Experimental Investigation on Graphene Oxide/SrCl2·6H2O Modified CaCl2·6H2O and the Resulting Thermal Performances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
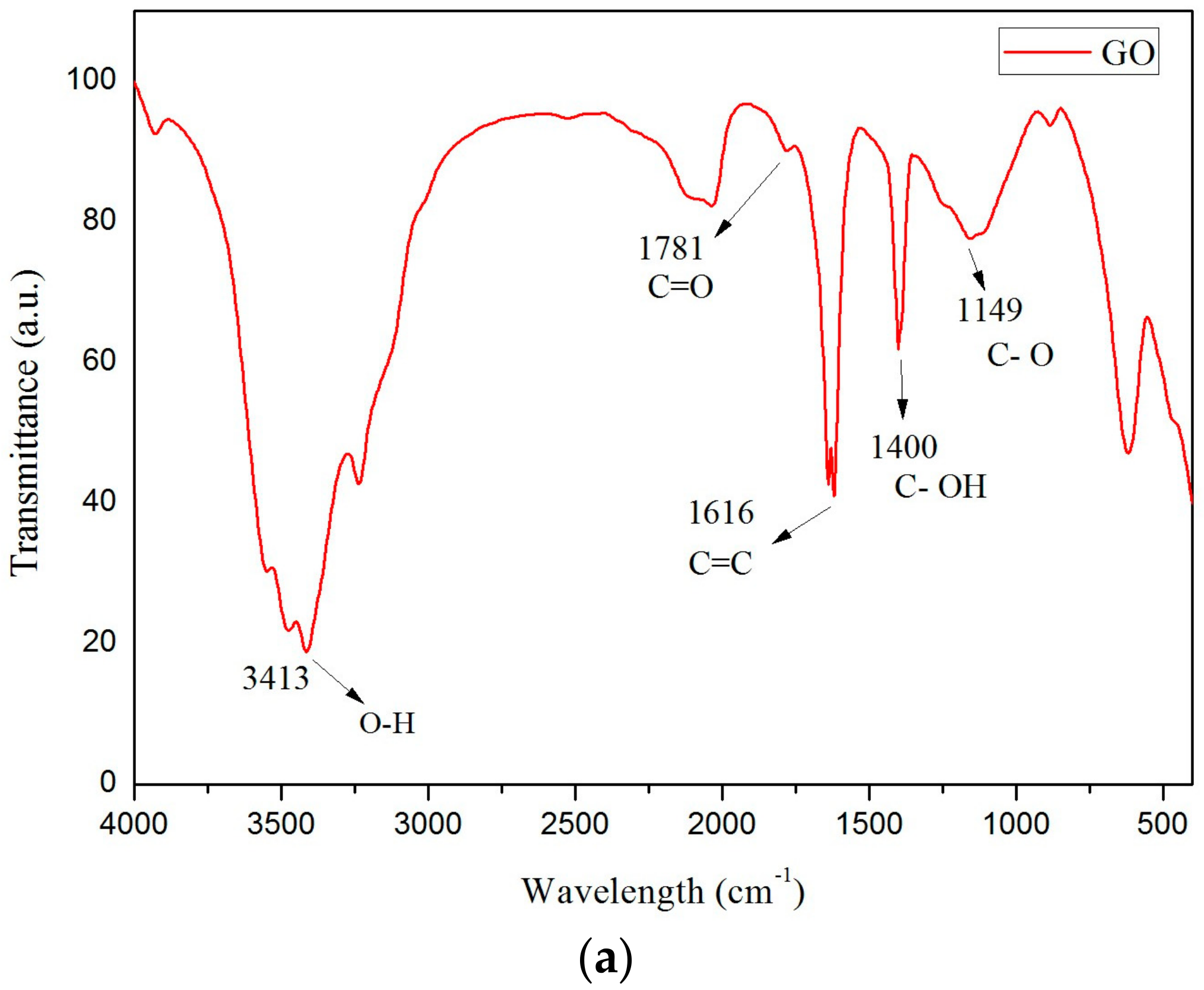
2.2. Preparation of Modified CaCl2·6H2O
