New Insights on the Photodegradation of Caffeine in the Presence of Bio-Based Substances-Magnetic Iron Oxide Hybrid Nanomaterials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
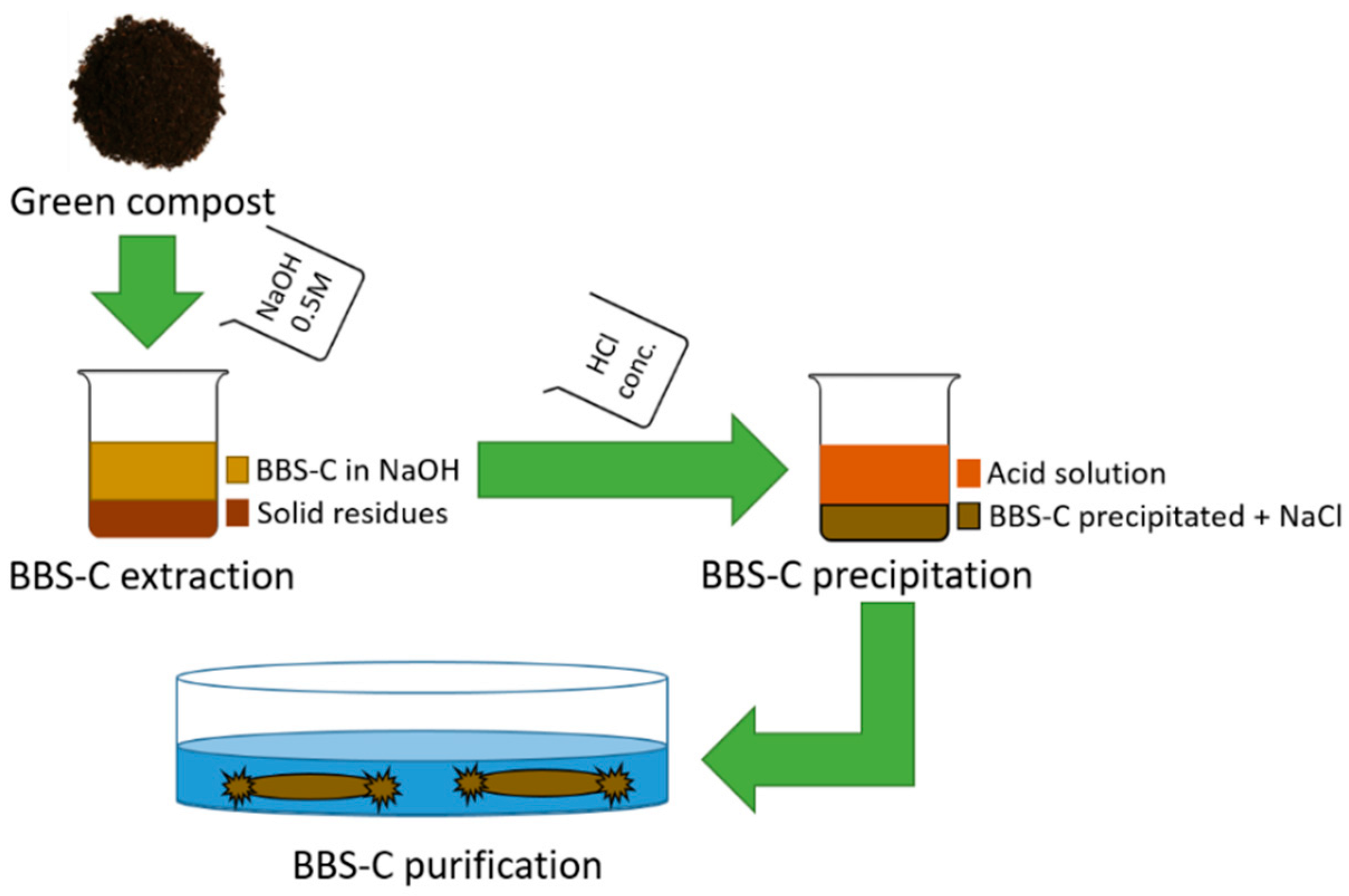
2.1.1. BBS-C Isolation from the Green Compost
2.1.2. HMNPs Synthesis
