A New Thermal Controlling Material with Positive Temperature Coefficient for Body Warming: Preparation and Characterization
Abstract
1. Introduction
2. Materials and Experiment
2.1. Materials
2.2. Preparation of PTC Composites
2.2.1. Pretreatment of Acetylene Black
2.2.2. Preparation of the Composites
2.3. Methods and Instruments
3. Results and Discussion
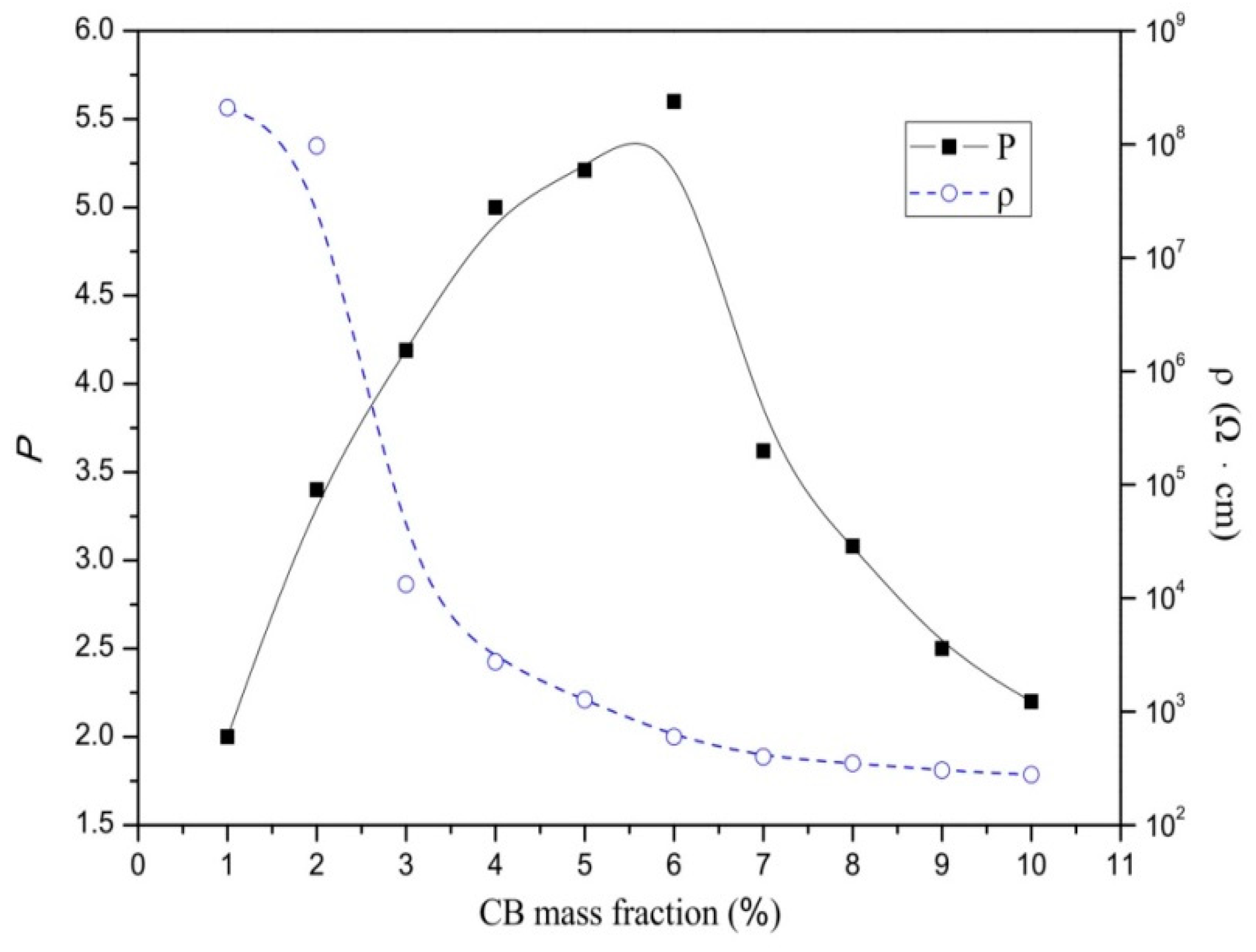
3.1. Optimal PTC Effect at Different Material Proportions
3.2. Repeatability of the PTC Effect for Composite 6C
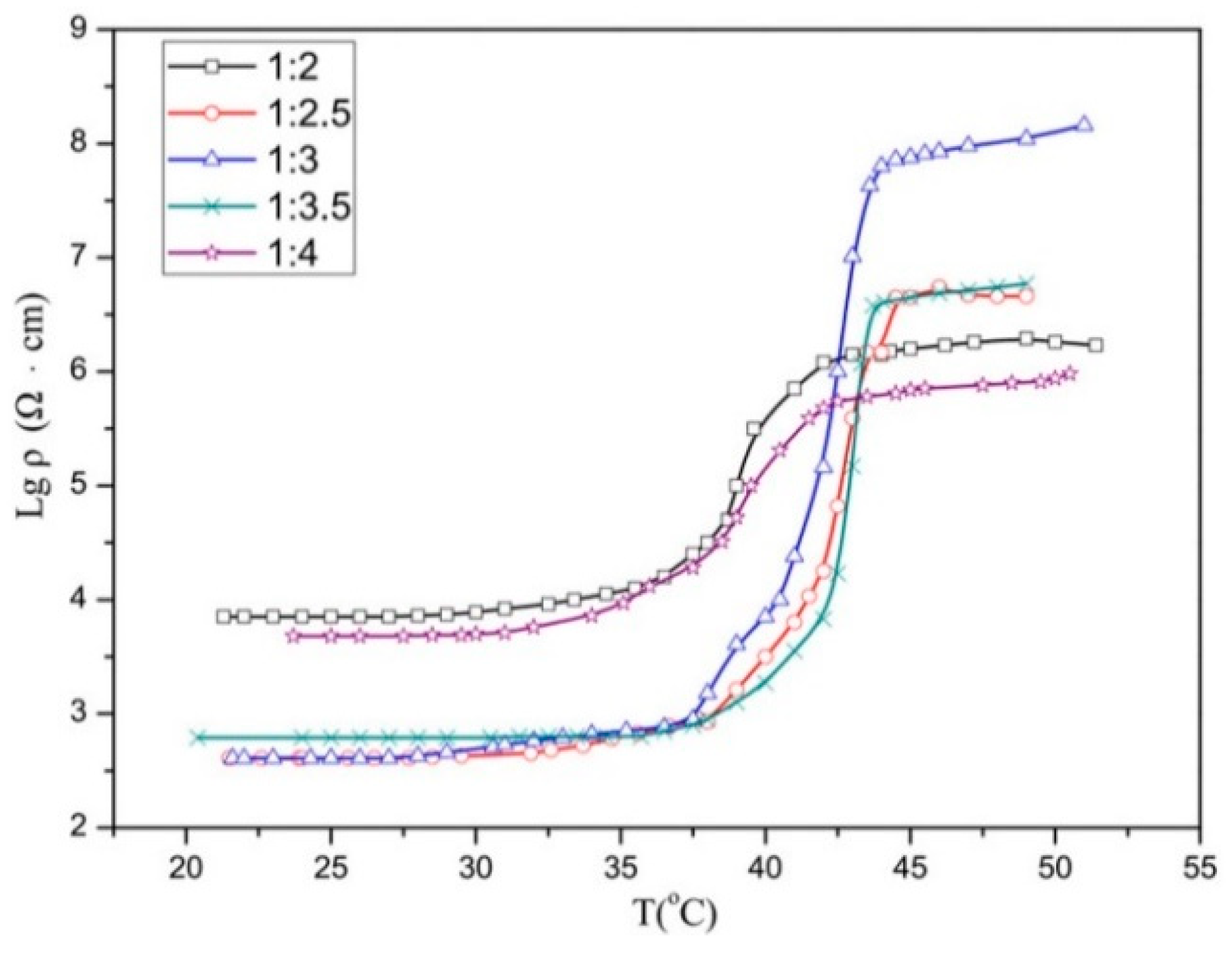
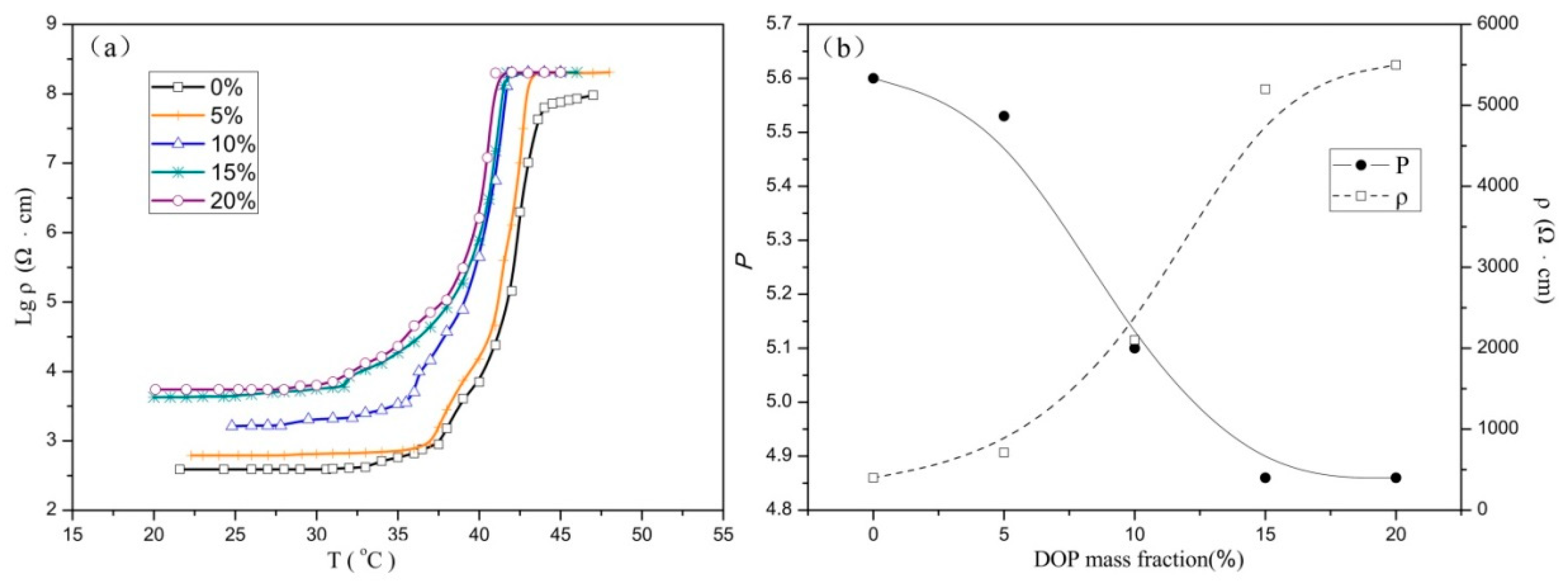
3.3. Curie Temperatures of PTC Composites
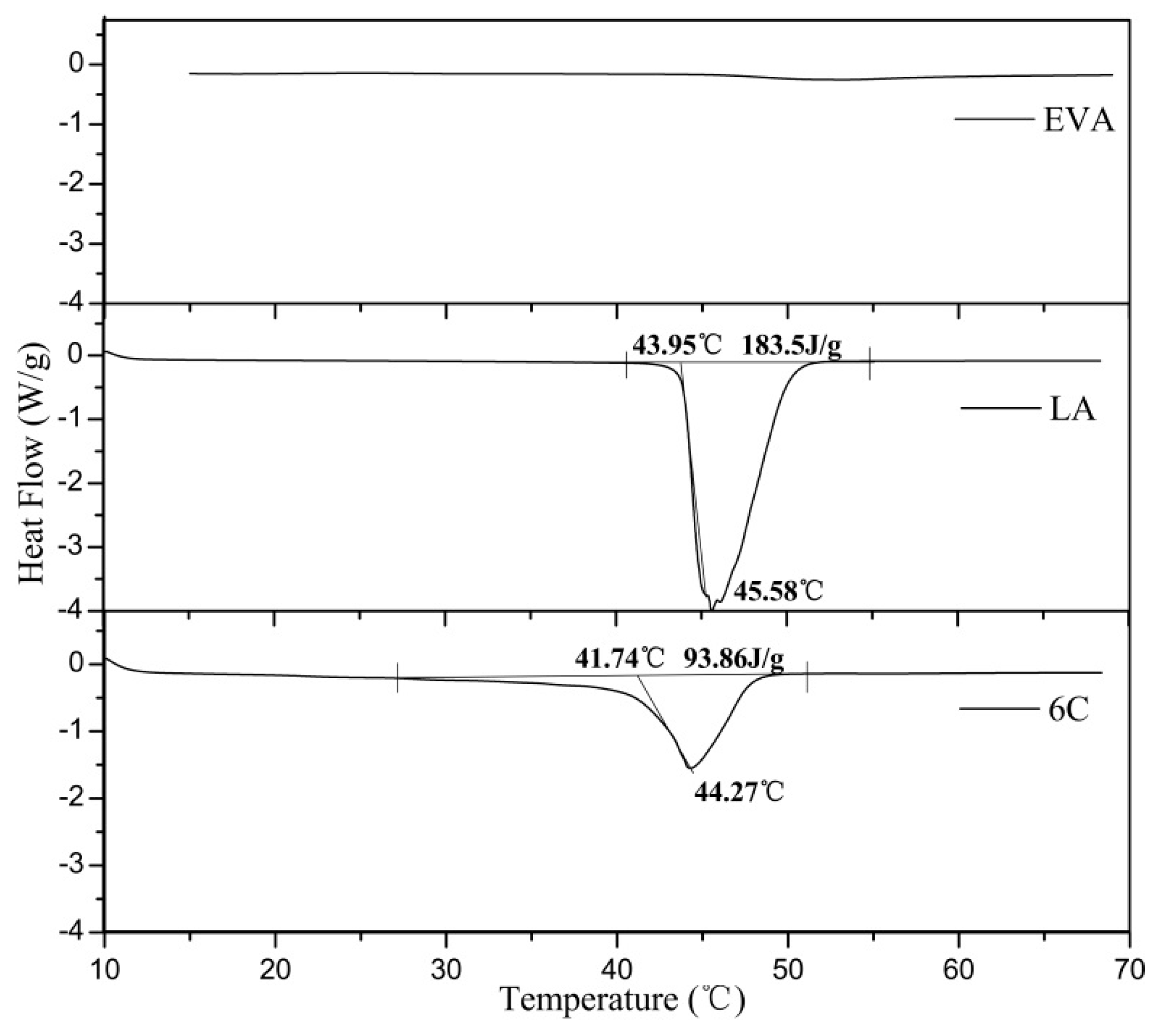
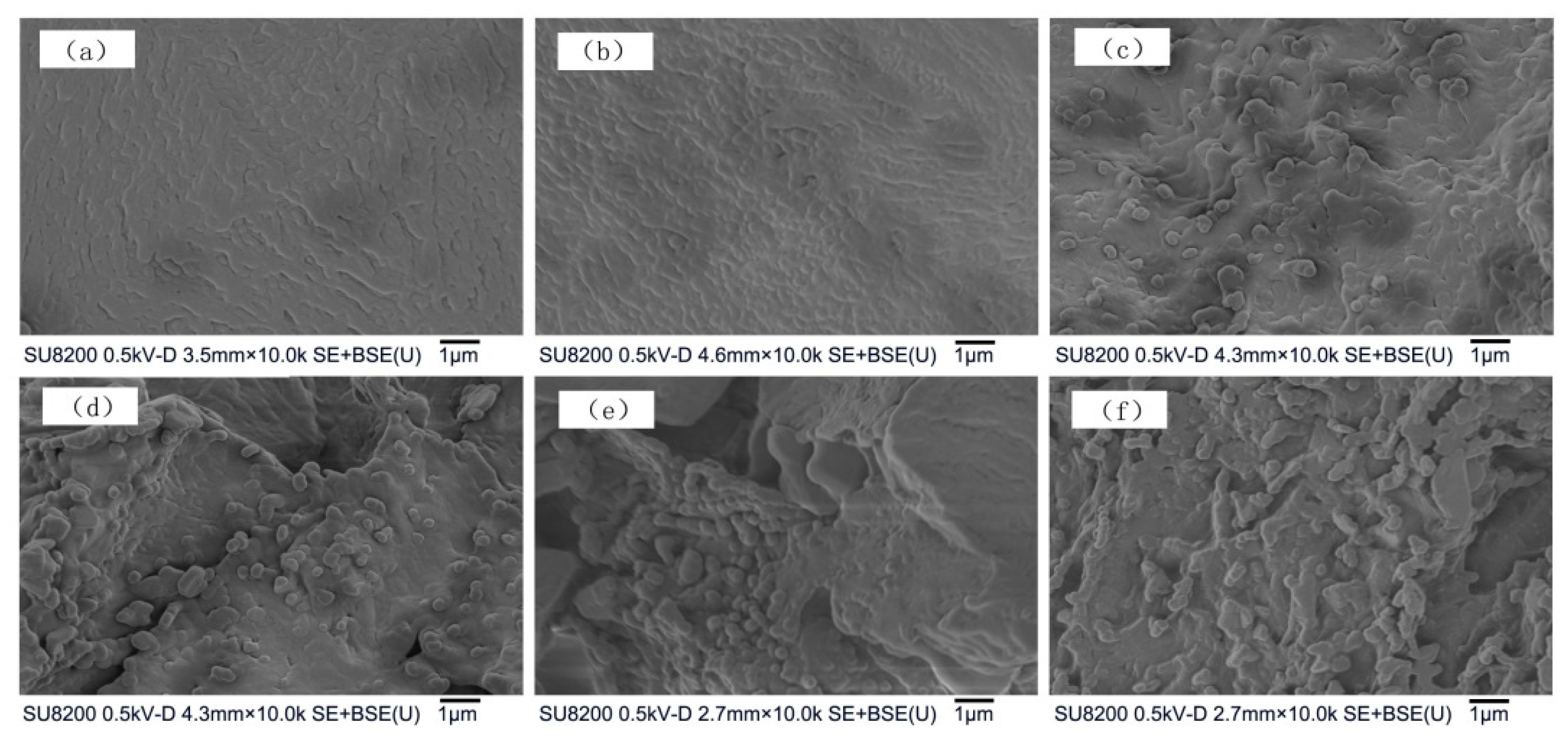
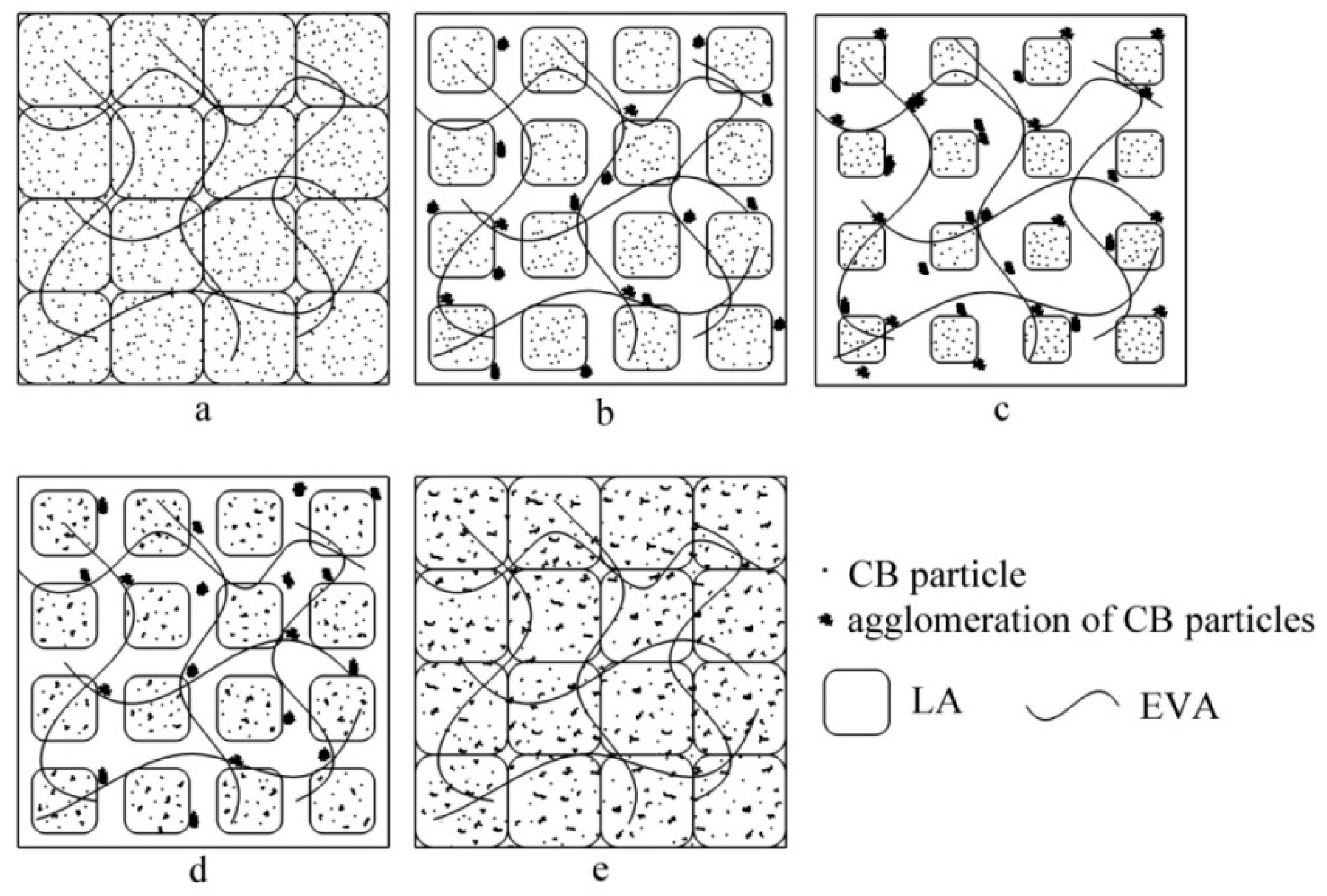
3.4. Microstructural and Model Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- He, X.J.; Du, J.H.; Ying, Z.; Cheng, H.M.; He, X.J. Positive temperature coefficient effect in multiwalled carbon nanotube/high-density polyethylene composites. Appl. Phys. Lett. 2005, 86, 1–3. [Google Scholar] [CrossRef]
