Investigations on the Electrochemical Atomic Layer Growth of Bi2Se3 and the Surface Limited Deposition of Bismuth at the Silver Electrode
Abstract
:1. Introduction
2. Materials and Methods
2.1. E-ALD Growth
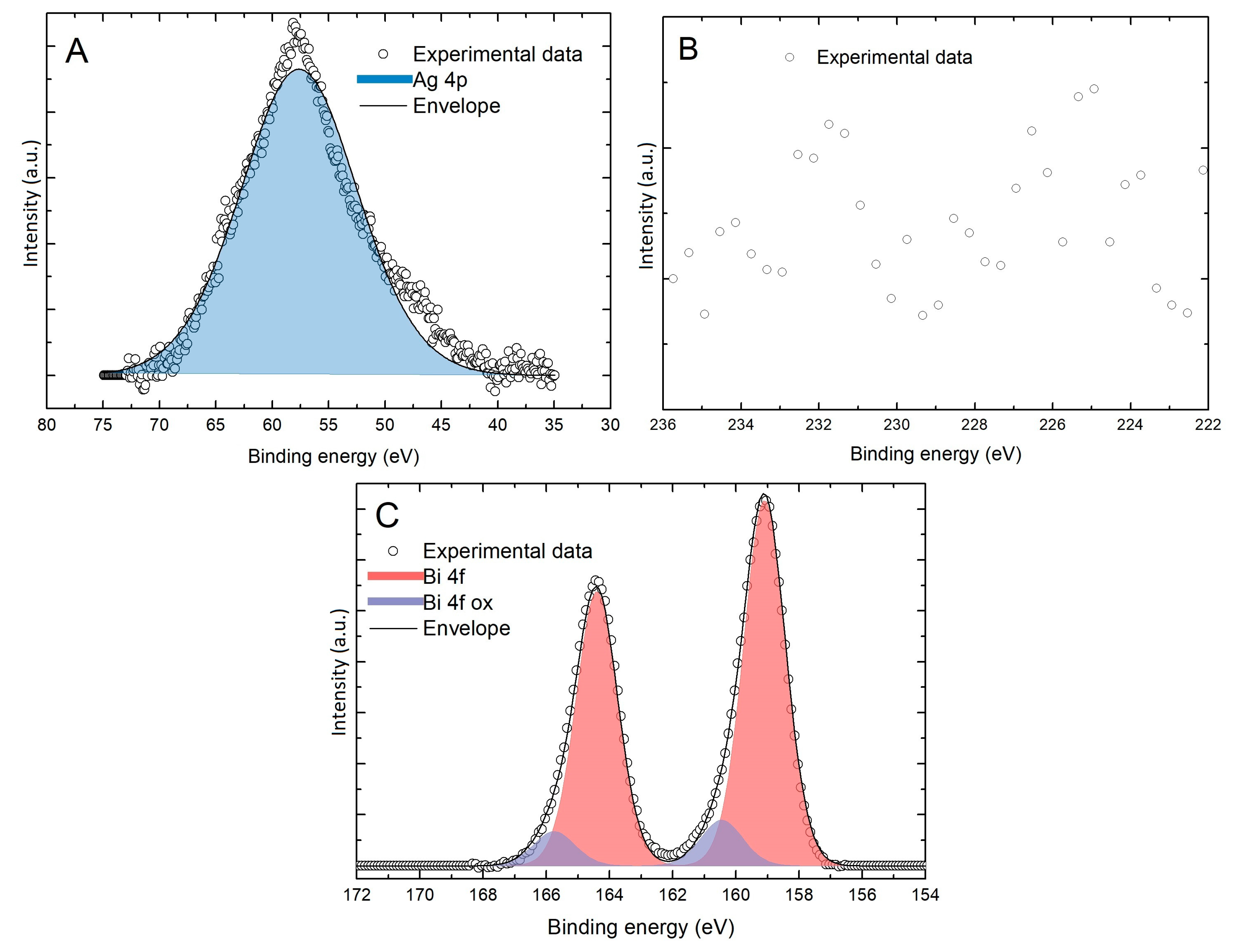
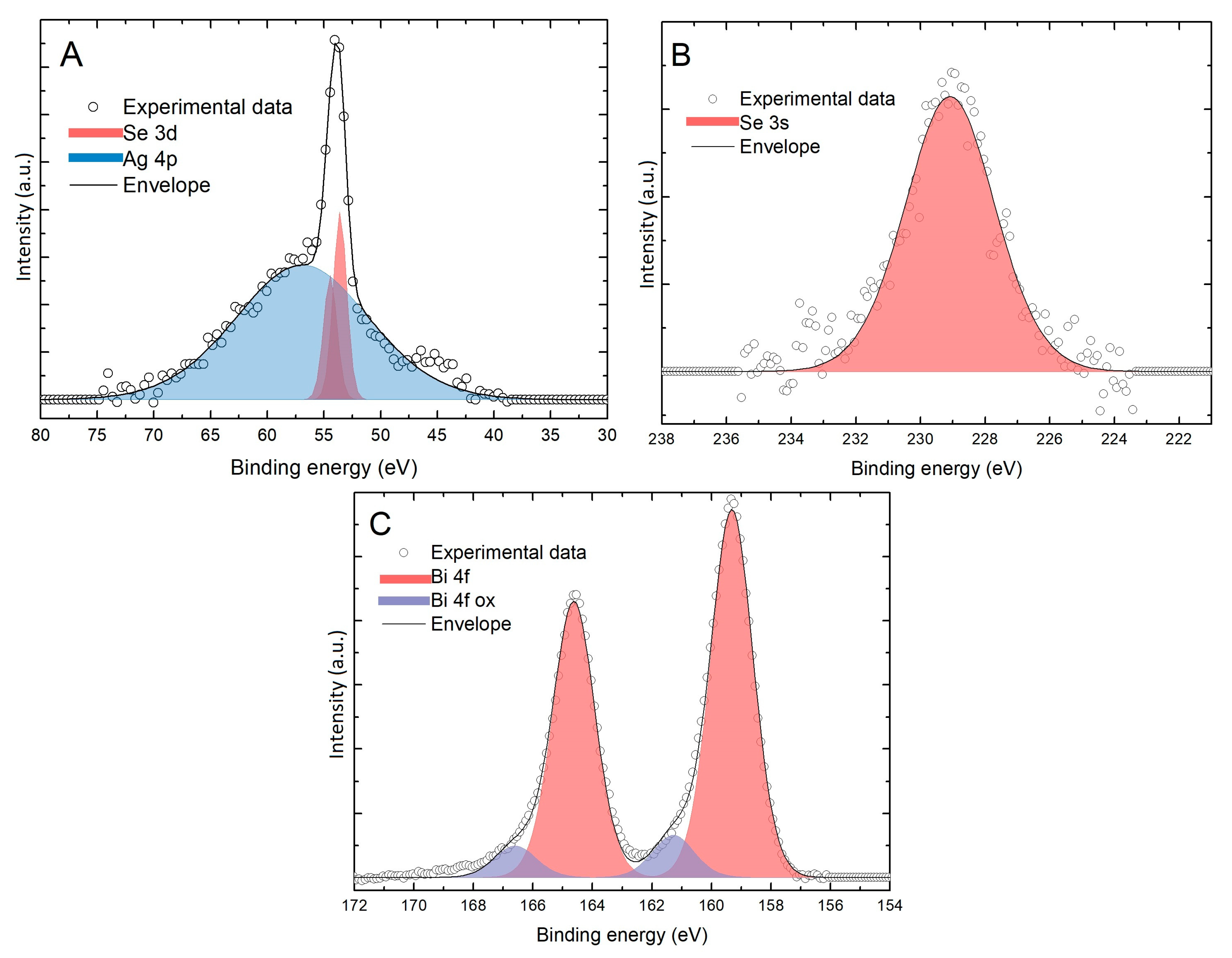
2.2. Spectroscopic Characterization
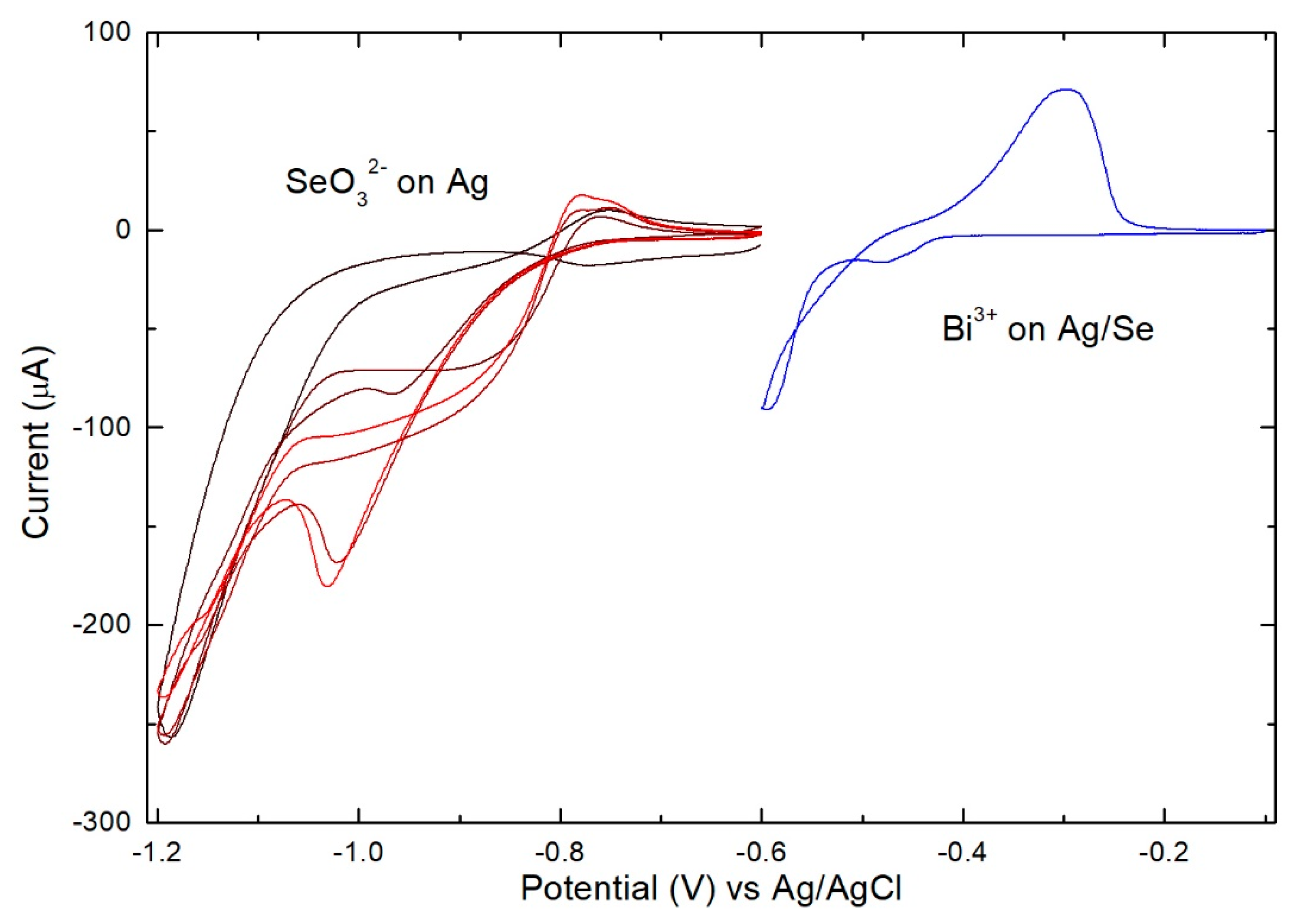
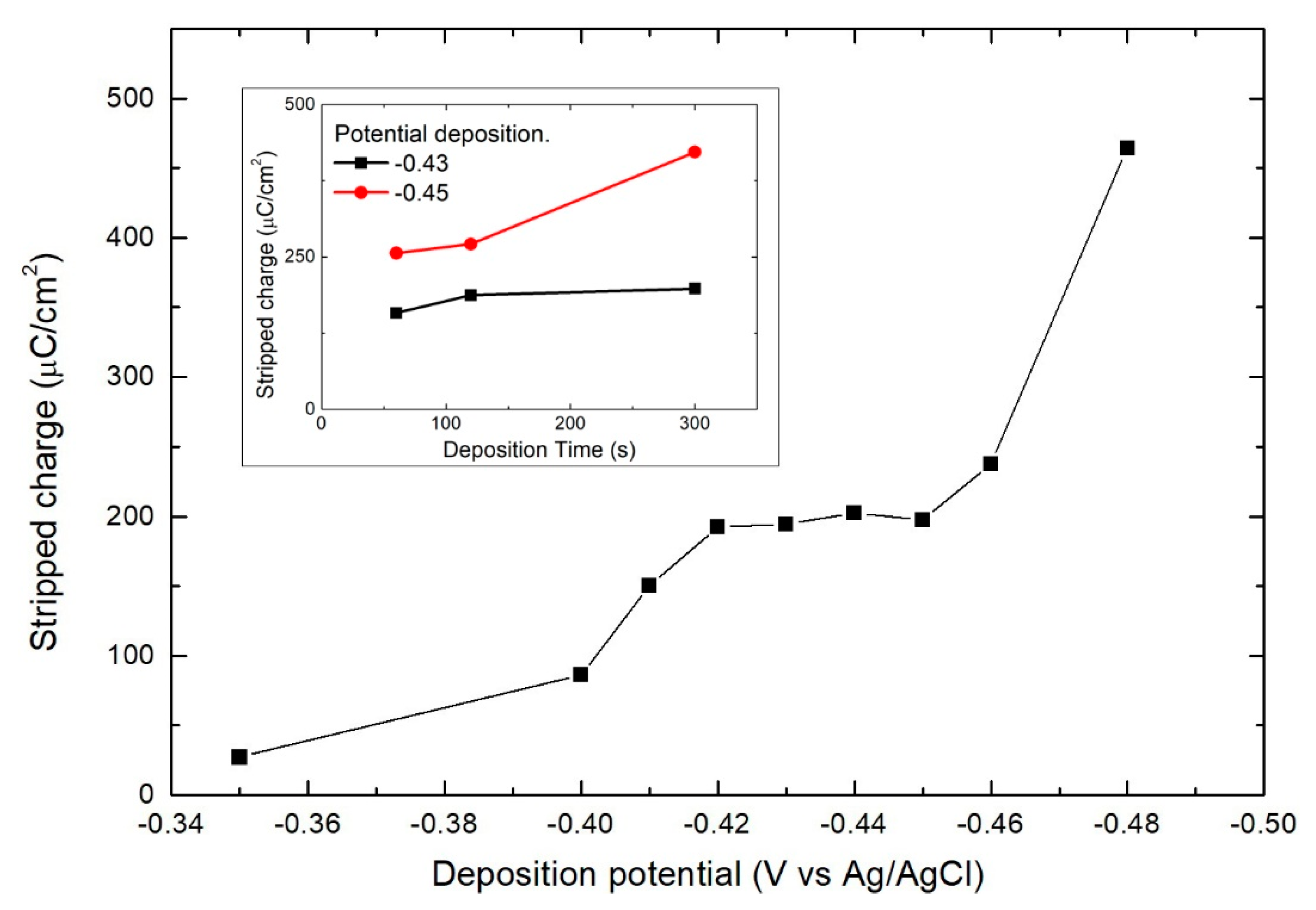
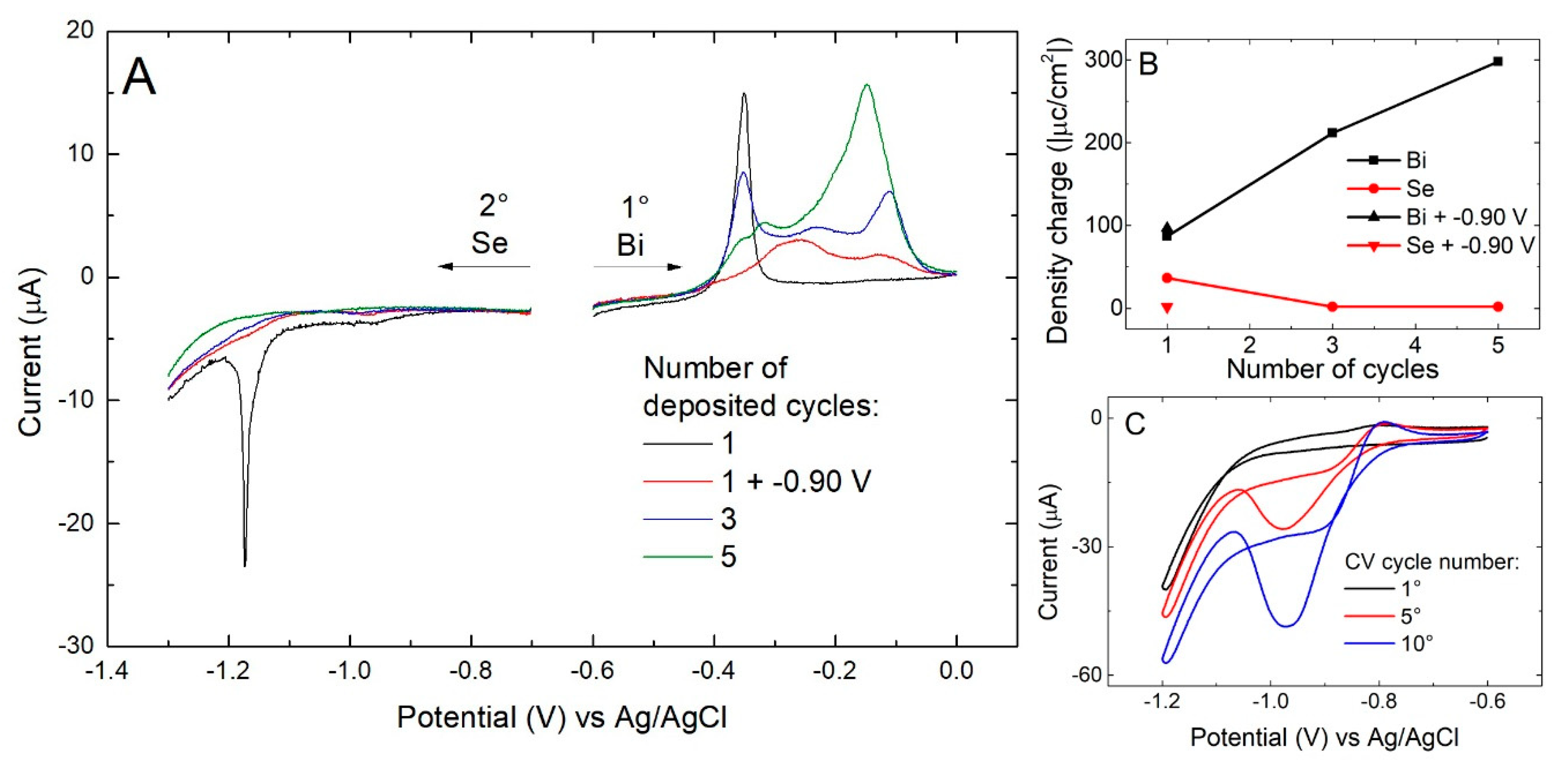
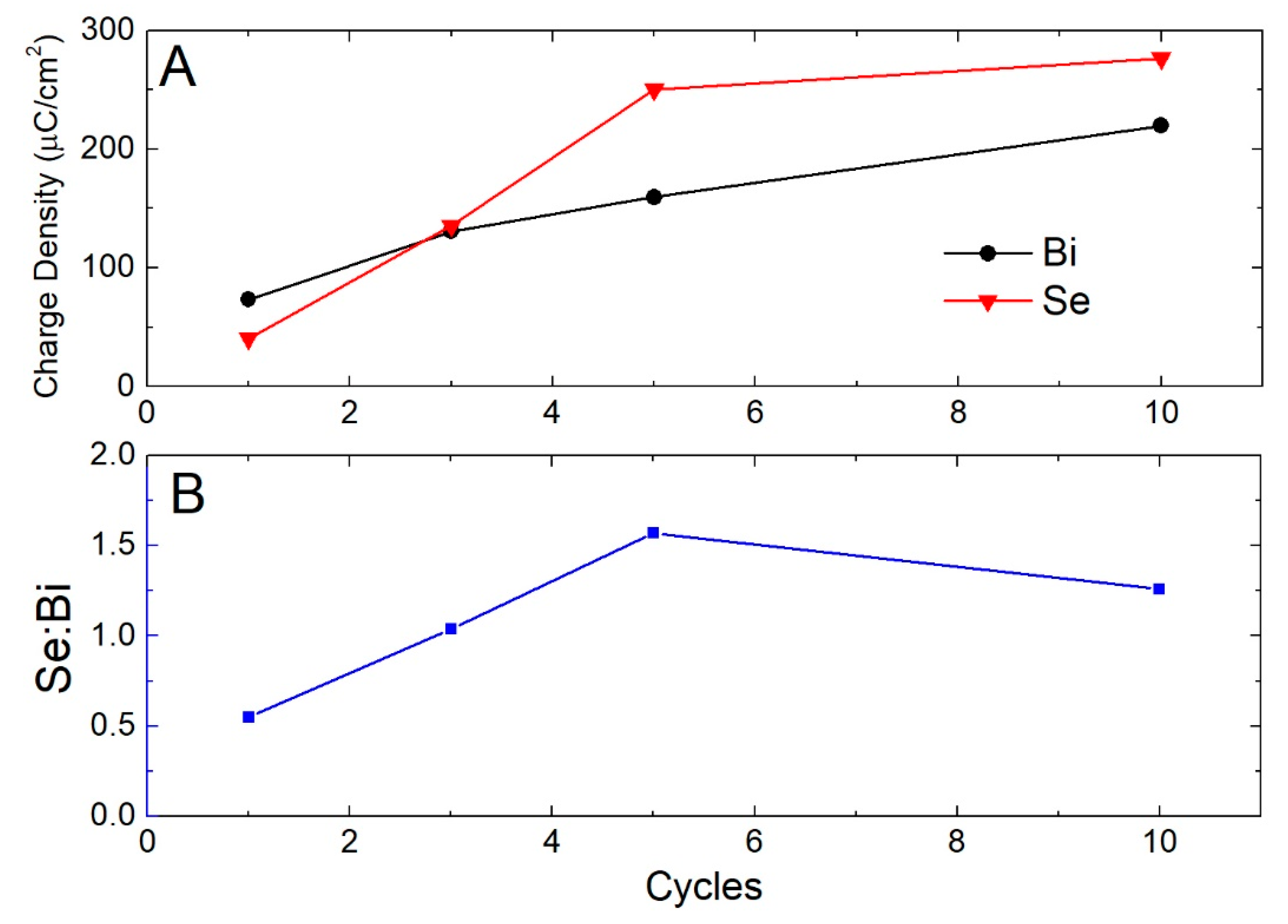
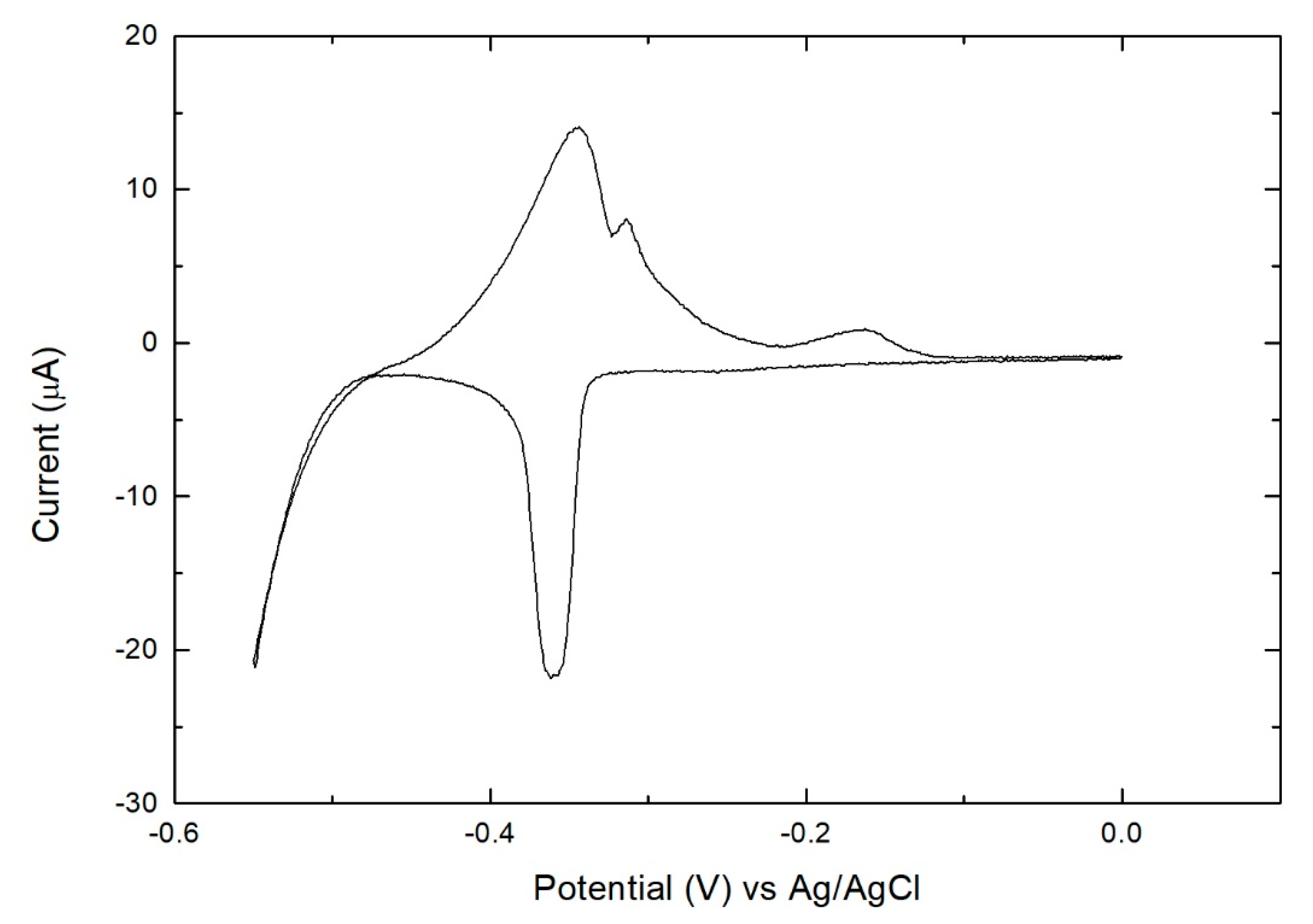
3. Results
- Se solution at −0.90 V for 60 s (growth of Se)
- Buffer solution at −0.90 V for 60 s (excess Se removal and rinse)
- Bi solution at −0.43 V for 60 s (growth of Bi)
- Washing with buffer solution (rinse)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wood, C. Materials for thermoelectric energy conversion. Inst. Phys. 1988, 51, 459–539. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.-X.; Qi, X.-L.; Dai, X.; Fang, Z.; Zhang, S.