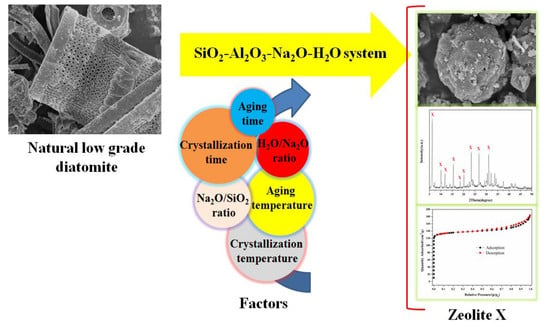
One-Step Hydrothermal Synthesis of Zeolite X Powder from Natural Low-Grade Diatomite
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Zeolite X
2.3. Characterization
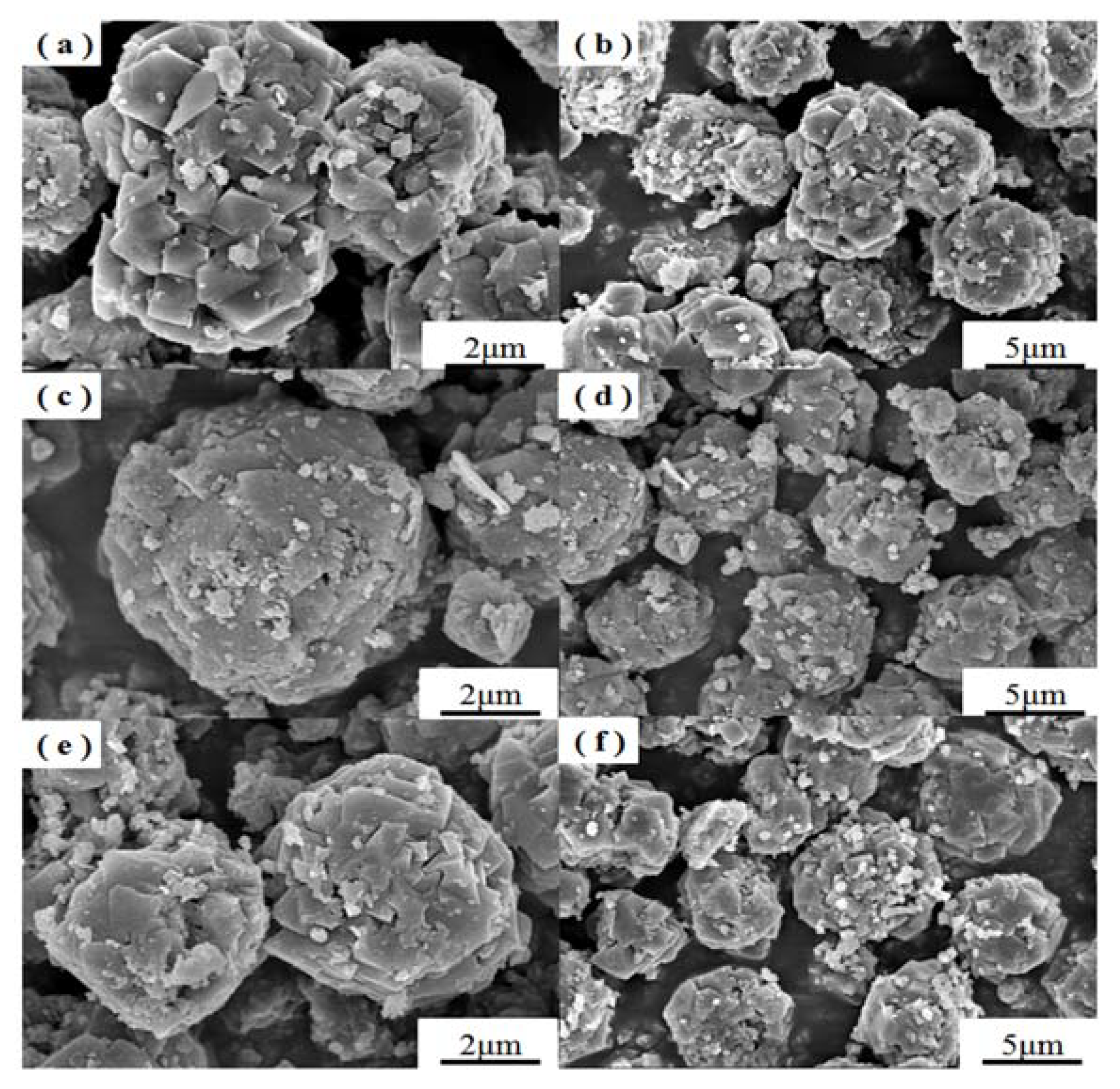
3. Results and Discussion
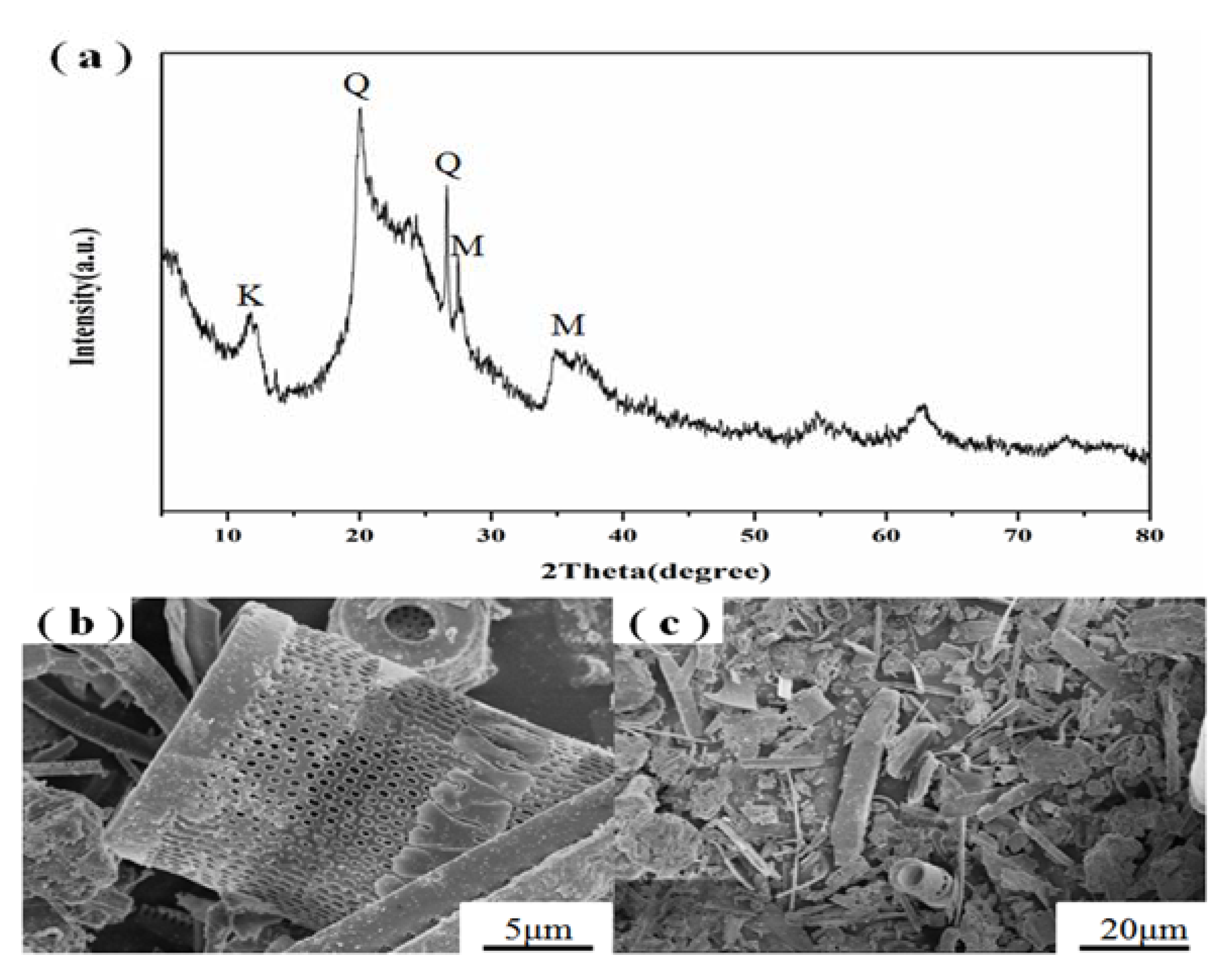
3.1. Starting Materials
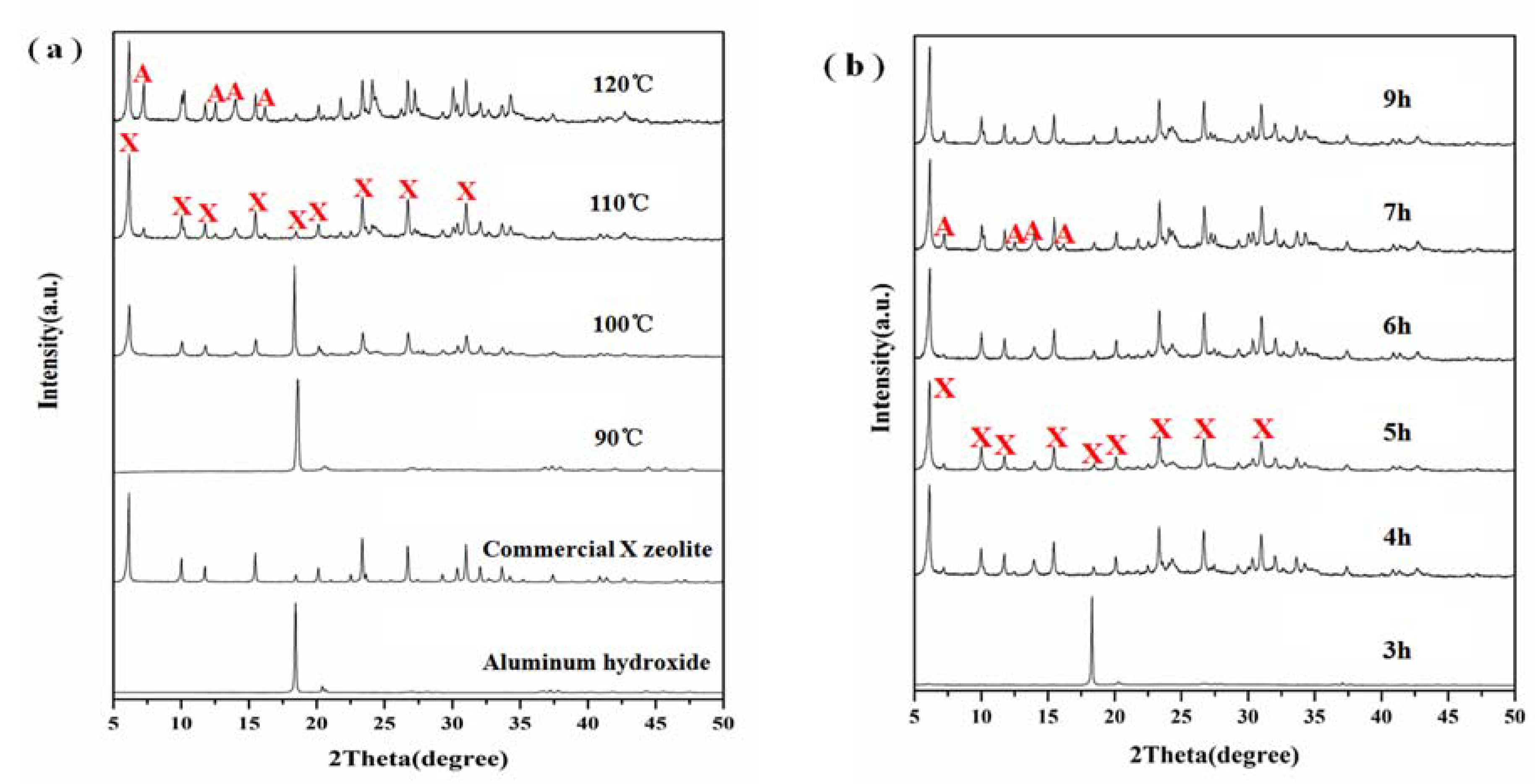
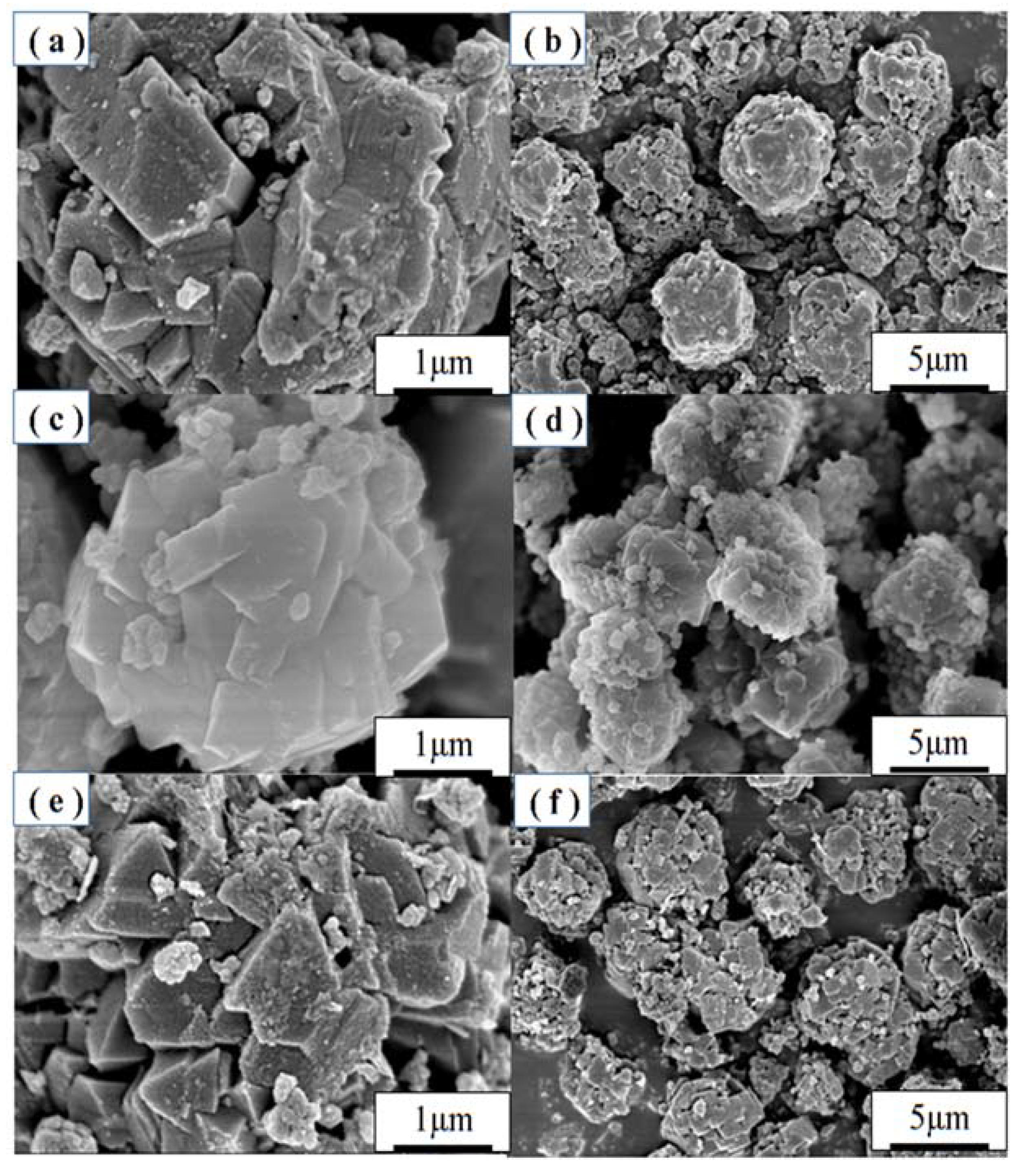
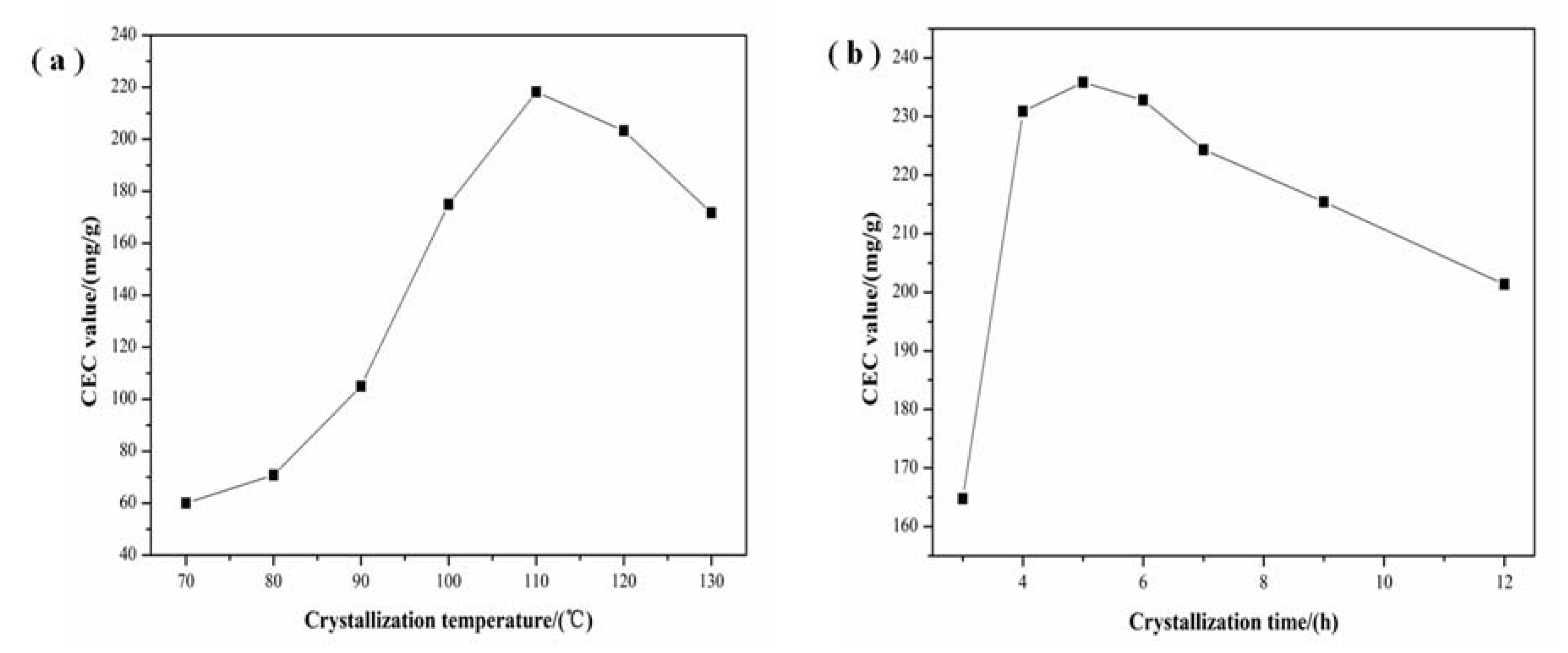
3.2. Effect of Crystallization
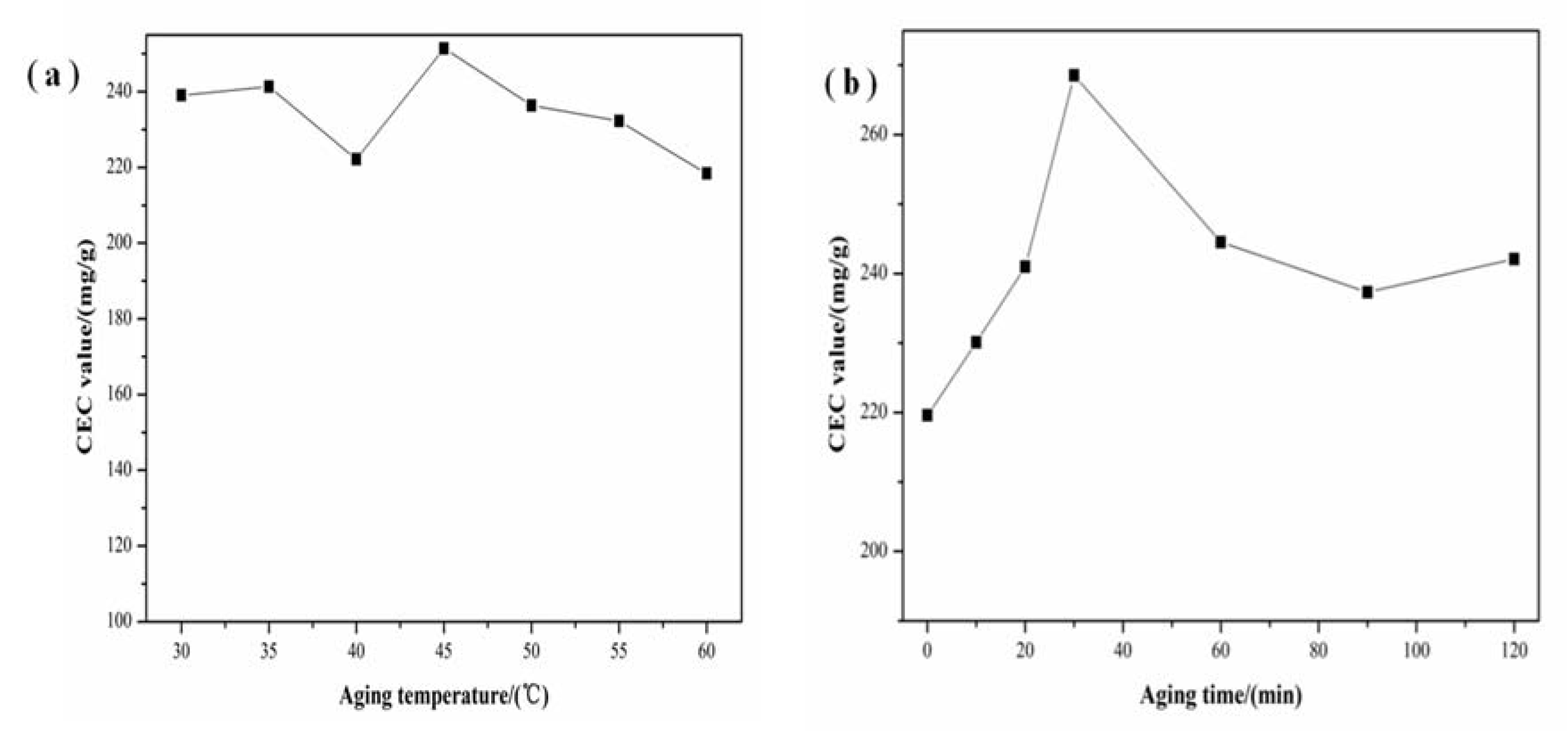
3.3. Effect of Aging
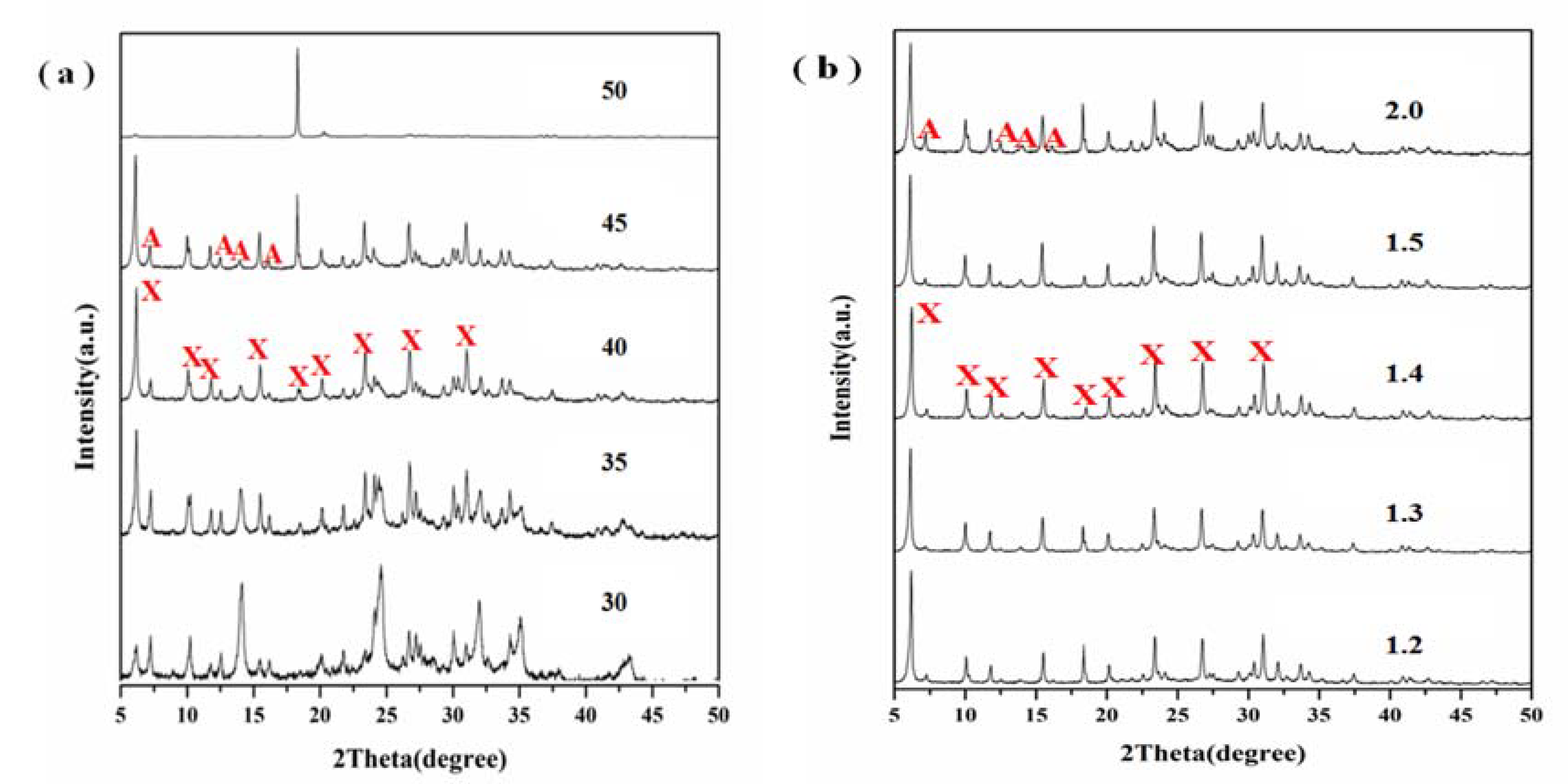
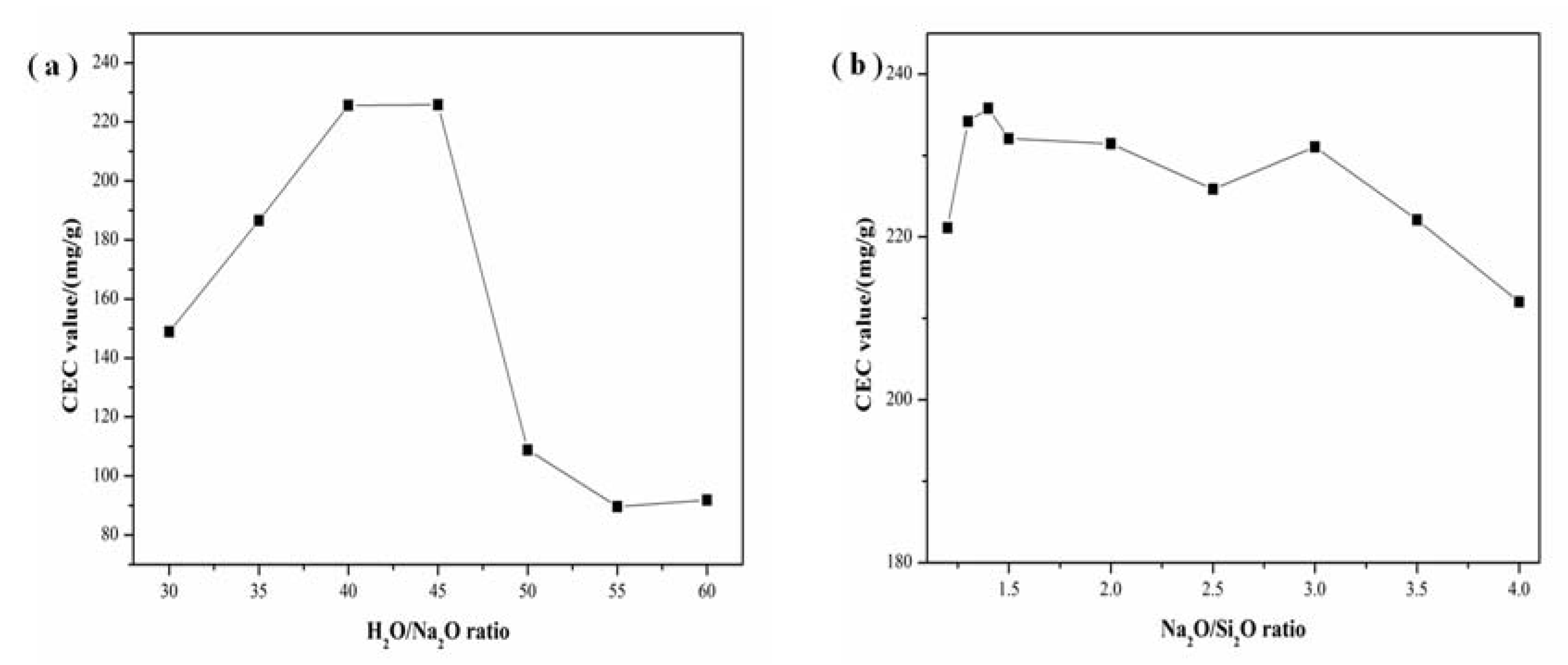
3.4. Effect of Alkalinity
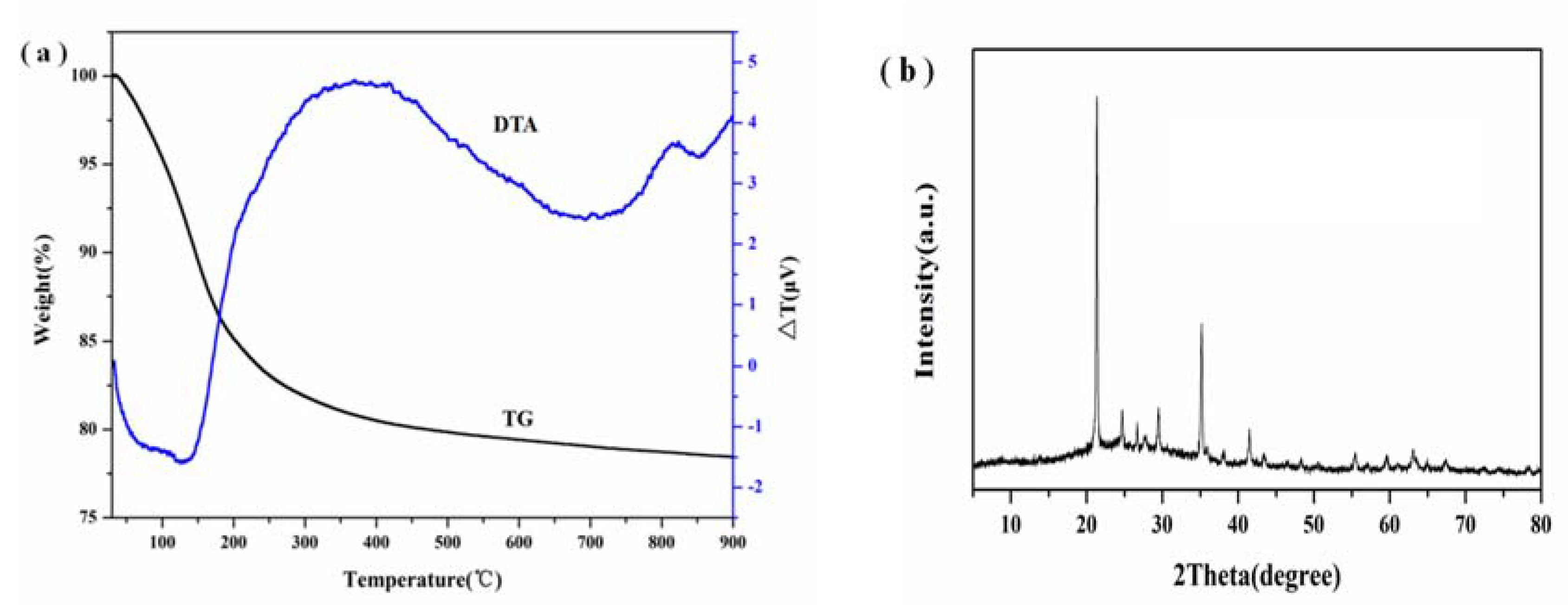
3.5. TG-DTA and XRF Analysis
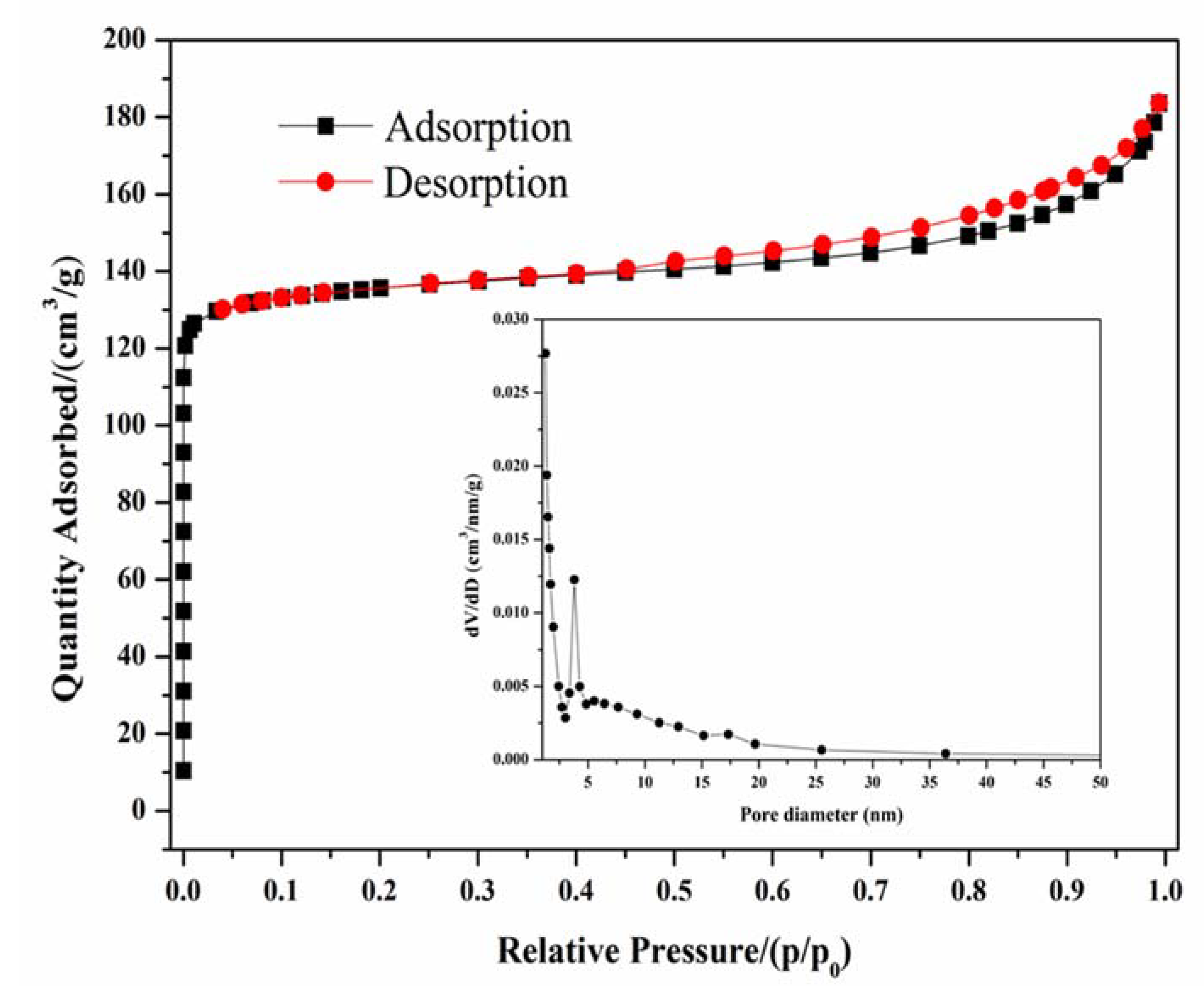
3.6. N2 Adsorption Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garcia, G.; Cardenas, E.; Cabrera, S.; Hedlund, J.; Mouzon, J. Synthesis of zeolite Y from diatomite as silica source. Microporous Mesoporous Mater. 2016, 219, 29–37. [Google Scholar] [CrossRef]
- Murad, S.; Jia, W.; Krishnamurthy, M. Molecular simulations of ion exchange in NaA zeolite membranes. Chem. Phys. Lett. 2003, 369, 402–408. [Google Scholar] [CrossRef]
- Fan, M.; Sun, J.; Bai, S.; Panezai, H. Size effects of extraframework monovalent cations on the thermal stability and nitrogen adsorption of LSX zeolite. Microporous Mesoporous Mater. 2015, 202, 44–49. [Google Scholar] [CrossRef]