2.1.3. Other Reagents
2.2. Methods
2.2.1. HMNPs Characterization
2.2.2. Caffeine Irradiation Test and Analysis
3. Results
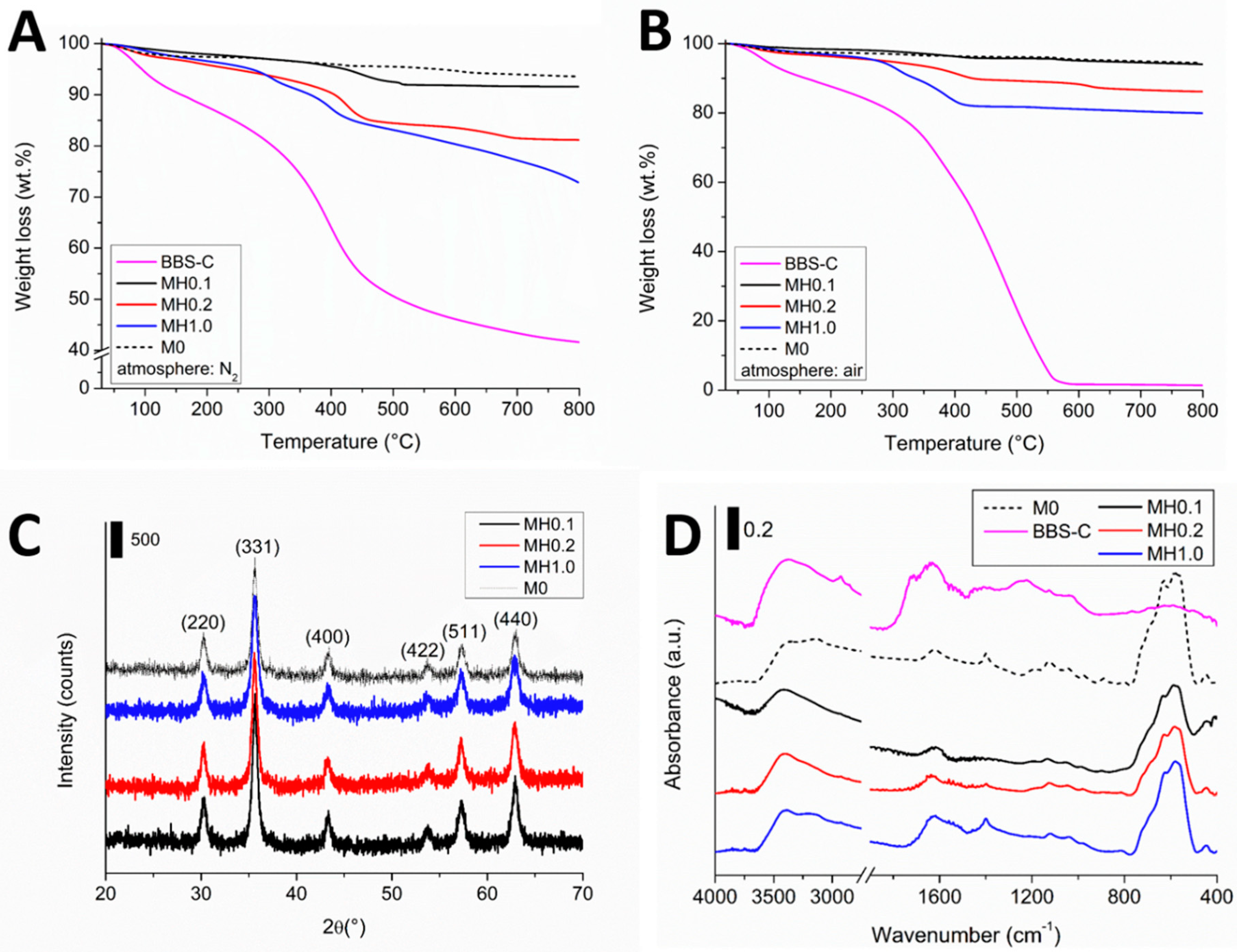
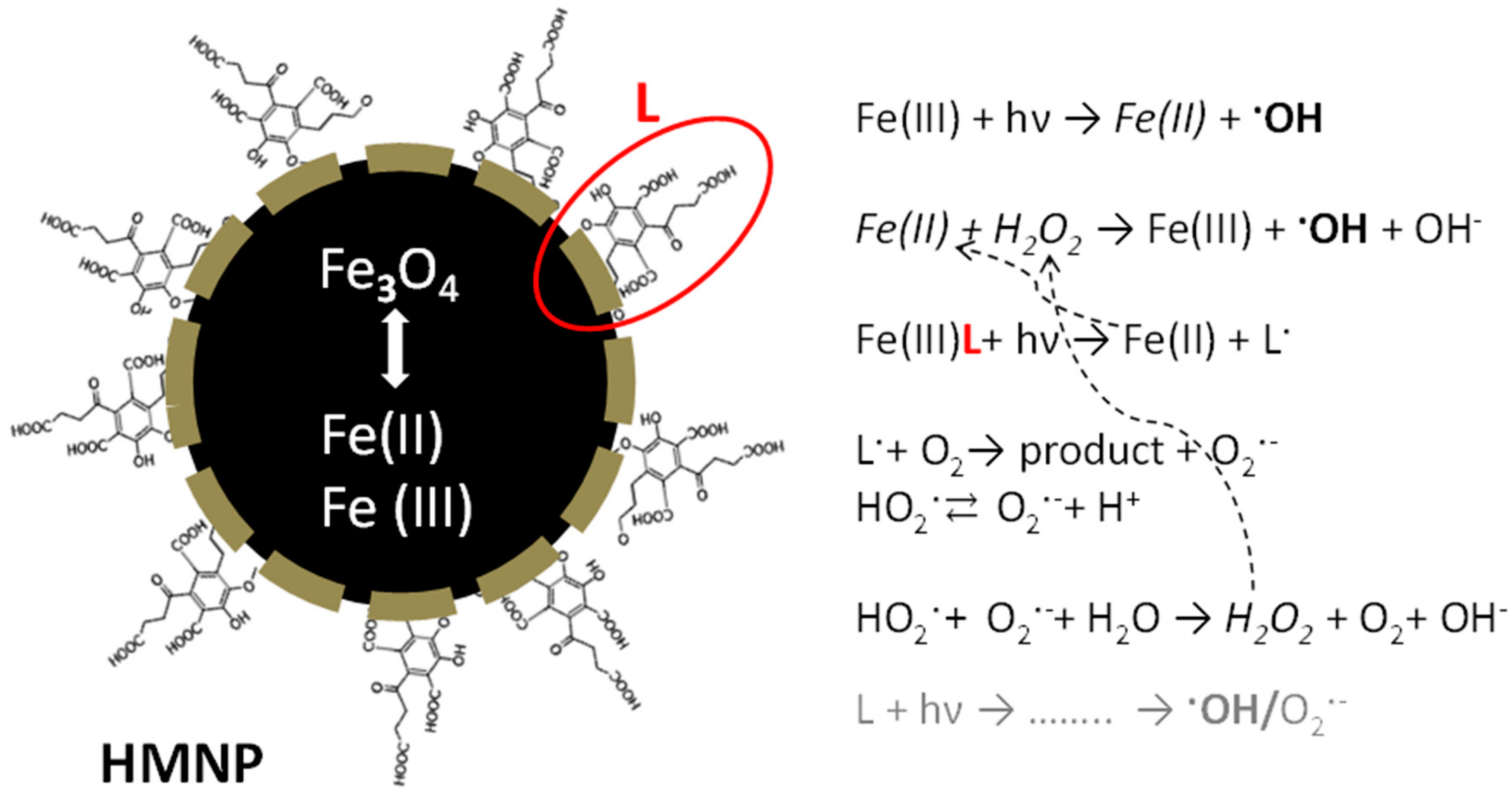
3.1. HMNPs Main Physico-Chemical Features
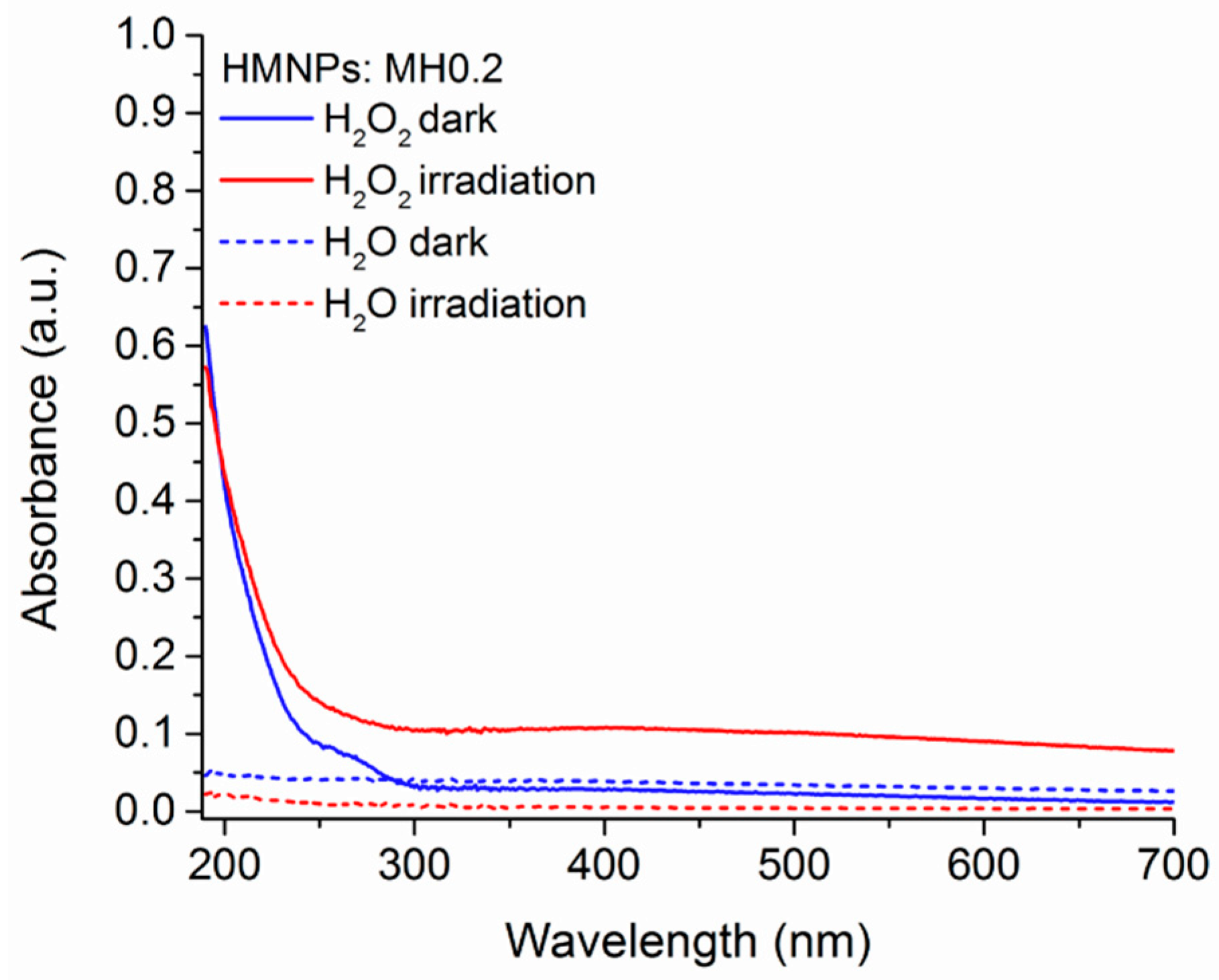
3.2. HMNPs Stability
