Effect of Additives on Stability of Alumina—Waste Alumina Suspension for Slip Casting: Optimization Using Box-Behnken Design
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preliminary Tests
2.2. Box-Behnken Response Surface Design
- 0.05 dwb.% to 0.15 dwb.% (“Dry Weight Basis”, i.e., weight content of dry powders) dispersant Tiron;
- 0.1 dwb.% to 0.5 dwb.% poly (vinyl alcohol) (PVA) as binder;
- 0.2 dwb.% to 1 dwb.% magnesium aluminate spinel (MgAl2O4) for the prevention the abnormal grain growth.
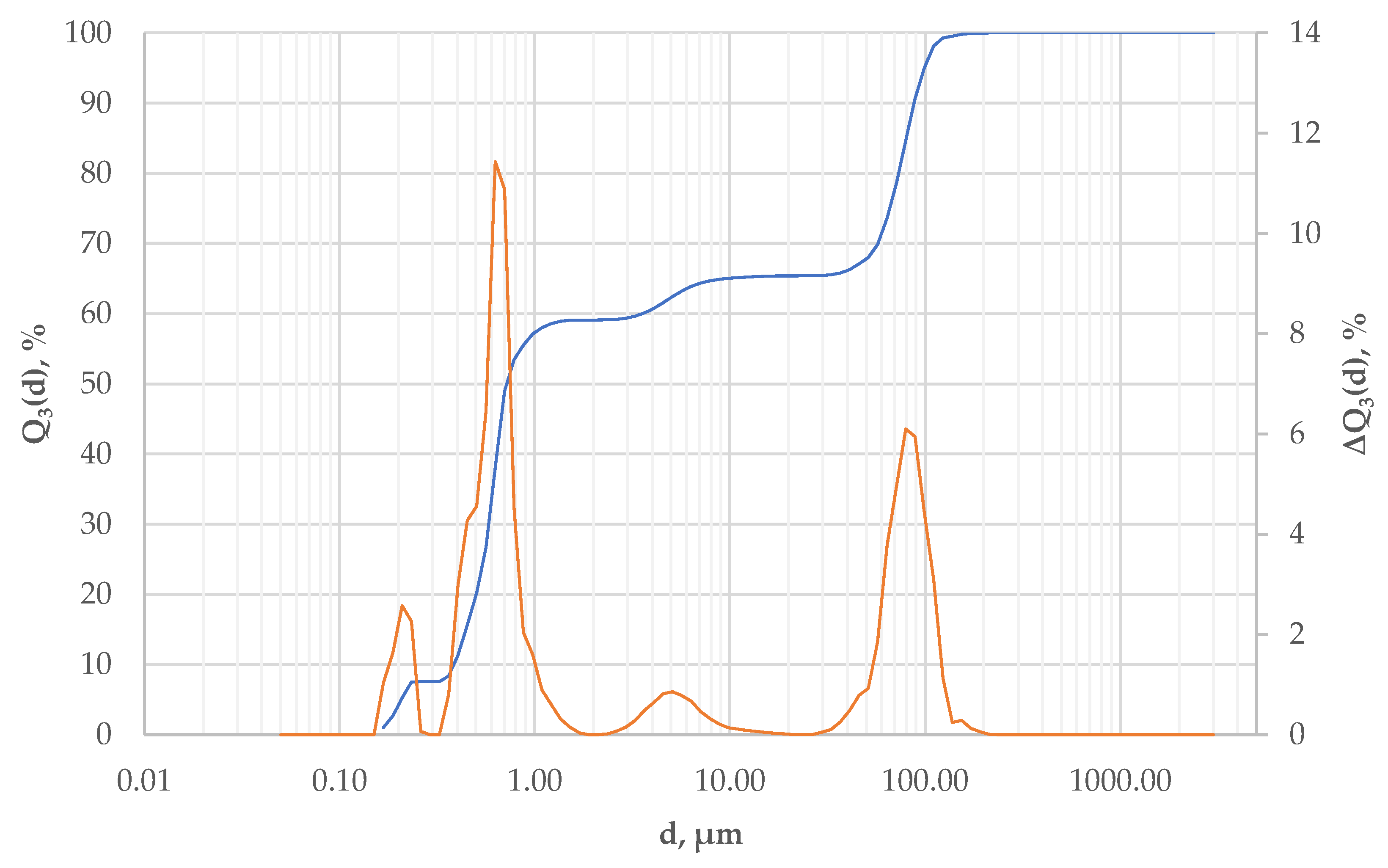
2.3. Preparation and Characterization of Al2O3—Waste Al2O3 Suspensions
3. Results and Discussion
3.1. Preliminary Results
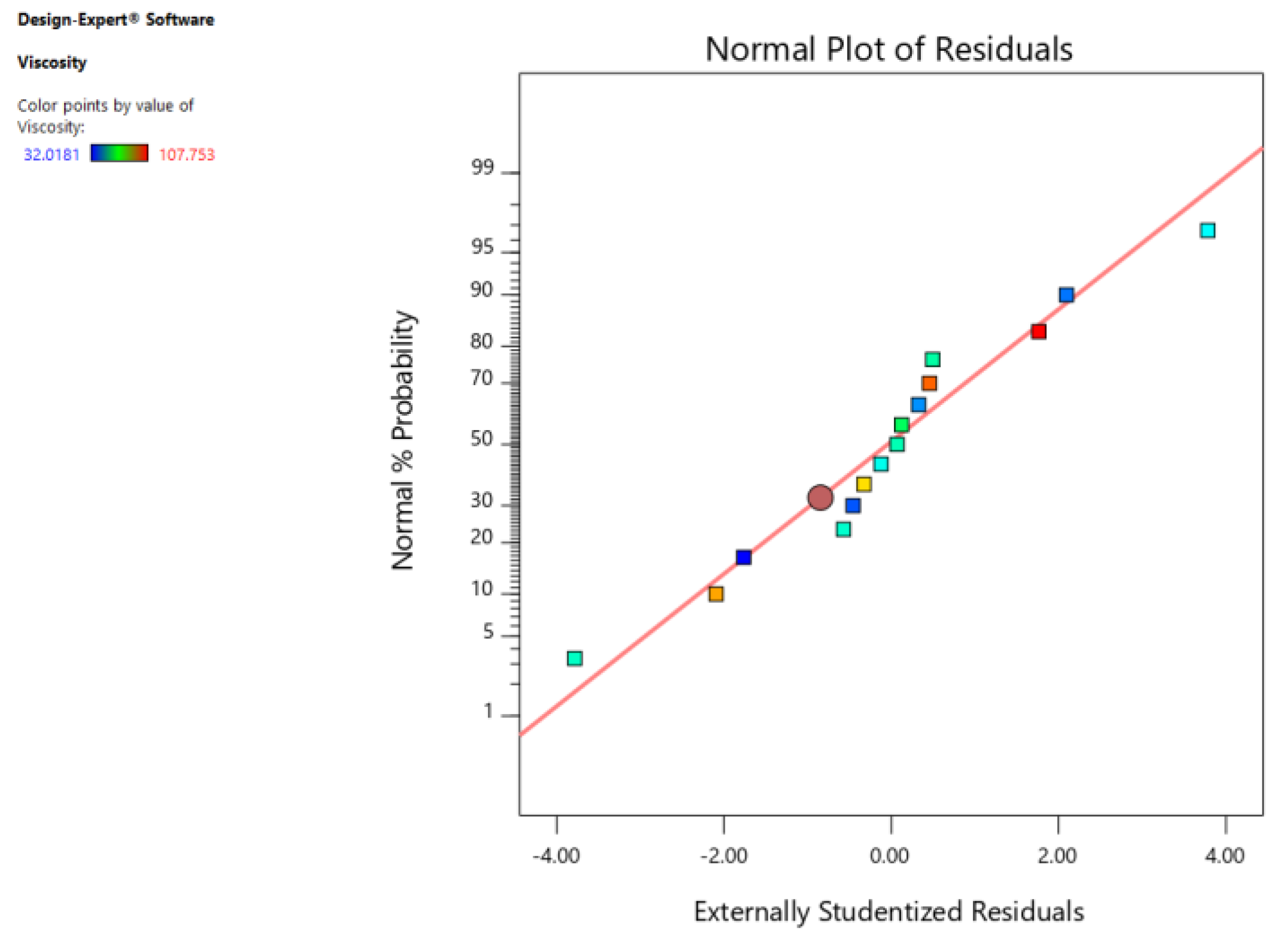
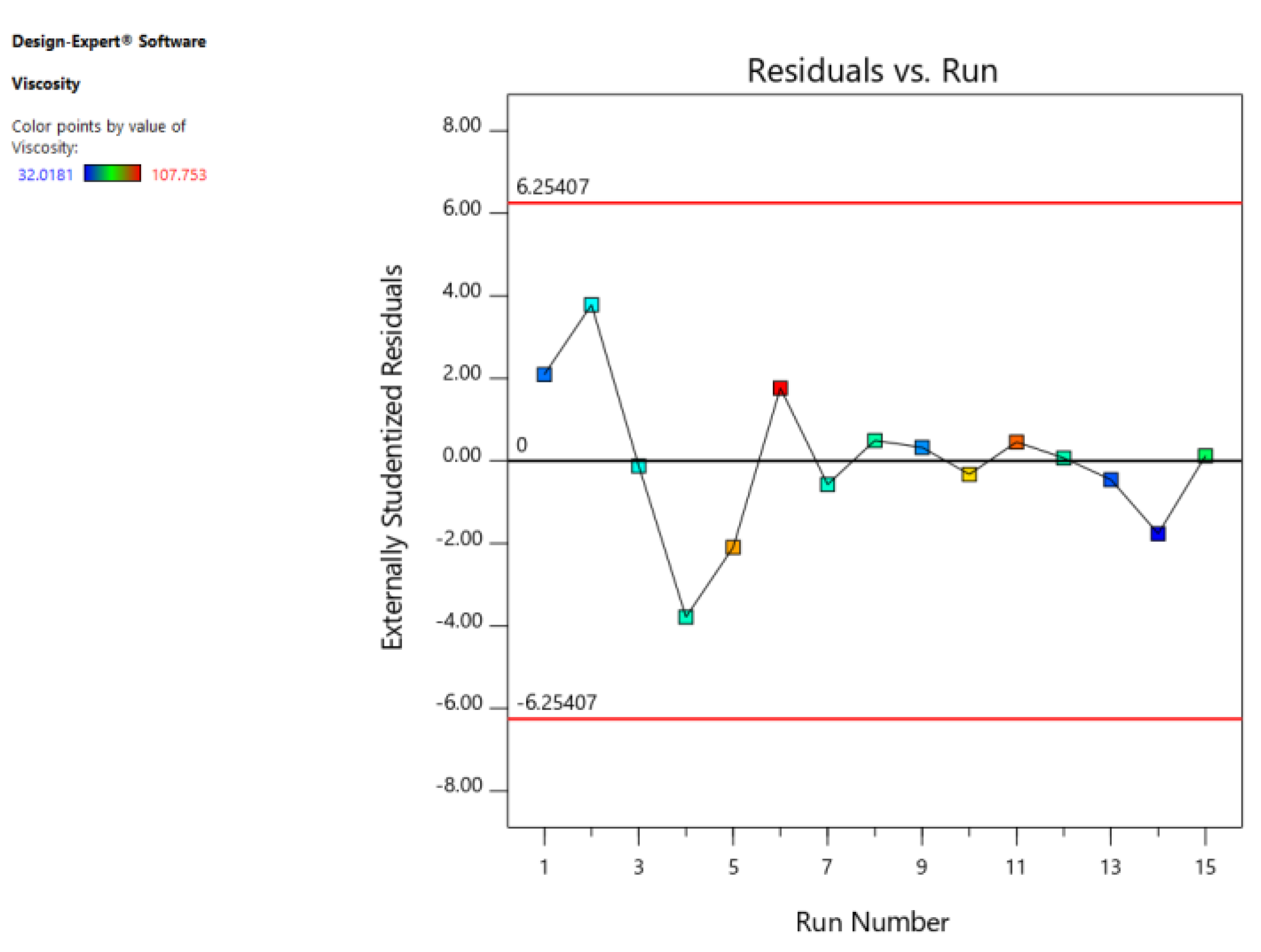
3.2. Results of Modeling
- A is the weight ratio of the dispersant Tiron (dwb.%),
- B is the weight ratio of the binder PVA (dwb.%),
- C is the weight ratio of the abnormal grain growth inhibitor MgAl2O4 (dwb.%).
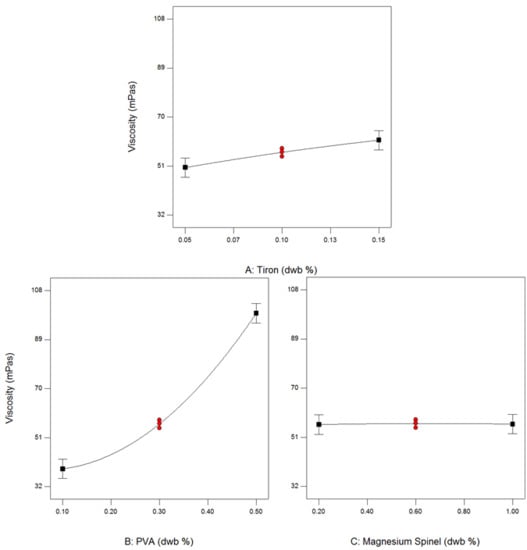
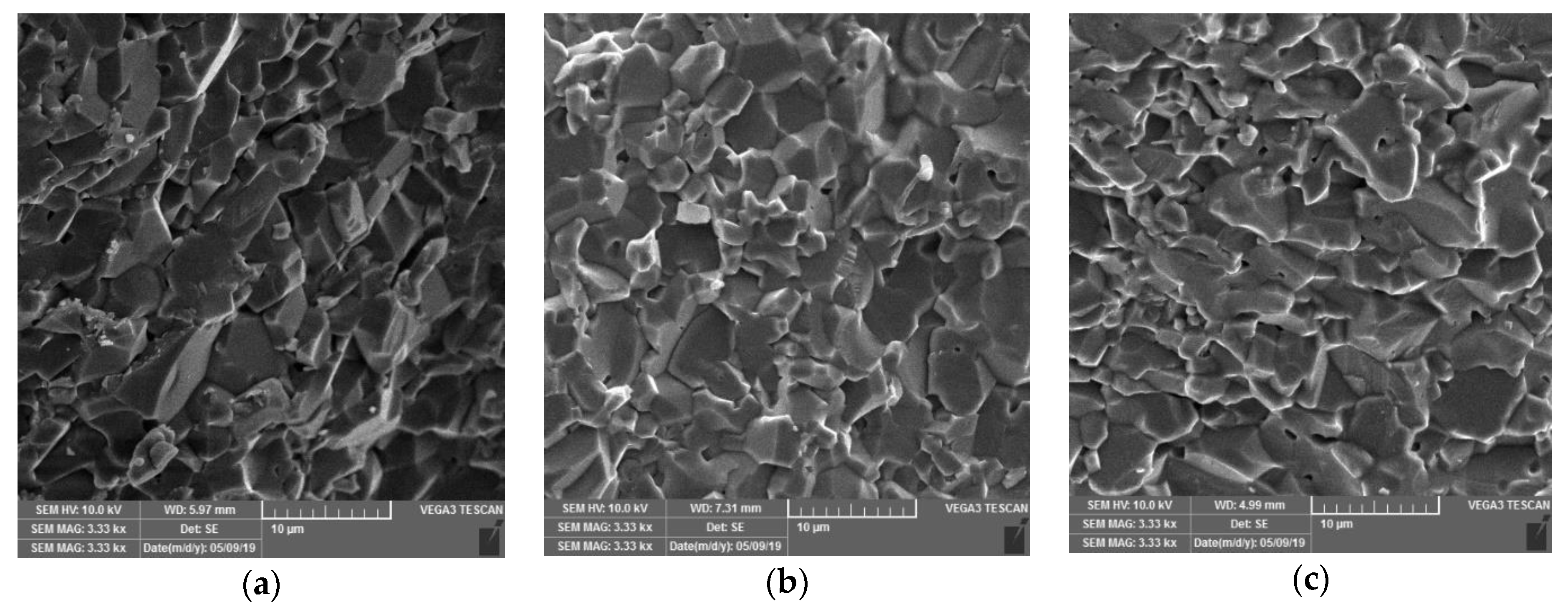
3.3. Effect of Additives on Stability of Alumina—Waste Alumina Suspension
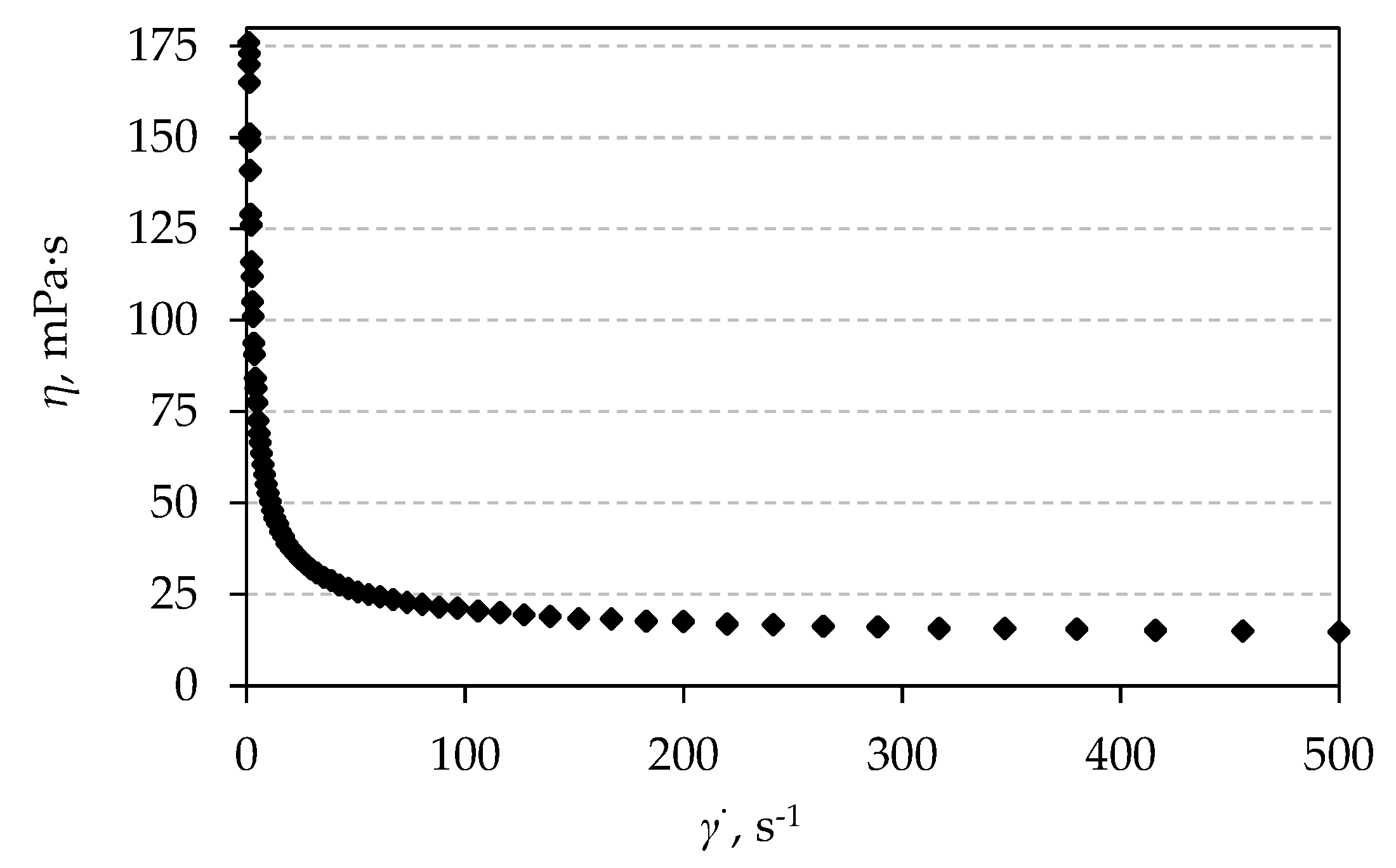
3.4. Rheological Properties
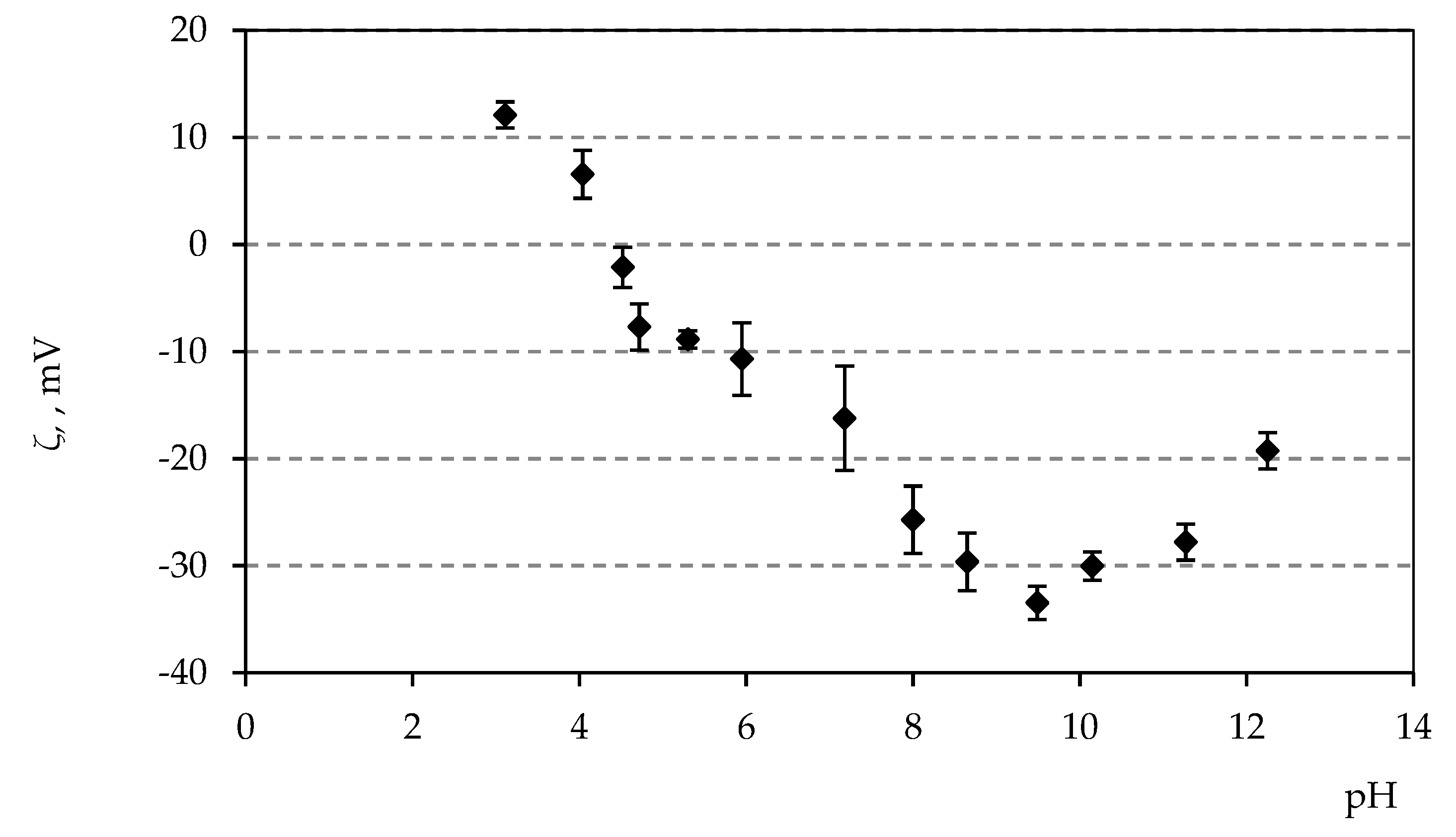
3.5. Zeta Potential Measurements
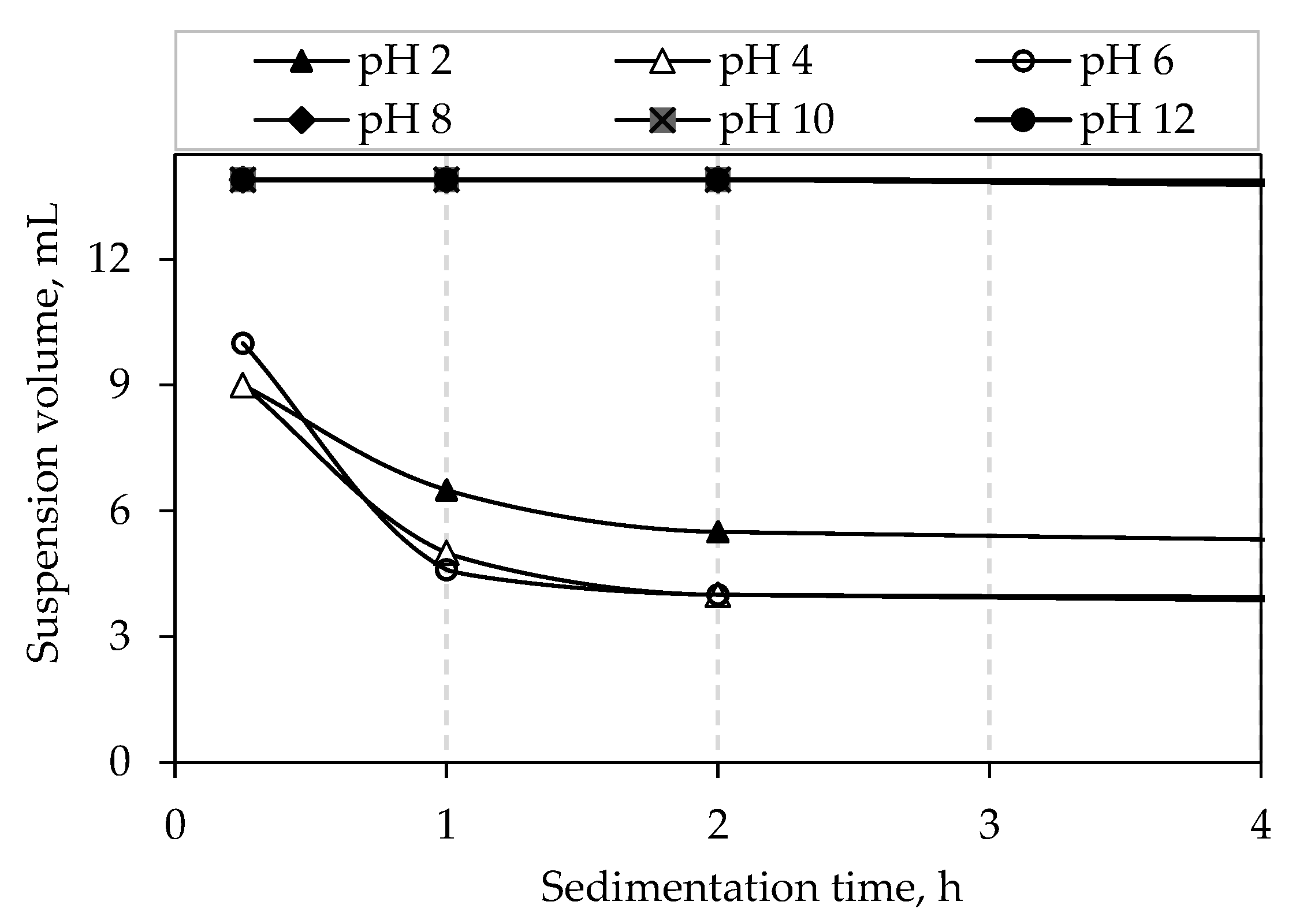
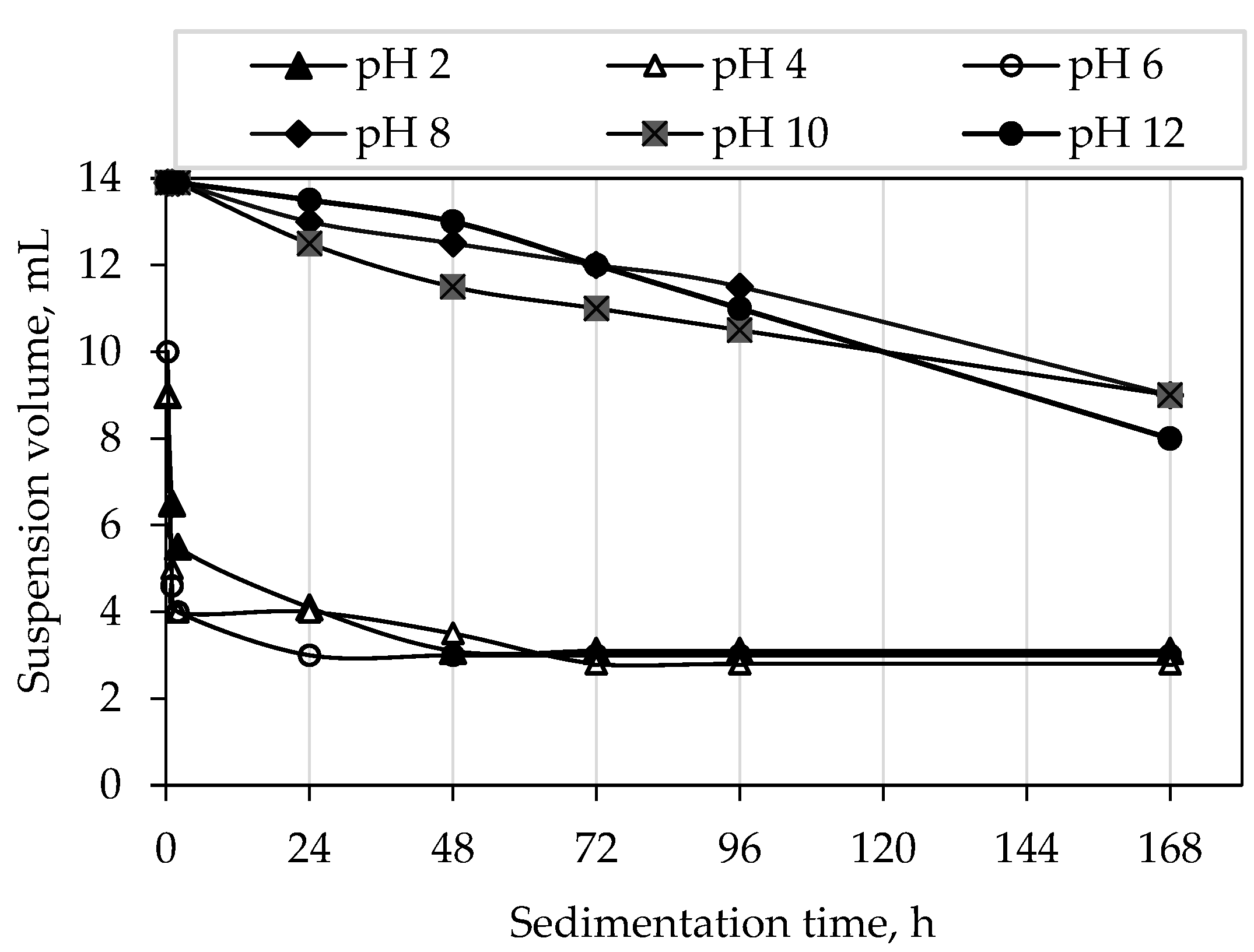
