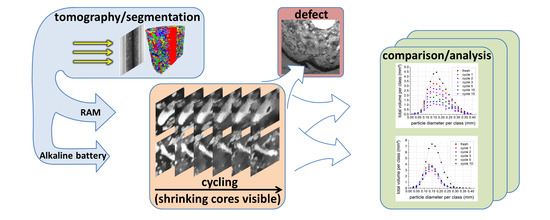
An X-ray Tomographic Study of Rechargeable Zn/MnO2 Batteries
Abstract
1. Introduction
2. Experimental Set-Up and Data Processing
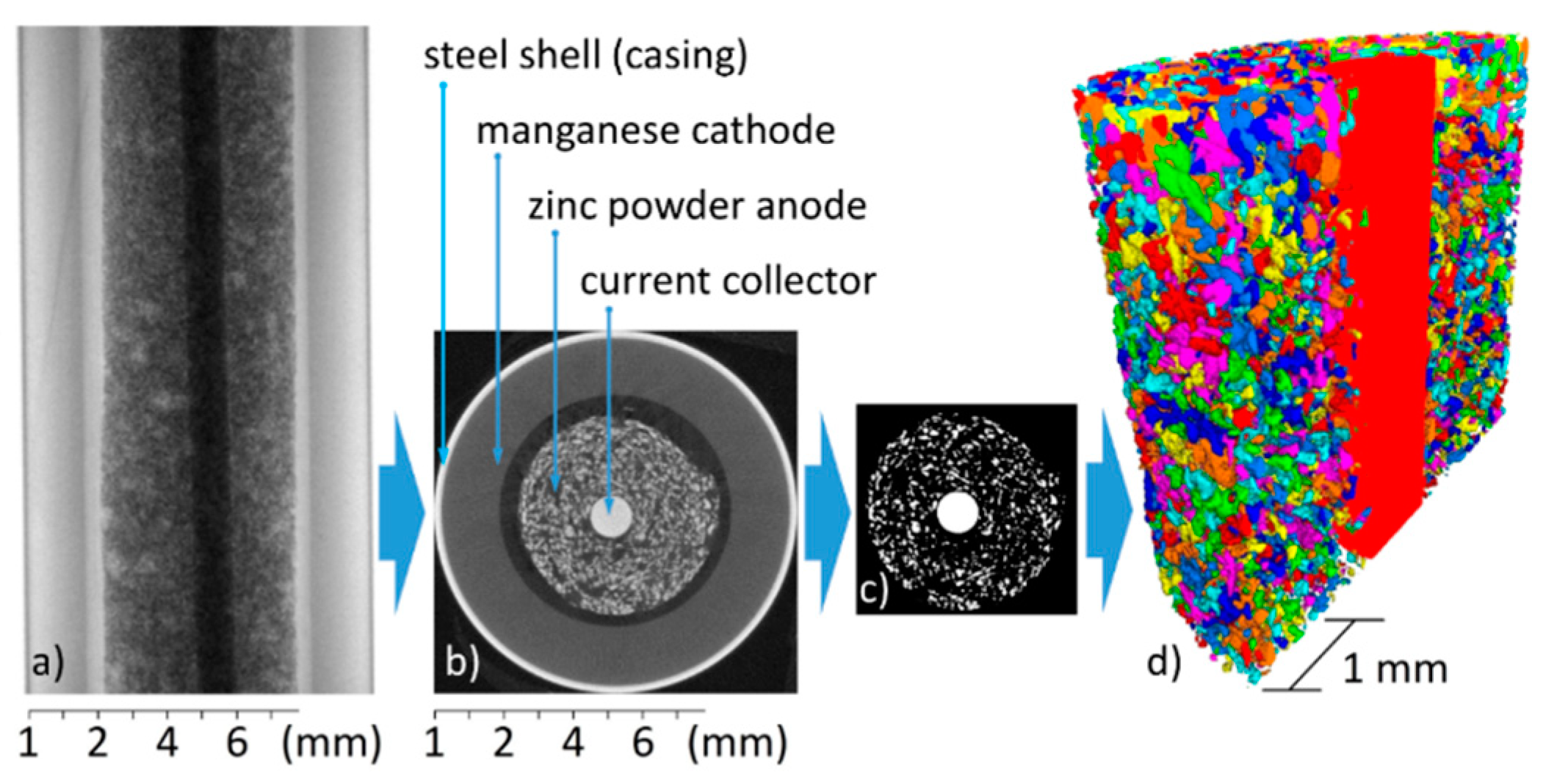
2.1. The Alkaline-Manganese Battery
2.1.1. Set-Up
2.1.2. Chemical Processes in an Alkaline-Manganese Battery
2.1.3. Setup for Charge and Discharge of Alkaline-Manganese Batteries
2.1.4. X-ray Tomography System and Measurement Procedure
2.2. Data Processing
2.2.1. Median Filter
2.2.2. Threshold Filter/Binarization
2.2.3. Erosion/Dilation
2.2.4. Watershed Transformation
2.2.5. Location Retrieval
3. Experimental Results and Discussion
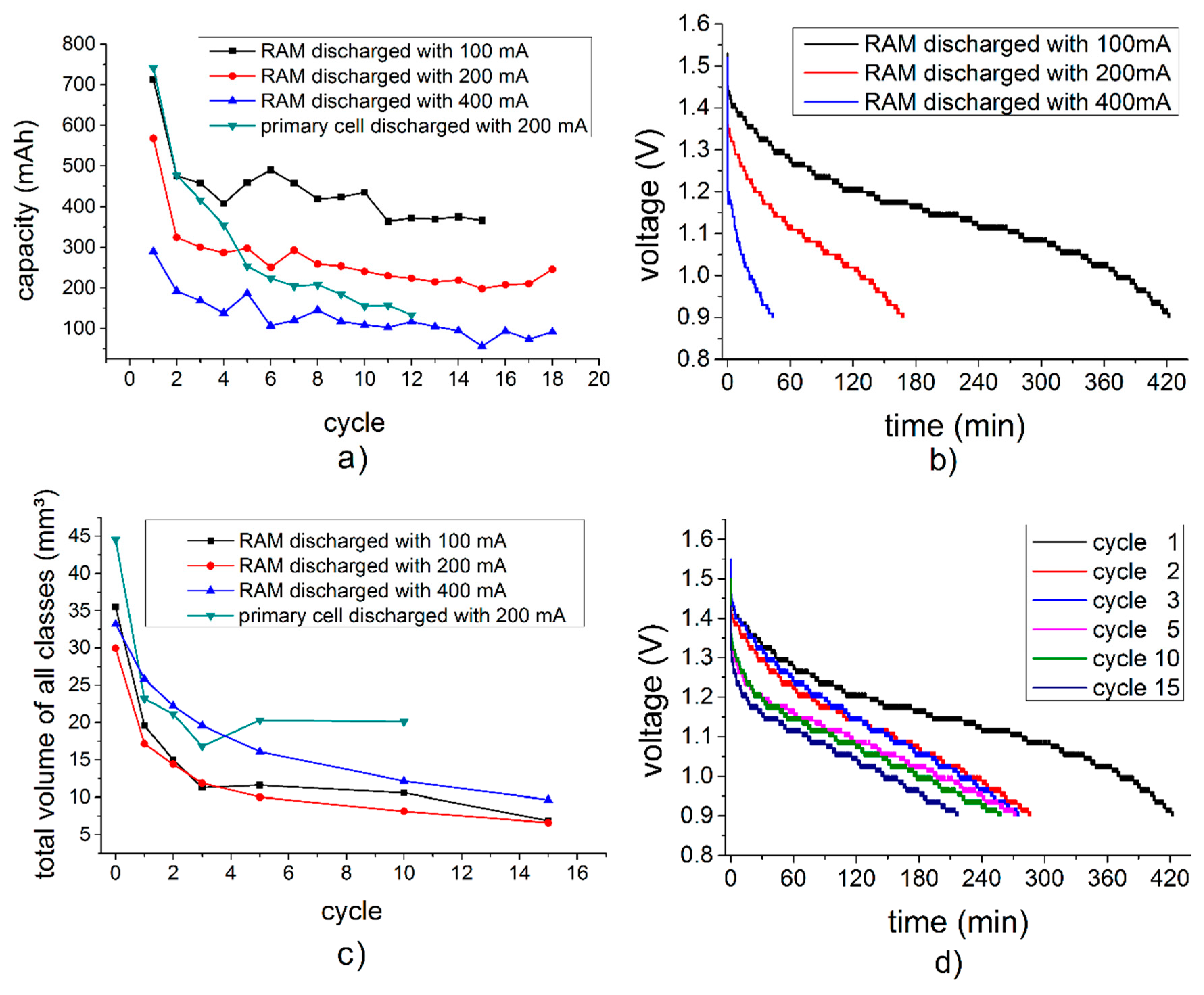
3.1. Properties of the Cells at Different Discharge Rates and Numbers of Cycles
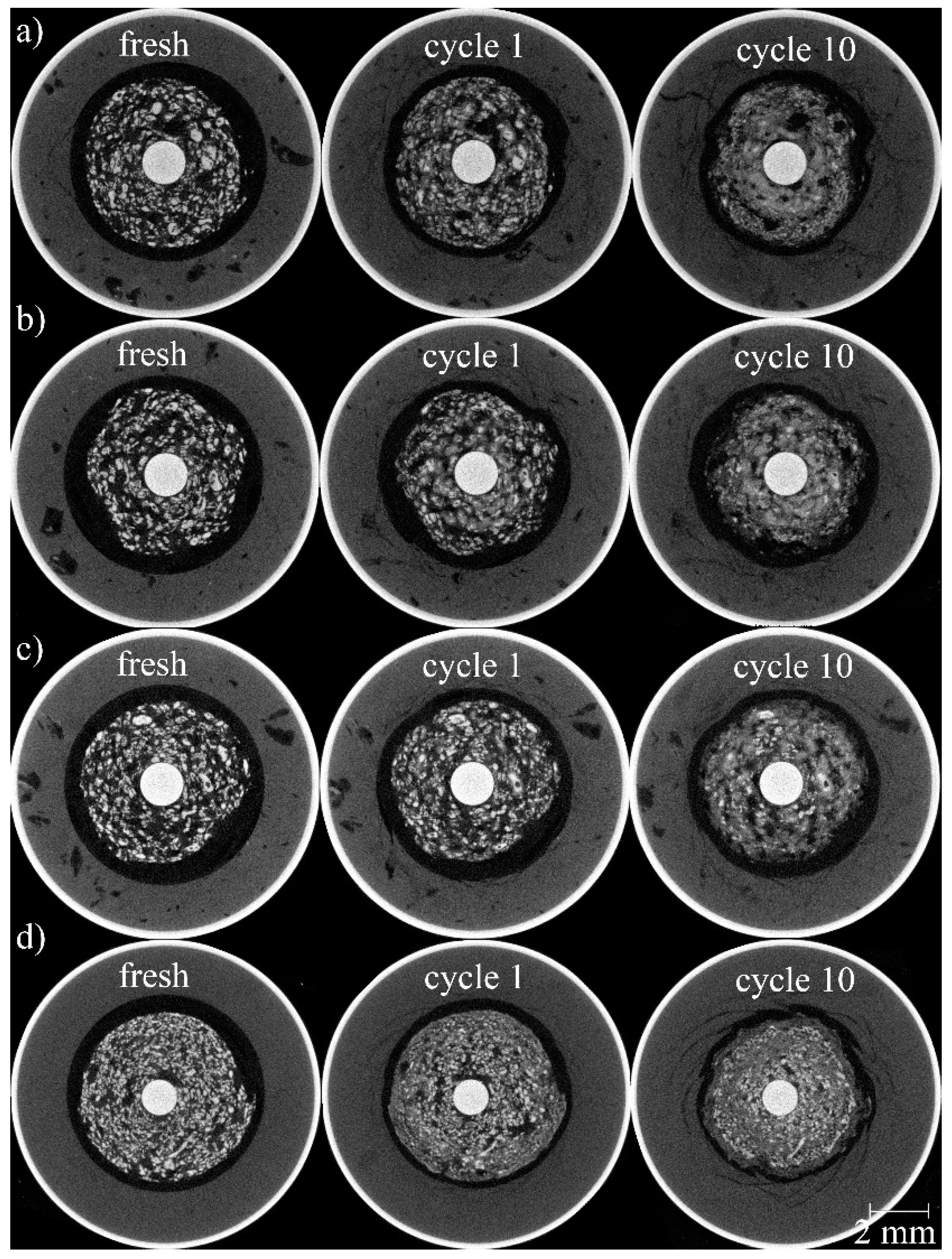
3.2. 3D Structural Analysis
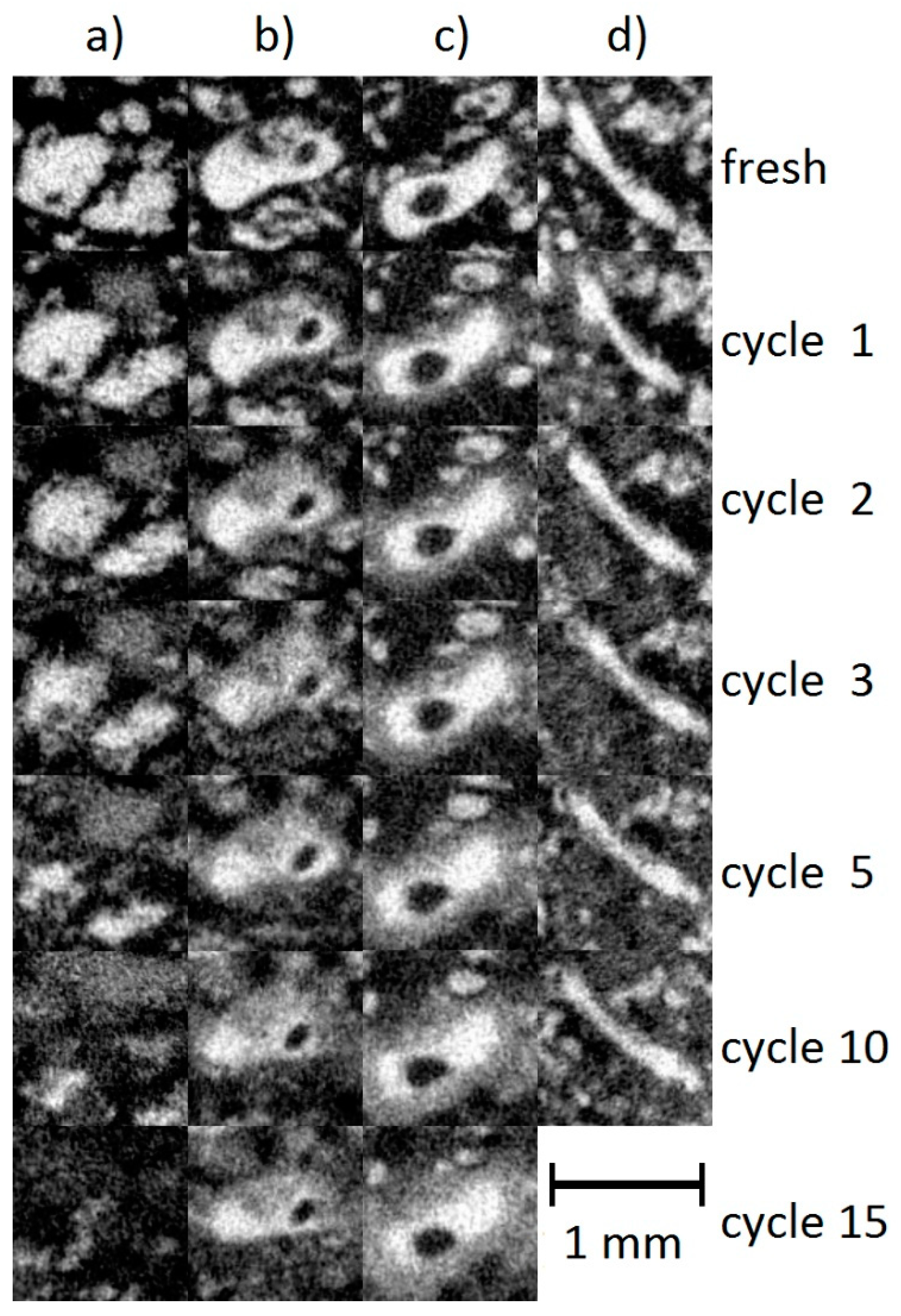
3.3. Dissolution of Individual Particles During Cycling
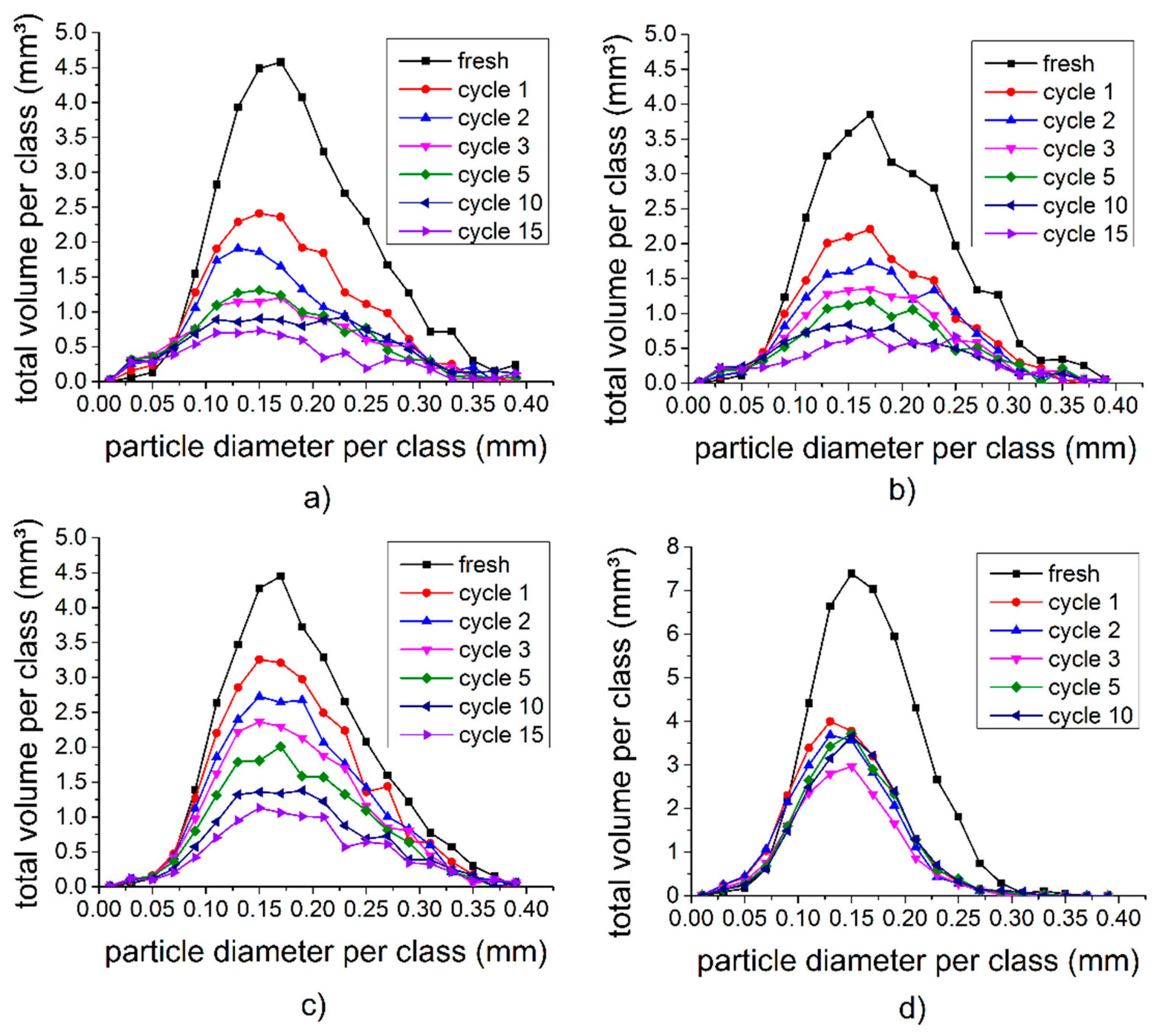
3.4. Quantitative 3D Analysis
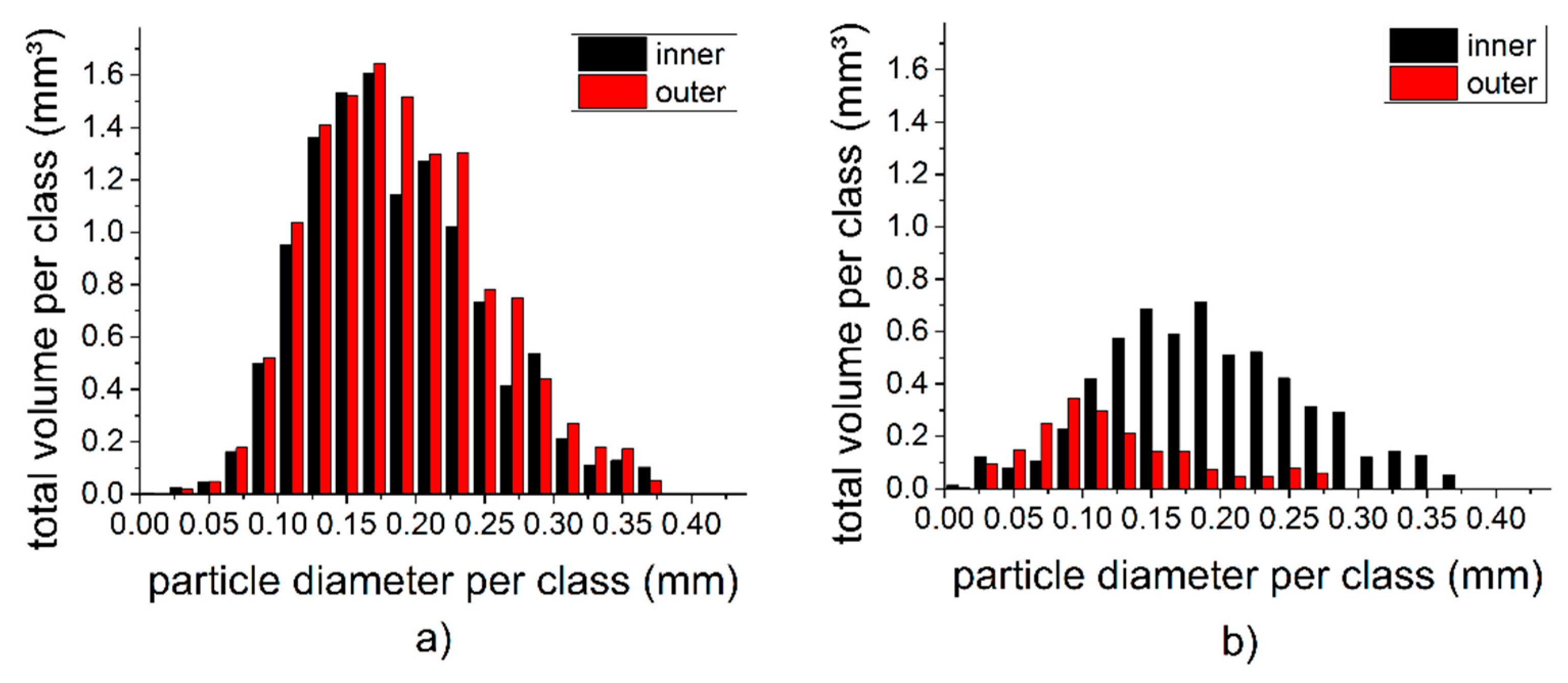
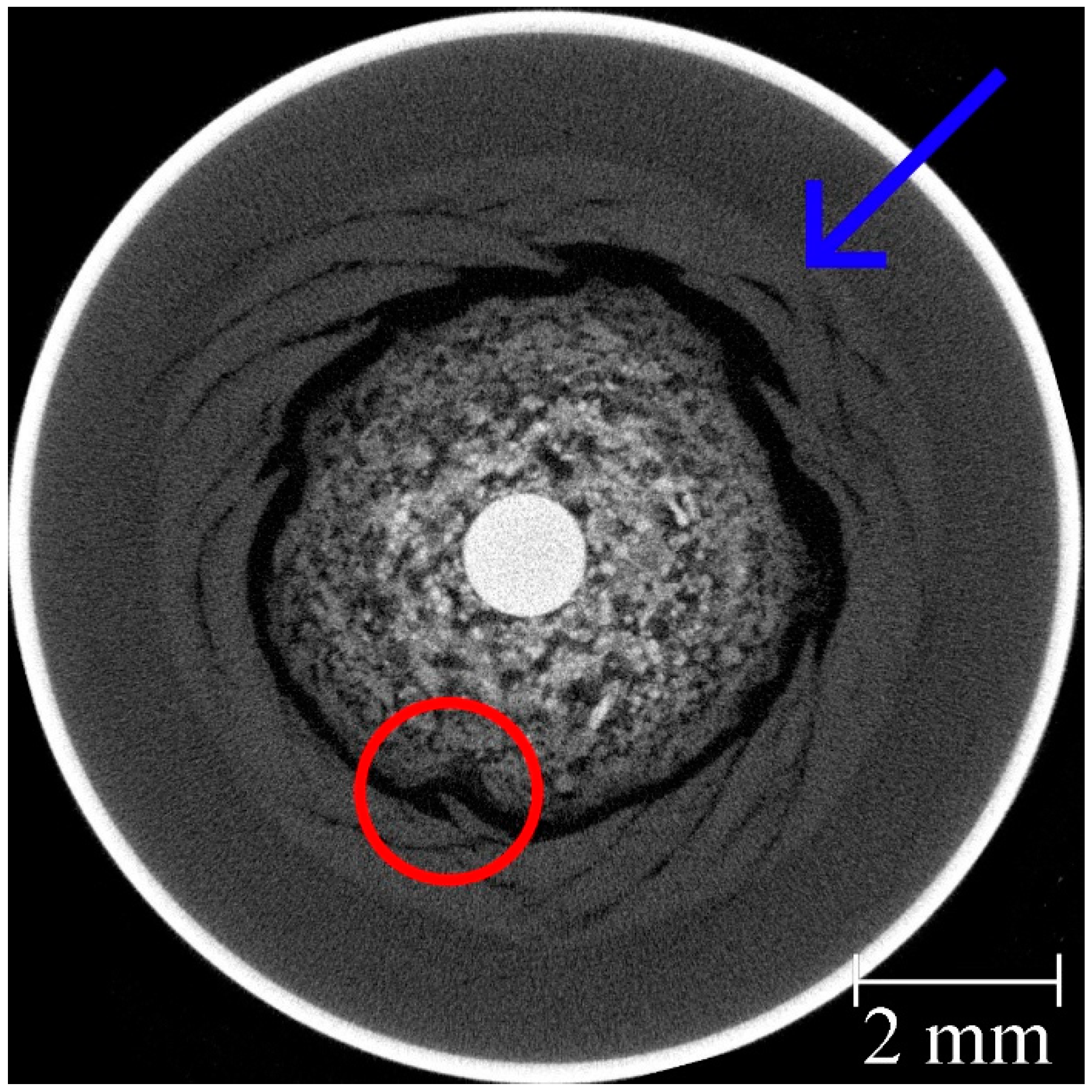
3.5. Local Effects
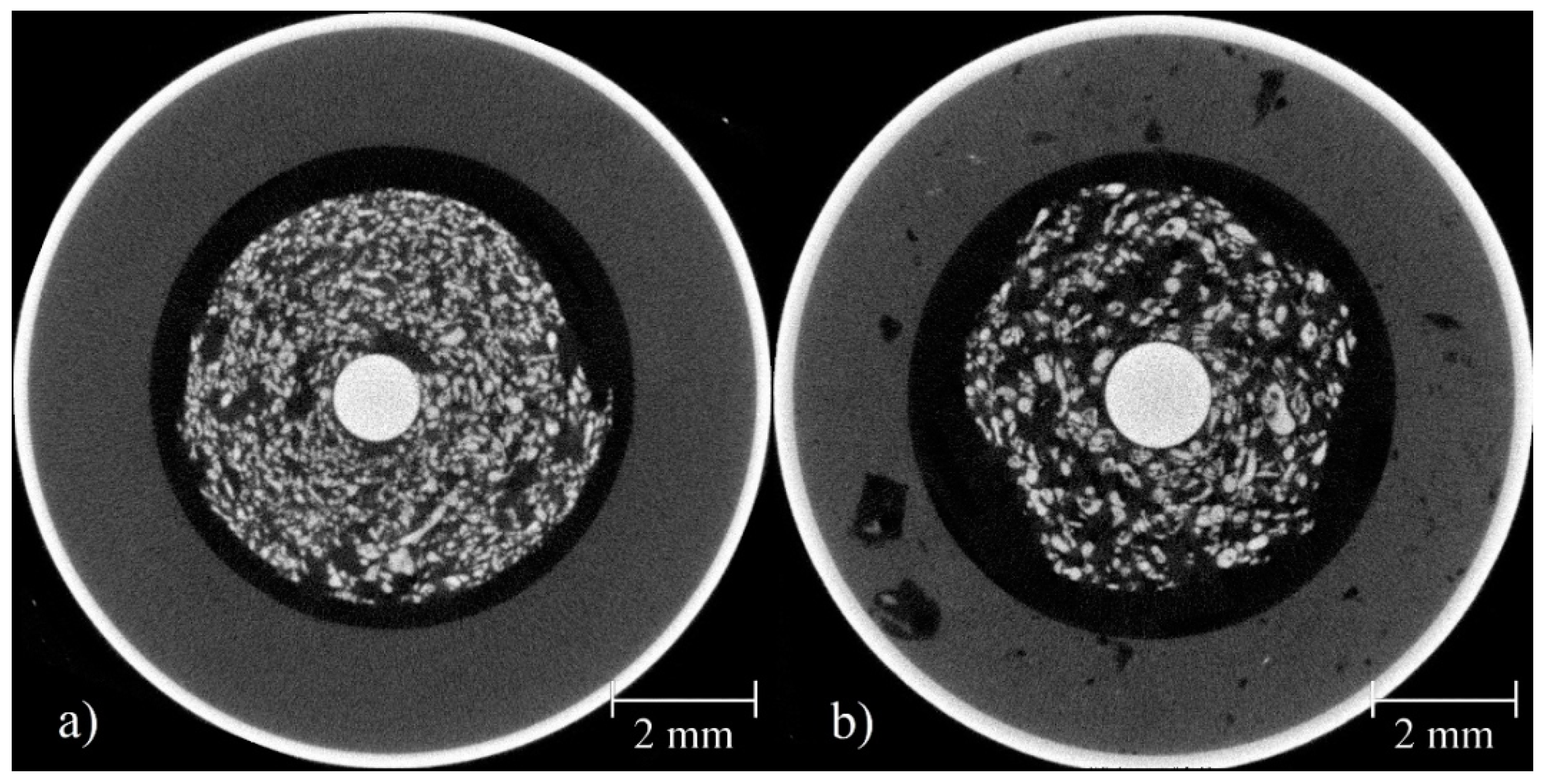
3.6. Comparison Between the Primary Cell and the RAM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Linden, D.; Reddy, T.B. Handbook of Batteries, 3rd ed.; McGraw-Hill: New York, NY, USA, 2002. [Google Scholar]
- Harting, K.; Kunz, U.; Turek, T. Zinc-Air Batteries: Prospects and Challenges for Future Improvement. Z. Phys. Chem. 2012, 226, 151–166. [Google Scholar] [CrossRef]