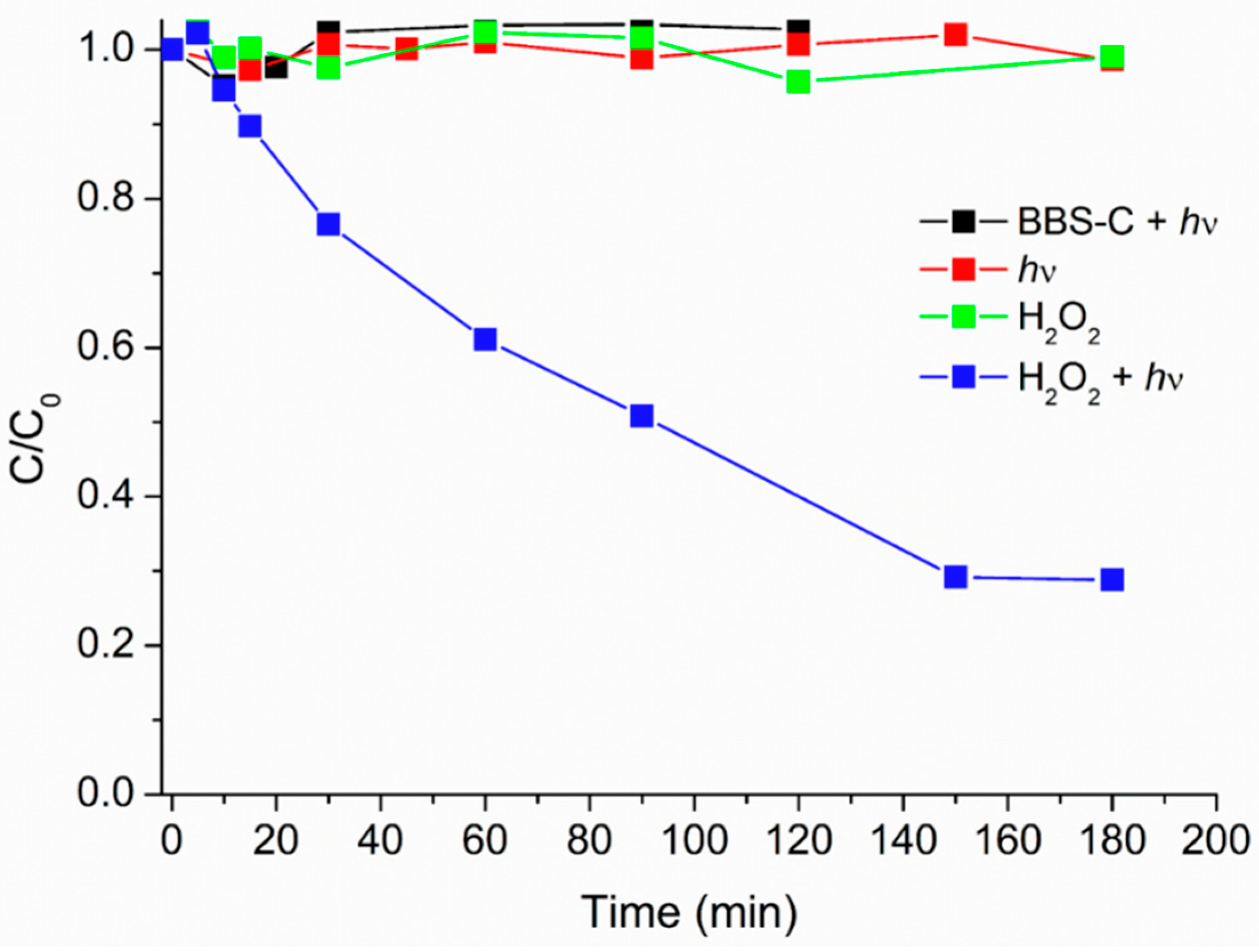
3.3. Preliminary Test on Caffeine (Photo)stability
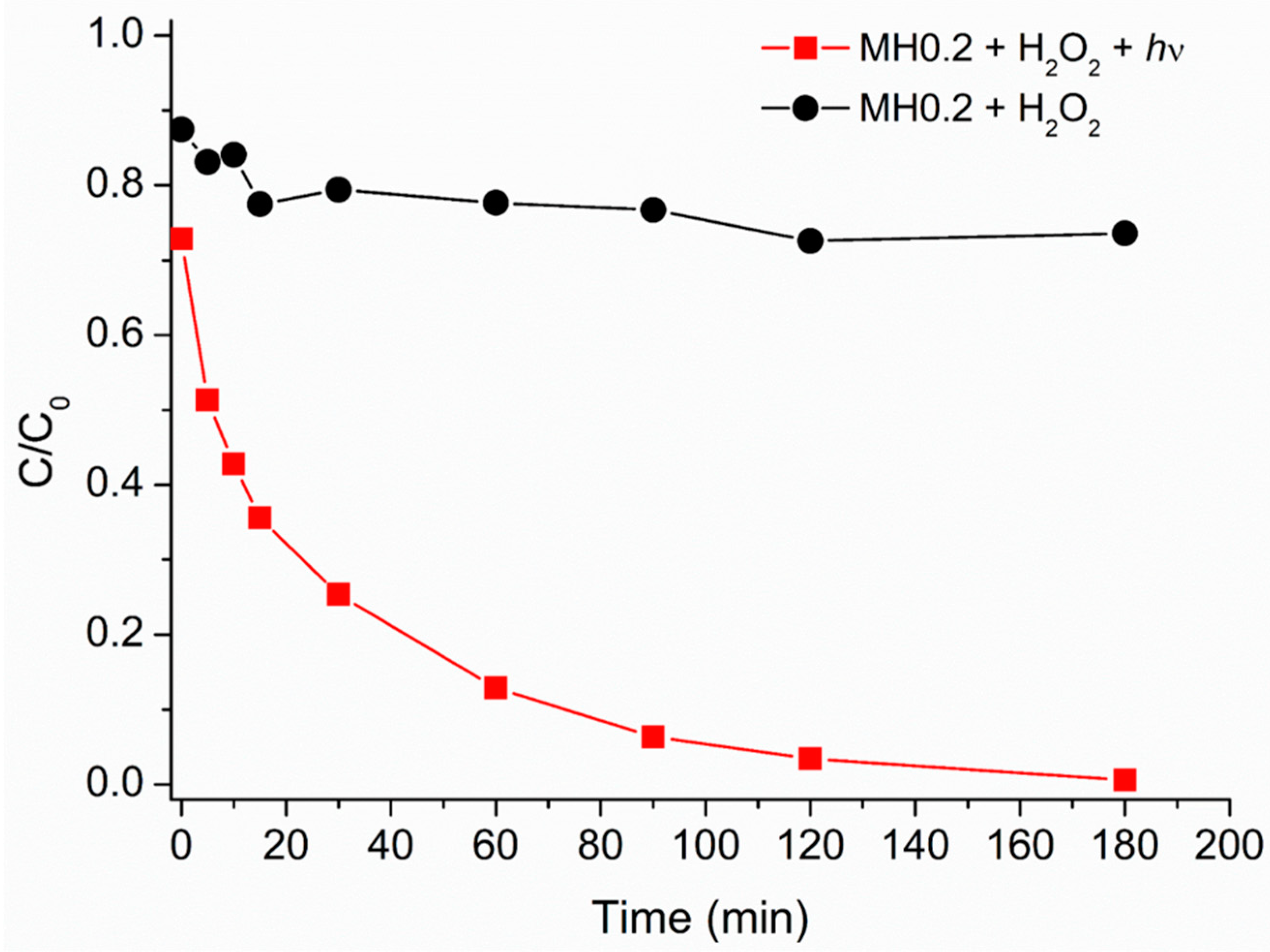
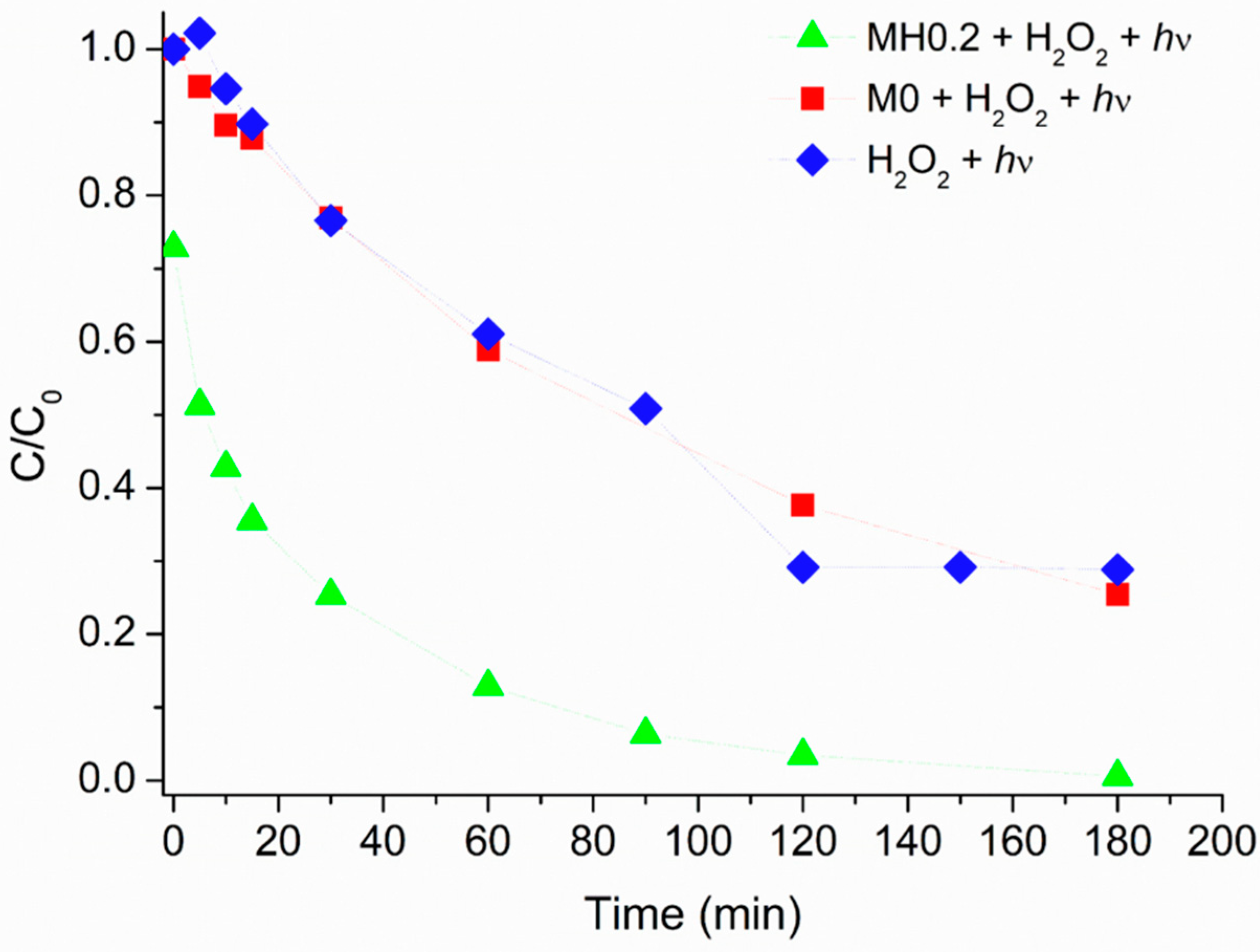
3.4. Heterogeneous Caffeine (Photo)degradation in the Presence of HMNPs
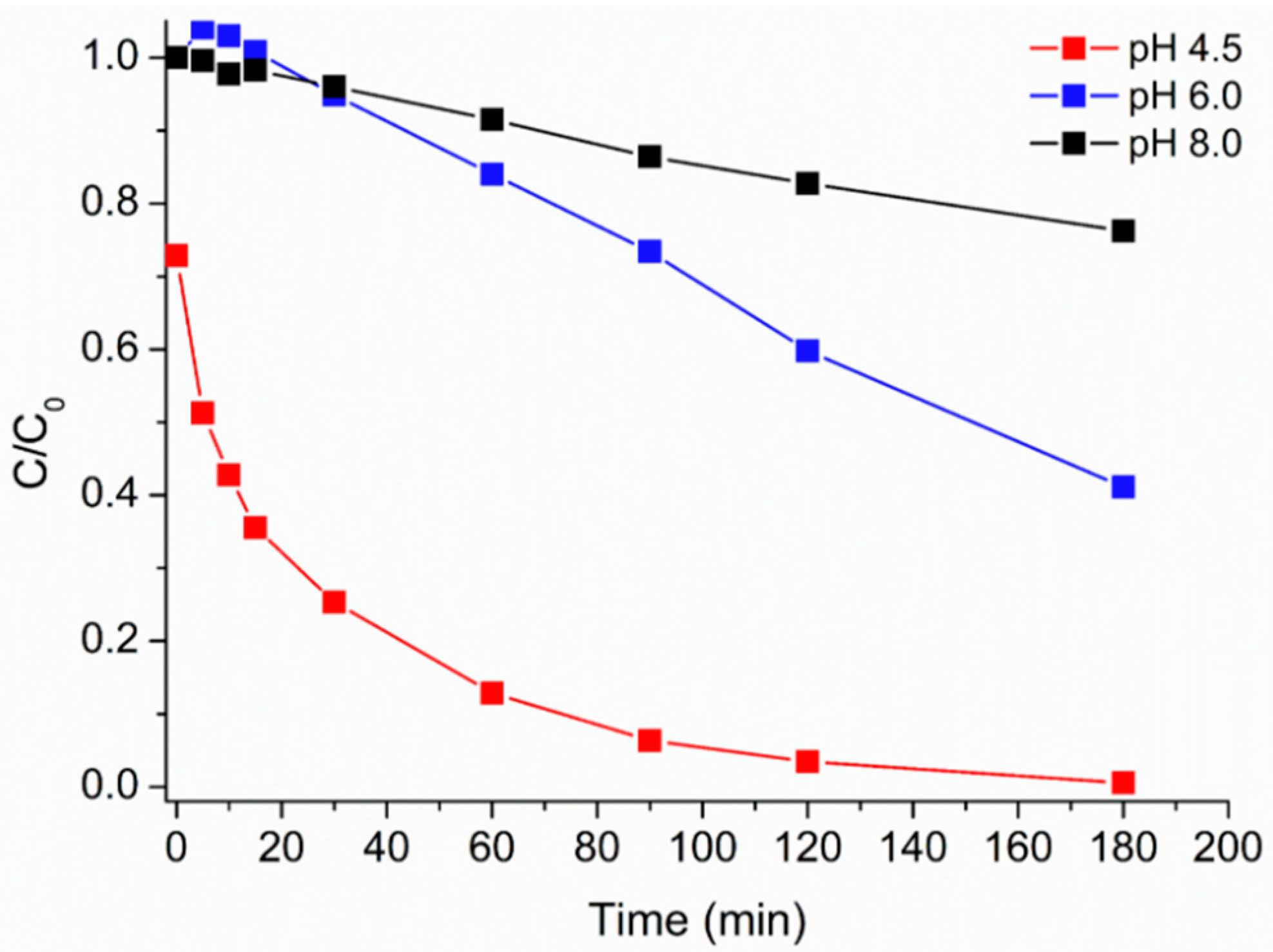