3.6. Sedimentation Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Dispersant | High-Purity Al2O3 Dry Powder Content (wt.%) | Waste Al2O3 Dry Powder Content (wt.%) | Water Content (wt.%) | * Dispersant Content (dwb.%) |
|---|---|---|---|---|
| Tiron | 55 | 15 | 30 | 0.04–0.1 |
| 50 | 20 | 30 | 0.04–0.1 | |
| 45 | 25 | 30 | 0.04–0.1 |
| Waste Al2O3 Dry Powder Content dwb.% | Tiron dwb.% | pH | η (mPa·s) | |
|---|---|---|---|---|
| γ = 50 s−1 | γ = 100 s−1 | |||
| 15 | 0.04 | 8.78 | 22.63 | 20.04 |
| 0.05 | 8.79 | 19.32 | 17.20 | |
| 0.06 | 8.75 | 17.93 | 15.97 | |
| 0.1 | 8.64 | 20.40 | 17.76 | |
| 0.2 | 8.41 | 25.62 | 21.19 | |
| 20 | 0.04 | 8.86 | 27.09 | 23.55 |
| 0.05 | 8.89 | 24.63 | 21.11 | |
| 0.06 | 8.86 | 26.71 | 22.83 | |
| 0.1 | 8.72 | 29.17 | 23.99 | |
| 0.2 | 8.57 | 40.10 | 29.18 | |
| 25 | 0.04 | 8.96 | 43.33 | 35.16 |
| 0.05 | 8.98 | 39.71 | 32.21 | |
| 0.06 | 8.92 | 36.48 | 29.34 | |
| 0.1 | 8.80 | 43.10 | 34.01 | |
| 0.2 | 8.43 | 54.79 | 39.21 | |
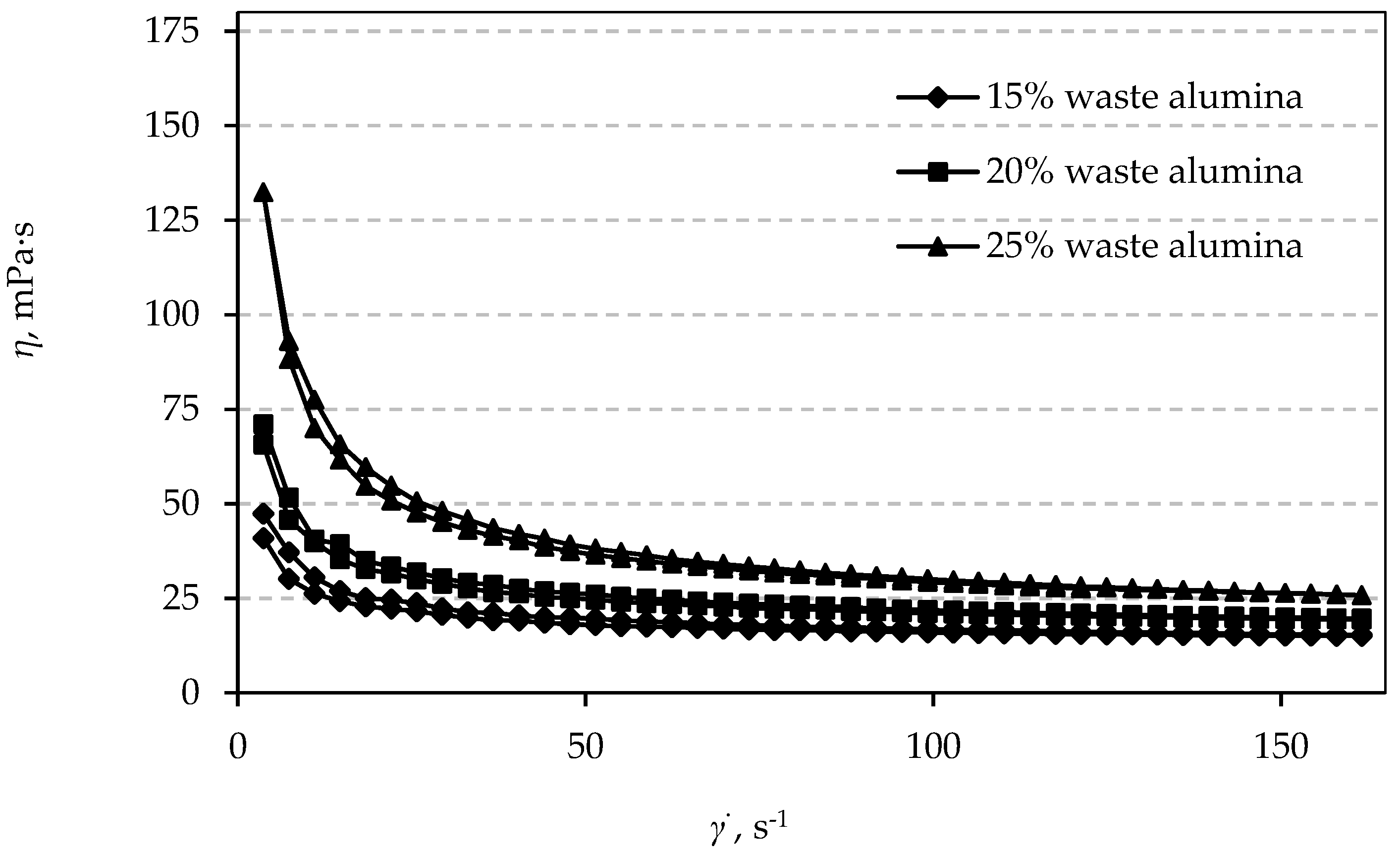
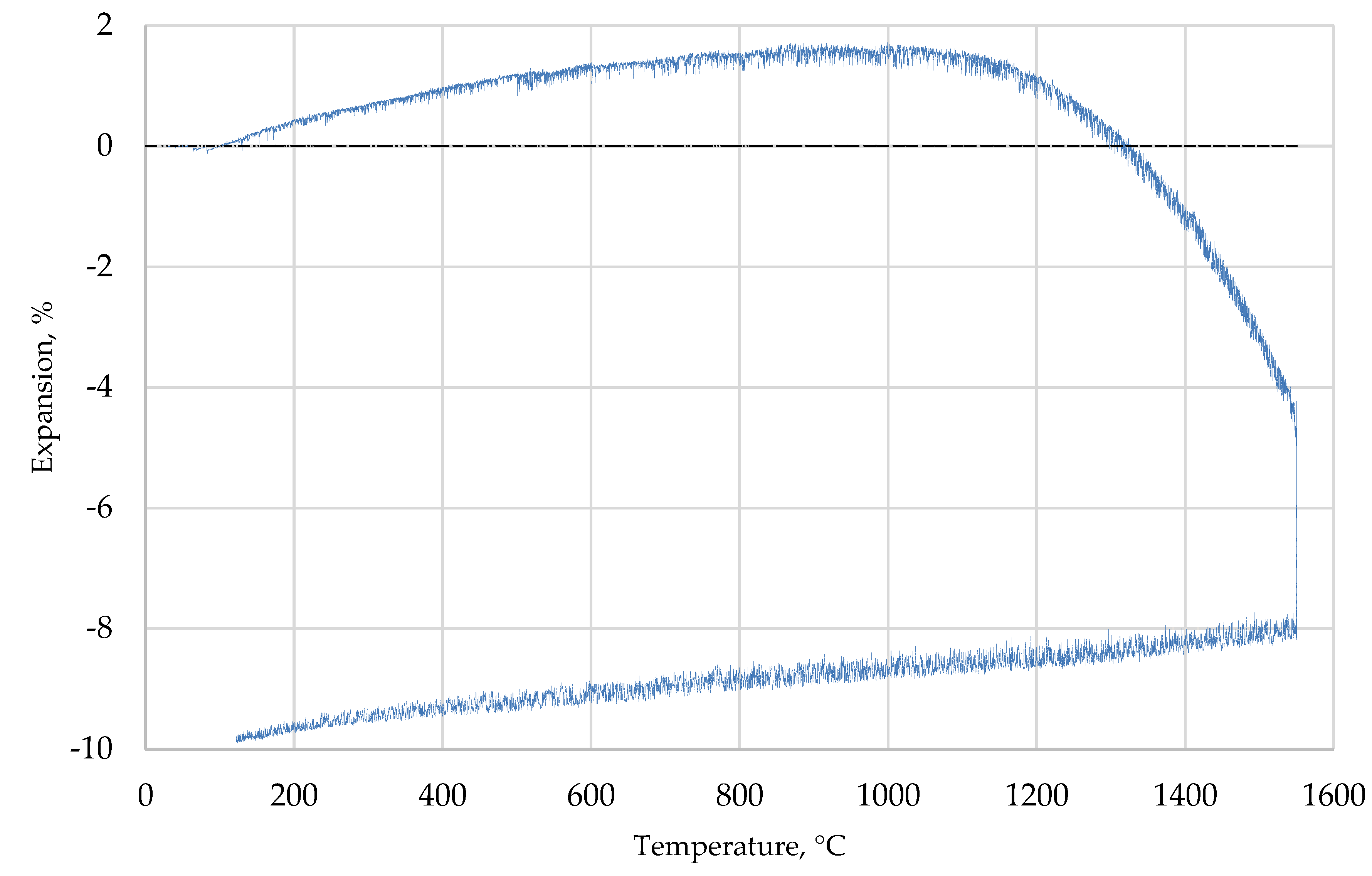
References
- Ruys, A.J. Alumina Ceramics: Biomedical and Clinical Applications; Woodhead Publishing: Sawston, UK, 2018; p. 580. [Google Scholar]
- Dentoni, V.; Grosso, B.; Massacci, G. Environmental Sustainability of the Alumina Industry in Western Europe. Sustainability 2014, 6. [Google Scholar] [CrossRef]