- Ohtsuka, S.; Souta, Y.; Abe, T.; Kimura, K.; Hikita, M. Switching phenomena of a new type PTC device as a reusable current-limiting fuse operated by dielectrophoresis. In Proceedings of the 17th Annual Meeting of the IEEE Lasers and Electro-Optics Society, 2004 (LEOS 2004), Boulder, CO, USA, 20 October 2004; pp. 592–595. [Google Scholar]
- Horn, M.; Umar, L.; Ruser, H. Self-controlled PTC sensor for reliable overfill protection of liquids. In Proceedings of the IMTC/2002. Proceedings of the 19th IEEE Instrumentation and Measurement Technology Conference (IEEE Cat. No.00CH37276), Anchorage, AK, USA, 21–23 May 2002; Volume 1, pp. 415–419. [Google Scholar]
- Wang, L. Differential Structure for Temperature Sensing Based on Conductive Polymer Composites. IEEE Trans. Electron Devices 2015, 62, 3025–3028. [Google Scholar] [CrossRef]
- Wang, J.; Guo, W.; Cheng, S.; Zhang, Z. Structure and applications of CB/crystal fluoride resin alloy in self-regulated heating cables. J. Appl. Polym. Sci. 2003, 88, 2664–2669. [Google Scholar] [CrossRef]