3.5. Effect of pH on the Photo-Fenton Caffeine Degradation Mediated by HMNPs
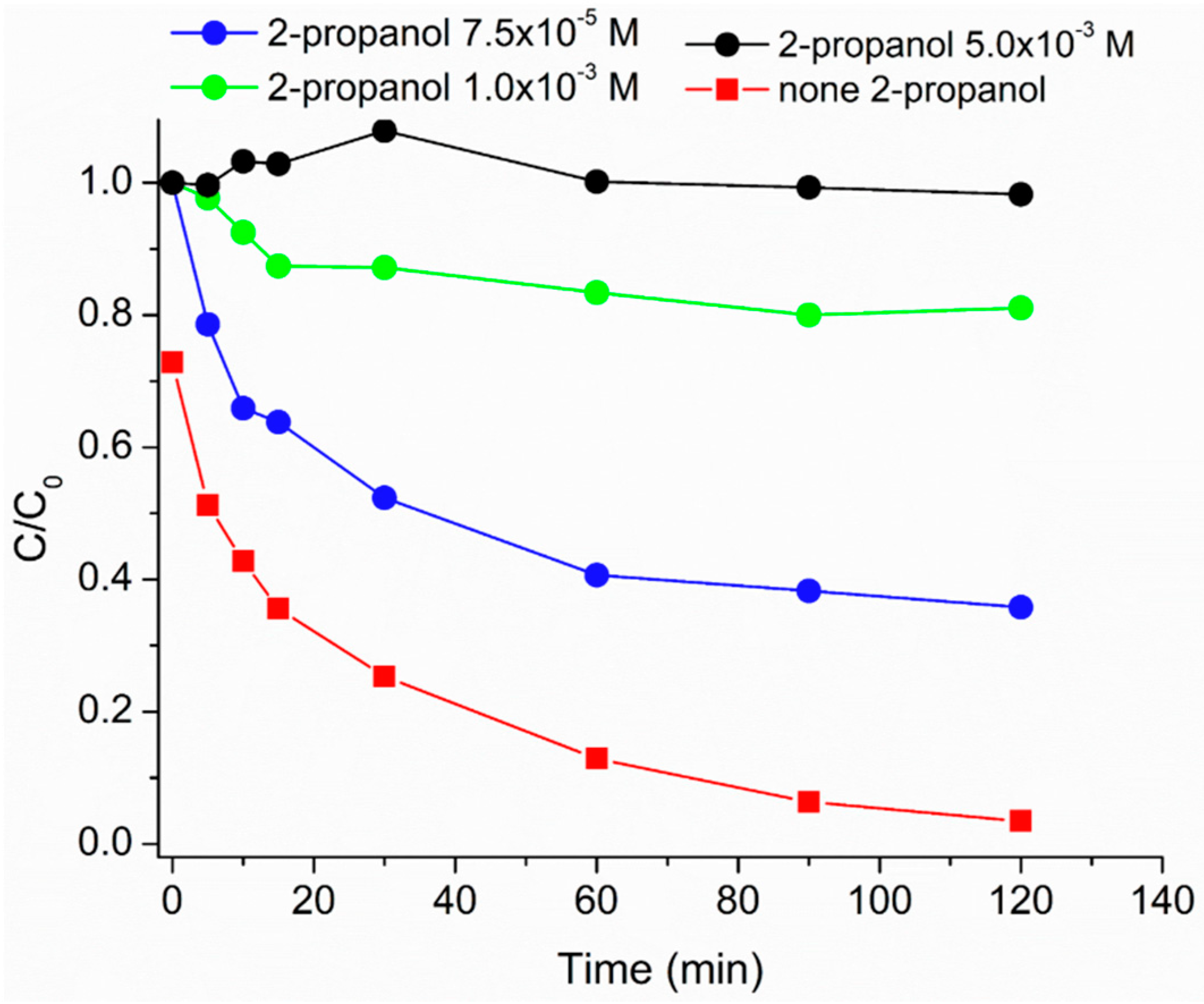
3.6. Effect of Hydroxyl Radical Scavenger and Dissolved Oxygen on the Caffeine Photodegradation Mediated by HMNPs
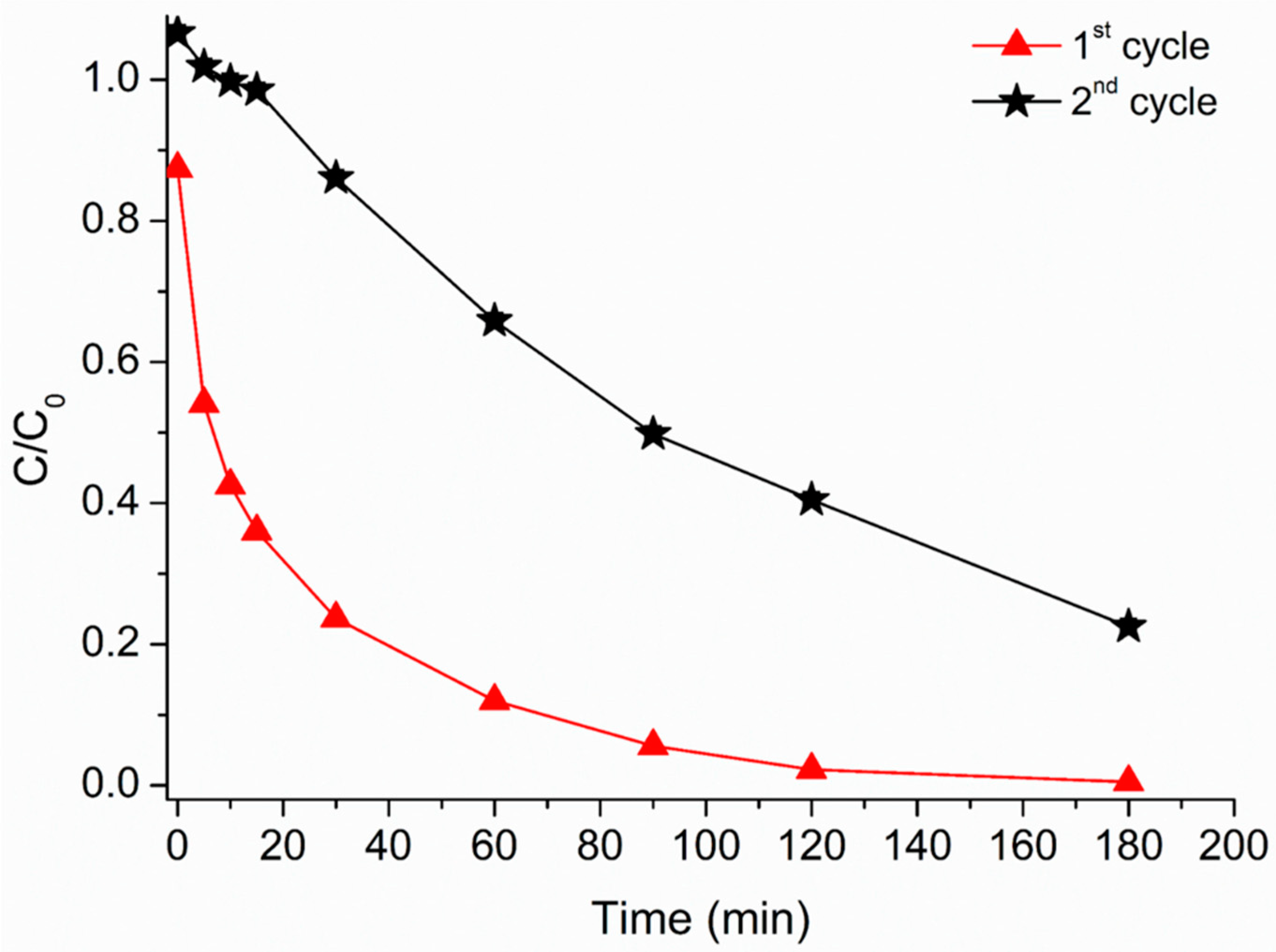
3.7. Re-Use of HMNPs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations General Assembly (UNGA). The Human Right to Water and Sanitation; Resolution 64/292; United Nations: New York, NY, USA, 2010. [Google Scholar]
- United Nations General Assembly (UNGA). Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Clean Water and Sanitation. Available online: http://www.un.org/sustainabledevelopment/water-and-sanitation/ (accessed on 1 March 2018).
- Applying the Circular Economy Lens to Water. Available online: http://circulatenews.org/2017/01/applying-the-circular-economy-lens-to-water/ (accessed on 1 March 2018).