- Commission Decision Establishing a List of Wastes 2000/532/EC. Available online: https://publications.europa.eu/en/publication-detail/-/publication/239a2785-9115-4e06-adae-66c8e08a5a42 (accessed on 13 May 2019).
- Commission Notice on Technical Guidance on the Classification of Waste, C/2018/1447. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?toc=OJ:C:2018:124:TOC&uri=uriserv:OJ.C_.2018.124.01.0001.01.ENG (accessed on 13 May 2019).
- Directive on Waste 2008/98/EC. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32008L0098 (accessed on 13 May 2019).
- Closing the Loop—An EU Action Plan for the Circular Economy, COM/2015/0614. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52015DC0614 (accessed on 13 May 2019).
- Calambás Pulgarin, H.L.; Garrido, L.B.; Albano, M.P. Rheological properties of aqueous alumina–alumina-doped Y-PSZ suspensions. Ceram. Int. 2012, 38, 1843–1849. [Google Scholar] [CrossRef]
- Ortega, F.S.; Castro, R.H.R.; Gouvêa, D.; Pandolfelli, V.C. The rheological behavior and surface charging of gelcasting alumina suspensions. Ceram. Int. 2008, 34, 237–241. [Google Scholar] [CrossRef]
- Yu, J.; Yang, J.; Huang, Y. The transformation mechanism from suspension to green body and the development of colloidal forming. Ceram. Int. 2011, 37, 1435–1451. [Google Scholar] [CrossRef]
- Tari, G.; Ferreira, J.M.F.; Lyckfeldt, O. Influence of the stabilising mechanism and solid loading on slip casting of alumina. J. Eur. Ceram. Soc. 1998, 18, 479–486. [Google Scholar] [CrossRef]