2.3. Thermo-Physical Performance of Modified CaCl2·6H2O
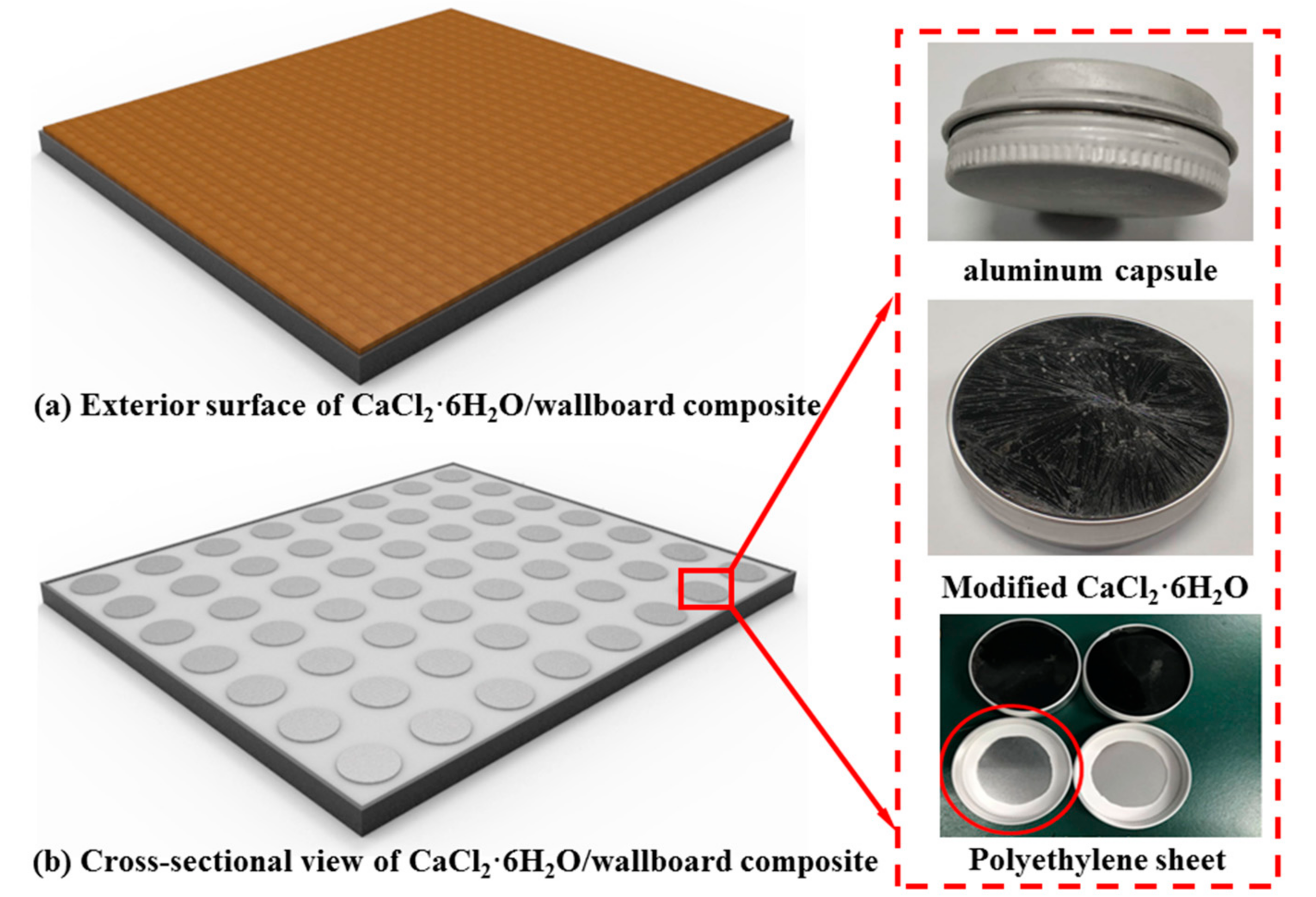
2.4. Encapsulation of Modified CaCl2·6H2O
2.5. Preparation of Modified CaCl2·6H2O/Wallboard
2.6. Thermo-Regulated Performance of Modified CaCl2·6H2O
3. Results and Discussions
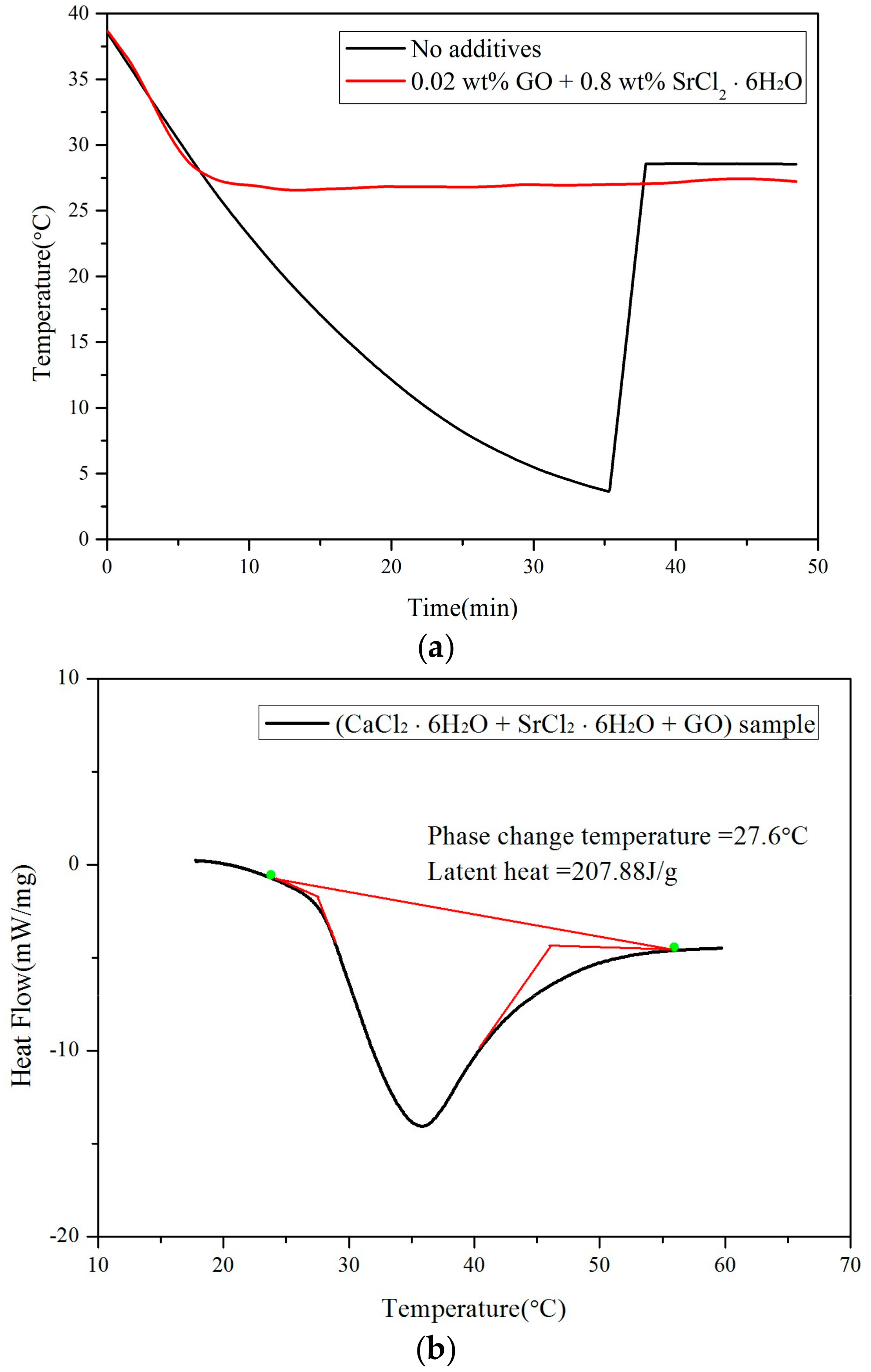
