Spherical Activated Carbons with High Mechanical Strength Directly Prepared from Selected Spherical Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Methodology
2.1.1. Carbonization Process
2.1.2. Activation Process
2.2. Characterization
2.2.1. Morphology
2.2.2. Surface Area and Pore Volumes
2.2.3. Mechanical Properties
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Wickramaratne, N.P.; Qiao, S.Z.; Jaroniec, M. Molecular-based design and emerging applications of nanoporous carbon spheres. Nat. Mater. 2015, 14, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ling, L.; Qiao, W.; Liu, L. Effect of hydrogen on the mesopore development of pitch-based spherical activated carbon containing iron during activation by steam. Carbon 1999, 37, 2063–2066. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, R.; Liu, C.; Liu, X.; Qiao, W.; Zhan, L.; Ling, L. Preparation of polystyrene-based activated carbon spheres and their adsorption of dibenzothiophene. New Carbon Mater. 2009, 9, 8–13. [Google Scholar] [CrossRef]
- Gryglewicz, G.; Grabas, K.; Lorenc-Grabowska, E. Preparation and characterization of spherical activated carbons from oil agglomerated bituminous coals for removing organic impurities from water. Carbon 2002, 40, 2403–2411. [Google Scholar] [CrossRef]
- Romero-Anaya, A.J.; Lillo-Ródenas, M.A.; Linares-Solano, A. Spherical activated carbons for low concentration toluene adsorption. Carbon 2010, 48, 2625–2633. [Google Scholar] [CrossRef]
- Yenisoy-Karakaş, S.; Aygün, A.; Güneş, M.; Tahtasakal, E. Physical and chemical characteristics of polymer-based spherical activated carbon and its ability to adsorb organics. Carbon 2004, 42, 477–484. [Google Scholar] [CrossRef]
- Lee, C.; Hsu, S. Preparation of spherical encapsulation of activated carbons and their adsorption capacity of typical uremic toxins. J. Biomed. Mater. Res. 1990, 24, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Wickramaratne, N.P.; Jaroniec, M. Activated carbon spheres for CO2 adsorption. Appl. Mater. Interfaces 2013, 5, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Romero-Anaya, A.J.; Lillo-Ródenas, M.A.; Linares-Solano, A. Activation of a spherical carbon for toluene adsorption at low concentration. Carbon 2014, 77, 616–626. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Rao, Y.; Zhao, X.; Wu, M. Superior CO2, CH4, and H2 uptakes over ultrahigh-surface-area carbon spheres prepared from sustainable biomass-derived char by CO2 activation. Carbon 2016, 105, 454–462. [Google Scholar] [CrossRef]