- Takao, Y.; Hotta, T.; Naito, M.; Shinohara, N.; Okumiya, M.; Uematsu, K. Microstructure of alumina compact body made by slip casting. J. Eur. Ceram. Soc. 2002, 22, 397–401. [Google Scholar] [CrossRef]
- Tari, G.; Ferreira, J.M.F.; Fonseca, A.T. Influence of particle size and particle size distribution on drying-shrinkage behaviour of alumina slip cast bodies. Ceram. Int. 1999, 25, 577–580. [Google Scholar] [CrossRef]
- Jaafar, M.; Fantozzi, G.; Reveron, H. Preparation and Characterization of Pressureless Sintered Alumina/5 vol % SiC Micro-Nanocomposites. Ceramics 2018, 1. [Google Scholar] [CrossRef]
- Yang, Y.; Sigmund, W.M. A new approach to prepare highly loaded aqueous alumina suspensions with temperature sensitive rheological properties. J. Eur. Ceram. Soc. 2003, 23, 253–261. [Google Scholar] [CrossRef]
- Tseng, W.J.; Wu, C.H. Sedimentation, rheology and particle-packing structure of aqueous Al2O3 suspensions. Ceram. Int. 2003, 29, 821–828. [Google Scholar] [CrossRef]
- Chou, K.-S.; Lee, L.-J. Effect of Dispersants on the Rheological Properties and Slip Casting of Concentrated Alumina Slurry. J. Am. Ceram. Soc. 1989, 72, 1622–1627. [Google Scholar] [CrossRef]
- Penard, A.L.; Rossignol, F.; Nagaraja, H.S.; Pagnoux, C.; Chartier, T. Dispersion of alpha-alumina ultrafine powders using 2-phosphonobutane-1,2,4-tricarboxylic acid for the implementation of a DCC process. J. Eur. Ceram. Soc. 2005, 25, 1109–1118. [Google Scholar] [CrossRef]
