds in the salt form. Corros. Sci. 2005, 47, 33–55. [Google Scholar] [CrossRef]
- Popova, A.; Christov, M.; Raicheva, S.; Sokolova, E. Adsorption and inhibitive properties of benzimidazole derivatives in acid mild steel corrosion. Corros. Sci. 2004, 46, 1333–1350. [Google Scholar] [CrossRef]
- Wang, H.-L.; Liu, R.-B.; Xin, J. Inhibiting effects of some mercapto-triazole derivatives on the corrosion of mild steel in 1.0 M HCl medium. Corros. Sci. 2004, 46, 2455–2466. [Google Scholar] [CrossRef]
- Gan, T.; Zhang, Y.; Yang, M.; Hu, H.; Huang, Z.; Feng, Z.; Chen, D.; Chen, C.; Liang, J. Synthesis, characterization, and application of a multifunctional cellulose derivative as an environmentally friendly corrosion and scale inhibitor in simulated cooling water systems. J. Taiwan. Inst. Chem. Eng. 2018, 57, 10786–10797. [Google Scholar] [CrossRef]
- Dong, S.; Yuan, X.; Chen, S.; Zhang, L.; Huang, T. A novel hpei-based hyperbranched scale and corrosion inhibitor: Construction, performance, and inhibition mechanism. Ind. Eng. Chem. Res. 2018, 57, 13952–13961. [Google Scholar] [CrossRef]
- Umoren, S.A.; Banera, M.J.; Alonso-Garcia, T.; Gervasi, C.A.; Mirífico, M.V. Inhibition of mild steel corrosion in HCl solution using chitosan. Cellulose 2013, 20, 2529–2545. [Google Scholar] [CrossRef]
- Jokar, M.; Farahani, T.S.; Ramezanzadeh, B. Electrochemical and surface characterizations of morus alba pendula leaves extract (maple) as a green corrosion inhibitor for steel in 1 M HCl. J. Taiwan. Inst. Chem. Eng. 2016, 63, 436–452. [Google Scholar] [CrossRef]
- Odewunmi, N.A.; Umoren, S.A.; Gasem, Z.M.; Ganiyu, S.A.; Muhammad, Q. L-citrulline: An active corrosion inhibitor component of watermelon rind extract for mild steel in HCl medium. J. Taiwan. Inst. Chem. Eng. 2015, 51, 177–185. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Lin, T.; Xie, X.; Du, G. Cassava starch-sodium allylsulfonate-acryl amide graft copolymer as an effective inhibitor of aluminum corrosion in HCl solution. J. Taiwan. Inst. Chem. Eng. 2018, 86, 252–269. [Google Scholar] [CrossRef]
- Yan, R.; He, W.; Zhai, T.; Ma, H. Anticorrosion organic–inorganic hybrid films constructed on iron substrates using self-assembled polyacrylic acid as a functional bottom layer. Electrochim. Acta 2019, 295, 942–955. [Google Scholar] [CrossRef]
- Elayyachy, M.; El Idrissi, A.; Hammouti, B. New thio-compounds as corrosion inhibitor for steel in 1 M HCl. Corros. Sci. 2006, 48, 2470–2479. [Google Scholar] [CrossRef]
- Eduok, U.; Ohaeri, E.; Szpunar, J. Electrochemical and surface analyses of x70 steel corrosion in simulated acid pickling medium: Effect of poly (N-vinyl imidazole) grafted carboxymethyl chitosan additive. Electrochim. Acta 2018, 278, 302–312. [Google Scholar] [CrossRef]
- Emregül, K.C.; Hayvalí, M. Studies on the effect of a newly synthesized schiff base compound from phenazone and vanillin on the corrosion of steel in 2 M HCl. Corros. Sci. 2006, 48, 797–812. [Google Scholar] [CrossRef]
- Moretti, G.; Guidi, F.; Fabris, F. Corrosion inhibition of the mild steel in 0.5 M HCl by 2-butyl-hexahydropyrrolo[1,2-b][1,2]oxazole. Corros. Sci. 2013, 76, 206–218. [Google Scholar] [CrossRef]
- Asadi, N.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Utilizing lemon balm extract as an effective green corrosion inhibitor for mild steel in 1 M HCl solution: A detailed experimental, molecular dynamics, monte carlo and quantum mechanics study. J. Taiwan. Inst. Chem. Eng. 2019, 95, 252–272. [Google Scholar] [CrossRef]
- Babic-Samardzija, K.; Lupu, C.; Hackerman, N.; Barron, A.R. Inhibitive properties, adsorption and surface study of butyn-1-ol and pentyn-1-ol alcohols as corrosion inhibitors for iron in HCl. J. Mater. Chem. 2005, 15, 1908–1916. [Google Scholar] [CrossRef]