- Liu, C.; Liang, X.; Liu, X.; Wang, Q.; Teng, N.; Zhan, L.; Zhang, R.; Qiao, W.; Ling, L. Wettability modification of pitch-based spherical activated carbon by air oxidation and its effects on phenol adsorption. Appl. Surf. Sci. 2008, 254, 2659–2665. [Google Scholar] [CrossRef]
- Xuan, H.; Wang, Y.; Lin, G.; Wang, F.; Zhou, L.; Dong, X.; Chen, Z. Air-assisted activation strategy for porous carbon spheres to give enhanced electrochemical performance. RSC Adv. 2016, 6, 15313–15319. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. A green approach to high-performance supercapacitor electrodes: The chemical activation of hydrochar with potassium bicarbonate. ChemSusChem 2016, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Tsivadze, A.Y.; Gur’yanov, V.V.; Petukhova, G.A. Preparation of spherical activated carbon from furfural, its properties and prospective applications in medicine and the national economy. Prot. Met. Phys. Chem. Surf. 2011, 47, 612–620. [Google Scholar] [CrossRef]
- Bo, B.; Wasserscheid, P.; Etzold, B.J. Polymer-based spherical activated carbon as easy-to-handle catalyst support for hydrogenation reactions. Chem. Eng. Technol. 2016, 39, 276–284. [Google Scholar] [CrossRef]
- Rufete-Beneite, M.; Román-Martínez, M.C.; Linares-Solano, A. Insight into the immobilization of ionic liquids on porous carbons. Carbon 2014, 77, 947–957. [Google Scholar] [CrossRef]
- Long, D.; Zhang, R.; Qiao, W.; Zhang, L.; Liang, X.; Ling, L. Biomolecular adsorption behavior on spherical carbon aerogels with various mesopore sizes. J. Colloid Interface Sci. 2009, 331, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.C.; Kim, J.G.; Kim, H.; Chen, M.L.; Zhang, F.J.; Zhang, K.; Meng, Z. Da Preparation of spherical activated carbon and their physicochemical properties. J. Korean Ceram. Soc. 2009, 46, 568–573. [Google Scholar] [CrossRef]