- Hou, Y.L.; Zhang, P.; Xie, M.M. Thermally induced double-positive temperature coefficients of electrical resistivity in combined conductive filler–doped polymer composites. J. Appl. Polym. Sci. 2017, 134, 1–11. [Google Scholar] [CrossRef]
- Li, X.; Sun, Z.; Gu, L.; Han, J.; Wang, J.; Wu, S. Research of fuel temperature control in fuel pipeline of diesel engine using positive temperature coefficient material. Adv. Mech. Eng. 2016, 8, 1–11. [Google Scholar] [CrossRef]
- Song, J.; Cheng, W.; Xu, Z.; Yuan, S.; Liu, M. Study on PID temperature control performance of a novel PTC material with room temperature Curie point. Int. J. Heat Mass Transf. 2016, 95, 1038–1046. [Google Scholar] [CrossRef]
- Cheng, W.; Song, J.; Liu, Y.; Yuan, S.; Wu, W.; Xu, Z. Theoretical and experimental studies on thermal control by using a novel PTC material with room temperature Curie point. Int. J. Heat Mass Transf. 2014, 74, 441–447. [Google Scholar] [CrossRef]
- Xu, X.B.; Li, Z.M.; Dai, K.; Yang, M.B. Anomalous attenuation of the positive temperature coefficient of resistivity in a carbon-black-filled polymer composite with electrically conductive in situ microfibrils. Appl. Phys. Lett. 2006, 89, 3–6. [Google Scholar] [CrossRef]
- Kirstein, K.; Reichmann, K.; Preis, W.; Mitsche, S. Effect of commercial anatase-TiO2raw materials on the electrical characteristics of ceramics with positive temperature coefficient of resistivity. J. Eur. Ceram. Soc. 2011, 31, 2339–2349. [Google Scholar] [CrossRef]
- Seo, M.K.; Rhee, K.Y.; Park, S.J. Influence of electro-beam irradiation on PTC/NTC behaviors of carbon blacks/HDPE conducting polymer composites. Curr. Appl. Phys. 2011, 11, 428–433. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, M.Q.; Zeng, H.M. Carbon-black-filled polyolefine as a positive temperature coefficient material: Effect of composition, processing, and filler treatment. J. Appl. Polym. Sci. 1998, 70, 559–566. [Google Scholar] [CrossRef]