- Jiang, L.; Gao, L. Effect of Tiron adsorption on the colloidal stability of nano-sized alumina suspension. Mater. Chem. Phys. 2003, 80, 157–161. [Google Scholar] [CrossRef]
- Gulicovski, J.J.; Čerović, L.S.; Milonjić, S.K. Stability of alumina suspensions in the presence of Tiron. Ceram. Int. 2008, 34, 23–26. [Google Scholar] [CrossRef]
- Briscoe, B.J.; Khan, A.U.; Luckham, P.F. Optimising the dispersion on an alumina suspension using commercial polyvalent electrolyte dispersants. J. Eur. Ceram. Soc. 1998, 18, 2141–2147. [Google Scholar] [CrossRef]
- Park, J.M.; Han, J.S.; Gal, C.W.; Oh, J.W.; Kate, K.H.; Atre, S.V.; Kim, Y.; Park, S.J. Effect of binder composition on rheological behavior of PMN-PZT ceramic feedstock. Powder Technol. 2018, 330, 19–26. [Google Scholar] [CrossRef]
- Taktak, R.; Baklouti, S.; Bouaziz, J. Effect of binders on microstructural and mechanical properties of sintered alumina. Mater. Charact. 2011, 62, 912–916. [Google Scholar] [CrossRef]
- Chovanec, J.; Ghillányová, K.; Ráheľ, J.; Šajgalík, P.; Galusek, D. The influence of dopants on loss tangent of polycrystalline alumina ceramics. Ceram. Int. 2012, 38, 2043–2049. [Google Scholar] [CrossRef]
- Barmala, M.; Moheb, A.; Emadi, R. Applying Taguchi method for optimization of the synthesis condition of nano-porous alumina membrane by slip casting method. J. Alloys Compd. 2009, 485, 778–782. [Google Scholar] [CrossRef]