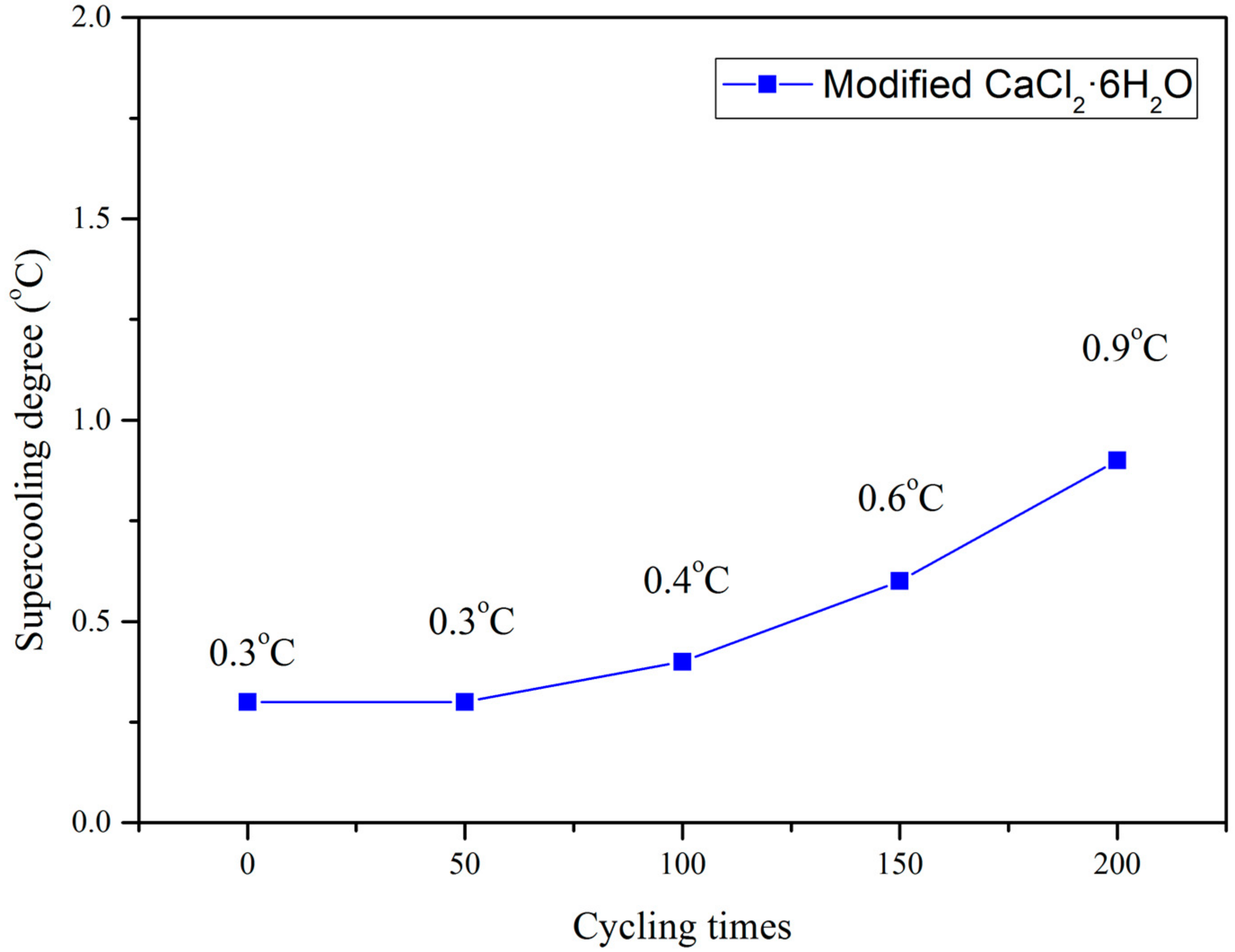
3.1. Thermo-Physical Performance of Modified CaCl2·6H2O
3.2. Thermo-Regulated Performance of Modified CaCl2·6H2O
4. Conclusions
- (1)
- In this study, graphene and SrCl2·6H2O were utilized as nano nucleating agents and successfully reduced the supercooling of pure CaCl2·6H2O to around 0.3 °C which was much lower than that of the original pure CaCl2·6H2O (25.4 °C). The latent heat value and phase change temperature of modified CaCl2·6H2O were 207.88 J/g and 27.6 °C, respectively.