- Li, Y.G.; Gong, M.; Liang, Y.Y.; Feng, J.; Kim, J.E.; Wang, H.L.; Hong, G.S.; Zhang, B.; Dai, H.J. Advanced zinc-air batteries based on high-performance hybrid electrocatalysts. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.U.; Park, H.W.; Higgins, D.; Nazar, L.; Chen, Z.W. Highly Active Graphene Nanosheets Prepared via Extremely Rapid Heating as Efficient Zinc-Air Battery Electrode Material. J. Electrochem. Soc. 2013, 160, F910–F915. [Google Scholar] [CrossRef]
- Chen, Z.; Choi, J.Y.; Wang, H.J.; Li, H.; Chen, Z.W. Highly durable and active non-precious air cathode catalyst for zinc air battery. J. Power Sources 2011, 196, 3673–3677. [Google Scholar] [CrossRef]
- Kordesch, K.; Daniel-Ivad, J. Advances in battery systems for energy storage. In Proceedings of the Second International Symposium on New Materials for Fuel Cell and Modern Battery Systems, Montréal, QC, Canada, 6–10 July 1997; Ecole polytechnique de Montreal: Montreal, QC, Canada, 1997; pp. 2–13. [Google Scholar]
- Daniel-Ivad, J.; Kordesch, K. The status of the rechargeable alkaline manganese dioxide zinc battery. In Proceedings of the Symposium on Aqueous Batteries; Bennett, P.D., Gross, S., Eds.; The Electrochemical Society, Inc.: Pennington, NJ, USA, 1997; Volume 96, pp. 11–22. [Google Scholar]