- Cheng, W.L.; Yuan, S.; Song, J.L. Studies on preparation and adaptive thermal control performance of novel PTC (positive temperature coefficient) materials with controllable Curie temperatures. Energy 2014, 74, 447–454. [Google Scholar] [CrossRef]
- Zheng, R.; Gao, J.; Wang, J.; Chen, G. Reversible temperature regulation of electrical and thermal conductivity using liquid-solid phase transitions. Nat. Commun. 2011, 2, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.C.; Wu, Y.L.; Gao, J.W.; Cheng, G.A.; Chen, G.; Zheng, R.T. Room temperature electrical and thermal switching CNT/hexadecane composites. Adv. Mater. 2013, 25, 4938–4943. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.L.; Wu, W.F.; Song, J.L.; Liu, Y.; Yuan, S.; Liu, N. A new kind of shape-stabilized PCMs with positive temperature coefficient (PTC) effect. Energy Convers. Manag. 2014, 79, 470–476. [Google Scholar] [CrossRef]
- Lai, F.; Wang, B.-B.; Zhang, P. Enhanced positive temperature coefficient in amorphous PS/CSPE-MWCNT composites with low percolation threshold. J. Appl. Polym. Sci. 2018, 136, 47053. [Google Scholar] [CrossRef]
- Dai, K.; Zhang, Y.; Tang, J.; Ji, X.; Li, Z. Anomalous attenuation and structural origin of positive temperature coefficient (PTC) effect in a carbon black (CB)/poly(ethylene terephthalate) (PET)/polyethylene (PE) electrically conductive microfibrillar polymer composite with a preferential CB distribution. J. Appl. Polym. Sci. 2012, 125, E561–E570. [Google Scholar]
- Zha, J.W.; Li, W.K.; Liao, R.J.; Bai, J.; Dang, Z.M. High performance hybrid carbon fillers/binary-polymer nanocomposites with remarkably enhanced positive temperature coefficient effect of resistance. J. Mater. Chem. A 2013, 1, 843–851. [Google Scholar] [CrossRef]
- Li, Q.; Kim, J.W.; Shim, T.H.; Jang, Y.K.; Lee, J.H. Positive Temperature Coefficient Behavior of the Graphite Nanofibre and Carbon Black Filled High-Density Polyethylene Hybrid Composites. Adv. Mater. Res. 2008, 47–50, 226–229. [Google Scholar] [CrossRef]

| No. | ω (CB) | ω (EVA)/ω (LA) | ω (DOP) | No. | ω (CB) | ω (EVA)/ω (LA) | ω (DOP) |
|---|---|---|---|---|---|---|---|