- Luo, G.; Shi, W.; Chen, X.; Ni, W.; Strong, P.J.; Jia, Y.; Wang, H. Hydrothermal conversion of water lettuce biomass at 473 or 523 K. Biomass Bioenergy 2011, 35, 4855–4861. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C. Hydrothermal Carbonization of Lignocellulosic Biomass. Energy Fuels 2014, 25, 1802–1810. [Google Scholar] [CrossRef]
- Sevilla, M.; Maciá-Agulló, J.A.; Fuertes, A.B. Hydrothermal carbonization of biomass as a route for the sequestration of CO2: Chemical and structural properties of the carbonized products. Biomass Bioenergy 2011, 35, 3152–3159. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y. Ga2O3 and GaN semiconductor hollow spheres. Angew. Chem. Int. Ed. 2004, 43, 3827–3831. [Google Scholar] [CrossRef] [PubMed]
- Bedin, K.C.; Cazetta, A.L.; Souza, I.P.A.F.; Pezoti, O.; Souza, L.S.; Souza, P.S.C.; Yokoyama, J.T.C.; Almeida, V.C. Porosity enhancement of spherical activated carbon: Influence and optimization of hydrothermal synthesis conditions using response surface methodology. J. Environ. Chem. Eng. 2018, 6, 991–999. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, A.; Yan, L.; Liu, F.; Zhang, Q. Preparation and characterization of highly mesoporous spherical activated carbons from divinylbenzene-derived polymer by ZnCl2 activation. J. Colloid Interface Sci. 2007, 316, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, M.; Wang, C.; Bai, J.; Zheng, J. Synthesis of carbon microspheres from urea formaldehyde resin. Mater. Lett. 2011, 65, 1069–1072. [Google Scholar] [CrossRef]
- Yao, C.; Shin, Y.; Wang, L.Q.; Windisch, C.F.; Samuels, W.D.; Arey, B.W.; Wang, C.; Risen, W.M.; Exarhos, G.J. Hydrothermal dehydration of aqueous fructose solutions in a closed system. J. Phys. Chem. C 2007, 111, 15141–15145. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- Wang, F.L.; Pang, L.L.; Jiang, Y.Y.; Chen, B.; Lin, D.; Lun, N.; Zhu, H.-L.; Liu, R.; Meng, X.L.; Wang, Y.; et al. Simple synthesis of hollow carbon spheres from glucose. Mater. Lett. 2009, 63, 2564–2566. [Google Scholar] [CrossRef]
- Romero-Anaya, A.J.; Ouzzine, M.; Lillo-Ródenas, M.A.; Linares-Solano, A. Spherical carbons: Synthesis, characterization and activation processes. Carbon 2014, 68, 296–307. [Google Scholar] [CrossRef]