- Uzunova, E.L.; Mikosch, H.; Hafner, J. Theoretical study of transition metal cation exchanged zeolites: Interaction with NO. J. Mol. Struct. THEOCHEM 2009, 912, 88–94. [Google Scholar] [CrossRef]
- Tekin, R.; Bac, N. Antimicrobial behavior of ion-exchanged zeolite X containing fragrance. Microporous Mesoporous Mater. 2016, 234, 55–60. [Google Scholar] [CrossRef]
- Doyle, A.M.; Alismaeel, Z.T.; Albayati, T.M.; Abbas, A.S. High purity FAU-type zeolite catalysts from shale rock for biodiesel production. Fuel 2017, 199, 394–402. [Google Scholar] [CrossRef]
- Abu-Zied, B.M. Cu2+-acetate exchanged X zeolites: Preparation, characterization and N2O decomposition activity. Microporous Mesoporous Mater. 2011, 139, 59–66. [Google Scholar] [CrossRef]
- Liu, H.; Lu, G.; Guo, Y.; Guo, Y.; Wang, J. Deactivation and regeneration of TS-1/diatomite catalyst for hydroxylation of phenol in fixed-bed reactor. Chem. Eng. J. 2005, 108, 187–192. [Google Scholar] [CrossRef]
- Maihom, T.; Wannakao, S.; Boekfa, B.; Limtrakul, J. Density functional study of the activity of gold-supported ZSM-5 zeolites for nitrous oxide decomposition. Chem. Phys. Lett. 2013, 556, 217–224. [Google Scholar] [CrossRef]
- Arefi Pour, A.; Sharifnia, S.; Neishabori Salehi, R.; Ghodrati, M. Adsorption separation of CO2/CH4 on the synthesized NaA zeolite shaped with montmorillonite clay in natural gas purification process. J. Nat. Gas Sci. Eng. 2016, 36, 630–643. [Google Scholar] [CrossRef]