- Kordesch, K.; Binder, L.; Taucher, W.; Faistauer, C.; Daniel-Ivad, J. The Rechargeable Alkaline MnO2-Zn System (New Theoretical and Technological Aspects). J. Power Sources 1993, 14, 193–216. [Google Scholar]
- Binder, L.; Odar, W.; Kordesch, K. A Study of Rechargeable Zinc Electrodes for Alkaline Cells Requiring Anodic Limitation. J. Power Sources 1981, 6, 271–289. [Google Scholar] [CrossRef]
- Binder, L.; Kordesch, K.; Urdl, P. Improvements of the rechargeable alkaline MnO2-Zn cell. J. Electrochem. Soc. 1996, 143, 13–17. [Google Scholar] [CrossRef]
- Stani, A.; Taucher-Mautner, W.; Kordesch, K.; Daniel-Ivad, J. Development of flat plate rechargeable alkaline manganese dioxide-zinc cells. J. Power Sources 2006, 153, 405–412. [Google Scholar] [CrossRef]
- Cheng, F.Y.; Chen, J.; Gou, X.L.; Shen, P.W. High-Power Alkaline Zn–MnO2 Batteries Using γ-MnO2 Nanowires/Nanotubes and Electrolytic Zinc Powder. Adv. Mater. 2005, 17, 2753–2756. [Google Scholar] [CrossRef]
- Im, D.; Manthiram, A. Role of bismuth and factors influencing the formation of Mn3O4 in rechargeable alkaline batteries based on bismuth-containing manganese oxides. J. Electrochem. Soc. 2003, 150, A68–A73. [Google Scholar] [CrossRef]
- Horn, Q.C.; Shao-Horn, Y. Morphology and spatial distribution of ZnO formed in discharged alkaline Zn/MnO2 AA cells. J. Electrochem. Soc. 2003, 150, A652–A658. [Google Scholar] [CrossRef]
- Turner, S.; Buseck, P.R. Defects in nsutite ([gamma]-MnO2) and dry-cell battery efficiency. Nature 1983, 304, 143–146. [Google Scholar] [CrossRef]