- (2)
- The supercooling degree of modified CaCl2·6H2O after 200 thermal cycles was still much lower than that of non-modified PCM.
- (3)
- Aluminum capsules wrapped with polypropylene bag can effectively prevent leakage and deliquescence of PCM.
- (4)
- The infrared thermography showed that the temperature difference between the top and bottom layers of modified CaCl2·6H2O/wallboard composite was 15.8 °C after heating for one hour, while it was 4.9 °C for control wallboard. The results further demonstrated the excellent thermal energy storage and thermal-regulated capacity of modified CaCl2·6H2O.
Author Contributions
Funding
Conflicts of Interest
References
- Souayfane, F.; Fardoun, F.; Biwole, P.H. Phase change materials (PCM) for cooling applications in buildings: A review. Energy Build. 2016, 129, 396–431. [Google Scholar] [CrossRef]
- Asimakopoulou, E.K.; Kolaitis, D.I.; Founti, M.A. Fire safety aspects of PCM-enhanced gypsum plasterboards: An experimental and numerical investigation. Fire Saf. J. 2015, 72, 50–58. [Google Scholar] [CrossRef]
- Li, T.X.; Wu, D.L.; He, F.; Wang, R.Z. Experimental investigation on copper foam/hydrated salt composite phase change material for thermal energy storage. Int. J. Heat Mass Transfer 2017, 115, 148–157. [Google Scholar] [CrossRef]
- Kasaeian, A.; Bahrami, L.; Pourfayaz, F.; Khodabandeh, E.; Yan, W.M. Experimental studies on the applications of PCMs and nano-PCMs in buildings: A critical review. Energy Build. 2017, 154, 96–112. [Google Scholar] [CrossRef]
- Veerakumar, C.; Sreekumar, A. Phase change material based cold thermal energy storage: Materials, techniques and applications—A review. Int. J. Refrig. 2016, 67, 271–289. [Google Scholar] [CrossRef]