- Selishchev, D.S.; Kolinko, P.A.; Kozlov, D.V. Adsorbent as an essential participant in photocatalytic processes of water and air purification: Computer simulation study. Appl. Catal. A Gen. 2010, 377, 140–149. [Google Scholar] [CrossRef]
- Knauth, M.; Vasenkov, S.; Kärger, J.; Fritzsche, S. Molecular dynamics study of sorbate diffusion in a simple porous membrane containing microporous nanocrystals and mesopores. Chem. Phys. Lett. 2009, 479, 95–99. [Google Scholar] [CrossRef]
- Jin, X.; Jiang, M.Q.; Shan, X.Q.; Pei, Z.G.; Chen, Z. Adsorption of methylene blue and orange II onto unmodified and surfactant-modified zeolite. J. Colloid Interface Sci. 2008, 328, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Nibou, D.; Mekatel, H.; Amokrane, S.; Barkat, M.; Trari, M. Adsorption of Zn2+ ions onto NaA and NaX zeolites: Kinetic, equilibrium and thermodynamic studies. J. Hazard. Mater. 2010, 173, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Alver, E.; Metin, A.Ü. Anionic dye removal from aqueous solutions using modified zeolite: Adsorption kinetics and isotherm studies. Chem. Eng. J. 2012, 200–202, 59–67. [Google Scholar] [CrossRef]
- Benaliouche, F.; Hidous, N.; Guerza, M.; Zouad, Y.; Boucheffa, Y. Characterization and water adsorption properties of Ag- and Zn-exchanged A zeolites. Microporous Mesoporous Mater. 2015, 209, 184–188. [Google Scholar] [CrossRef]