- Powers, R.W.; Breiter, M.W. The Anodic Dissolution and Passivation of Zinc in Concentrated Potassium Hydroxide Solutions. J. Electrochem. Soc. 1969, 116, 719–729. [Google Scholar] [CrossRef]
- Zielke, L.; Hutzenlaub, T.; Wheeler, D.R.; Chao, C.-W.; Manke, I.; Hilger, A.; Paust, N.; Zengerle, R.; Thiele, S. Three-Phase Multiscale Modeling of a LiCoO2 Cathode: Combining the Advantages of FIB-SEM Imaging and X-Ray Tomography. Adv. Energy Mater. 2015, 5, 1401612. [Google Scholar] [CrossRef]
- Ebner, M.; Geldmacher, F.; Marone, F.; Stampanoni, M.; Wood, V. X-Ray Tomography of Porous, Transition Metal Oxide Based Lithium Ion Battery Electrodes. Adv. Energy Mater. 2013, 3, 845–850. [Google Scholar] [CrossRef]
- Shearing, P.R.; Howard, L.E.; Jorgensen, P.S.; Brandon, N.P.; Harris, S.J. Characterization of the 3-dimensional microstructure of a graphite negative electrode from a Li-ion battery. Electrochem. Commun. 2010, 12, 374–377. [Google Scholar] [CrossRef]
- Stenzel, O.; Westhoff, D.; Manke, I.; Kasper, M.; Kroese, D.P.; Schmidt, V. Graph-based simulated annealing: A hybrid approach to stochastic modeling of complex microstructures. Model. Simul. Mater. Sci. Eng. 2013, 21, 055004. [Google Scholar] [CrossRef]
- Ebner, M.; Marone, F.; Stampanoni, M.; Wood, V. Visualization and Quantification of Electrochemical and Mechanical Degradation in Li Ion Batteries. Science 2013, 342, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Mitsch, T.; Kraemer, Y.; Feinauer, J.; Gaiselmann, G.; Markoetter, H.; Manke, I.; Hintennach, A.; Schmidt, V. Preparation and Characterization of Li-Ion Graphite Anodes Using Synchrotron Tomography. Materials 2014, 7, 4455–4472. [Google Scholar] [CrossRef] [PubMed]
- Zielke, L.; Hutzenlaub, T.; Wheeler, D.R.; Manke, I.; Arlt, T.; Paust, N.; Zengerle, R.; Thiele, S. A Combination of X-ray Tomography and Carbon Binder Modeling: Reconstructing the Three Phases of LiCoO2 Li-Ion Battery Cathodes. Adv. Energy Mater. 2014, 4, 1301617. [Google Scholar] [CrossRef]
- Zielke, L.; Barchasz, C.; Waluś, S.; Alloin, F.; Leprêtre, J.C.; Spettl, A.; Schmidt, V.; Hilger, A.; Manke, I.; Banhart, J.; et al. Degradation of Li/S Battery Electrodes On 3D Current Collectors Studied Using X-ray Phase Contrast Tomography. Sci. Rep. 2015, 5, 10921. [Google Scholar] [CrossRef] [PubMed]
- Eastwood, D.S.; Yufit, V.; Gelb, J.; Gu, A.; Bradley, R.S.; Harris, S.J.; Brett, D.J.L.; Brandon, N.P.; Lee, P.D.; Withers, P.J.; et al. Lithiation- Induced Dilation Mapping in a Lithium- Ion Battery Electrode by 3D X-Ray Microscopy and Digital Volume Correlation. Adv. Energy Mater. 2014, 4, 1300506. [Google Scholar] [CrossRef]
- Weker, J.N.; Liu, N.; Misra, S.; Andrews, J.C.; Cui, Y.; Toney, M.F. In situ nanotomography and operando transmission X-ray microscopy of micron-sized Ge particles. Energy Environ. Sci. 2014, 7, 2771–2777. [Google Scholar] [CrossRef]