- Jin, X.; Medina, M.A.; Zhang, X.S. On the importance of the location of PCMs in building walls for enhanced thermal performance. Appl. Energy 2013, 106, 72–78. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Cheng, Z.D.; Jia, L.S.; Mo, S.P.; Liu, Z.W. Ultrahigh specific surface area of graphene for eliminating subcooling of water. Appl. Energy 2014, 130, 824–829. [Google Scholar] [CrossRef]
- Liu, Y.D.; Su, C.J.; Hu, P.; Peng, Q.G.; Wei, L.Z.; Wang, J.Q. Containerless nucleation behavior and supercooling degree of acoustically levitated graphene oxide nanofluid PCM. Int. J. Refrig. 2015, 60, 70–80. [Google Scholar]
- Liu, Y.D.; Li, X.; Hu, P.F.; Hu, G.H. Study on the supercooling degree and nucleation behavior of water-based graphene oxide nanofluids PCM. Int. J. Refrig. 2015, 50, 80–86. [Google Scholar] [CrossRef]
- Li, G.; Zhang, B.B.; Li, X.; Zhou, Y.; Sun, Q.G.; Yun, Q. The preparation, characterization and modification of a new phase change material: CaCl2·6H2O-MgCl2·6H2O eutectic hydrate salt. Sol. Energy Mater. Sol. Cells 2014, 126, 51–55. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Nian, H.E.; Ren, X.F.; Dong, O.Y.; Hai, C.X.; Shen, Y.; Zeng, J.B. Phase change behavior of latent heat storage media based on calcium chloride hexahydrate composites containing strontium chloride hexahydrate and oxidation expandable graphite. Appl. Therm. Eng. 2016, 102, 38–44. [Google Scholar] [CrossRef]
- Cui, H.; Liao, W.; Mi, X.; Lo, T.Y.; Chen, D. Study on functional and mechanical properties of cement mortar with graphite-modified microencapsulated phase-change materials. Energy Build. 2015, 105, 273–284. [Google Scholar] [CrossRef]