-C. Topological insulators in Bi2Se3, Bi2Te3 and Sb2Te3 with a single Dirac cone on the surface. Nat. Phys. 2009, 5, 438–442. [Google Scholar] [CrossRef]
- Tumelero, M.A.; Faccio, R.; Pasa, A.A. Unraveling the native conduction of trichalcogenides and its ideal band alignment for new photovoltaic interfaces. J. Phys. Chem. C 2016, 120, 1390–1399. [Google Scholar] [CrossRef]
- Tumelero, M.A.; Benetti, L.C.; Isoppo, E.; Faccio, R.; Zangari, G.; Pasa, A.A. Electrodeposition and ab Initio Studies of Metastable Orthorhombic Bi2Se3: A Novel Semiconductor with Bandgap for Photovoltaic Applications. J. Phys. Chem. C 2016, 120, 11797–11806. [Google Scholar] [CrossRef]
- Ahmed, R.; Xu, Y.; Sales, M.G.; Lin, Q.; McDonnell, S.J.; Zangari, G. Synthesis and Material Properties of Bi2Se3 Nanostructures Deposited by SILAR. J. Phys. Chem. C 2018, 122, 12052–12060. [Google Scholar] [CrossRef]
- Gregory, B.W.; Stickney, J.L. Electrochemical atomic layer epitaxy (ECALE). J. Electroanal. Chem. Interfacial Electrochem. 1991, 300, 543–561. [Google Scholar] [CrossRef]
- Giaccherini, A.; Felici, R.; Innocenti, M. Operando structural characterization of the E-ALD process ultra-thin films growth. In X-ray Characterization of Nanostructured Energy Materials by Synchrotron Radiation; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Giaccherini, A.; Russo, F.; Carlà, F.; Guerri, A.; Picca, R.A.; Cioffi, N.; Cinotti, S.; Montegrossi, G.; Passaponti, M.; Di Benedetto, F.; et al. Operando SXRD of E-ALD deposited sulphides ultra-thin films: Crystallite strain and size. Appl. Surf. Sci. 2018, 432, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Giaccherini, A.; Cinotti, S.; Guerri, A.; Carlà, F.; Montegrossi, G.; Vizza, F.; Lavacchi, A.; Felici, R.; Di Benedetto, F.; Innocenti, M. Operando SXRD study of the structure and growth process of Cu2S ultra-thin films. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Giaccherini, A.; Montegrossi, G.; Di Benedetto, F.; Innocenti, M. Thermochemistry of the E-ALD process for the growth of CuxZnyS on Ag(111): Interpretation of experimental data. Electrochim. Acta 2018, 262, 135–143. [Google Scholar] [CrossRef]
- Caporali, S.; Tolstogouzov, A.; Teodoro, O.M.N.D.; Innocenti, M.; Di Benedetto, F.; Cinotti, S.; Picca, R.A.; Sportelli, M.C.; Cioffi, N. Sn-deficiency in the electrodeposited ternary CuxSnySz thin films by ECALE. Sol. Energy Mater. Sol. Cells 2015, 138, 9–16. [Google Scholar] [CrossRef]
- Innocenti, M.; Becucci, L.; Bencistà, I.; Carretti, E.; Cinotti, S.; Dei, L.; Di Benedetto, F.; Lavacchi, A.; Marinelli, F.; Salvietti, E.; et al. Electrochemical growth of Cu-Zn sulfides. J. Electroanal. Chem. 2013, 710, 17–21. [Google Scholar] [CrossRef]
- Innocenti, M.; Bencistà, I.; Bellandi, S.; Bianchini, C.; Di Benedetto, F.; Lavacchi, A.; Vizza, F.; Foresti, M.L. Electrochemical layer by layer growth and characterization of copper sulfur thin films on Ag(111). Electrochim. Acta 2011, 58, 599–605. [Google Scholar] [CrossRef]