- Feng, P.; Zhang, G.; Zang, K.; Li, X.; Xu, L.; Chen, X. A theoretical study on the selective adsorption behavior of dimethyl ether and carbon monoxide on H-FER zeolites. Chem. Phys. Lett. 2017, 684, 279–284. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Lettino, A.; Fiore, S. A and X-type zeolites synthesised from kaolinite at low temperature. Appl. Clay Sci. 2013, 80–81, 162–168. [Google Scholar] [CrossRef]
- Garshasbi, V.; Jahangiri, M.; Anbia, M. Equilibrium CO2 adsorption on zeolite 13X prepared from natural clays. Appl. Surf. Sci. 2017, 393, 225–233. [Google Scholar] [CrossRef]
- Alaba, P.A.; Sani, Y.M.; Mohammed, I.Y.; Abakr, Y.A.; Daud, W.M.A.W. Synthesis and characterization of sulfated hierarchical nanoporous faujasite zeolite for efficient transesterification of shea butter. J. Clean. Prod. 2017, 142, 1987–1993. [Google Scholar] [CrossRef]
- Su, S.; Ma, H.; Chuan, X. Hydrothermal synthesis of zeolite A from K-feldspar and its crystallization mechanism. Adv. Powder Technol. 2016, 27, 139–144. [Google Scholar] [CrossRef]
- Qian, T.; Li, J. Synthesis of Na-A zeolite from coal gangue with the in-situ crystallization technique. Adv. Powder Technol. 2015, 26, 98–104. [Google Scholar] [CrossRef]
- Chen, D.; Hu, X.; Shi, L.; Cui, Q.; Wang, H.; Yao, H. Synthesis and characterization of zeolite X from lithium slag. Appl. Clay Sci. 2012, 59–60, 148–151. [Google Scholar] [CrossRef]
- Purnomo, C.W.; Salim, C.; Hinode, H. Synthesis of pure Na–X and Na–A zeolite from bagasse fly ash. Microporous Mesoporous Mater. 2012, 162, 6–13. [Google Scholar] [CrossRef]