| 1 | 1% | 1:3 | – | 6A | 6% | 1:4 | – |
| 2 | 2% | 1:3 | – | 6B | 6% | 1:3.5 | – |
| 3 | 3% | 1:3 | – | 6C | 6% | 1:3 | – |
| 4 | 4% | 1:3 | – | 6D | 6% | 1:2.5 | – |
| 5 | 5% | 1:3 | – | 6E | 6% | 1:2 | – |
| 6 | 6% | 1:3 | – | A6 | 6% | 1:3 | – |
| 7 | 7% | 1:3 | – | B6 | 6% | 1:3 | 5% |
| 8 | 8% | 1:3 | – | C6 | 6% | 1:3 | 10% |
| 9 | 9% | 1:3 | – | D6 | 6% | 1:3 | 15% |
| 10 | 10% | 1:3 | – | E6 | 6% | 1:3 | 20% |
| Composites No. | 6A | 6B | 6C | 6D | 6E |
|---|---|---|---|---|---|
| EVA/LA ratio | 1:4 | 1:3.5 | 1:3 | 1:2.5 | 1:2 |
| 25 °C resistivity (Ω·cm) | 4800 | 610 | 390 | 410 | 7070 |
| PTC intensity P | 2.3 | 3.92 | 5.6 | 4.13 | 2.44 |
| Composites No. | A6 | B6 | C6 | D6 | E6 |
|---|---|---|---|---|---|
| DOP content | 0 | 5 | 10 | 15 | 20 |
| Curie temperature (°C) | 38 | 37 | 35 | 33 | 32 |
| Sample Name or No. | LA | 6A | 6B | 6C | 6D | 6E |
|---|---|---|---|---|---|---|
| Peak temperature (°C) | 44.0 | 41.9 | 42.9 | 41.7 | 41.1 | 40.3 |
| Resistivity jump point (°C) | – | 37.1 | 37.5 | 37.5 | 37.7 | 37 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Chang, C.; Li, X.; Li, Y.; Guan, G. A New Thermal Controlling Material with Positive Temperature Coefficient for Body Warming: Preparation and Characterization. Materials 2019, 12, 1758. https://doi.org/10.3390/ma12111758
Li J, Chang C, Li X, Li Y, Guan G. A New Thermal Controlling Material with Positive Temperature Coefficient for Body Warming: Preparation and Characterization. Materials. 2019; 12(11):1758. https://doi.org/10.3390/ma12111758
Chicago/Turabian StyleLi, Jing, Chang Chang, Xue Li, Yang Li, and Guoqiang Guan. 2019. "A New Thermal Controlling Material with Positive Temperature Coefficient for Body Warming: Preparation and Characterization" Materials 12, no. 11: 1758. https://doi.org/10.3390/ma12111758
APA StyleLi, J., Chang, C., Li, X., Li, Y., & Guan, G. (2019). A New Thermal Controlling Material with Positive Temperature Coefficient for Body Warming: Preparation and Characterization. Materials, 12(11), 1758. https://doi.org/10.3390/ma12111758