- Fu, L.L.; Wang, Q.H.; Ye, R.D.; Fang, X.M.; Zhang, Z.G. A calcium chloride hexahydrate/expanded perlite composite with good heat storage and insulation properties for building energy conservation. Renew. Energy 2017, 114, 733–743. [Google Scholar] [CrossRef]
- Fu, L.L.; Ling, Z.Y.; Fang, X.M.; Zhang, Z.G. Thermal performance of CaCl2·6H2O/expanded perlite composite phase change boards embedded in aluminous gusset plates for building energy conservation. Energy Build. 2017, 155, 484–491. [Google Scholar] [CrossRef]
- Xu, X.; Cui, H.; Memon, S.A.; Yang, H.; Tang, W. Development of novel composite PCM for thermal energy storage using CaCl2·6H2O with graphene oxide and SrCl2·6H2O. Energy Build. 2017, 156, 163–172. [Google Scholar] [CrossRef]
- Liu, Y.S.; Yang, Y.Z. Form-stable phase change material based on Na2CO3·10H2O-Na2HPO4·12H2O eutectic hydrated salt/expanded graphite oxide composite: The influence of chemical structures of expanded graphite oxide. Renew. Energy 2018, 115, 734–740. [Google Scholar] [CrossRef]
- Chen, W.F.; Yan, L.F.; Bangal, P.R. Preparation of graphene by the rapid and mild thermal reduction of graphene oxide induced by microwaves. Carbon 2010, 48, 1146–1152. [Google Scholar] [CrossRef]
- Nethravathi, C.; Rajamathi, M. Chemically modified graphene sheets produced by the solvothermal reduction of colloidal dispersions of graphite oxide. Carbon 2008, 46, 1994–1998. [Google Scholar] [CrossRef]
- Jiao, X.; Zhang, L.Y.; Qiu, Y.S.; Guan, J.F. Comparison of the adsorption of cationic blue onto graphene oxides prepared from natural graphites with different graphitization degrees. Colloids Surf. A. Physicochem. Eng. Aspects 2017, 529, 292–301. [Google Scholar] [CrossRef]