- Ma, D.; Wang, Z.; Guo, M.; Zhang, M.; Liu, J. Feasible conversion of solid waste bauxite tailings into highly crystalline 4A zeolite with valuable application. Waste Manag. 2014, 34, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhong, H.; Wang, S.; Xue, J.; Zhang, Z. Removal of basic dye (methylene blue) from aqueous solution using zeolite synthesized from electrolytic manganese residue. J. Ind. Eng. Chem. 2015, 23, 344–352. [Google Scholar] [CrossRef]
- Musyoka, N.M.; Ren, J.; Langmi, H.W.; North, B.C.; Mathe, M. A comparison of hydrogen storage capacity of commercial and fly ash-derived zeolite X together with their respective templated carbon derivatives. Int. J. Hydrog. Energy 2015, 40, 12705–12712. [Google Scholar] [CrossRef]
- Babajide, O.; Musyoka, N.; Petrik, L.; Ameer, F. Novel zeolite Na-X synthesized from fly ash as a heterogeneous catalyst in biodiesel production. Catal. Today 2012, 190, 54–60. [Google Scholar] [CrossRef]
- Volli, V.; Purkait, M.K. Selective preparation of zeolite X and A from flyash and its use as catalyst for biodiesel production. J. Hazard. Mater. 2015, 297, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Manadee, S.; Sophiphun, O.; Osakoo, N.; Supamathanon, N.; Kidkhunthod, P.; Chanlek, N.; Wittayakun, J.; Prayoonpokarach, S. Identification of potassium phase in catalysts supported on zeolite NaX and performance in transesterification of Jatropha seed oil. Fuel Process. Technol. 2017, 156, 62–67. [Google Scholar] [CrossRef]
- Li, T.; Liu, H.; Fan, Y.; Yuan, P.; Shi, G.; Bi, X.T.; Bao, X. Synthesis of zeolite Y from natural aluminosilicate minerals for fluid catalytic cracking application. Green Chem. 2012, 14, 3255–3259. [Google Scholar] [CrossRef]