- Pal, S.; Bandyopadhyay, A.K.; Pal, P.G.; Mukherjee, S.; Samaddar, B.N. Sintering behaviour of spinel-alumina composites. Bull. Mater. Sci. 2009, 32, 169–176. [Google Scholar] [CrossRef]
- Billotte, C.; Fotsing, E.R.; Ruiz, E. Optimization of Alumina Slurry for Oxide-Oxide Ceramic Composites Manufactured by Injection Molding. Adv. Mater. Sci. Eng. 2017, 2017, 9. [Google Scholar] [CrossRef]
- Yalamaç, E. Effect of spinel addition on the sintering behavior and microstructure of alumina-spinel ceramics. Ceram. Silikáty 2014, 58, 314–319. [Google Scholar]
- Majić Renjo, M.; Lalić, M.; Ćurković, L.; Matijašić, G. Rheological properties of aqueous alumina suspensions. Mater. Werkst. 2012, 43, 979–983. [Google Scholar] [CrossRef]
- Thorat, N.; Khot, V.; Salunkhe, A.; Ningthoujam, F.N.A.S.R.; Pawar, S. Surface Functionalized LSMO Nanoparticles with Improved Colloidal Stability for Hyperthermia Applications. J. Phys. D Appl. Phys 2013, 46, 105003. [Google Scholar] [CrossRef]
- Mikkola, P.; Ylhä, P.; Levänen, E.; Rosenholm, J.B. Effect of impurities on dispersion properties of alpha-alumina powder. Ceram. Int. 2004, 30, 291–299. [Google Scholar] [CrossRef]
- Singh, B.P.; Menchavez, R.; Takai, C.; Fuji, M.; Takahashi, M. Stability of dispersions of colloidal alumina particles in aqueous suspensions. J. Colloid Interface Sci. 2005, 291, 181–186. [Google Scholar] [CrossRef]