- Steinbock, L.; Dustmann, C.H. Investigation of the inner structures of ZEBRA cells with a microtomograph. J. Electrochem. Soc. 2001, 148, A132–A136. [Google Scholar] [CrossRef]
- Gonzalez, J.; Sun, K.; Huang, M.; Dillon, S.; Chasiotis, I.; Lambros, J. X-ray microtomography characterization of Sn particle evolution during lithiation/delithiation in lithium ion batteries. J. Power Sources 2015, 285, 205–209. [Google Scholar] [CrossRef]
- Gonzalez, J.; Sun, K.; Huang, M.; Lambros, J.; Dillon, S.; Chasiotis, I. Three dimensional studies of particle failure in silicon based composite electrodes for lithium ion batteries. J. Power Sources 2014, 269, 334–343. [Google Scholar] [CrossRef]
- Hoch, C.; Schier, H.; Kallfass, C.; Totzke, C.; Hilger, A.; Manke, I. Electrode deterioration processes in lithium ion capacitors monitored by in situ X-ray radiography on micrometre scale. Micro-Nano Lett. 2012, 7, 262–264. [Google Scholar] [CrossRef]
- Tariq, F.; Yufit, V.; Eastwood, D.S.; Merla, Y.; Biton, M.; Wu, B.; Chen, Z.; Freedman, K.; Offer, G.; Peled, E.; et al. In-Operando X-ray Tomography Study of Lithiation Induced Delamination of Si Based Anodes for Lithium-Ion Batteries. ECS Electrochem. Lett. 2014, 3, A76–A78. [Google Scholar] [CrossRef]
- Sun, F.; Markötter, H.; Dong, K.; Manke, I.; Hilger, A.; Kardjilov, N.; Banhart, J. Investigation of failure mechanisms in silicon based half cells during the first cycle by micro X-ray tomography and radiography. J. Power Sources 2016, 321, 174–184. [Google Scholar] [CrossRef]