- Magaña, S.M.; Quintana, P.; Aguilar, D.H.; Toledo, J.A.; Ángeles-Chávez, C.; Cortés, M.A.; León, L.; Freile-Pelegrín, Y.; López, T.; Sánchez, R.M.T. Antibacterial activity of montmorillonites modified with silver. J. Mol. Catal. A Chem. 2008, 281, 192–199. [Google Scholar] [CrossRef]
- Ogura, M.; Kawazu, Y.; Takahashi, H.; Okubo, T. Aluminosilicate species in the hydrogel phase formed during the aging process for the crystallization of FAU zeolite. Chem. Mater. 2003, 15, 2661–2667. [Google Scholar] [CrossRef]
- Liu, L.; Du, T.; Li, G.; Yang, F.; Che, S. Using one waste to tackle another: Preparation of a CO2 capture material zeolite X from laterite residue and bauxite. J. Hazard. Mater. 2014, 278, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Ltaief, O.O.; Siffert, S.; Fourmentin, S.; Benzina, M. Synthesis of Faujasite type zeolite from low grade Tunisian clay for the removal of heavy metals from aqueous waste by batch process: Kinetic and equilibrium study. C. R. Chim. 2015, 18, 1123–1133. [Google Scholar] [CrossRef]
- Derkowski, A.; Franus, W.; Waniak-Nowicka, H.; Czímerová, A. Textural properties vs. CEC and EGME retention of Na–X zeolite prepared from fly ash at room temperature. Int. J. Miner. Process. 2007, 82, 57–68. [Google Scholar] [CrossRef]
- Izidoro, J.D.C.; Fungaro, D.A.; Abbott, J.E.; Wang, S. Synthesis of zeolites X and A from fly ashes for cadmium and zinc removal from aqueous solutions in single and binary ion systems. Fuel 2013, 103, 827–834. [Google Scholar] [CrossRef]