- Rodriguez-Reinoso, F.; Linares-Solano, A. Microporous Structure of Activated Carbons as Revealed by Adsorption Methods; Thrower, P.A., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1989; Volume 21, ISBN 0-8247-7939-8. [Google Scholar]
- Linares-Solano, Á.; Salinas-Martínez de Lecea, C.; Alcañiz-Monge, J.; Cazorla-Amorós, D. Further advances in the characterization of microporous carbons by physical adsorption of gases. Tanso 1998, 185, 316–325. [Google Scholar] [CrossRef]
- Cazorla-Amorós, D.; Alcañiz-Monge, J.; Linares-Solano, A. Characterization of activated carbon fibers by CO2 adsorption. Langmuir 1996, 12, 2820–2824. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Marco-Lozar, J.P.; Cazorla-Amorós, D.; Linares-Solano, A. Activated carbons prepared by pyrolysis of mixtures of carbon precursor/alkaline hydroxide. J. Anal. Appl. Pyrolysis 2007, 80, 166–174. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F.; Molina-Sabio, M. Activated carbons from lignocellulosic materials by chemical and/or physical activation: An overview. Carbon 1992, 30, 1111–1118. [Google Scholar] [CrossRef]
- Contreras, M.S.; Páez, C.A.; Zubizarreta, L.; Léonard, A.; Blacher, S.; Olivera-Fuentes, C.G.; Arenillas, A.; Pirard, J.P.; Job, N. A comparison of physical activation of carbon xerogels with carbon dioxide with chemical activation using hydroxides. Carbon 2010, 48, 3157–3168. [Google Scholar] [CrossRef]
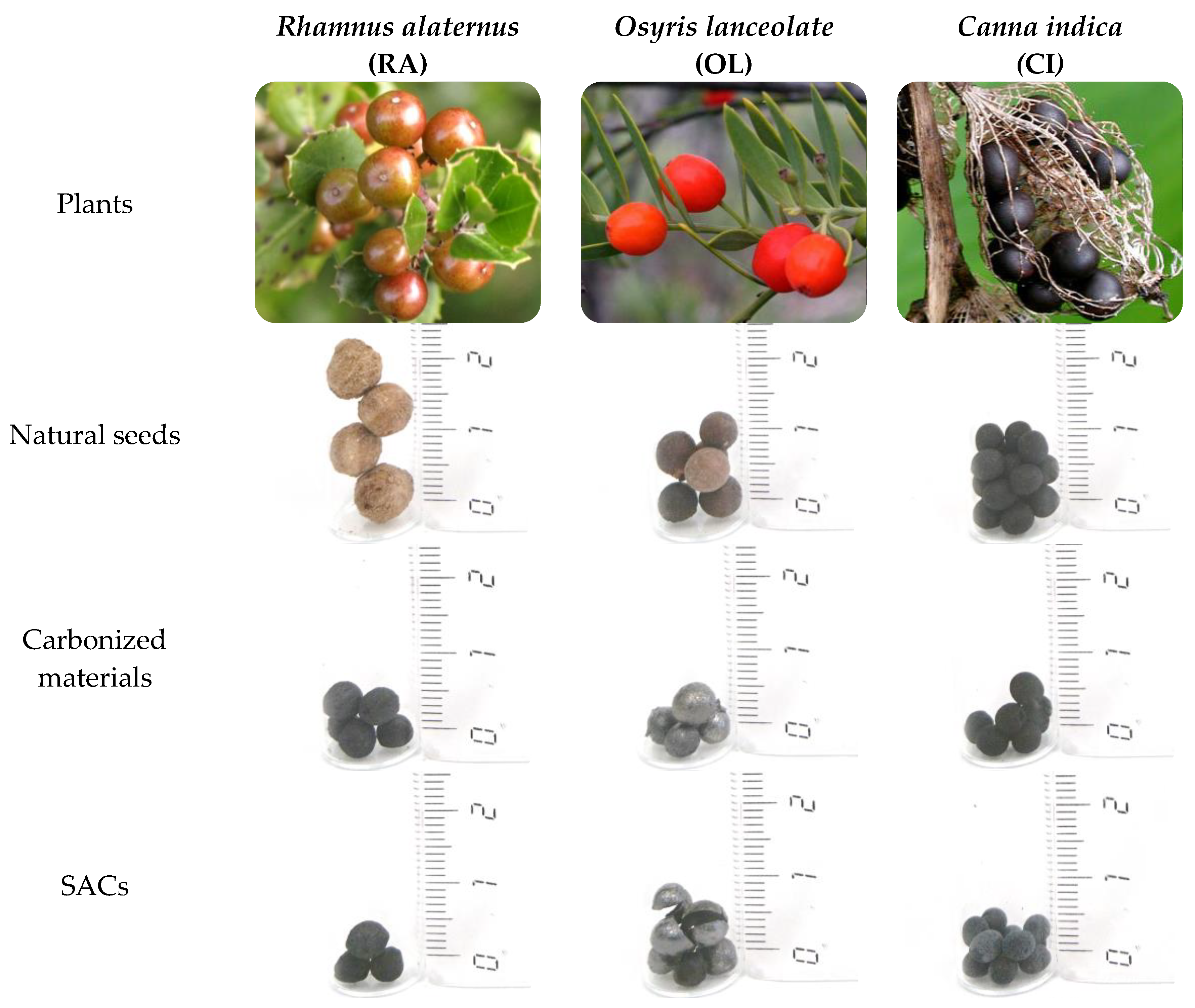

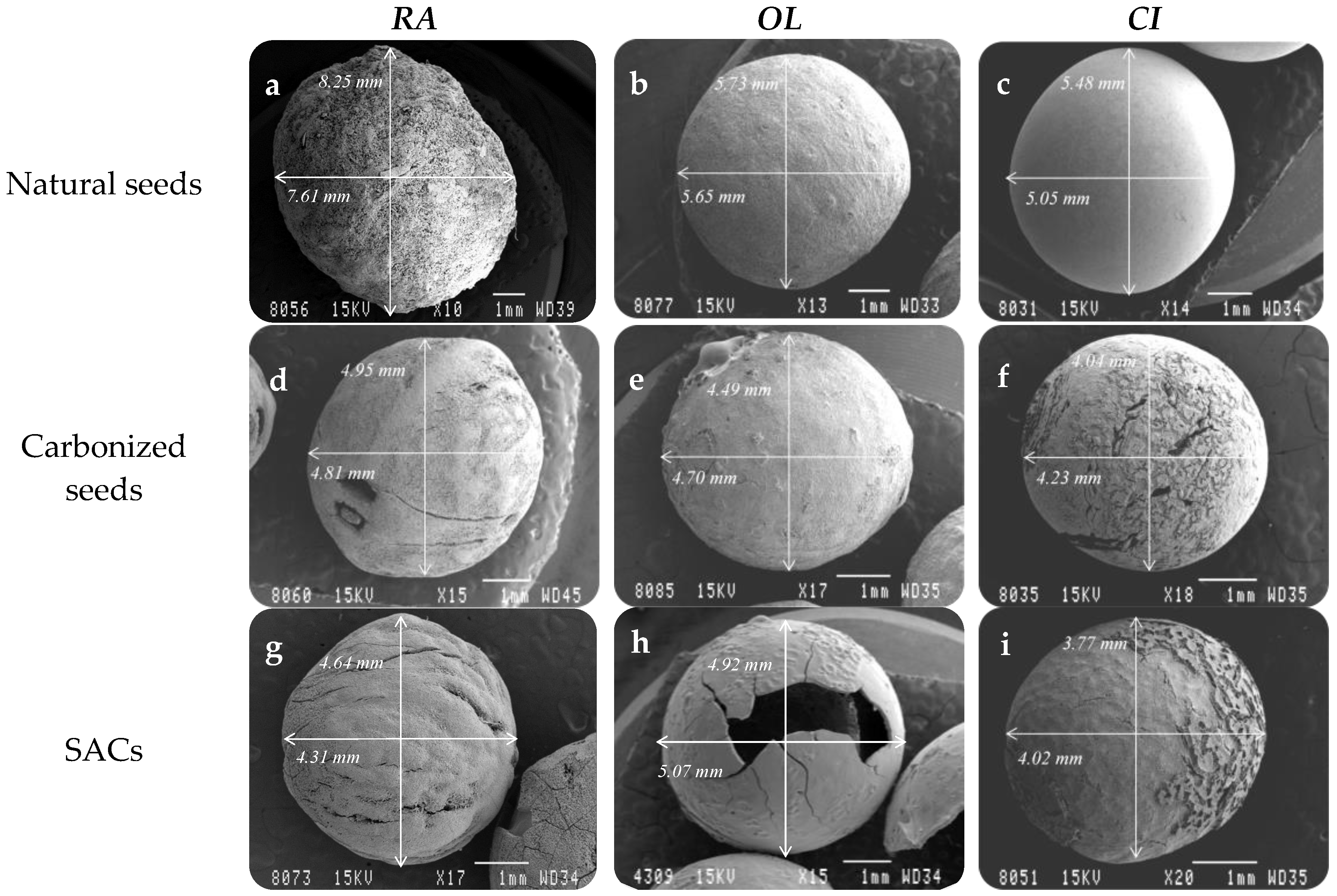

| Common Name | Scientific Name | Mean Diameter (mm) |
|---|---|---|
| Poppy | Papaver rhoeas | 1 |
| Amaranth | Amaranthus hypochondriacus | 1 |
| Millet | Panicum miliaceum | 2 |
| Mustard | Sinapis alba | 3 |
| Black pepper | Piper nigrum | 4 |
| False pepper | Schinus molle | 4 |
| Palm | Phoenix dactylifera | 5 |
| Indian shot | Canna indica | 5 |
| African sandalwood | Osyris lanceolate | 5 |
| Phoenicean juniper | Juniperus phoenicea | 6 |
| Mediterranean buckthorn | Rhamnus alaternus | 7 |
| Prickly juniper | Juniperus oxycedrus | 7 |
| Precursor | SRM a (%) | Yield b (%) | VDR (N2) c (cm3/g) | VDR (CO2) d (cm3/g) | SRM e (%) |
|---|---|---|---|---|---|
| RA | 99.1 | 30 | 0.01 | 0.18 | 98.8 |
| OL | 99.9 | 21 | 0.01 | 0.19 | 99.4 |
| CI | 99.0 | 22 | 0.02 | 0.20 | 98.7 |