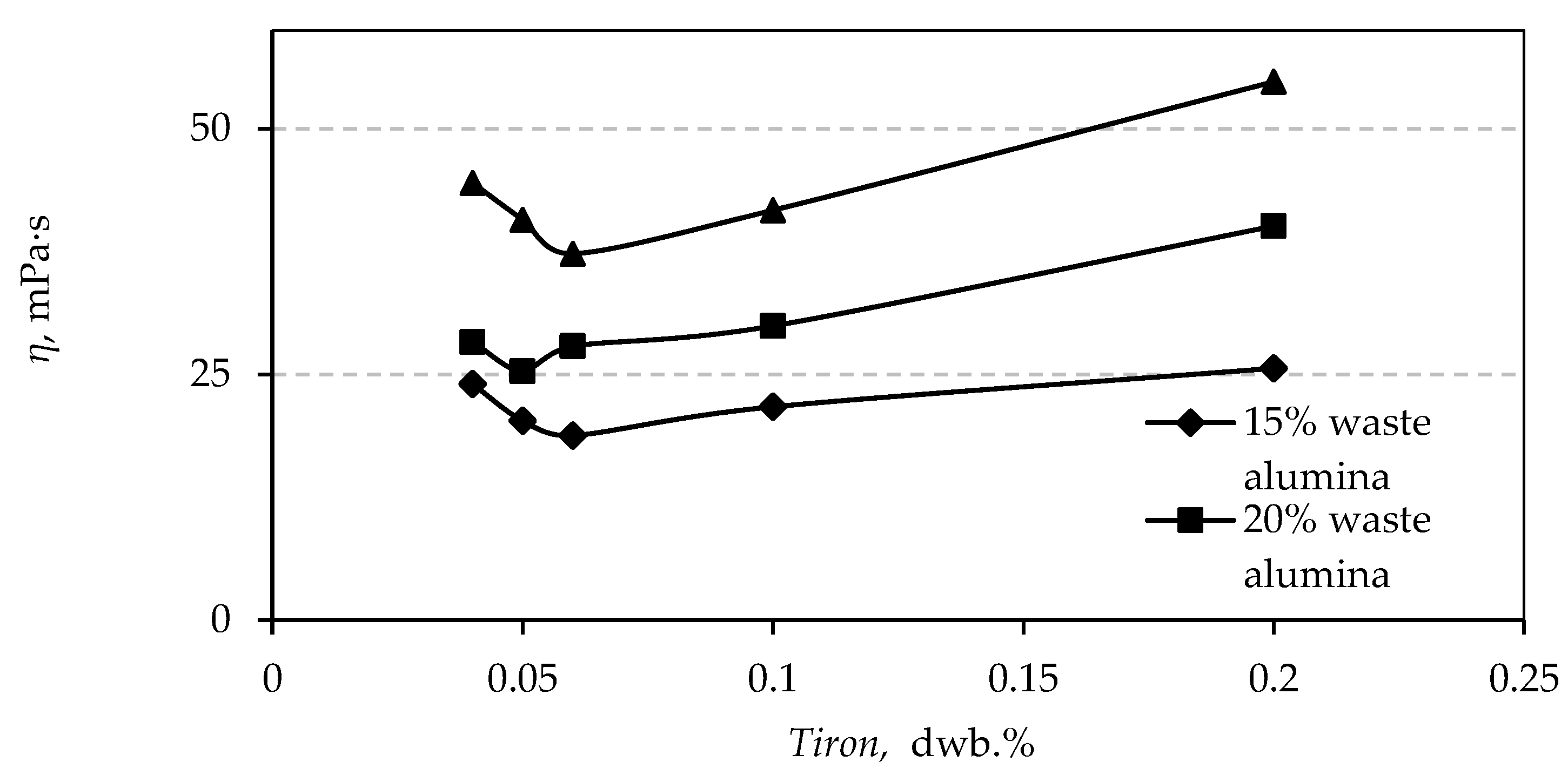
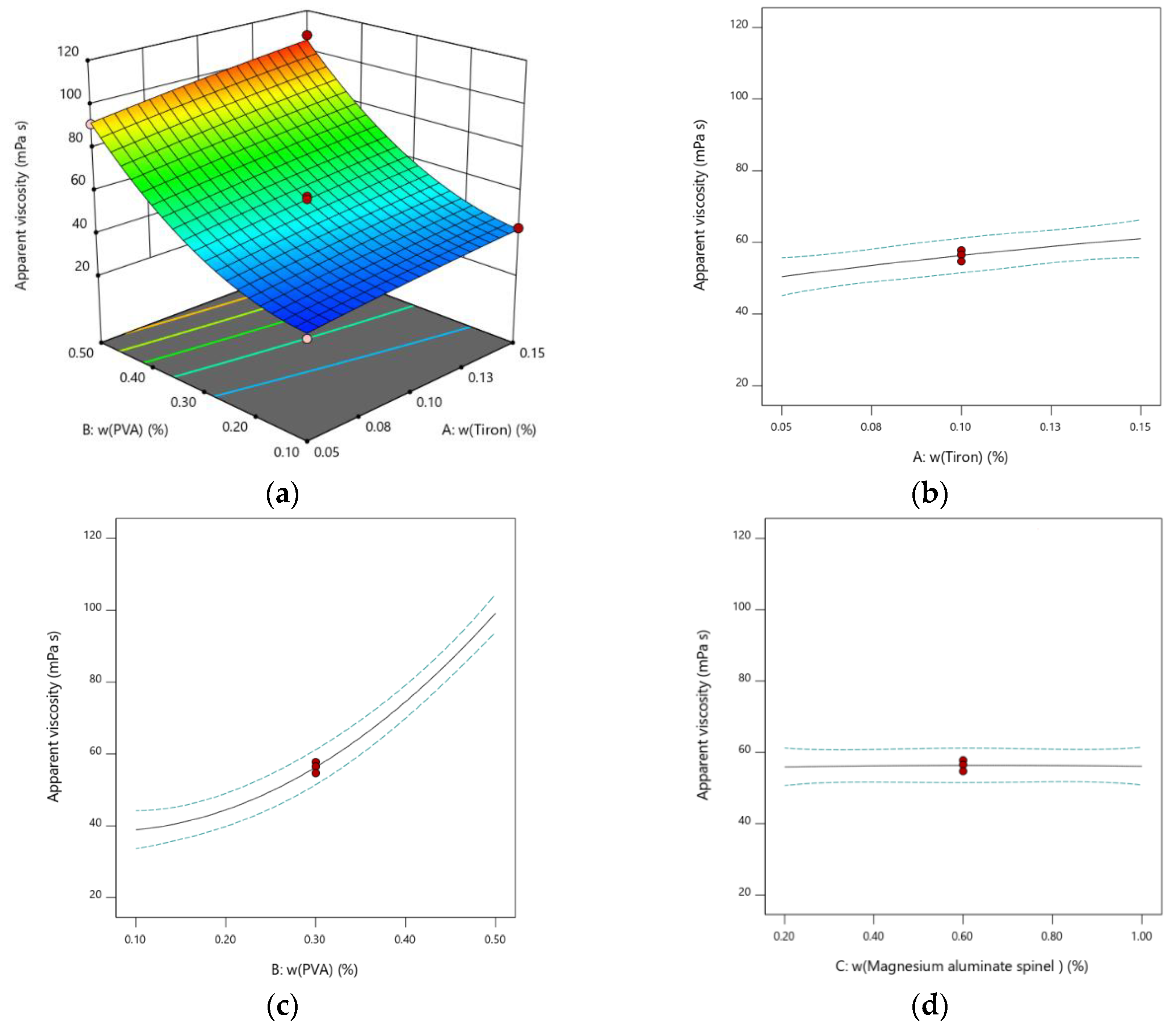
| Experiment | Factor A: Tiron, dwb.% | Factor B: PVA dwb.% | Factor C: MgAl2O4 dwb.% | Response Apparent Viscosity mPa·s | Predicted Apparent Viscosity mPa·s |
|---|---|---|---|---|---|
| 1 | 0.1 | 0.1 | 1 | 40.72 | 38.06 |
| 2 | 0.05 | 0.3 | 0.2 | 51.03 | 47.79 |
| 3 | 0.05 | 0.3 | 1 | 52.22 | 52.45 |
| 4 | 0.15 | 0.3 | 1 | 55.34 | 58.58 |
| 5 | 0.1 | 0.5 | 0.2 | 95.51 | 98.16 |
| 6 | 0.15 | 0.5 | 0.6 | 107.75 | 105.33 |
| 7 | 0.1 | 0.3 | 0.6 | 54.68 | 56.33 |
| 8 | 0.1 | 0.3 | 0.6 | 57.76 | 56.33 |
| 9 | 0.15 | 0.1 | 0.6 | 42.79 | 42.20 |
| 10 | 0.05 | 0.5 | 0.6 | 91.26 | 91.86 |
| 11 | 0.1 | 0.5 | 1 | 100.41 | 99.59 |
| 12 | 0.1 | 0.3 | 0.6 | 56.53 | 56.33 |
| 13 | 0.1 | 0.1 | 0.2 | 38.33 | 39.15 |
| 14 | 0.05 | 0.1 | 0.6 | 32.02 | 34.44 |
| 15 | 0.15 | 0.3 | 0.2 | 63.11 | 62.89 |
| Source | Sum of Squares | df * | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 8132.05 | 9 | 903.56 | 84.05 | < 0.0001 |
| A (Tiron) | 225.42 | 1 | 225.42 | 20.97 | 0.0060 |
| B (PVA) | 7264.81 | 1 | 7264.81 | 675.76 | < 0.0001 |
| C (MgAl2O4) | 0.062 | 1 | 0.062 | 0.005738 | 0.9426 |
| AB | 8.16 | 1 | 8.16 | 0.76 | 0.4234 |
| AC | 20.07 | 1 | 20.07 | 1.87 | 0.2300 |
| BC | 1.58 | 1 | 1.58 | 0.15 | 0.7171 |
| A2 | 1.29 | 1 | 1.29 | 0.12 | 0.7428 |