- Srivastava, V.; Chauhan, D.S.; Joshi, P.G.; Maruthapandian, V.; Sorour, A.A.; Quraishi, M.A. Peg-functionalized chitosan: A biological macromolecule as a novel corrosion inhibitor. ChemistrySelect. 2018, 3, 1990–1998. [Google Scholar] [CrossRef]
- Haque, J.; Srivastava, V.; Verma, C.; Lgaz, H.; Salghi, R.; Quraishi, M.A. N-methyl-N,N,N-trioctylammonium chloride as a novel and green corrosion inhibitor for mild steel in an acid chloride medium: Electrochemical, dft and md studies. New. J. Chem. 2017, 41, 13647–13662. [Google Scholar] [CrossRef]
- Rahim, A.A.; Rocca, E.; Steinmetz, J.; Jain Kassim, M. Inhibitive action of mangrove tannins and phosphoric acid on pre-rusted steel via electrochemical methods. Corros. Sci. 2008, 50, 1546–1550. [Google Scholar] [CrossRef]
- Ochoa, N.; Bello, M.; Sancristóbal, J.; Balsamo, V.; Albornoz, A.; Brito, J.L. Modified cassava starches as potential corrosion inhibitors for sustainable development. Mater. Res. 2013, 16, 1209–1219. [Google Scholar] [CrossRef][Green Version]
- Menaka, R.; Subhashini, S. Chitosan schiff base as eco-friendly inhibitor for mild steel corrosion in 1 M HCl. J. Adhes. Sci. Techonl. 2016, 30, 1622–1640. [Google Scholar] [CrossRef]
- Abiola, O.K.; James, A.O. The effects of aloe vera extract on corrosion and kinetics of corrosion process of zinc in HCl solution. Corros. Sci. 2010, 52, 661–664. [Google Scholar] [CrossRef]
- Garai, S.; Garai, S.; Jaisankar, P.; Singh, J.K.; Elango, A. A comprehensive study on crude methanolic extract of artemisia pallens (asteraceae) and its active component as effective corrosion inhibitors of mild steel in acid solution. Corros. Sci. 2012, 60, 193–204. [Google Scholar] [CrossRef]
- Li, X.; Deng, S. Cassava starch graft copolymer as an eco-friendly corrosion inhibitor for steel in H2SO4 solution. Korean. J. Chem. Eng. 2015, 32, 2347–2354. [Google Scholar] [CrossRef]
- Kalaiselvi, P.; Chellammal, S.; Palanichamy, S.; Subramanian, G. Artemisia pallens as corrosion inhibitor for mild steel in HCl medium. Mater. Chem. Phys. 2010, 120, 643–648. [Google Scholar] [CrossRef]
- Eddy, N.O.; Ebenso, E.E.; Ibok, U.J. Adsorption, synergistic inhibitive effect and quantum chemical studies of ampicillin (amp) and halides for the corrosion of mild steel in H2SO4. J. Appl. Electrochem. 2009, 40, 445–456. [Google Scholar] [CrossRef]
- Sangeetha, Y.; Meenakshi, S.; Sairam Sundaram, C. Corrosion inhibition of aminated hydroxyl ethyl cellulose on mild steel in acidic condition. Carbohyd. Polym. 2016, 150, 13–20. [Google Scholar] [CrossRef]
- Ren, Y.; Luo, Y.; Zhang, K.; Zhu, G.; Tan, X. Lignin terpolymer for corrosion inhibition of mild steel in 10% hydrochloric acid medium. Corros. Sci. 2008, 50, 3147–3153. [Google Scholar] [CrossRef]
- Stewart, D. Lignin as a base material for materials applications: Chemistry, application and economics. Carbohyd. Polym. 2008, 27, 202–207. [Google Scholar] [CrossRef]
- Fox, S.C.; McDonald, A.G. Chenical and thermal characterization of three inndustrial lignins and their corresponding lignin esters. Bioresources 2010, 5, 990–1009. [Google Scholar]
- Hussin, M.H.; Rahim, A.A.; Mohamad Ibrahim, M.N.; Brosse, N. Improved corrosion inhibition of mild steel by chemically modified lignin polymers from elaeis guineensis agricultural waste. Mater. Chem. Phys. 2015, 163, 201–212. [Google Scholar] [CrossRef]
- Akbarzadeh, E.; Ibrahim, M.N.M.; Rahim, A.A. Monomers of lignin as corrosion inhibitors for mild steel: Study of their behaviour by factorial experimental design. Corros. Eng. Sci. Technol. 2013, 47, 302–311. [Google Scholar] [CrossRef]
- Li, L.; Dong, C.; Liu, L.; Li, J.; Xiao, K.; Zhang, D.; Li, X. Preparation and characterization of pH-controlled-release intelligent corrosion inhibitor. Mater. Lett. 2014, 116, 318–321. [Google Scholar] [CrossRef]