- Sun, F.; Markötter, H.; Zhou, D.; Alrwashdeh, S.S.S.; Hilger, A.; Kardjilov, N.; Manke, I.; Banhart, J. In Situ Radiographic Investigation of (De)Lithiation Mechanisms in a Tin-Electrode Lithium-Ion Battery. ChemSusChem 2016, 9, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Manke, I.; Banhart, J.; Haibel, A.; Rack, A.; Zabler, S.; Kardjilov, N.; Hilger, A.; Melzer, A.; Riesemeier, H. In situ investigation of the discharge of alkaline Zn–MnO2 batteries with synchrotron X-ray and neutron tomographies. Appl. Phys. Lett. 2007, 90, 214102. [Google Scholar] [CrossRef]
- Haibel, A.; Manke, I.; Melzer, A.; Banhart, J. In Situ Microtomographic Monitoring of Discharging Processes in Alkaline Cells. J. Electrochem. Soc. 2010, 157, A387–A391. [Google Scholar] [CrossRef]
- Schröder, D.; Arlt, T.; Krewer, U.; Manke, I. Analyzing transport paths in the air electrode of a zinc air battery using X-ray tomography. Electrochem. Commun. 2014, 40, 88–91. [Google Scholar] [CrossRef]
- Arlt, T.; Schroeder, D.; Krewer, U.; Manke, I. In operando monitoring of the state of charge and species distribution in zinc air batteries using X-ray tomography and model-based simulations. Phys. Chem. Chem. Phys. 2014, 16, 22273–22280. [Google Scholar] [CrossRef] [PubMed]
- Riley, G.V.; Hussey, D.S.; Jacobson, D. In Situ Neutron Imaging of Alkaline and Lithium Batteries. ECS Trans. 2010, 25, 75–83. [Google Scholar]