- Foresti, M.L.; Milani, S.; Loglio, F.; Innocenti, M.; Pezzatini, G.; Cattarin, S. Ternary CdSxSe1-x deposited on Ag(111) by ECALE: Synthesis and characterization. Langmuir 2005, 21, 6900–6907. [Google Scholar] [CrossRef] [PubMed]
- Loglio, F.; Innocenti, M.; D’Acapito, F.; Felici, R.; Pezzatini, G.; Salvietti, E.; Foresti, M.L. Cadmium selenide electrodeposited by ECALE: Electrochemical characterization and preliminary results by EXAFS. J. Electroanal. Chem. 2005, 575, 161–167. [Google Scholar] [CrossRef]
- Cecconi, T.; Atrei, A.; Bardi, U.; Forni, F.; Innocenti, M.; Loglio, F.; Foresti, M.L.; Rovida, G. X-ray photoelectron diffraction (XPD) study of the atomic structure of the ultrathin CdS phase deposited on Ag(111) by electrochemical atomic layer epitaxy (ECALE). J. Electron Spectrosc. Relat. Phenom. 2001, 114–116, 563–568. [Google Scholar] [CrossRef]
- Cavallini, M.; Facchini, M.; Albonetti, C.; Biscarini, F.; Innocenti, M.; Loglio, F.; Salvietti, E.; Pezzatini, G.; Foresti, M.L. Two-dimensional self-organization of CdS ultra thin films by confined electrochemical atomic layer epitaxy growth. J. Phys. Chem. C 2007, 111, 1061–1064. [Google Scholar] [CrossRef]
- Xiao, C.; Yang, J.; Zhu, W.; Peng, J.; Zhang, J. Electrodeposition and characterization of Bi2Se3 thin films by electrochemical atomic layer epitaxy (ECALE). Electrochim. Acta 2009, 54, 6821–6826. [Google Scholar] [CrossRef]
- Forni, F.; Innocenti, M.; Pezzatini, G.; Foresti, M.L. Electrochemical aspects of CdTe growth on the face (111) of silver by ECALE. Electrochim. Acta 2000, 45, 3225–3231. [Google Scholar] [CrossRef]
- Foresti, M.L.; Capolupo, F.; Innocenti, M.; Loglio, F. Visual Detection of Crystallographic Orientations of Face-Centered Cubic Single Crystals. Cryst. Growth Des. 2002, 2, 73–77. [Google Scholar] [CrossRef]
- Innocenti, M.; Cattarin, S.; Cavallini, M.; Loglio, F.; Foresti, M.L. Characterisation of thin films of CdS deposited on Ag(111) by ECALE. A morphological and photoelectrochemical investigation. J. Electroanal. Chem. 2002, 532, 219–225. [Google Scholar] [CrossRef]
- Shirley, D.A. High-resolution x-ray photoemission spectrum of the valence bands of gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef]
- Susi, T.; Pichler, T.; Ayala, P. X-ray photoelectron spectroscopy of graphitic carbon nanomaterials doped with heteroatoms. Beilstein J. Nanotechnol. 2015, 6, 177–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pezzatini, G.; Loglio, F.; Innocenti, M.; Foresti, M.L. Selenium(IV) Electrochemistry on Silver: A Combined Electrochemical Quartz-Crystal Microbalance and Cyclic Voltammetric Investigation. Collect. Czech. Chem. Commun. 2003, 68, 1579–1595. [Google Scholar] [CrossRef]
- NIST NIST X-ray Photoelectron Spectroscopy Database. Available online: Srdata.nist.gov/xps (accessed on 13 August 2018).