| Precursor | T (°C) | t (h) | Burn-off (%) | SBET a (m2/g) | VDR (N2) b (cm3/g) | VDR (CO2) c (cm3/g) | Vmeso d (cm3/g) | SRM e (%) | VN2–VCO2 g (cm3/g) |
|---|---|---|---|---|---|---|---|---|---|
| RA | 800 | 10 | 6 | 492 | 0.20 | 0.25 | 0.03 | NMf | < 0 |
| 800 | 30 | 26 | 812 | 0.28 | 0.36 | 0.02 | 97.8 | < 0 | |
| 800 | 40 | 33 | 889 | 0.40 | 0.33 | 0.03 | NMf | 0.07 | |
| 850 | 10 | 33 | 874 | 0.39 | 0.37 | 0.02 | NMf | 0.02 | |
| CI | 800 | 5 | 33 | 856 | 0.39 | 0.35 | 0.05 | 94.9 | 0.04 |
| 880 | 3 | 89 | 1616 | 0.64 | 0.37 | 0.19 | 85.3 | 0.27 |
| Name | Commercial Name | Morphology and Size | SBET a (m2/g) | VDR (N2) b (cm3/g) | VDR (CO2) c (cm3/g) | Vmeso d (cm3/g) | SRM (%) |
|---|---|---|---|---|---|---|---|
| CW | Mead Westvaco, WVA1100 | Granular (10 × 25 mesh) | 1796 | 0.72 | 0.34 | 0.42 | 72 |
| CK | Kureha Corporation carbon from petroleum pith | Spherical (0.75 µm) | 1185 | 0.57 | 0.42 | 0.02 | 97 |
| ROX | NORIT® ROX | Pellets (0.8 mm) | 1354 | 0.60 | 0.40 | 0.07 | 92 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amorós-Pérez, A.; Cano-Casanova, L.; Ouzzine, M.; Rufete-Beneite, M.; Romero-Anaya, A.J.; Lillo-Ródenas, M.Á.; Linares-Solano, Á. Spherical Activated Carbons with High Mechanical Strength Directly Prepared from Selected Spherical Seeds. Materials 2018, 11, 770. https://doi.org/10.3390/ma11050770
Amorós-Pérez A, Cano-Casanova L, Ouzzine M, Rufete-Beneite M, Romero-Anaya AJ, Lillo-Ródenas MÁ, Linares-Solano Á. Spherical Activated Carbons with High Mechanical Strength Directly Prepared from Selected Spherical Seeds. Materials. 2018; 11(5):770. https://doi.org/10.3390/ma11050770
Chicago/Turabian StyleAmorós-Pérez, Ana, Laura Cano-Casanova, Mohammed Ouzzine, Mónica Rufete-Beneite, Aroldo José Romero-Anaya, María Ángeles Lillo-Ródenas, and Ángel Linares-Solano. 2018. "Spherical Activated Carbons with High Mechanical Strength Directly Prepared from Selected Spherical Seeds" Materials 11, no. 5: 770. https://doi.org/10.3390/ma11050770
APA StyleAmorós-Pérez, A., Cano-Casanova, L., Ouzzine, M., Rufete-Beneite, M., Romero-Anaya, A. J., Lillo-Ródenas, M. Á., & Linares-Solano, Á. (2018). Spherical Activated Carbons with High Mechanical Strength Directly Prepared from Selected Spherical Seeds. Materials, 11(5), 770. https://doi.org/10.3390/ma11050770