| B2 | 597.62 | 1 | 597.62 | 55.59 | 0.0007 |
| C2 | 0.35 | 1 | 0.35 | 0.033 | 0.8635 |
| Residual | 53.75 | 5 | 10.75 | ||
| Pure Error | 4.80 | 2 | 2.40 | ||
| Cor. Total | 8185.80 | 14 | R2 | 0.9934 | |
| Std. Dev. | 3.28 | Adjusted R2 | 0.9816 | ||
| Mean | 62.63 | Predicted R2 | 0.9030 | ||
| C.V. % | 5.24 | Adequate Precision | 26.478 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vukšić, M.; Žmak, I.; Ćurković, L.; Ćorić, D. Effect of Additives on Stability of Alumina—Waste Alumina Suspension for Slip Casting: Optimization Using Box-Behnken Design. Materials 2019, 12, 1738. https://doi.org/10.3390/ma12111738
Vukšić M, Žmak I, Ćurković L, Ćorić D. Effect of Additives on Stability of Alumina—Waste Alumina Suspension for Slip Casting: Optimization Using Box-Behnken Design. Materials. 2019; 12(11):1738. https://doi.org/10.3390/ma12111738
Chicago/Turabian StyleVukšić, Milan, Irena Žmak, Lidija Ćurković, and Danko Ćorić. 2019. "Effect of Additives on Stability of Alumina—Waste Alumina Suspension for Slip Casting: Optimization Using Box-Behnken Design" Materials 12, no. 11: 1738. https://doi.org/10.3390/ma12111738
APA StyleVukšić, M., Žmak, I., Ćurković, L., & Ćorić, D. (2019). Effect of Additives on Stability of Alumina—Waste Alumina Suspension for Slip Casting: Optimization Using Box-Behnken Design. Materials, 12(11), 1738. https://doi.org/10.3390/ma12111738