- Zeng, S.; Wang, R.; Zhang, Z.; Qiu, S. Solventless green synthesis of sodalite zeolite using diatomite as silica source by a microwave heating technique. Inorg. Chem. Commun. 2016, 70, 168–171. [Google Scholar] [CrossRef]
- Sanhueza, V.; Kelm, U.; Cid, R.; López-Escobar, L. Synthesis of ZSM-5 from diatomite: A case of zeolite synthesis from a natural material. J. Chem. Technol. Biotechnol. 2004, 79, 686–690. [Google Scholar] [CrossRef]
- Du, Y.; Shi, S.; Dai, H. Water-bathing synthesis of high-surface-area zeolite P from diatomite. Particuology 2011, 9, 174–178. [Google Scholar] [CrossRef]
- Wajima, T.; Haga, M.; Kuzawa, K.; Ishimoto, H.; Tamada, O.; Ito, K.; Nishiyama, T.; Downs, R.T.; Rakovan, J.F. Zeolite synthesis from paper sludge ash at low temperature (90 degrees C) with addition of diatomite. J. Hazard. Mater. 2006, 132, 244–252. [Google Scholar] [CrossRef] [PubMed]


| Chemical Composition | SiO2 | Al2O3 | Na2O | Fe2O3 | H2O | n(Si/Al) |
|---|---|---|---|---|---|---|
| Optimal X zeolite | 35.31 | 24.87 | 15.17 | 0.96 | 22.90 | 1.21 |
| SBET (m2/g) | Smicro (m2/g) | Vtotal (cm3/g) | Vmicro (cm3/g) |
|---|---|---|---|
| 453 | 399 | 0.2838 | 0.1866 |
| Zeolite | Si and Al Source | BET Surface Area (m2/g) | Refs. |
|---|---|---|---|
| X | Fly ash | 344 | [36] |
| X | Feldspar | 472 | [19] |
| X | Bentonite | 505 | [19] |
| X | Kaolinite | 591 | [19] |