- Yang, Y.; Ok, Y.S.; Kim, K.-H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Tiedeken, E.J.; Tahar, A.; McHugh, B.; Rowan, N.J. Monitoring, sources, receptors, and control measures for three European Union watch list substances of emerging concern in receiving waters—A 20year systematic review. Sci. Total Environ. 2017, 574, 1140–11673. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.O.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Silva, A.M.T. Occurrence and removal of organic micropollutants: An overview of the watch list of EU Decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Esrafili, A.; Gholami, M.; Jonidi Jafari, A.; Rezaei Kalantary, R.; Farzadkia, M.; Kermani, M.; Sobhi, H.R. Contaminants of emerging concern: A review of new approach in AOP technologies. Environ. Monit. Assess. 2017, 189, 414. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, A.; Vercher, R.F.; Santos-Juanes, L.; Simón, P.J.; Lardín, C.; Martínez, M.A.; Vicente, J.A.; Gonzalez, R.; Llosa, C.; Arques, A.; et al. Solar photocatalysis as a tertiary treatment to remove emerging pollutants from wastewater treatment plant effluents. Catal. Today 2011, 161, 235–240. [Google Scholar] [CrossRef]
- Borowska, E.; Bourgin, M.; Hollender, J.; Kienle, C.; McArdell, C.S.; Von Gunten, U. Oxidation of cetirizine, fexofenadine and hydrochlorothiazide during ozonation: Kinetics and formation of transformation products. Water Res. 2016, 94, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Bouafıa-Cherguı, S.; Zemmourı, H.; Chabanı, M.; Bensmaılı, A. TiO2-photocatalyzed degradation of tetracycline: Kinetic study, adsorption isotherms, mineralization and toxicity reduction. Desalin. Water Treat. 2016, 57, 16670–16677. [Google Scholar] [CrossRef]
- Casas, M.E.; Bester, K. Can those organic micro-pollutants that are recalcitrant in activated sludge treatment be removed from wastewater by biofilm reactors (slow sand filters)? Sci. Total Environ. 2015, 506–507, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, R.; Franzoso, F.; Cesano, F.; Scarano, D.; Magnacca, G.; Parolo, M.E.; Carlos, L. Chitosan-derived iron oxide systems for magnetically guided and efficient water purification processes from polycyclic aromatic hydrocarbons. ACS Sustain. Chem. Eng. 2017, 5, 793–801. [Google Scholar] [CrossRef]
- De la Cruz, N.; Esquius, L.; Grandjean, D.; Magnet, A.; Tungler, A.; de Alencastro, L.F.; Pulgarin, C. Degradation of emergent contaminants by UV, UV/H2O2 and neutral photo-Fenton at pilot scale in a domestic wastewater treatment plant. Water. Res. 2013, 47, 5836–5845. [Google Scholar] [CrossRef] [PubMed]
- Demarchis, L.; Minella, M.; Nisticò, R.; Maurino, V.; Minero, C.; Vione, D. Photo-Fenton reaction in the presence of morphologically controlled hematite as iron source. J. Photochem. Photobiol. A 2015, 307–308, 99–107. [Google Scholar] [CrossRef]
- Nisticò, R. Magnetic materials and water treatments for a sustainable future. Res. Chem. Intermed. 2017, 43, 6911–6949. [Google Scholar] [CrossRef]
- Klamerth, N.; Malato, S.; Maldonado, M.I.; Aguera, A.; Fernández-Alba, A.R. Application of photo-Fenton as a tertiary treatment of emerging contaminants in municipal wastewater. Environ. Sci. Technol. 2010, 44, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Klamerth, N.; Rizzo, L.; Malato, S.; Maldonado, M.I.; Aguera, A.; Fernández-Alba, A.R. Degradation of fifteen emerging contaminants at μgL−1 initial concentrations by mild solar photo-Fenton in MWTP effluents. Water Res. 2010, 44, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.; Fallmann, H. The photo-Fenton oxidation—A cheap and efficient wastewater treatment method. Res. Chem. Intermed. 1997, 23, 341–354. [Google Scholar] [CrossRef]
- Wu, Y.L.; Passananti, M.; Brigante, M.; Dong, W.B.; Mailhot, G. Fe(III)-EDDS complex in Fenton and photo-Fenton processes: From the radical formation to the degradation of a target compound. Environ. Sci. Pollut. Res. 2014, 21, 12154–12162. [Google Scholar] [CrossRef] [PubMed]
- Montoneri, E.; Mainero, D.; Boffa, V.; Perrone, D.G.; Montoneri, C. Biochemenergy: A project to turn an urban wastes treatment plant into biorefinery for the production of energy, chemicals and consumer’s products with friendly environmental impact. Int. J. Glob. Environ. Issues 2011, 11, 170–196. [Google Scholar] [CrossRef]
- Palma, D.; Bianco Prevot, A.; Celi, L.; Martin, M.; Fabbri, D.; Magnacca, G.; Chierotti, M.R.; Nisticò, R. Isolation, characterization, and environmental application of bio-based materials as auxiliaries in photocatalytic processes. Catalysts 2018, 8, 197. [Google Scholar] [CrossRef]
- Nisticò, R.; Barrasso, M.; Carrillo Le Roux, G.A.; Seckler, M.M.; Sousa, W.; Malandrino, M.; Magnacca, G. Biopolymers from composted biowaste as stabilizers for the synthesis of spherical and homogeneously sized silver nanoparticles for textile applications on natural fibers. ChemPhysChem 2015, 16, 3902–3909. [Google Scholar] [CrossRef] [PubMed]
- Avetta, P.; Bella, F.; Bianco Prevot, A.; Laurenti, E.; Montoneri, E.; Arques, A.; Carlos, L. Waste cleaning waste: Photodegradation of monochlorophenols in the presence of waste derived organic catalysts. ACS Sustain. Chem. Eng. 2013, 1, 1545–1550. [Google Scholar] [CrossRef]
- Montoneri, E.; Bianco Prevot, A.; Avetta, P.; Arques, A.; Carlos, L.; Magnacca, G.; Laurenti, E.; Tabasso, S. Food wastes conversion to products for use in chemical and environmental technology, material science and agriculture. In The Economic Utilisation of Food Co-Products; Kazmi, A., Shuttleworth, P., Eds.; Royal Society of Chemistry Publishing: Cambridge, UK, 2013; pp. 64–109. ISBN 978-1-84973-615-2. [Google Scholar]
- Nisticò, R.; Cesano, F.; Franzoso, F.; Magnacca, G.; Scarano, D.; Funes, I.G.; Carlos, L.; Parolo, M.E. From biowaste to magnet-responsive materials for water remediation from polycyclic aromatic hydrocarbons. Chemosphere 2018, 202, 686–693. [Google Scholar] [CrossRef] [PubMed]
- What is Circular Economy? Available online: https://www.ellenmacarthurfoundation.org/circular-economy (accessed on 10 March 2018).