- Umoren, S.A.; Ogbobe, O.; Igwe, I.O.; Ebenso, E.E. Inhibition of mild steel corrosion in acidic medium using synthetic and naturally occurring polymers and synergistic halide additives. Corros. Sci. 2008, 50, 1998–2006. [Google Scholar] [CrossRef]
- Benali, O.; Larabi, L.; Traisnel, M.; Gengembre, L.; Harek, Y. Electrochemical, theoretical and XPS studies of 2-mercapto-1-methylimidazole adsorption on carbon steel in 1m HClO4. Appl. Sure. Sci. 2007, 253, 6130–6139. [Google Scholar] [CrossRef]
- Hussin, M.H.; Rahim, A.A.; Mohamad Ibrahim, M.N.; Brosse, N. The capability of ultrafiltrated alkaline and organosolv oil palm (elaeis guineensis) fronds lignin as green corrosion inhibitor for mild steel in 0.5 M HCl solution. Measurement 2016, 78, 90–103. [Google Scholar] [CrossRef]
- Feng, R.; Beck, J.; Ziomek-Moroz, M.; Lvov, S.N. Electrochemical corrosion of ultra-high strength carbon steel in alkaline brines containing hydrogen sulfide. Electrochim. Acta 2016, 212, 998–1009. [Google Scholar] [CrossRef]
- Yan, Y.; Li, W.; Cai, L.; Hou, B. Electrochemical and quantum chemical study of purines as corrosion inhibitors for mild steel in 1 M HCl solution. Electrochim. Acta 2008, 53, 5953–5960. [Google Scholar] [CrossRef]
- Xia, G.; Wan, J.; Zhang, J.; Zhang, X.; Xu, L.; Wu, J.; He, J.; Zhang, J. Cellulose-based films prepared directly from waste newspapers via an ionic liquid. Carbohyd polym. 2016, 151, 223–229. [Google Scholar] [CrossRef]
- Yahya, S.; Othman, N.K.; Daud, A.R.; Jalar, A.; Ismail, R. The influence of temperature on the inhibition of carbon steel corrosion in acidic lignin. Anti-Corros. Method. Mater. 2015, 62, 301–306. [Google Scholar] [CrossRef]
- Cheng, S.; Chen, S.; Liu, T.; Chang, X.; Yin, Y. Carboxymenthyl chitosan as an ecofriendly inhibitor for mild steel in 1 M HCl. Mater. Lett. 2007, 61, 3276–3280. [Google Scholar] [CrossRef]





| Concentration (ppm) | CR (mg /(cm2·h)) | IE (%) |
|---|---|---|
| 0 | 2.65 | 0.00 |
| 50 | 0.80 | 69.81% |
| 75 | 0.33 | 87.54% |
| 100 | 0.37 | 86.04% |
| 125 | 0.71 | 73.21% |
| C (mg/L) | Ecorr (mV/SCE) | icorr (uA/cm2) | ba (mV) | bc (mV) | IE (%) |
|---|---|---|---|---|---|
| 0 | −461.80 | 891 | 154 | 185 | 0.00 |
| 50 | −427.60 | 246 | 164 | 148 | 72.39% |
| 75 | −460.30 | 45 | 94 | 114 | 94.95% |
| 100 | −508.40 | 78 | 104 | 125 | 91.25% |
| 125 | −488.30 | 231 | 122 | 147 | 74.07% |
| Concentration(mg/L) | Rs(ohm) | Cc(uF) | Rc(ohm) | Rct(ohm) | Cdl(uF) | IE(%) |
|---|---|---|---|---|---|---|
| 0 | 5.729 | – | – | 29.60 | 254.60 | – |
| 50 | 4.701 | – | – | 59.74 | 240.60 | 50.45% |
| 75 | 5.508 | 91.19 | 178.90 | 227.00 | 34.09 | 86.96% |
| 100 | 4.760 | 180.70 | 96.97 | 101.00 | 45.91 | 70.69% |
| 125 | 4.780 | 231.90 | 50.32 | 74.24 | 42.70 | 60.12% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, C.; Wang, S.; Dong, X.; Liu, K.; Zhao, X.; Kong, F. Construction of a Novel Lignin-Based Quaternary Ammonium Material with Excellent Corrosion Resistant Behavior and Its Application for Corrosion Protection. Materials 2019, 12, 1776. https://doi.org/10.3390/ma12111776
Gao C, Wang S, Dong X, Liu K, Zhao X, Kong F. Construction of a Novel Lignin-Based Quaternary Ammonium Material with Excellent Corrosion Resistant Behavior and Its Application for Corrosion Protection. Materials. 2019; 12(11):1776. https://doi.org/10.3390/ma12111776
Chicago/Turabian StyleGao, Chao, Shoujuan Wang, Xinyu Dong, Keyin Liu, Xin Zhao, and Fangong Kong. 2019. "Construction of a Novel Lignin-Based Quaternary Ammonium Material with Excellent Corrosion Resistant Behavior and Its Application for Corrosion Protection" Materials 12, no. 11: 1776. https://doi.org/10.3390/ma12111776
APA StyleGao, C., Wang, S., Dong, X., Liu, K., Zhao, X., & Kong, F. (2019). Construction of a Novel Lignin-Based Quaternary Ammonium Material with Excellent Corrosion Resistant Behavior and Its Application for Corrosion Protection. Materials, 12(11), 1776. https://doi.org/10.3390/ma12111776