- Kim, F.H.; Penumadu, D.; Gregor, J.; Kardjilov, N.; Manke, I. High-Resolution Neutron and X-Ray Imaging of Granular Materials. J. Geotech. Geoenviron. Eng. 2013, 139, 715–723. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Banhart, J. Advanced Tomographic Methods in Materials Research and Engineering; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Sun, F.; Markoetter, H.; Manke, I.; Hilger, A.; Kardjilov, N.; Banhart, J. Three-Dimensional Visualization of Gas Evolution and Channel Formation inside a Lithium-Ion Battery. ACS Appl. Mater. Interfaces 2016, 8, 7156–7164. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osenberg, M.; Manke, I.; Hilger, A.; Kardjilov, N.; Banhart, J. An X-ray Tomographic Study of Rechargeable Zn/MnO2 Batteries. Materials 2018, 11, 1486. https://doi.org/10.3390/ma11091486
Osenberg M, Manke I, Hilger A, Kardjilov N, Banhart J. An X-ray Tomographic Study of Rechargeable Zn/MnO2 Batteries. Materials. 2018; 11(9):1486. https://doi.org/10.3390/ma11091486
Chicago/Turabian StyleOsenberg, Markus, Ingo Manke, André Hilger, Nikolay Kardjilov, and John Banhart. 2018. "An X-ray Tomographic Study of Rechargeable Zn/MnO2 Batteries" Materials 11, no. 9: 1486. https://doi.org/10.3390/ma11091486
APA StyleOsenberg, M., Manke, I., Hilger, A., Kardjilov, N., & Banhart, J. (2018). An X-ray Tomographic Study of Rechargeable Zn/MnO2 Batteries. Materials, 11(9), 1486. https://doi.org/10.3390/ma11091486