- Gomis, J.; Bianco Prevot, A.; Montoneri, E.; Gonzalez, M.C.; Amat, A.M.; Martire, D.O.; Arques, A.; Carlos, L. Waste sourced bio-based substances for solar-driven wastewater remediat ion: Photodegradation of emerging pollutants. Chem. Eng. J. 2014, 235, 236–243. [Google Scholar] [CrossRef]
- Gomis, J.; Carlos, L.; Bianco Prevot, A.; Teixeira, A.C.S.C.; Mora, M.; Amat, A.M.; Vicente, R.; Arques, A. Bio-based substances from urban waste as auxiliaries for solar photo-Fenton treatment under mild conditions: Optimization of operational variables. Catal. Today 2015, 240, 39–45. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Nisticò, R.; Celi, L.R.; Bianco Prevot, A.; Carlos, L.; Magnacca, G.; Zanzo, E.; Martin, M. Sustainable magnet-responsive nanomaterials for the removal of arsenic from contaminated water. J. Hazard. Mater. 2018, 342, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Minella, M.; Marchetti, G.; De Laurentiis, E.; Malandrino, M.; Maurino, V.; Minero, C.; Vione, D.; Hanna, K. Photo-Fenton oxidation of phenol with magnetite as iron source. Appl. Catal. B Environ. 2014, 154–155, 102–109. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, D.; Dong, M.; Chai, Z.; Fu, G. Facile and green synthesis of core-shell structured magnetic chitosan submicrospheres and their surface functionalization. Langmuir 2013, 29, 11770–11778. [Google Scholar] [CrossRef] [PubMed]
- Magnacca, G.; Allera, A.; Montoneri, E.; Celi, L.; Benito, D.E.; Gagliardi, L.G.; González, M.C.; Mártire, D.O.; Carlos, L. Novel magnetite nanoparticles coated with waste-sourced biobased substances as sustainable and renewable adsorbing materials. ACS Sustain. Chem. Eng. 2014, 2, 1518–1524. [Google Scholar] [CrossRef]
- Bianco Prevot, A.; Baino, F.; Fabbri, D.; Franzoso, F.; Magnacca, G.; Nisticò, R.; Arques, A. Urban biowaste-derived sensitizing materials for caffeine photodegradation. Environ. Sci. Pollut. Res. 2017, 24, 12599–12607. [Google Scholar] [CrossRef] [PubMed]
- Franzoso, F.; Nisticò, R.; Cesano, F.; Corazzari, I.; Turci, F.; Scarano, D.; Bianco Prevot, A.; Magnacca, G.; Carlos, L.; Martire, D.O. Biowaste-derived substances as a tool for obtaining magnet-sensitive materials for environmental applications in wastewater treatments. Chem. Eng. J. 2017, 310, 307–316. [Google Scholar] [CrossRef]
- Dafouz, R.; Cáceres, N.; Rodríguez-Gil, J.L.; Mastroianni, N.; López de Alda, M.; Barceló, D.; Gil de Miguel, A.; Valcárcel, Y. Does the presence of caffeine in the marine environment represent an environmental risk? A regional and global study. Sci. Total Environ. 2018, 615, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Florawiva Compost di Qualità. Available online: http://ambiente.aceapinerolese.it/Florawiva_prese.html (accessed on 24 November 2017).
- Cesano, F.; Fenoglio, G.; Carlos, L.; Nisticò, R. One-step synthesis of magnetic chitosan polymer composite films. Appl. Surf. Sci. 2015, 345, 175–181. [Google Scholar] [CrossRef]
- Sodano, M.; Lerda, C.; Nisticò, R.; Martin, M.; Magnacca, G.; Celi, L.; Said-Pullicino, D. Dissolved organic carbon retention by coprecipitation during the oxidation of ferrous iron. Geoderma 2017, 307, 19–29. [Google Scholar] [CrossRef]
- Peuravuori, J.; Pihlaja, K. Molecular size distribution and spectroscopic properties of aquatic humic substances. Anal. Chim. Acta 1997, 337, 133–149. [Google Scholar] [CrossRef]
- Franzoso, F.; Vaca-Garcia, C.; Rouilly, A.; Evon, P.; Montoneri, E.; Persico, P.; Mendichi, R.; Nisticò, R.; Francavilla, M. Extruded versus solvent cast blends of poly(vinyl alcohol-co-ethylene) and biopolymers isolated from municipal biowaste. J. Appl. Polym. Sci. 2016, 133, 43009. [Google Scholar] [CrossRef]
- Avetta, P.; Berto, S.; Bianco Prevot, A.; Minella, M.; Montoneri, E.; Persico, D.; Vione, D.; Gonzalez, M.C.; Mártire, D.O.; Carlos, L.; et al. Photoinduced transformation of waste-derived soluble bio-based substances. Chem. Eng. J. 2015, 274, 247–255. [Google Scholar] [CrossRef]
- Vione, D.; Maurino, V.; Minero, C.; Pelizzetti, E. Phenol photonitration upon UV irradiation of nitrite in aqueous solution I: Effects of oxygen and 2-propanol. Chemosphere 2001, 45, 893–902. [Google Scholar] [CrossRef]
- Huang, W.Y.; Luo, M.Q.; Wei, C.S.; Wang, Y.H.; Hanna, K.; Mailhot, G. Enhanced heterogeneous photo-Fenton process modified by magnetite and EDDS: BPA degradation. Environ. Sci. Pollut. Res. 2017, 24, 10421–10429. [Google Scholar] [CrossRef] [PubMed]
- Amine-Khodja, A.; Trubetskaya, O.; Trubetskoj, O.; Cavani, C.; Ciavatta, C.; Guyot, G.; Richard, C. Humic-like substances extracted from composts can promote the photodegradation of Irgarol 1051 in solar light. Chemosphere 2005, 62, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Calza, P.; Di Sarro, J.; Magnacca, G.; Bianco Prevot, A.; Laurenti, E. Low-cost magnetic materials containing waste derivatives as catalyst for removal of organic pollutants: Insights into the reaction mechanism and odd aspects. In Proceedings of the 6th International Conference on Sustainable Solid Waste Management, Naxos Island, Greece, 13–16 June 2018. [Google Scholar]
| pH | Fe(II) (mg·L−1) | Fe(III) (mg·L−1) | Fe Tot. (mg·L−1) |
|---|---|---|---|
| 3.0 | 0.837 | 0.153 | 0.993 |
| 5.0 | 0.036 | 0.014 | 0.049 |
| 7.0 | 0.137 | 0.051 | 0.189 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palma, D.; Bianco Prevot, A.; Brigante, M.; Fabbri, D.; Magnacca, G.; Richard, C.; Mailhot, G.; Nisticò, R. New Insights on the Photodegradation of Caffeine in the Presence of Bio-Based Substances-Magnetic Iron Oxide Hybrid Nanomaterials. Materials 2018, 11, 1084. https://doi.org/10.3390/ma11071084
Palma D, Bianco Prevot A, Brigante M, Fabbri D, Magnacca G, Richard C, Mailhot G, Nisticò R. New Insights on the Photodegradation of Caffeine in the Presence of Bio-Based Substances-Magnetic Iron Oxide Hybrid Nanomaterials. Materials. 2018; 11(7):1084. https://doi.org/10.3390/ma11071084
Chicago/Turabian StylePalma, Davide, Alessandra Bianco Prevot, Marcello Brigante, Debora Fabbri, Giuliana Magnacca, Claire Richard, Gilles Mailhot, and Roberto Nisticò. 2018. "New Insights on the Photodegradation of Caffeine in the Presence of Bio-Based Substances-Magnetic Iron Oxide Hybrid Nanomaterials" Materials 11, no. 7: 1084. https://doi.org/10.3390/ma11071084
APA StylePalma, D., Bianco Prevot, A., Brigante, M., Fabbri, D., Magnacca, G., Richard, C., Mailhot, G., & Nisticò, R. (2018). New Insights on the Photodegradation of Caffeine in the Presence of Bio-Based Substances-Magnetic Iron Oxide Hybrid Nanomaterials. Materials, 11(7), 1084. https://doi.org/10.3390/ma11071084