- Kenisarin, M.; Mahkamov, K. Salt hydrates as latent heat storage materials: Thermophysical properties and costs. Sol. Energy Mater. Sol. Cells 2016, 145, 255–286. [Google Scholar] [CrossRef]

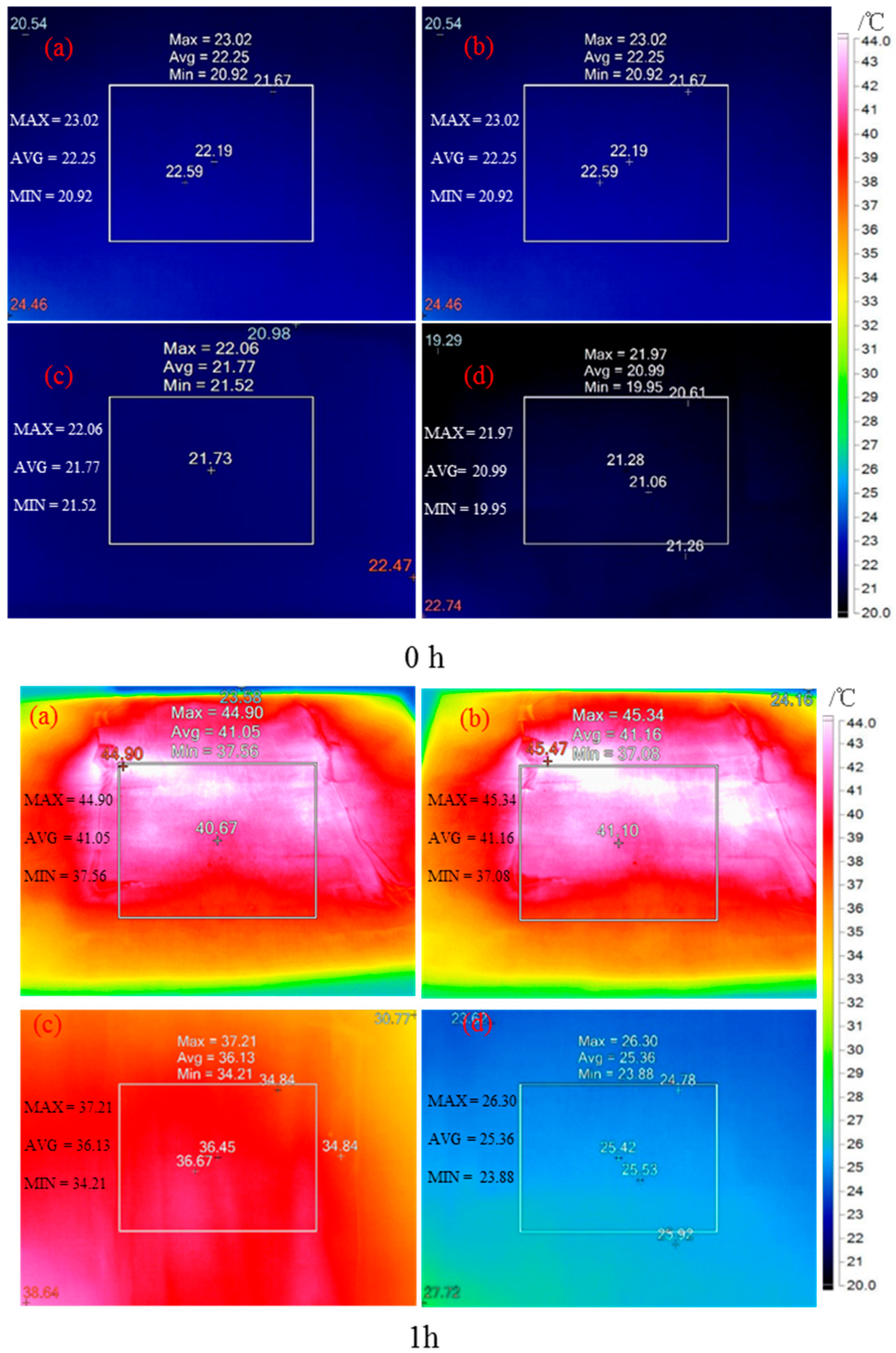
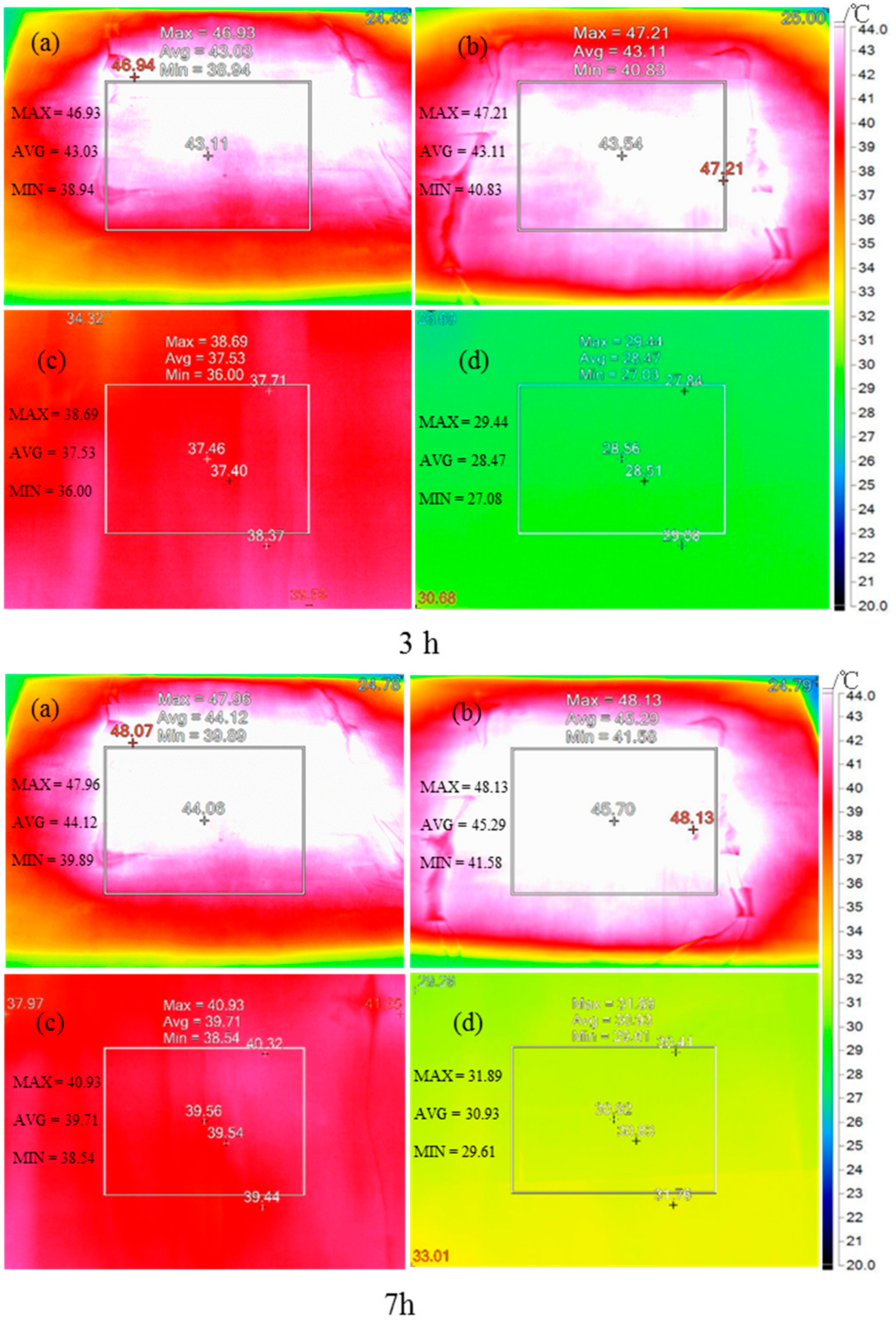
| No. | Taverage | ||||
|---|---|---|---|---|---|
| Surface | 0 | 1 h | 3 h | 7 h | |
| Control group | Top | 22.3 | 41.1 | 43.0 | 44.1 |
| Bottom | 21.8 | 36.1 | 37.5 | 39.7 | |
| CaCl2·6H2O/woodboard | Top | 22.3 | 41.2 | 43.1 | 45.3 |
| Bottom | 21.0 | 25.4 | 28.5 | 30.9 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Z.; Tian, Y.; Xu, X.; Cui, H.; Tang, W.; Yun, Y.; Sun, G. Experimental Investigation on Graphene Oxide/SrCl2·6H2O Modified CaCl2·6H2O and the Resulting Thermal Performances. Materials 2018, 11, 1507. https://doi.org/10.3390/ma11091507
Jin Z, Tian Y, Xu X, Cui H, Tang W, Yun Y, Sun G. Experimental Investigation on Graphene Oxide/SrCl2·6H2O Modified CaCl2·6H2O and the Resulting Thermal Performances. Materials. 2018; 11(9):1507. https://doi.org/10.3390/ma11091507
Chicago/Turabian StyleJin, Zhiyang, Yuanyuan Tian, Xiaoxiao Xu, Hongzhi Cui, Waiching Tang, Yanchun Yun, and Guoxing Sun. 2018. "Experimental Investigation on Graphene Oxide/SrCl2·6H2O Modified CaCl2·6H2O and the Resulting Thermal Performances" Materials 11, no. 9: 1507. https://doi.org/10.3390/ma11091507
APA StyleJin, Z., Tian, Y., Xu, X., Cui, H., Tang, W., Yun, Y., & Sun, G. (2018). Experimental Investigation on Graphene Oxide/SrCl2·6H2O Modified CaCl2·6H2O and the Resulting Thermal Performances. Materials, 11(9), 1507. https://doi.org/10.3390/ma11091507