- Dharmadhikari, V.S.; Sainkar, S.R.; Badrinarayan, S.; Goswami, A. Characterisation of thin films of bismuth oxide by X-ray photoelectron spectroscopy. J. Electron Spectrosc. Relat. Phenom. 1982, 25, 181–189. [Google Scholar] [CrossRef]
- Innocenti, M.; Forni, F.; Pezzatini, G.; Raiteri, R.; Loglio, F.; Foresti, M.L. Electrochemical behavior of As on silver single crystals and experimental conditions for InAs growth by ECALE. J. Electroanal. Chem. 2001, 514, 75–82. [Google Scholar] [CrossRef]
- Hatchett, D.W.; Gao, X.; Catron, S.W.; White, H.S. Electrochemistry of sulfur adlayers on Ag(111). Evidence for a concentration- and potential-dependent surface-phase transition. J. Phys. Chem. 1996, 100, 331–338. [Google Scholar] [CrossRef]
- Innocenti, M.; Bellandi, S.; Lastraioli, E.; Loglio, F.; Foresti, M.L. Selective electrodesorption based atomic layer deposition (SEBALD): A novel electrochemical route to deposit metal clusters on Ag(111). Langmuir 2011, 27, 11704–11709. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, M.; Zangari, G.; Zafferoni, C.; Bencistà, I.; Becucci, L.; Lavacchi, A. Selective electrodesorption based atomic layer deposition (SEBALD) modifications of silver surfaces for enhancing oxygen reduction reaction activity. J. Power Sources 2013, 241, 80–86. [Google Scholar] [CrossRef]
- Giurlani, W.; Giaccherini, A.; Salvietti, E.; Passaponti, M.; Comparini, A.; Morandi, V.; Liscio, F.; Cavallini, M.; Innocenti, M. Selective Electrodesorption Based Atomic Layer Deposition (SEBALD) of Bismuth under morphological control. Electrochem. Soc. Interface 2018, 77–81. [Google Scholar] [CrossRef]
- Nascimento, V.B.; de Carvalho, V.E.; Paniago, R.; Soares, E.A.; Ladeira, L.O.; Pfannes, H.D. XPS and EELS study of the bismuth selenide. J. Electron Spectrosc. Relat. Phenom. 1999, 104, 99–107. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giurlani, W.; Giaccherini, A.; Calisi, N.; Zangari, G.; Salvietti, E.; Passaponti, M.; Caporali, S.; Innocenti, M. Investigations on the Electrochemical Atomic Layer Growth of Bi2Se3 and the Surface Limited Deposition of Bismuth at the Silver Electrode. Materials 2018, 11, 1426. https://doi.org/10.3390/ma11081426
Giurlani W, Giaccherini A, Calisi N, Zangari G, Salvietti E, Passaponti M, Caporali S, Innocenti M. Investigations on the Electrochemical Atomic Layer Growth of Bi2Se3 and the Surface Limited Deposition of Bismuth at the Silver Electrode. Materials. 2018; 11(8):1426. https://doi.org/10.3390/ma11081426
Chicago/Turabian StyleGiurlani, Walter, Andrea Giaccherini, Nicola Calisi, Giovanni Zangari, Emanuele Salvietti, Maurizio Passaponti, Stefano Caporali, and Massimo Innocenti. 2018. "Investigations on the Electrochemical Atomic Layer Growth of Bi2Se3 and the Surface Limited Deposition of Bismuth at the Silver Electrode" Materials 11, no. 8: 1426. https://doi.org/10.3390/ma11081426
APA StyleGiurlani, W., Giaccherini, A., Calisi, N., Zangari, G., Salvietti, E., Passaponti, M., Caporali, S., & Innocenti, M. (2018). Investigations on the Electrochemical Atomic Layer Growth of Bi2Se3 and the Surface Limited Deposition of Bismuth at the Silver Electrode. Materials, 11(8), 1426. https://doi.org/10.3390/ma11081426