| X | Fly ash | 404 | [27] |
| X | Fly ash and sodium aluminate | 397 | [37] |
| X | Diatomite and aluminum hydroxide | 453 | This work |
| Zeolite | Al Source | Hydrothermal Time (h) | Synthenic Method | Refs. |
|---|---|---|---|---|
| Sodalite | / | / | Microwave heating methods | [38] |
| Y | Al2(SO4)3 | 6–48 | H2SO4 activation and hydrothermal methods | [1] |
| ZSM-5 | NaAlO2 | 40 | Templation and hydrothermal methods | [39] |
| P | Aluminum hydroxide | 6–24 | Water-bathing methods | [40] |
| P | Paper sludge ash | 24 | Low-temperature methods | [41] |
| X | Aluminum hydroxide | 5 | Hydrothermal methods | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, G.; Lei, J.; Zhang, X.; Sun, Z.; Zheng, S. One-Step Hydrothermal Synthesis of Zeolite X Powder from Natural Low-Grade Diatomite. Materials 2018, 11, 906. https://doi.org/10.3390/ma11060906
Yao G, Lei J, Zhang X, Sun Z, Zheng S. One-Step Hydrothermal Synthesis of Zeolite X Powder from Natural Low-Grade Diatomite. Materials. 2018; 11(6):906. https://doi.org/10.3390/ma11060906
Chicago/Turabian StyleYao, Guangyuan, Jingjing Lei, Xiaoyu Zhang, Zhiming Sun, and Shuilin Zheng. 2018. "One-Step Hydrothermal Synthesis of Zeolite X Powder from Natural Low-Grade Diatomite" Materials 11, no. 6: 906. https://doi.org/10.3390/ma11060906
APA StyleYao, G., Lei, J., Zhang, X., Sun, Z., & Zheng, S. (2018). One-Step Hydrothermal Synthesis of Zeolite X Powder from Natural Low-Grade Diatomite. Materials, 11(6), 906. https://doi.org/10.3390/ma11060906